Abstract
Hypertensive disorders of pregnancy (HDP) are one of the leading causes of maternal and fetal morbidity and mortality. We aimed to estimate the prevalence of each HDP in France and to study their associations. All pregnant women who delivered in France between 2010 and 2018 were included in a cohort and followed during their pregnancy and 6 weeks of postpartum. Each HDP occurring during the follow‐up was identified. Prevalence of each HDP and cumulative incidence by gestational age were estimated. Incidence rate ratio (IRR) and 95% confidence interval (CI) for preeclampsia among women with preexisting or gestational hypertension (GH) were estimated using Poisson regression and adjusted for age were estimated. Between 2010 and 2018, 6 302 810 deliveries were included. HDP complicated 7.4% of pregnancies. Preeclampsia and GH complicated 2.0% and 4.2% of pregnancies, respectively. Most of preeclampsia cases occurred without a prior HDP. HELLP syndrome represented 10.4% of preeclampsia cases. Compared to nulliparous pregnancies without HDP prior preeclampsia, the age‐adjusted IRR of preeclampsia was 6.2 [95% CI: 6.1‐6.4] in nulliparous pregnancies with preexisting hypertension and 2.9 [95% CI: 2.8‐3.0] in nulliparous pregnancies with GH. In France, HDP occurred in 7.4% of all pregnancies. Women with preexisting chronic hypertension are at high risk to present preeclampsia during pregnancy. Preeclampsia complicated 2.0% of pregnancies in France. Tailoring management of women according to the HDP is a major challenge to avoid complications related to these disorders.
Keywords: France, gestational hypertension, HELLP syndrome, preeclampsia, prevalence
Short abstract
HDP complicated 7.4% of pregnancies. Preeclampsia and GH complicated 2.0% and 4.2% of pregnancies, respectively. HELLP syndrome represented 10.4% of preeclampsia cases. Compared to nulliparous pregnancies without HDP prior preeclampsia, the incidence rate ratio of preeclampsia was 6.2 [95% CI: 6.1‐6.4] in nulliparous pregnancies with preexisting hypertension and 2.9 [95% CI: 2.8‐3.0] in nulliparous pregnancies with GH.
1. INTRODUCTION
Hypertensive disorders of pregnancy (HDP), including chronic hypertension (persistent or not during pregnancy), gestational hypertension, and preeclampsia/eclampsia, represent a major cause of maternal and fetal morbidity and mortality. 1 In addition to the potentially serious complications during pregnancy and postpartum, hypertensive disorders are associated with an increased risk of cardiovascular and renal diseases long after delivery. 2 , 3 HDP include different entities of variable severity with their classification varying across different countries. Several studies have estimated that HDP could affect between 5% and 10% of pregnancies, with an incidence of preeclampsia ranging from 1% to 7% depending on the countries and characteristics of the women selected and the parity. 1 , 4 , 5 A higher risk of HDP has been described in primiparous women as compared with multiparous women. 4 , 6 However, because of the low frequency of some of these disorders, especially HELLP syndrome and eclampsia, accurate estimates of the incidence are difficult to obtain despite the large number of studies. In industrialized countries, due to the increasing age at first pregnancy as well as the increasing prevalence of obesity—two major risk factors for HDP—several countries have highlighted the unfavorable trends of these HDP in recent periods. 7 In France, no large‐scale studies have been conducted to estimate the incidence of HDP. Most of the studies, conducted on a limited number and selected population of women (low risk, nulliparous, etc), have estimated the incidence of preeclampsia to be between 0.5% and 3%. 8 , 9 , 10 , 11 In France, the development and improvement of a national health insurance database covering almost the entire French population offers a unique opportunity to provide the first national accurate estimate of the incidence of all subtype of HDP.
2. MATERIALS AND METHODS
2.1. Data source
The CONCEPTION (Cohort of Cardiovascular diseases in Pregnancy) study is a prospective cohort study conducted using the French National Health Insurance Information System (Système national des données de santé, SNDS). 12 This database contains the health care expenditure reimbursed by the national health insurance. For the present study, two databases of the SNDS were used: the national hospital discharge database, which contains informations about all hospital stays diagnoses coded using ICD‐10 codes in public and private hospitals; and the national health insurance database, which records reimbursements for health care expenditure such as drugs and outpatient medical care prescribed or performed by health care professionals.
2.2. Study population
The population of the present study was composed of general health scheme beneficiaries, including local mutualist sections representing almost 90% of the French population. All deliveries, occurring in this population, between January 1, 2010, and December 31, 2018, were identified using hospital stay information mentioning a delivery procedure with a gestational age over 22 weeks of gestation. Women not living in France, those who delivered anonymously, those for whom we were unable to match data from the two SNDS databases and those not affiliated to the general or local mutual scheme were excluded from the study population because of possible missing data in their medical history. All women were followed up during their pregnancy and 6 weeks of postpartum.
2.3. Outcomes
HDP were identified in the database using different algorithms, based on the delivered antihypertensive medication delivered and/or hospital diagnosis as follows. Pregnancy start date was computed as the date of delivery minus the gestational age at delivery.
