Abstract
Inconsistent findings on the association between urine albumin‐to‐creatinine ratio (UACR) and risk of hypertension have been reported. This meta‐analysis sought to evaluate the association between the elevated level of UACR within the normal range and incident hypertension in the general population. We comprehensively searched PubMed and Embase databases until July 31, 2020. All longitudinal observational studies that assessed the association of elevated baseline level of UACR within the normal range with incident hypertension in the general population were included. The predictive value was estimated by pooling risk ratio (RR) with 95% confidence intervals (CI) for the highest versus the lowest category of UACR level. Nine articles (10 studies) involving 27 771 individuals were identified and analyzed. When compared with the lowest category of UACR, individuals with the highest UACR had a 1.75‐fold (RR 1.75; 95% CI 1.47–2.09; p < .001) higher risk of hypertension in a random effect model. Gender‐specific analysis indicated that the impact of UACR on the development of hypertension seemed to be stronger in women (RR 2.47; 95% CI 1.10–5.55; p = .029) than in men (RR 1.88; 95% CI 1.35–2.61; p < .001). An increased UACR within the normal range is independently associated with a higher risk of hypertension in the general population. Baseline UACR can be served as a predictor of incident hypertension in the general population.
Keywords: general population, hypertension, meta‐analysis, urine albumin‐to‐creatinine ratio
1. INTRODUCTION
Hypertension remains a worldwide public health burden. The global prevalence of hypertension is rising mainly due to the population aging and obesity. 1 Estimated number of hypertensive adults will reach 1.56 billion by 2025. 2 Hypertension is a well‐established risk factor for cardiovascular disease, renal disease, and premature death. In order to improve the secondary prevention and minimize its complications, early prediction of individuals at risk of hypertension is of great importance.
Albumin detected in the urine may represent a sign of kidney disease. Urine albumin‐to‐creatinine ratio (UACR) is most frequently applied to diagnose albuminuria. In spot urine specimens, normal level of UACR is below 30 mg/g. The normal UACR value is less than or equal to 17 mg/g in men but in women, the level is observed to be higher ranging around 25 mg/g. 3 Value of 30 to 300 mg/g in the spot urine is considered as presence of microalbuminuria. Microalbuminuria has been identified as an independent predictor of coronary artery disease, cardiovascular, and all‐cause death in the general population. 4 Several observational studies 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 have provided evidence of an association between a slight increase in UACR within the normal range and incident hypertension with conflicting results. Particularly, one study 12 reported that the association of UACR with incident hypertension was female‐specific, whereas other 5 did not find the significant association in women. These conflicting findings may be correlated with the impact of gender‐specific cutoff value of UACR. 13
To the best of our knowledge, no previous systematic review and meta‐analysis have assessed the association between elevated UACR within the normal range and development of hypertension. To address this knowledge gap, we perform this meta‐analysis to answer whether elevated level of albuminuria within the normal range can predict incident hypertension in the general population.
2. METHODS
2.1. Data sources and searches
We performed this meta‐analysis according to the guideline of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses Statement. 14 A comprehensive literature search was conducted on PubMed and Embase databases until July 31, 2020, using the following terms in combination: “albumin creatinine ratio” AND “hypertension” AND “follow‐up”. We also manually scanned the reference lists of included articles and pertinent reviews to identify additional studies.
2.2. Study selection
Two authors independently selected the eligible studies according to the following inclusion criteria: (a) longitudinal observational studies that enrolled the participants in the general population; (b) exposure: baseline UACR within the normal range (spot urine UACR from 0 to 30 mg/g); (c) comparison: the highest versus the lowest category of UACR level; (d) outcome measure: incident hypertension; and (e) provided multivariable‐adjusted risk summary of hypertension. Hypertension was defined as a systolic blood pressure of at least 140 mm Hg and/or diastolic blood pressure of at least 90 mm Hg, or use of antihypertensive drugs. Exclusion criteria were as follows: (a) participants with hypertension at baseline; (b) albuminuria as exposure; and (c) analysis of UACR level by continuous value.
