Abstract
The aim of the present study was to explore the relationship between androgen and LVH in postmenopausal hypertensive women. Enrolled in this study were 378 postmenopausal hypertensive women who were admitted to the department of cardiology between December 2018 and December 2020. According to left ventricular mass index (LVMI) evaluated by echocardiography, the patients were divided into LVH group (n = 172) and non‐LVH group (n = 206). Their clinical characteristics were collected. Based on the result of propensity score matching analysis, 160 cases in each group were matched successfully. After correcting for confounding factors by various models, the results showed that free androgen index (FAI) and sex hormone–binding globulin (SHBG) were the influencing factors of LVH in postmenopausal women with hypertension. Patients with elevated SHBG were 5% less likely to develop LVH than those without elevated SHBG (OR: 0.950, 95% CI 0.922‐1.578). Postmenopausal hypertensive patients with elevated FAI were 16% more likely to have LVH than those without elevated FAI (OR: 1.608, 95% CI 0.807‐3.202). Multiple linear regression showed that LVMI increased by 61.82g/m2 for every 1 unit increase in FAI. In addition, SHBG decreased by 1 nmol/l, and LVMI increased by 0.177g/m2. Subgroup analysis showed that patients in the controlled BP group had a lower risk of LVH for every additional unit of SHBG compared with the uncontrolled BP group. The risk of LVH for each additional unit of FAI in the uncontrolled BP group was higher than that in the controlled BP group. The results of this present study showed that the occurrence of LVH was positively correlated with FAI and negatively correlated with SHBG in postmenopausal women with hypertension. The increase in FAI level and the decrease in SHBG level may be related to the occurrence and development of LVH in postmenopausal hypertension.
Keywords: postmenopausal hypertension, left ventricular hypertrophy, free androgen index, sex hormone–binding globulin
The prevalence of left ventricular hypertrophy increases dramatically in women after menopause, leading to an increased risk of death from cardiovascular events. Androgen was found to be associated with blood pressure regulation in animal models. However, there has been no clinical study to address the association between androgen and LVH in postmenopausal women.
1. INTRODUCTION
Left ventricular hypertrophy (LVH) is one of the important signs of early target organ damage in patients with hypertension. 1 Studies have shown that the incidence of hypertension with LVH is 20%‐40%. 2 The mortality rate of hypertensive patients with LVH is 2‐5 times that of patients without LVH. 3 In fact, sex affects the formation and development of LVH. The incidence of LVH in postmenopausal women is much higher than that of men in the same age group. 4
The prevalence of hypertension and related target organ damage in postmenopausal women increase significantly, which is believed to be related to the change in the sex hormone axis in women after menopause. 5 , 6 Direct and indirect clinical evidence suggest that androgen plays a key role in the risk of cardiovascular disease in both men and women. 7 , 8 Although there is limited knowledge about the role of testosterone elevation in postmenopausal women with hypertension, women are extremely sensitive to testosterone alteration in their bodies. 9
However, most current studies have focused on the relationship between serum total testosterone (TT) and blood pressure (BP) regulation in male patients. 10 , 11 Studies on the role of bioavailable testosterone (BioT) in postmenopausal hypertensive women with LVH have rarely been reported. By analyzing the relationship between androgen and LVH in postmenopausal hypertensive women, this study aims to provide a new predictor and theoretical basis for target organ damage in postmenopausal hypertensive patients.
2. MATERIALS AND METHODS
2.1. Patients and study design
The study recruited postmenopausal female hypertensive patients from December 2018 to December 2020 at the Lanzhou University Second Hospital (Lanzhou, China). This study was reviewed and approved by the Ethics Review Committee of Lanzhou University Second Hospital (2020A‐283). Patients and their family members were informed of the study content and signed informed consent.