Preexisting chronic hypertension was considered if women have had at least three dispensations of antihypertensive medication at three different dates in the year preceding the pregnancy (or on two dates, if at least one large package (for 3 months) of antihypertensive drugs was dispensed), or if they were hospitalized with a primary diagnosis mentioning a preexisting hypertension (ICD‐10 codes: O10, O11) during pregnancy or postpartum. Preexisting chronic hypertension before pregnancy was considered persistent if women have had at least one delivery of antihypertensive medication during pregnancy or were hospitalized with a primary diagnosis of hypertension during pregnancy (ICD‐10 codes: O10, O11, O13, O16, I10). Nonpersistent preexisting chronic hypertension could be due to the lowering level of blood pressure during pregnancy.
In women without preexisting chronic hypertension, gestational hypertension was identified in our database by a hospitalization with a primary diagnosis of gestational hypertension during pregnancy (ICD‐10 code: O13) or at least one delivery of antihypertensive medication between 20 weeks of gestation and 6 weeks of postpartum. 13 In France, a large proportion of women (70.2% in our study) who took antihypertensive treatment received a calcium channel blocker that could be given both as tocolytic or antihypertensive drugs (mainly Nicardipine and Nifedipine) (supplemental files). Since antihypertensive drugs can also be given for an indication of preterm labor, we excluded from the gestational hypertension group all women who had both delivery of antihypertensive medication and hospitalization with a primary diagnosis of preterm labor (ICD‐10 codes: O47, O60.0‐O60.2, O60.9).
Preeclampsia, HELLP syndrome, and eclampsia were identified by a hospitalization with a diagnosis of preeclampsia (O14), HELLP syndrome (O14.2), or eclampsia (O15). In our study, severity of preeclampsia was considered if it was reported in the hospital diagnosis (O14.1: severe preeclampsia) or in cases of HELLP syndrome or eclampsia. All preeclampsia without severity criteria were considered moderate. Early‐onset preeclampsia was considered if the diagnosis was made before 34 weeks of gestation.
2.4. Individual covariates
For each delivery included in the study, information relating to women and pregnancies was recorded. Multiple pregnancy, delivery mode (vaginal or cesarean section), fetal death, and obesity were identified from the hospital discharge summaries of the delivery stay. Parity was coded in the delivery hospital stay for women who delivered vaginally exclusively. For women who had a cesarean section, their childbirth history since 2006 was searched to identify parity. Smoking was identified by specific coding at hospital or by the reimbursement of nicotine replacement treatments before or during pregnancy. Pregnancy or postpartum hemorrhages were identified during pregnancy and postpartum hospital stays or during pregnancy and postpartum or during the delivery stay. Personal history of cardiovascular disease was identified in all hospital discharge summaries of each women between 2006 and their pregnancy with the following ICD‐10 codes: I20.0 and I21‐I23 for acute coronary syndrome, I60‐I64 for stroke, I50 for heart failure, and I26 for pulmonary embolism. We used an algorithm to identify maternal preexisting diabetes using the delivery of three antidiabetic medication on three different dates in the year preceding pregnancy (or on two dates, if at least one large package of antidiabetic drugs was dispensed). Gestational diabetes was identified using an algorithm based on dispensation of insulin and glucose strips or the diagnosis of diabetes during the pregnancy without a history of diabetes before pregnancy. 14
2.5. Statistical analysis
The prevalence of each HDP between 2010 and 2018 was estimated by dividing the number of women with the disorder by the number of women who delivered during the same period.
Cumulative incidence of gestational hypertension and preeclampsia by gestational age was estimated by dividing the cumulative number of women developing one of these two HDP during the pregnancy period, by the number of women still pregnant during this period.
To allow comparison of the prevalence of HDP between nulliparous and multiparous pregnancies, rates were age standardized using the direct method by applying the 5‐year age prevalence rates to the age structure of all the women who delivered between 2010 and 2018.
Incidence rate ratio (IRR) and 95% confidence interval (CI) for preeclampsia among women with preexisting chronic hypertension or gestational hypertension were estimated using Poisson regression and adjusted for age.
Statistical analyses were performed with SAS software (version 7.1, SAS Institute).
2.6. Ethics approval
In line with French governmental regulations and the National Ethics Committee, no patient consent was required. Santé publique France, as the national public health agency had full and permanent access to the SNDS database (Delibération no 2016‐316 of October 13th 2016).
2.7. Patient involvement
We acknowledge the importance of public involvement. Nevertheless, no patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
3. RESULTS
Between January 1, 2010, and December 31, 2018, 7 022 789 deliveries were identified in the hospital discharge database. After excluding women who did not meet the inclusion criteria, 6 302 810 deliveries, corresponding to 4 459 322 women, were included in our study (Figure 1). Almost 45% of the pregnancies included were nulliparous (N = 2 833 376).
FIGURE 1.