2.3. Data extraction and quality assessment
The following relevant data were extracted by two independent authors: first author's last name, publication year, study design, source of population, participants, sample sizes, gender distribution, age at baseline, cutoff of UACR elevation, definition of hypertension, case of hypertension event, length of follow‐up, multivariable‐adjusted risk ratio (RR) or hazard ratio (HR) with their 95% confidence intervals (CI), and adjusted confounders. Methodological quality of included studies was assessed with a 9‐point Newcastle‐Ottawa Scale (NOS). 15 Those with a score of 7 points or more were deemed as high‐quality studies. Any disagreement was resolved by consulting with the third author.
2.4. Statistical analysis
The data were analyzed using Stata 12.0 (Stata Corporation). The pooling risk estimate of hypertension was calculated for the highest versus the lowest category of UACR. Heterogeneity between studies was explored using the I2 statistics (significance set at ≥50%) and Cochrane Q test (significance set at p < .10). A random effect model was selected when there was statistically significant heterogeneity; otherwise, we choose a fixed‐effect model. In case of random effect model analysis, we also estimated a 95% predictive interval using the “rfdist” option under the metan command. In order to observe the credibility of the pooled risk summary, we conducted a sensitivity analysis by removal of any single study each time. Subgroup analyses were conducted according to study design, number of participants, gender, menopausal status, duration of follow‐up, and whether adjusted estimated glomerular filtration rate (eGFR). Begg's test 16 and Egger's test 17 were used to examine the publication bias. A trim‐and‐fill analysis was conducted to investigate the impact of publication bias when the publication bias was observed.
3. RESULTS
3.1. Search results and studies' characteristics
Of 1052 potential records identified, 37 full‐text articles were retrieved for assessment of eligibility. Twenty‐eight articles were removed with various reasons (Figure 1). Forman and colleagues article 5 included first Nurses' Health Study (NHS I) and the second Nurses' Health Study (NHS II). Thus, nine articles (10 studies) 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 18 were finally included in this analysis.
FIGURE 1.
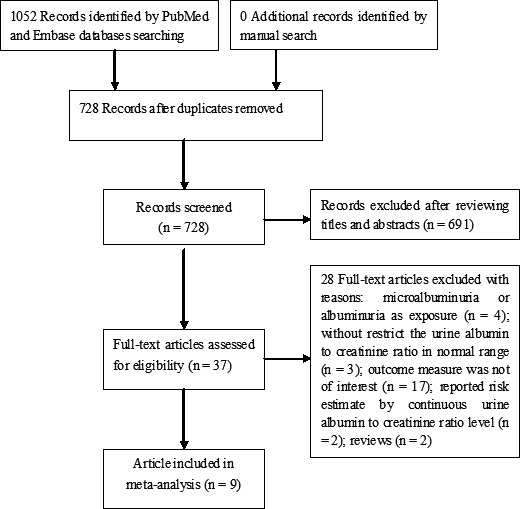
Flow chart of studies selection process
Table 1 describes the main characteristic of the included studies. These studies were published from 2008 to 2017 and performed in the United States, 10 , 18 Pakistan, 7 Japan, 6 , 9 , 18 and Korea. 8 , 11 , 12 Two articles 9 , 11 were retrospective designs and others were prospective studies. Sample sizes ranged from 412 to 9,102 individuals. The overall number was 27 771 participants. The follow‐up length ranged between 2.0 and 11 years. Eight studies 5 , 8 , 9 , 10 , 11 , 12 , 18 were classified as high‐quality studies according to the NOS criteria.
TABLE 1.