Menopause was defined as the permanent cessation of menstruation with serum follicle‐stimulating hormone (FSH) level greater than 40 U/L and estradiol level lower than 30pg/ml. The diagnosis of hypertension was based on the criteria provided by the 2018 ESC/ESH Guidelines for the management of arterial hypertension, with office systolic blood pressure (SBP)≥140 and (or) diastolic blood pressure (DBP)≥90mmhg. 12 Postmenopausal hypertension refers to high BP occurring one year after female physiological menopause.
The inclusion criteria of this study were patients (1) who met the diagnostic criteria of hypertension in postmenopausal women; (2) with complete clinical data; and (3) who agreed to sign informed consent form and cooperate to complete data collection. The exclusion criteria were patients (1) with premenopausal definitive diagnosis of hypertension or receiving antihypertensive treatment; (2) with secondary hypertension; (3) with refractory hypertension; (4) who underwent hysterectomy or bilateral ovariectomy; (5) who received any form of hormone replacement therapy; (6) with diabetes mellitus, malignant tumor, severe neurological or psychological disease; and (7) with serious primary heart, kidney, and hematopoietic diseases.
Age, height, weight, waist circumference, hip circumference, menopause onset, and educational level were recorded routinely. The formula was used to calculate the body mass index (BMI) and waist‐hip ratio (WHR) (BMI = height/weight2 and WHR=waist circumference/ hip circumference). Fasting cubital fossa vein blood was extracted on the morning of the second day of admission. The serum was separated and stored at −80℃ for unified detection. The sex hormone levels were determined by chemiluminescence. The internal coefficient of variation (CV) of this method was 3.8%‐5.4%, and the inter‐batch CV was 4.3%‐6.4%. Mammalian target of rapamycin (mTOR), phosphorylated ribosome S6 protein kinase (PS6K), and androgen receptor (AR) was detected by enzyme‐linked immunosorbent assay (ELISA) (mTOR: Abcam ab45996 Shanghai, China; PS6K: Abcam ab9366 Shanghai, China; AR: JL12564 Shanghai, China). Free testosterone (FT) and BioT levels were calculated from the known TT, sex hormone–binding globulin (SHBG), and albumin (ALB) values, using a simple formula and a calculator widely used on the Internet (http://www.issam.ch/freetesto.htm). 13 The free androgen index (FAI) was calculated by the ratio of TT levels to SHBG.
A non‐invasive portable BP detector was used to measure and record the 24‐h BP. There were no restrictions on patients’ daily activities. According to the nocturnal BP decline rate, the patient's BP was further divided into three patterns: normal BP dipping (average asleep BP dropped by 10%‐20% against awake BP), non‐dipping (mean asleep BP declined by less than 10% against awake BP), and reverse dipping (average asleep BP was higher than awake BP). 14 Systolic or diastolic pressure load was defined as the percentage of the number of times the BP level reaches or exceeds normal BP in the same time period. Arterial stiffness index was defined as the ratio of the mean value to standard deviation of the decrease in BP per hour. The CV of systolic or diastolic BP was obtained from the ratio of standard deviation to mean systolic or diastolic blood pressure at the same time period.
The Philips IE33 ultrasound system was used to measure the cardiac structure and function. The measurements were based on the guidelines from the American Society of Echocardiography and the European Society of Cardiovascular Imaging. The mean value of three cardiac cycles was used as the final measurement for all the indexes. 15 Left ventricular mass index (LVMI) was calculated by LVMI=0.8 × 1.04× [(left ventricular end‐diastolic diameter (LVDd) + interventricular septal thickness +posterior wall thickness)3‐LVDd3] + 0.6/body surface area. 16 According to the definition of LVH in the 2018 ESC/ESH Guidelines for the management of arterial hypertension, postmenopausal hypertensive patients were classified as a LVH group (LVMI>95 g/m2) and a non‐LVH group (LVMI≤95 g/m2). 12 The indicators and the results were measured and recorded by professional technicians.