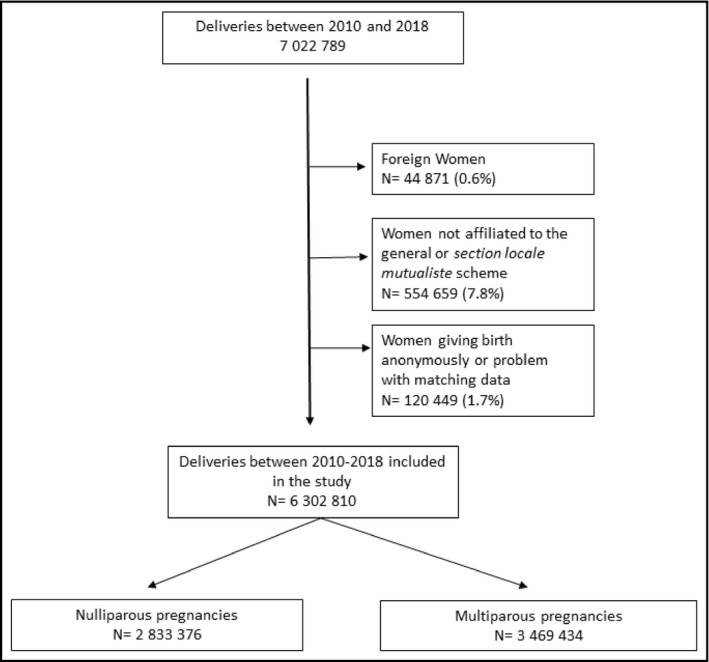
Flowchart of the study population
Characteristics of women and pregnancies by HDP were provided in Table 1. Women with HDP were significantly older, irrespective of parity and HDP (p < .0001). Maternal low‐socioeconomic status, obesity, diabetes, gestational diabetes, and personal history of cardiovascular diseases were more frequently observed in pregnancies with HDP (p < .0001). Tobacco use was less frequently reported in pregnancies with severe preeclampsia (7.8%) as compared with pregnancies without HDP (8.9%) or pregnancies with preexisting chronic hypertension (11.3%) (p < .0001). The prevalence of cardiovascular and inflammatory diseases, stroke, and pulmonary embolism was higher in pregnancies with HDP. The proportions of cesarean sections, multiple pregnancies, and in utero death were particularly high in nulliparous and multiparous pregnancies with early preeclampsia as compared with pregnancies without HDP (respectively, 88.5% vs. 25.0% for cesarean section, 11.3% vs. 1.9% for multiple pregnancies, and 0.2% vs. 0.04% for in utero death [p < .0001]).
TABLE 1.
Characteristics of women included in the study by parity and hypertensive disorders
| Nulliparous pregnancies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Preexisting hypertension | Gestational hypertension | Preeclampsia | Severe preeclampsia | Early onset preeclampsia | |||||||
| N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | |
| Age (years) | 2 594 032 | 28.2 | 43 122 | 31.5 | 130 151 | 29.0 | 83 045 | 29.5 | 34 479 | 29.7 | 16 547 | 30.1 |
| Gestational age (weeks of amenorrhea) | 2 594 032 | 39.0 | 43 122 | 37.8 | 130 151 | 38.6 | 83 045 | 36.2 | 34 479 | 34.3 | 16 547 | 31.2 |
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Women with complementary universal health insurance a | 355 480 | 13.7 | 6280 | 14.6 | 17 933 | 13.8 | 13 563 | 16.3 | 6087 | 17.7 | 3231 | 19.5 |
| Obesity | 94 511 | 3.6 | 6288 | 14.6 | 9809 | 7.5 | 9613 | 11.6 | 3801 | 11.0 | 2528 | 15.3 |
| Tobacco | 230 609 | 8.9 | 4881 | 11.3 | 11 621 | 8.9 | 7135 | 8.6 | 2693 | 7.8 | 1647 | 10.0 |
| Multiple pregnancy | 49 235 | 1.9 | 1438 | 3.3 | 4336 | 3.3 | 6957 | 8.4 | 3056 | 8.9 | 1869 | 11.3 |
| Cesarean section | 647 565 | 25.0 | 21 249 | 49.3 | 49 122 | 37.7 | 53 560 | 64.5 | 27 981 | 81.2 | 14 649 | 88.5 |
| In utero death | 1049 | 0.04 | 38 | 0.1 | 78 | 0.1 | 99 | 0.1 | 69 | 0.2 | 40 | 0.2 |
| Gestational diabetes | 207 489 | 8.0 | 7240 | 16.8 | 14 862 | 11.4 | 10 632 | 12.8 | 3945 | 11.4 | 2056 | 12.4 |
| Pregnancy hemorrhage | 21 879 | 0.8 | 482 | 1.1 | 1536 | 1.2 | 924 | 1.1 | 432 | 1.3 | 276 | 1.7 |
| Postpartum hemorrhage | 124 353 | 4.8 | 2612 | 6.1 | 6858 | 5.3 | 7407 | 9.0 | 3483 | 10.1 | 1182 | 7.1 |
| Postpartum infection | 138 585 | 5.3 | 2625 | 6.1 | 7550 | 5.8 | 4942 | 6.0 | 1977 | 5.7 | 950 | 5.7 |
| Diabetes | 10 558 | 0.4 | 1779 | 4.1 | 1283 | 1.0 | 1642 | 2.0 | 646 | 1.9 | 414 | 2.5 |
| Personal history of CVD | 646 | 0.02 | 508 | 1.2 | 77 | 0.1 | 94 | 0.1 | 40 | 0.1 | 35 | 0.2 |
| Personal history of stroke | 1698 | 0.1 | 223 | 0.5 | 109 | 0.1 | 108 | 0.1 | 47 | 0.1 | 33 | 0.2 |
| Personal history of pulmonary embolism | 1498 | 0.1 | 73 | 0.2 | 73 | 0.1 | 98 | 0.1 | 64 | 0.2 | 39 | 0.2 |
| Cardiac malformations | 2272 | 0.1 | 330 | 0.8 | 166 | 0.1 | 120 | 0.1 | 53 | 0.2 | 23 | 0.1 |
| Personal history of inflammatory disease | 21 684 | 0.8 | 1 070 | 2.5 | 1250 | 1.0 | 1 025 | 1.2 | 431 | 1.3 | 259 | 1.6 |
| Personal history of HIV | 2737 | 0.1 | 166 | 0.4 | 152 | 0.1 | 203 | 0.2 | 87 | 0.3 | 58 | 0.4 |
| Multiparous pregnancies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Preexisting hypertension | Gestational hypertension | Preeclampsia | Severe preeclampsia | Early onset preeclampsia | |||||||