Main characteristic of the included studies
| First author/ Year | Country | Study design | Participants | Sample size (% males) | Median/mean age (years) b | UACR cutoff (mg/g) | HYP case | RR/HR (95% CI) | Follow‐up (years) | Adjustment for covariates | Total NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Forman. 2008 5 | USA | P | Postmenopausal women | 1065 (0) | 65 (60‐70) | Quartile 4 vs. 1; >6.5 vs.<1.0 | 271 | 5.4 (3.7–23.8) | 4.0 | Age, BMI, eGFR, baseline BP, physical activity, smoking, family history of hypertension | 7 |
| Forman. 2008 5 | USA | P | Premenopausal women | 1114 (0) | 44 (41‐47) | Quartile 4 vs. 1; >6.5 vs.<1.0 | 296 | 1.35 (0.97–1.91) | 8.0 | Age, BMI, eGFR, baseline BP, physical activity, smoking, family history of hypertension | 8 |
| Jessani 2012 7 | Pakistan | P | Normotensive non‐diabetic adults | 920 (52.4) | 48.8 ± 9.4 | Quartile 4 vs. 1; >7.0 vs.<2.0 | 105 | 2.45 (1.21–4.98) | 2.0 | Age, sex, census, cluster assignment to home health education | 6 |
| Park 2014 8 | Korea | P | Men on medical check‐up | 1284 (100) | 50.9 ± 10.5 | Quartile 4 vs. 1; ≥5.54 vs.<2.84 | 284 | 1.89 (1.31–2.71) | 3.2 | BMI, DM, SBP, TC, hsCRP, eGFR, recent smoking, alcohol intake, regular exercise. | 7 |
| Takase 2015 9 | Japan | R | Normotensive on medical check‐up | 6205 (61.8) | 53.4 ± 11.4. | Quartile 4 vs. 1; >7.04 vs.<1.82 | 1184 | 1.53 (1.30–1.80) | 3.0 | BMI, SBP, heart rate, eGFR, uric acid, fasting plasma glucose, LDL, TG, current smoking, family history of hypertension | 8 |
| Hirayama 2015 6 | Japan | P | Normotensive community‐based persons | 412 (39.3) | 56.9 ± 8.6 | Tertile 3 vs.1; >10 vs.<5 | 133 | 2.67 (1.36–5.38) | 6.7 | Age, sex, alcohol, smoking, BMI, 24‐h urinary excretion of sodium, HbA1c, BP categories at baseline | 6 |
| Huang 2015 10 | USA | P | Normotensive general population | 5215 (NP) | 62.6 ± 5.6 | Quintile 5 vs. 1; ≥9.14 vs.<1.36 | 2175 | 1.26 (1.08‐1.46) a | 9.8 | Age, sex, race, BMI, alcohol, smoking, DM, prevalent stroke, TC, education, physical activity, eGFR, SBP, DBP | 8 |
| Sung 2016 11 | Korea | R | Individuals on health screening | 9102 (53.3) | 42.7 ± 10.3 | Quartile 4 vs. 1; ≥7.4 vs.<3.4 | 963 | 1.97 (1.52–2.55) | 11 | Age, sex, center, screening examination, smoking, alcohol, regular exercise, education level, SBP, BMI, eGFR, LDL, HDL | 8 |
| Yadav 2016 12 | Korea | P | Normotensive general population | 1173 (37.7) | 53.5 ± 8.4 | Tertile 3 vs.1; ≥9.37 vs.< 5.38 (M); ≥11.6 vs.< 6.17 (W) | 123 |
1.83 (0.85–3.94) M 2.69 (1.27–5.73) W |
2.6 | Age, BMI, muscle mass, SBP, DM, smoking, alcohol consumption, regular exercise, fasting serum glucose, TG, HDL, hs‐CRP, eGFR | 8 |
| Munakata 2017 18 | Japan | P | Normotensive general population | 1281 (34.3) | 58 ± 12.3 | Quartile 4 vs. 1 | 315 | 1.67 (1.15–2.42) | 3.7 | Age, sex, BMI, SBP, DBP, TG, LDL, HbA1c, uric acid, eGFR, smoking, exercise habits, heavy drinking | 7 |
Abbreviations: BP, blood pressure; CAD, coronary artery disease; CI, confidence intervals; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; HYP, hypertension; LDL, low‐density lipoprotein; M, men; NOS, Newcastle‐Ottawa Scale; P, prospective; R, retrospective; RR, risk ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UACR, urine albumin‐to‐creatinine ratio; W, women.