| N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | |
| Age (years) | 3 243 821 | 31.2 | 61 231 | 33.7 | 134 617 | 31.9 | 42 258 | 32.4 | 14 661 | 32.4 | 7543 | 32.5 |
| Gestational age (weeks of amenorrhea) | 3 243 821 | 39.0 | 61 231 | 38.1 | 134 617 | 38.7 | 42 258 | 36.4 | 14 661 | 34.5 | 7543 | 31.8 |
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Women with complementary universal health insurance a | 660 997 | 20.4 | 15 775 | 25.8 | 29 620 | 22.0 | 11 429 | 27.1 | 4 278 | 29.2 | 2433 | 32.3 |
| Obesity | 154 738 | 4.8 | 9979 | 16.3 | 10 943 | 8.1 | 6944 | 16.4 | 2264 | 15.4 | 1627 | 21.6 |
| Tobacco | 318 287 | 9.8 | 6586 | 10.8 | 13 619 | 10.1 | 3755 | 8.9 | 1151 | 7.9 | 752 | 10.0 |
| Multiple pregnancy | 51 694 | 1.6 | 1344 | 2.2 | 3258 | 2.4 | 2723 | 6.4 | 978 | 6.7 | 604 | 8.0 |
| Cesarean section | 451 056 | 13.9 | 17 087 | 27.9 | 25 701 | 19.1 | 19 795 | 46.8 | 9 718 | 66.3 | 5 670 | 75.2 |
| In utero death | 1233 | 0.04 | 80 | 0.1 | 100 | 0.1 | 70 | 0.2 | 41 | 0.3 | 24 | 0.3 |
| Gestational diabetes | 329 696 | 10.2 | 12 658 | 20.7 | 18 295 | 13.6 | 7837 | 18.6 | 2 391 | 16.3 | 1 438 | 19.1 |
| Pregnancy hemorrhage | 22 280 | 0.7 | 563 | 0.9 | 1293 | 1.0 | 430 | 1.0 | 146 | 1.0 | 119 | 1.6 |
| Postpartum hemorrhage | 252 715 | 7.8 | 5960 | 9.7 | 10 792 | 8.0 | 5602 | 13.3 | 2216 | 15.1 | 878 | 11.6 |
| Postpartum infection | 168 826 | 5.2 | 3611 | 5.9 | 7730 | 5.7 | 2653 | 6.3 | 869 | 5.9 | 432 | 5.7 |
| Diabetes | 12 577 | 0.4 | 2299 | 3.8 | 1078 | 0.8 | 1037 | 2.5 | 345 | 2.4 | 270 | 3.6 |
| Personal history of CVD | 1359 | 0.04 | 768 | 1.3 | 122 | 0.1 | 117 | 0.3 | 51 | 0.4 | 38 | 0.5 |
| Personal history of stroke | 2444 | 0.1 | 294 | 0.5 | 139 | 0.1 | 89 | 0.2 | 36 | 0.3 | 27 | 0.4 |
| Personal history of pulmonary embolism | 2742 | 0.1 | 156 | 0.3 | 143 | 0.1 | 92 | 0.2 | 29 | 0.2 | 28 | 0.4 |
| Cardiac malformations | 3242 | 0.1 | 377 | 0.6 | 155 | 0.1 | 60 | 0.1 | 21 | 0.1 | 11 | 0.2 |
| Personal history of inflammatory disease | 28 452 | 0.9 | 1 166 | 1.9 | 1 318 | 1.0 | 612 | 1.5 | 258 | 1.8 | 190 | 2.5 |
| Personal history of HIV | 5205 | 0.2 | 349 | 0.6 | 284 | 0.2 | 198 | 0.5 | 73 | 0.5 | 50 | 0.7 |
Abbreviations: CVD: cardiovascular diseases including acute coronary syndrome and heart failure; HIV: human immunodeficiency virus.
Providing free access to health care for people with an annual income less than 60% of the poverty threshold (CMUc).
3.1. Prevalence of HD
At least one HDP occurred in 7.4% of pregnancies (8.4% of nulliparous and 6.5% of multiparous pregnancies) (Table 2). The prevalence of chronic hypertension was 1.5% and 1.8% in nulliparous and multiparous pregnancies, respectively (p < .0001). After adjustment for age by standardization, the prevalence of preexisting chronic hypertension was higher in nulliparous than in multiparous pregnancies (1.8% vs. 1.6% respectively) (p < .0001). Treatment of preexisting chronic hypertension persisted throughout pregnancy in 59.0% of cases regardless of parity. Gestational hypertension occurred in 4.6% and 3.9% of nulliparous pregnancies and multiparous pregnancies, respectively. Preeclampsia was 2.5 times more frequent in nulliparous than in multiparous pregnancies (2.9% vs. 1.2%, p < .0001). Early‐onset preeclampsia represented 20% of all preeclampsia cases in nulliparous pregnancies. In 40% of cases, preeclampsia was severe (similar rate in nulliparous and multiparous pregnancies). HELLP syndrome represented 10.0% of preeclampsia cases with a prevalence of 0.2%. Finally, eclampsia occurred in 0.1% of nulliparous and multiparous pregnancies.
TABLE 2.
Crude and age‐standardized prevalence of hypertensive disorders during pregnancy and 6 weeks of postpartum by parity, France, 2010‐2018
| Hypertensive disorders |
Nulliparous pregnancies N = 2 833 376 |
Multiparous pregnancies N = 3 469 434 |
Total Pregnancies N = 6 302 810 |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Crude prevalence | Standardized prevalence | N | Crude prevalence | Standardized prevalence | N | Crude prevalence | |
| None | 2 594 032 | 91.6 | 91.1 | 3 243 821 | 93.5 | 93.8 | 5 837 853 | 92.6 |
| Preexisting hypertension | 43 122 | 1.5 | 1.8 | 61 231 | 1.8 | 1.6 | 104 353 | 1.7 |
| Persistent preexisting hypertension | 25 777 | 0.9 | 1.1 | 38 967 | 1.1 | 1.0 | 64 744 | 1.0 |
| Gestational hypertension a | 130 151 | 4.6 | 4.8 | 134 617 | 3.9 | 3.8 | 264 768 | 4.2 |
| Preeclampsia | 83 045 | 2.9 | 3.1 | 42 258 | 1.2 | 1.2 | 125 303 | 2.0 |
| Early onset preeclampsia | 16 547 | 0.6 | 0.6 | 7543 | 0.2 | 0.2 | 24 090 | 0.4 |
| Severe preeclampsia | 34 479 | 1.2 | 1.3 | 14 661 | 0.4 | 0.4 | 49 140 | 0.8 |
| HELLP Syndrome | 9160 | 0.3 | 0.4 | 3413 | 0.1 | 0.1 | 12 573 | 0.2 |
| Eclampsia | 3354 | 0.1 | 0.1 | 1455 | 0.04 | 0.04 | 4809 | 0.1 |
Excluding gestational hypertension complicated by preeclampsia which are included in the preeclampsia group.
Among nulliparous preeclamptic pregnancies, 8.9% had preeclampsia superimposed on preexisting chronic hypertension and 11.6% had gestational hypertension before preeclampsia (Table 3). Indeed, in a large majority of cases, the preeclampsia was the first HDP occurring, with no evidence of previous HDP earlier in the pregnancy. In multiparous pregnancies, the proportion of inaugural preeclampsia was lower than in nulliparous (70.4%), because of a higher of superimposed preeclampsia on preexisting hypertension (17.2%) than in nulliparous women (8.9%) (p < .0001).
TABLE 3.
Hypertensive disorders preceding preeclampsia in nulliparous and multiparous pregnancies
| Nulliparous | Multiparous | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N preeclampsia = 83 045 | N preeclampsia = 42 258 | |||||||||||
| Total preeclampsia | Early onset preeclampsia | Severe preeclampsia | Total preeclampsia | Early onset preeclampsia | Severe preeclampsia | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| None | 66 071 | 79.5 | 12 077 | 73.0 | 27 378 | 79.4 | 29 765 | 70.4 | 4509 | 59.8 | 10 197 | 69.6 |
| Preexisting hypertension | 7361 | 8.9 | 2439 | 14.7 | 3210 | 9.3 | 7277 | 17.2 | 2081 | 27.6 | 2654 | 18.1 |
| Persistent preexisting hypertension | 5394 | 6.5 | 1868 | 11.3 | 2440 | 7.1 | 5791 | 13.7 | 1688 | 22.4 | 2196 | 15.0 |
| Gestational hypertension | 9613 | 11.6 | 2031 | 12.3 | 3891 | 3,0 | 5216 | 12.3 | 953 | 12.6 | 1810 | 12.3 |
| Total | 83 045 | 100.0 | 16 547 | 100.0 | 34 479 | 100.0 | 42 258 | 100.0 | 7543 | 100.0 | 14 661 | 100.0 |
In nulliparous pregnancies, the age‐adjusted risk (aIRR) of preeclampsia was more than sixfold higher for pregnancies with preexisting hypertension compared to those without HDP (Table 4). The IRR of preeclampsia was higher when preexisting hypertension persisted during pregnancy (aIRR = 7.5 [95% CI 7.3‐7.7]) as compared with pregnancies without HDP (p < .0001). The age‐adjusted risk of preeclampsia reached 2.9 (95% CI 2.8‐3.0) in pregnancies with gestational hypertension.
TABLE 4.