Excluding those with ACR ≥300 mg/g.
Age was expressed by median (interquartile range) or mean (standard deviation).
3.2. Hypertension
All the included studies reported the association between UACR and incident hypertension. As shown in Figure 2, a random effect model was applied because there was significant heterogeneity (I2 = 61.0%; p = .004). Meta‐analysis showed that individuals with the highest UACR had a 75% higher risk of incident hypertension (RR 1.75; 95% CI 1.47–2.09) when compared with those with the lowest UACR. The predictive interval for the RR of hypertension was 1.06 to 2.89. Sensitivity analysis further confirmed the robustness of the original predictive value (data not shown). Table 2 summarizes the results of subgroup analysis. Both the Begg's test (p = .062) and the Egger's test (p = .004) suggested the presence of publication bias. However, the “trim‐and‐fill” analysis indicates that the pooling risk estimate of hypertension remained statistically significant (RR 1.42; 95% CI 1.03–1.90) when imputed these 6 missing studies (Figure 3).
FIGURE 2.
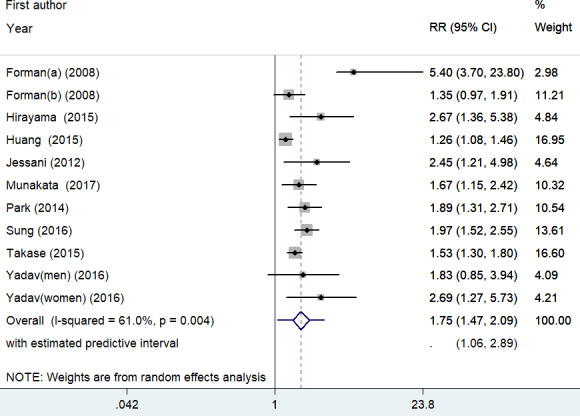
Forest plots showing pooled RR with 95% CI of incident hypertension for the highest versus the lowest category of UACR. Circle and squares represent the estimated effect size and percentage of weight of individual studies, respectively. Diamonds represent the pooled effect sizes from all the studies
TABLE 2.
Results of subgroup analysis
| Subgroup | Number of studies | Pooled RR | 95% CI | Heterogeneity between studies |
|---|---|---|---|---|
| Study design | ||||
| Prospective | 8 | 1.85 | 1.44–2.37 | p = .005; I2 = 63.2% |
| Retrospective | 2 | 1.70 | 1.33–2.17 | p = .105; I2 = 61.9% |
| Region | ||||
| Asia | 7 | 1.75 | 1.55–1.97 | p = .407; I2 = 3.0% |
| USA | 3 | 1.64 | 1.03–2.59 | p = .010; I2 = 78.92% |
| Sample sizes | ||||
| ≥1000 | 8 | 1.68 | 1.40–2.00 | p = .006; I2 = 62.7% |
| <1000 | 2 | 2.56 | 1.56–4.19 | p = .864; I2 = 0.0% |
| Follow‐up duration | ||||
| ≥5 years | 4 | 1.60 | 1.19–2.15 | p = .008; I2 = 74.7% |
| <5 years | 6 | 1.90 | 1.51–2.39 | p = .111; I2 = 42.0% |
| Adjusted eGFR | ||||
| Yes | 8 | 1.68 | 1.40–2.00 | p = .006; I2 = 62.7% |
| No | 2 | 2.56 | 1.56–4.19 | p = .864; I2 = 0.0% |
| Menopausal status | ||||
| Postmenopausal | 2 | 4.62 | 2.38–8.96 | p = .639; I2 = 0.0% |
| Premenopausal | 2 | 1.51 | 0.76–3.01 | p = .274; I2 = 16.4% |
Abbreviations: CI, confidence intervals; eGFR, estimated glomerular filtration rate; RR, risk ratio.