Age‐adjusted Incidence rate ratio (IRR) and 95% confidence interval of preeclampsia in nulliparous and multiparous pregnancies among women with preexisting hypertension or gestational hypertension
| N preeclampsia/ N total | Preeclampsia (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nulliparous | N preeclampsia/ N total | Multiparous | ||||||||||
| % | Crude IRR | 95% CI | Adj IRR | 95% CI | % | Crude IRR | 95% CI | Adj IRR | 95% CI | |||
| None | 66 071/2 594 032 | 2.5 | ref | ref | 22 488/3 243 821 | 0.9 | ref | ref | ||||
| Preexisting hypertension | 7361/43 122 | 17.1 | 6.9 | 6.7‐7.0 | 6.2 | 6.1‐6.4 | 7277/61 231 | 11.9 | 13.1 | 12.7‐13.4 | 12.2 | 11.9‐12.5 |
| Persistent preexisting hypertension | 5394/25 777 | 20.9 | 8.4 | 8.2‐8.7 | 7.5 | 7.3‐7.7 | 5791/38 967 | 14.9 | 16.3 | 15.9‐16.8 | 15.1 | 14.6‐15.5 |
| Gestational hypertension | 9613/130 151 | 7.4 | 3.0 | 2.9‐3.0 | 2.9 | 2.8‐3.0 | 5216/134 617 | 3.9 | 4.3 | 4.1‐4.4 | 4.2 | 4.0‐4.3 |
3.2. Incidence of HDP according to gestational and maternal age
The cumulative incidence of preeclampsia increased throughout pregnancy from 0.44% of pregnancies in the 25th week of gestation to 3.8% of pregnancies in the 41th week of gestation (Figure 2). Preeclampsia diagnosed in the postpartum was rare (<0.5% of all preeclampsia). The incidence of gestational hypertension increased earlier in pregnancy with 50% of cases of gestational hypertension occurring before the 35th week of gestation (vs. the 37th week for preeclampsia).
FIGURE 2.
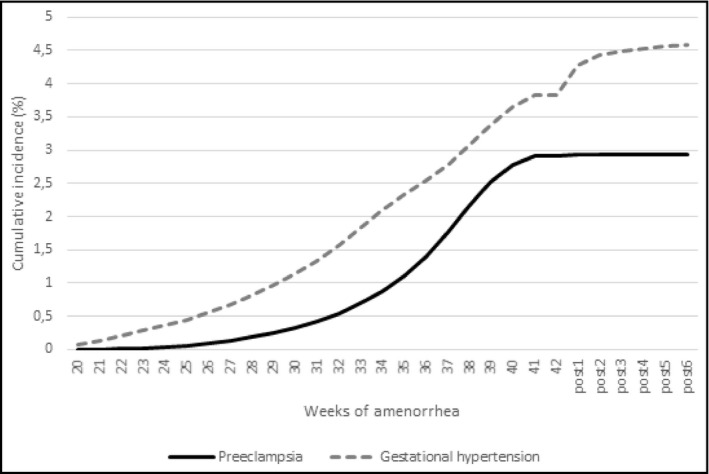
Cumulative incidence of preeclampsia and gestational hypertension by gestational age in nulliparous women
The incidence of gestational hypertension and preeclampsia increased slightly with maternal age before 27 years old and almost exponentially afterward. Thus, between 27 and 40 years of age, the incidence doubled for preeclampsia and multiplied by 1.7 for gestational hypertension (Figure 3).
FIGURE 3.
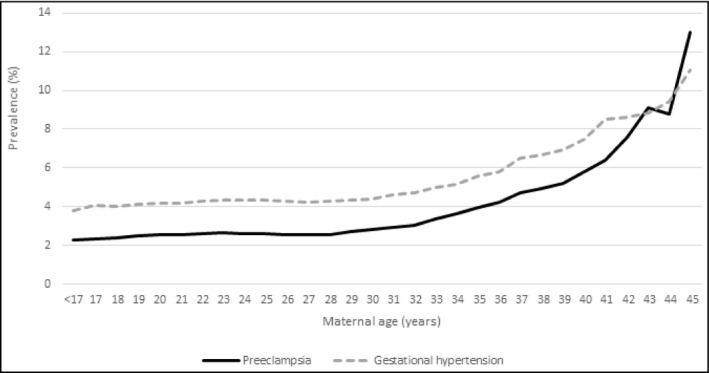
Prevalence of hypertensive disorders by maternal age in nulliparous women
3.3. Antihypertensive medication delivered during pregnancy and postpartum
Antihypertensive medications delivered to women with persistent preexisting chronic hypertension or gestational hypertension are described in Table S1. In women with gestational hypertension, the class of calcium channel blockers (ATC classification: C08) was the most frequently delivered followed by central‐acting antihypertensives (C02) and beta‐blockers (C07). By contrast, in women with persistent preexisting hypertension, beta‐blockers (C07) were most frequently delivered.
4. DISCUSSION
4.1. Main finding
Our nationwide study allowed us to evaluate more than 6 million pregnancies in France over a period of 9 years and provided recent results on the prevalence of each HDP. We showed that HDP were common in France, occurring in almost 8% of all pregnancies. Preeclampsia complicated 2.9% of nulliparous and 1.2% of multiparous pregnancies. Women with preexisting chronic hypertension were at high risk developing preeclampsia. Most of the cases of preeclampsia occurred without a prior HDP. All HDP studied here were positively associated with maternal age.