FIGURE 3.
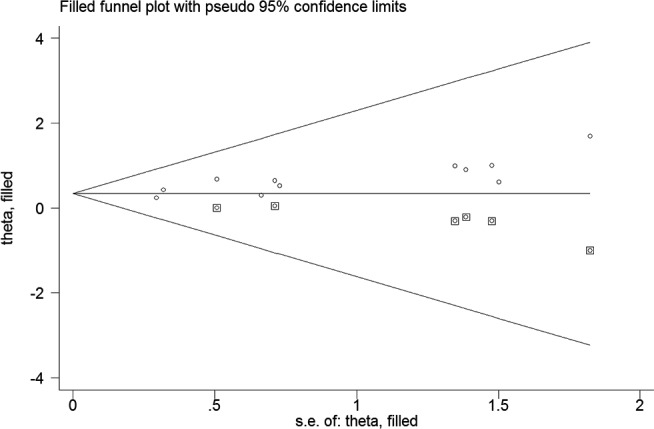
Funnel plot showing the association of urine albumin‐to‐creatinine ratio (UACR) with incident hypertension. The circles alone are real studies and the circles enclosed in boxes are “filled” studies
3.3. Gender‐stratified analysis
One study 12 reported the risk estimates by gender, one study 8 recruited men only, and one article 5 only enrolled women. As shown in Figure 4, when the highest UACR were compared with the lowest UACR, the pooled RR of hypertension was 2.47 (95% CI: 1.10–5.55; I2 = 78.1%, p = .010) for women and 1.88 (95% CI: 1.35–2.61; I2 = 0.0%, p = .941) for men in a random effect model.
FIGURE 4.
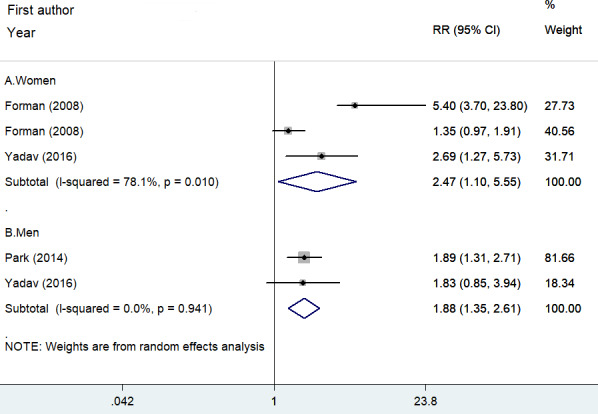
Forest plots showing pooled RR with 95% CI of incident hypertension by gender. Circle and squares represent the estimated effect size and percentage of weight of individual studies, respectively. Diamonds represent the pooled effect sizes from all the studies
4. DISCUSSION
This is the first meta‐analysis to examine the association between UACR level at baseline and development of hypertension. The main finding of this meta‐analysis is that elevated UACR even within the normal range is independently associated with an increased risk of hypertension in the general population. The pooled multivariable‐adjusted risk estimate of the association of UACR with incident hypertension showed that individuals with the highest UACR level had a 75% higher risk of future hypertension compared to those with the lowest UACR. This finding reveals that baseline UACR can be served as a predictor of incident hypertension in the general population.
Recently, albuminuria has been proposed as a cause of hypertension. 19 However, there are different degrees of albuminuria according to the amounts of protein in the urine. Clinicians usually examine the ratio of albumin to creatinine for the detection of albuminuria. Lower amounts of albumin constitute a condition known as microalbuminuria. Our meta‐analysis specially focused on the predictive role of elevated UACR within the normal range in the general population. Apart from the categorical analysis of UACR, per 10‐fold increase of baseline UAER level was also significantly associated with higher risk of incident hypertension (RR 2.29; 95%CI 1.77‐ 2.95) in the general population. 20 Also, normal‐range UACR level in healthy individuals increased over time was associated with an increased risk of hypertension. 21 Taken together, these findings support that elevated UACR conferred an increased risk of incident hypertension.