4.2. Interpretation
The prevalence of preeclampsia, described in our study, was in accordance with that reported in the French National Perinatal survey, conducted in 2016 on 13 400 live births and in industrialized countries in other studies. 4 , 6 , 11 The higher risk of HDP in nulliparous pregnancies reported in our study has been well described, with the risk of gestational hypertension and preeclampsia being respectively 1.5 and 1.3‐ to 4.75 times higher. 4 In addition, our study provided, for the first time in France, an estimation of HELLP syndrome, eclampsia, and gestational hypertension prevalence in a large study of women.
Few studies to date have analyzed all HDP in both nulliparous and multiparous pregnancies. Our data enabled us to study, with high statistical power, the risk of preeclampsia for all types of HDP. Preexisting chronic hypertension increased significantly the risk of preeclampsia (2.7‐fold in nulliparous pregnancies), the number of cases of preeclampsia preceded by gestational hypertension was higher than the one preceded by preexisting hypertension. Furthermore, most of preeclampsia occurred without a prior HDP both in nulliparous and multiparous pregnancies.
In our study, we found that the risk of preeclampsia increased significantly with maternal age, especially from 27 to 30 years, but we did not highlight an increased risk in the younger age groups. While the higher risk of HDP with increased maternal age has been well described, the relation between young maternal age and HDP is still being debated with discordant results. In a large database gathering data from 29 countries, age under 17 years old was not associated with a higher risk of preeclampsia. 7 , 15
Our results showed that gestational hypertension and preeclampsia increased with gestational age as previously described. 16 However, we observed a significant increase in the incidence of hypertension in the early postpartum. This increase could be partly due to untreated gestational or preexisting hypertension, not identified by our algorithm based on treatment delivery.
Our study also described the risk of preeclampsia according to the presence or absence of a preexisting HDP. This analysis, taking into account all hypertensive disorders, allowed a stratification of preeclampsia risk. Our results showed the need for close monitoring and management of women with preexisting or gestational hypertension, given their particularly high‐relative risk of preeclampsia. In women with inaugural preeclampsia, further studies are needed to understand if the absence of preexisting HDP was due to the lack of monitoring of these women with undiagnosed preexisting hypertension or gestational hypertension or if preeclampsia occurred suddenly, without warning hypertensive signs.
Concerning the classes of drugs prescribed to hypertensive women during pregnancy, although a majority of delivered drugs correspond to classes recommended during pregnancy, several hundred prescriptions of non‐indicated or even contraindicated agents, particularly agents acting on the renin‐angiotensin‐aldosterone system, raised questions taking into account the risk of congenital malformation in children with regard to the use of this therapeutic class during pregnancy. 17
4.3. Strengths and limitations
This first French nationwide study, using medico‐administrative database, estimating all HDP prevalence during pregnancy, including HELLP syndrome and eclampsia, have several strengths. The completeness of the discharge data used in our study to identify pregnancies was evaluated to be high (99.6% of live births in France). 18 Consequently, the number of pregnancies included, more than 6 million, allowed an accurate estimation. All dispensations of antihypertensive drugs were also registered in an exhaustive manner, for each women, in our database. 19 In addition, the positive predictive value (PPV) and sensibility of the preeclampsia and HELLP syndrome diagnoses in the hospital discharges summaries were evaluated in a validation study and found to be high (PPV = 100% and sensibility = 81.3%). 20 This same validation study showed that the gestational hypertension's hospital diagnosis was valid (VPP = 100%) although the burden of gestational hypertension was underestimated (sensibility = 63%). To avoid this underestimation of gestational hypertension, we added antihypertensive medication dispensations to our algorithm to identify cases in our study. However, finally, for eclampsia, a previous study showed the possible over reporting of this disorder in the hospital discharge diagnosis with cases of severe preeclampsia or HELLP syndrome being coded as eclampsia. 21 Regarding preexisting hypertension, we cannot exclude that its prevalence reported in our study could have been overestimated by our algorithm. Indeed, women receiving antihypertensive medication, especially beta‐blockers, for an indication other than hypertension (chronic stress, migraine, etc) in the year preceding pregnancy could have been wrongly considered to have hypertension. However, it was not possible to exclude these women from preexisting hypertension group with certainty. Finally, women receiving antihypertensive medication for threatened premature delivery without hospitalization have been considered as gestational hypertension and could participate to an overestimation of this disorder.
5. CONCLUSIONS
HDP were common in France, occurring in 7.4% of all pregnancies. Preeclampsia complicated 2.0% of pregnancies. Women with preexisting hypertension are at high risk to develop preeclampsia during pregnancy. The identification of different HDP and their association is still a major issue for risk stratification in pregnant women in order to reduce the risk of complications related to these HDP by tailoring monitoring and management of these women.
CONFLICT OF INTEREST
VO, AG, CG, GPB, CDT, EM, VT, and NR have nothing to disclose. SK reports, outside the submitted work, non‐financial support from Lilly France, Novonordisk, Novartis pharma, Roche diabetes care, Lifescan, Abbott france, Sanofi, ViiV healthcare, Servier, Becton Dickinson and personal fees from icomed, Pascaleo, BT3SI, M3global research. CMV reports, outside the submitted work, personal fees and non‐financial support from Servier, Lundbeck, Astrazeneca, MSD, Boehringer. JB reports, outside the submitted work, personal fees from Abbott, Bayer, Bottu, Egis, Ferring, Steripharma, Kantar, Teriak personal fees and non‐financial support from Pfizer, Quantum Genomics, personal fees from Sanofi, Servier.