There may be a gender‐specific association of elevated UACR within the normal range with incident hypertension. 12 Our subgroup analysis showed that elevated UACR level was associated with an increased risk of hypertension in both men and women. However, this conclusion was established in a small number of studies. Future well‐designed studies are required to further confirm this finding. Women had higher normal UACR level than men. 12 , 22 Gender‐specific reference value of UACR may be contributed to the different predictive role. Menopausal status may affect the association between UACR level and risk of hypertension. Stratified analysis suggested that postmenopausal women but not premenopausal women exhibit a significant association of elevated UACR with incident hypertension. This finding may be explained by lack of protection by estrogen in postmenopausal women.
The association between UACR and incident hypertension may be affected by renal function. Subgroup analysis indicated that the predictive value of elevated UACR was slightly attenuated after adjusting for the eGFR. However, this association remained statistically significant after adjustment of eGFR. These findings suggest that high normal UACR is still a predictor of incident hypertension even in the absence of kidney disease.
The mechanisms underlying the association of UACR with incident hypertension are largely unknown. Increased urinary albumin excretion can reflect glomerular and systemic vascular endothelial dysfunction. 23 On the other hand, low‐grade inflammation is determinants of the development of elevated UAER. 24 , 25 Glomerular hyperfiltration induced by inflammation may be linked between the albumin excretion and hypertension.
Nevertheless, this meta‐analysis has several limitations. First, specimen for analyzing UACR was collected from a single spot urine sample rather than a 24‐h collection, which may increase the possibility of misclassification of participants. Second, cutoff values of elevated UACR were not uniform in the analyzed studies. Notably, majority of included studies did not use the gender‐specific UACR 26 to assess the association between UACR and incident hypertension which may have affected their predictive values. Third, significant heterogeneity was observed in pooling the overall predictive role of UACR. Different cutoffs of UACR elevation, duration of follow‐up, gender distribution, and degrees of the adjusted model may be partly responsible for the significant heterogeneity. Finally, the pooling risk summary may be slightly overestimated due to the impact of publication bias.
5. CONCLUSIONS
This meta‐analysis indicates that even within the normal range of urinary albumin excretion, elevated UACR at baseline is associated with an increased risk of hypertension in the general population. For individuals with high UACR, closer monitoring of blood pressure is highly recommended. However, further studies are warranted to investigate the gender‐specific predictive role of UACR.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
YL Teng contributed to study and guaranteed the integrity of study. F Ren and MZ Li searched the literature, extracted data, and assessed the study quality. H Xu and XW Qin performed the statistical analysis. F Ren wrote the manuscript, and MZ Li revised the manuscript. All the authors approved the final version of manuscript to be published.
ACKNOWLEDGEMENTS
None.
Ren F, Li M, Xu H, Qin X, Teng Y. Urine albumin‐to‐creatinine ratio within the normal range and risk of hypertension in the general population: A meta‐analysis. J Clin Hypertens. 2021;23:1284–1290. 10.1111/jch.14263
Fei Ren and Mingzhu Li: Contribute equally to this work.