AUTHOR CONTRIBUTIONS
All authors met the ICMJE criteria for authorship:
-
‐
VO and JB were at the origin of the conception and design of the work.
-
‐
EM, CG, and AG performed the acquisition and analysis of the data.
-
‐
All authors made substantial contributions to the interpretation of data.
-
‐
JB and VO drafted the manuscript and all authors revised it critically for important intellectual content.
-
‐
All authors approved the final version of the manuscript
JB and VO (both guarantors) accept full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. JB and VO attest that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
The corresponding author (VO) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
Table S1
ACKNOWLEDGMENT
We thank the Fédération Française de Cardiologie (FFC), the Société Française d'Hypertension Artérielle (SFHTA) and the Fondation de Recherche sur l'hypertension artérielle (FRHTA) and their donators for their financial support.
Olié V, Moutengou E, Grave C, et al. Prevalence of hypertensive disorders during pregnancy in France (2010‐2018): The Nationwide CONCEPTION Study. J Clin Hypertens. 2021;23:1344–1353. 10.1111/jch.14254
Funding information
Fédération Française de Cardiologie (French Cardiology Federation), Société Française d’Hypertension Artérielle (SFHTA) and the Fondation de Recherche sur l'hypertension artérielle (FRHTA). The French Cardiology Federation funded this study through a call for scientific projects “thematic grant 2019: cardiovascular diseases in women”. Funders neither participated in the drafting of the study protocol, nor in the collection, exploitation and interpretation of the data.
REFERENCES
- 1. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391‐403. [DOI] [PubMed] [Google Scholar]
- 2. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70(4):798‐803. [DOI] [PubMed] [Google Scholar]
- 4. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213‐220. [DOI] [PubMed] [Google Scholar]
- 5. Panaitescu AM, Ciobanu AM, Popescu MR, et al. Incidence of hypertensive disorders of pregnancy in Romania. Hypertens Pregnancy. 2020;39(4):423‐428. [DOI] [PubMed] [Google Scholar]
- 6. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 7. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987‐2004. Am J Hypertens. 2008;21(5):521‐526. [DOI] [PubMed] [Google Scholar]
- 8. Goffinet F, Aboulker D, Paris‐Llado J, et al. Screening with a uterine Doppler in low risk pregnant women followed by low dose aspirin in women with abnormal results: a multicenter randomised controlled trial. BJOG. 2001;108(5):510‐518. [DOI] [PubMed] [Google Scholar]
- 9. Subtil D, Goeusse P, Puech F, et al. Aspirin (100 mg) used for prevention of pre‐eclampsia in nulliparous women: the Essai Regional Aspirine Mere‐Enfant study (Part 1). BJOG. 2003;110(5):475‐484. [DOI] [PubMed] [Google Scholar]
- 10. Haelterman E, Breart G, Paris‐Llado J, Dramaix M, Tchobroutsky C. Effect of uncomplicated chronic hypertension on the risk of small‐for‐gestational age birth. Am J Epidemiol. 1997;145(8):689‐695. [DOI] [PubMed] [Google Scholar]
- 11. Blondel B, Coulm B, Bonnet C, Goffinet F, Le Ray C, National Coordination Group of the National Perinatal S . Trends in perinatal health in metropolitan France from 1995 to 2016: results from the French National Perinatal Surveys. J Gynecol Obstet Hum Reprod. 2017;46(10):701‐713. [DOI] [PubMed] [Google Scholar]
- 12. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58(4):286‐290. [DOI] [PubMed] [Google Scholar]
- 13. WHO Technical Consultation on Postpartum and Postnatal Care . WHO Guidelines Approved by the Guidelines Review Committee. Geneva; 2010. [PubMed] [Google Scholar]
- 14. Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mostello D, Kallogjeri D, Tungsiripat R, Leet T. Recurrence of preeclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol. 2008;199(1):55.e1‐55.e7. [DOI] [PubMed] [Google Scholar]
- 16. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early‐ versus late‐onset disease. Am J Obstet Gynecol. 2013;209(6):544.e1‐544.e12. [DOI] [PubMed] [Google Scholar]
- 17. Cooper WO, Hernandez‐Diaz S, Arbogast PG, et al. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443‐2451. [DOI] [PubMed] [Google Scholar]
- 18. Quantin C, Cottenet J, Vuagnat A, et al. Quality of perinatal statistics from hospital discharge data: comparison with civil registration and the 2010 National Perinatal Survey. J Gynecol Obstet Biol Reprod (Paris). 2014;43(9):680‐690. [DOI] [PubMed] [Google Scholar]
- 19. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the systeme national d'information interregimes de l'Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149‐S167. [DOI] [PubMed] [Google Scholar]
- 20. Pierron A, Revert M, Goueslard K, et al. Evaluation of the metrological quality of the medico‐administrative data for perinatal indicators: a pilot study in 3 university hospitals. Rev Epidemiol Sante Publique. 2015;63(4):237‐246. [DOI] [PubMed] [Google Scholar]
- 21. Chantry AA, Deneux‐Tharaux C, Cans C, Ego A, Quantin C, Bouvier‐Colle MH. Hospital discharge data can be used for monitoring procedures and intensive care related to severe maternal morbidity. J Clin Epidemiol. 2011;64(9):1014‐1022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