REFERENCES
- 1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 3. Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13(4):1034‐1039. [DOI] [PubMed] [Google Scholar]
- 4. Xia F, Liu G, Shi Y, Zhang Y. Impact of microalbuminuria on incident coronary heart disease, cardiovascular and all‐cause mortality: a meta‐analysis of prospective studies. Int J Clin Exp Med. 2015;8(1):1‐9. [PMC free article] [PubMed] [Google Scholar]
- 5. Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19(10):1983‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirayama A, Konta T, Hozawa A, et al. Slight increase in urinary albumin excretion within the normal range predicts incident hypertension in a community‐based Japanese population: the Takahata study. Hypertens Res. 2015;38(1):56‐60. [DOI] [PubMed] [Google Scholar]
- 7. Jessani S, Levey AS, Chaturvedi N, Jafar TH. High normal levels of albuminuria and risk of hypertension in Indo‐Asian population. Nephrol Dial Transplant. 2012;27(Suppl 3):iii58‐iii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SK, Moon SY, Oh CM, Ryoo JH, Park MS. High normal urine albumin‐to‐creatinine ratio predicts development of hypertension in Korean men. Circ J. 2014;78(3):656‐661. [DOI] [PubMed] [Google Scholar]
- 9. Takase H, Sugiura T, Ohte N, Dohi Y. Urinary albumin as a marker of future blood pressure and hypertension in the general population. Medicine (Baltimore). 2015;94(6):e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang M, Matsushita K, Sang Y, Ballew SH, Astor BC, Coresh J. Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2015;65(1):58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sung KC, Ryu S, Lee JY, et al. Urine albumin/creatinine ratio below 30 mg/g is a predictor of incident hypertension and cardiovascular mortality. J Am Heart Assoc. 2016;5(9):e00325. 10.1161/JAHA.116.003245e003245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yadav D, Kang DR, Koh SB, Kim JY, Ahn SV. Association between urine albumin‐to‐creatinine ratio within the normal range and incident hypertension in men and women. Yonsei Med J. 2016;57(6):1454‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connell SJ, Hollis S, Tieszen KL, McMurray JR, Dornan TL. Gender and the clinical usefulness of the albumin: creatinine ratio. Diabet Med. 1994;11(1):32‐36. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. [DOI] [PubMed] [Google Scholar]
- 15. Wells G, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 28, 2020.
- 16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munakata M, Hattori T, Konno S. Relationship between subtle urinary albumin excretion and risk of incident hypertension: modification by glomerular filtration rate. Hypertens Res. 2017;40(12):994‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gansevoort RT, Snieder H. Albuminuria as a cause of hypertension. Nat Rev Nephrol. 2019;15(1):6‐8. [DOI] [PubMed] [Google Scholar]
- 20. Brantsma AH, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J Am Soc Nephrol. 2006;17(2):331‐335. [DOI] [PubMed] [Google Scholar]
- 21. Grupper A, Schwartz D, Berliner S, et al. Normal‐range albuminuria in healthy subjects increases over time in association with hypertension and metabolic outcomes. J Am Soc Hypertens. 2018;12(11):759‐767. [DOI] [PubMed] [Google Scholar]
- 22. Xu R, Zhang L, Zhang P, et al. Gender‐specific reference value of urine albumin‐creatinine ratio in healthy Chinese adults: results of the Beijing CKD survey. Clin Chim Acta. 2008;398(1–2):125‐129. [DOI] [PubMed] [Google Scholar]
- 23. Ritz E. Albuminuria and vascular damage–the vicious twins. N Engl J Med. 2003;348(23):2349‐2352. [DOI] [PubMed] [Google Scholar]
- 24. Jager A, van Hinsbergh VW, Kostense PJ, et al. C‐reactive protein and soluble vascular cell adhesion molecule‐1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol. 2002;22(4):593‐598. [DOI] [PubMed] [Google Scholar]
- 25. Zambrano‐Galvan G, Rodriguez‐Moran M, Simental‐Mendia LE, Lazalde B, Reyes‐Romero MA, Guerrero‐Romero F. C‐reactive protein is directly associated with urinary albumin‐to‐creatinine ratio. Arch Med Res. 2011;42(6):451‐456. [DOI] [PubMed] [Google Scholar]
- 26. Arnlov J, Evans JC, Meigs JB, et al. Low‐grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969‐975. [DOI] [PubMed] [Google Scholar]