2.2. Statistical analysis
SPSS20.0 and R software were used for statistical processing. Normally distributed data were expressed as the mean±standard deviation (SD). The independent samples t test was used for inter‐group comparison. Non‐normally distributed data were represented by median and interquartile range (P25, P75). The Mann‐Whitney U test was used for comparison between groups. Categorical variables were expressed in frequency (percentage) and were compared using the chi‐square or Fisher's exact tests. The risk factors of LVH in postmenopausal hypertension patients were screened by multivariable logistics regression analysis. Multivariable linear regression was used to investigate the relationship between androgen and LVH. The standard regression coefficient represented the degree of impact of independent variables on dependent variables, and the regression coefficient represented the parameters of impact size of independent variables on dependent variables. The Matchit package of R software V3.4.4 was conducted to achieve the propensity score matching (PSM) analysis. P < .05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
A total of 378 postmenopausal women with hypertension were enrolled in this study, including 172 LVH patients and 206 non‐LVH patients. The two groups of patients were matched 1:1 by PSM method. The matching conditions included age and BMI. The matching tolerance was set to 0.1. The result showed that a total of 160 pairs were successfully matched. The patient screening and research process were shown in Figure 1.
FIGURE 1.
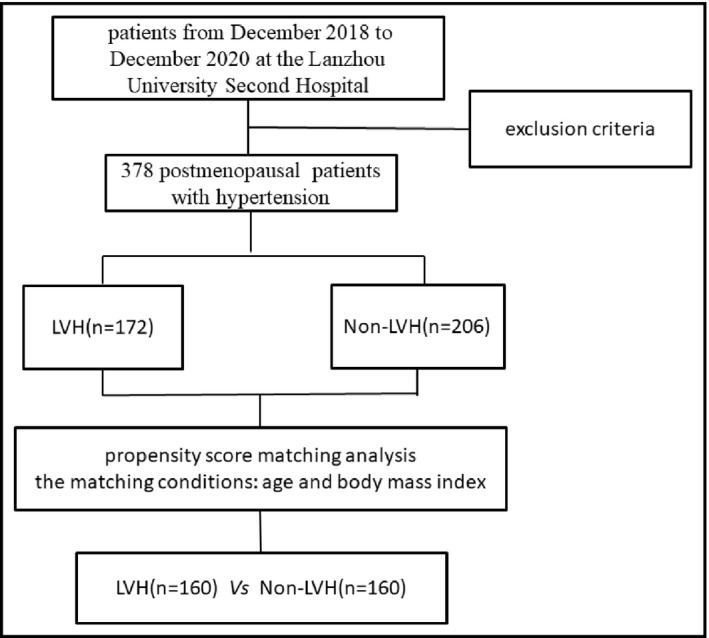
The patient screening and research process
3.2. Clinical characteristics
The utilization rate of angiotensin‐converting enzyme inhibitors and angiotensin receptor blocker of antihypertensive drugs in non‐LVH group was significantly higher than that in LVH group (Table 1). The serological indexes FT, BioT, FAI, SHBG, total cholesterol, mTOR, and PS6K were significantly different between the two groups (Table 2). 24h mean SBP, DBP, load SBP, and load DBP in LVH group were greater than those in non‐LVH group. 24h CV of SBP and DBP in LVH group were lower than those in non‐LVH group (Table 3).
TABLE 1.
Characteristics of the study population
| LVH (N = 160) | non‐LVH (N = 160) | P value | |
|---|---|---|---|
| Demographic characteristic | |||
| Age | 54.14 ± 3.35 | 54.21 ± 3.86 | .891 |
| BMI (kg/m2) | 23.55 ± 2.50 | 23.65 ± 2.60 | .790 |
| WHR | 0.83 ± 0.06 | 0.83 ± 0.66 | .712 |
| Smoking, n (%) | 2 (1%) | 0 (0%) | .316 |
| Alcohol drinking, n (%) | 28 (18%) | 23 (14%) | .329 |
| The course of hypertension | 5.02 ± 2.48 | 5.34 ± 2.53 | .368 |
| Menopausal interval | |||
| ≥5 years, n (%) | 110 (69%) | 93 (58%) | .106 |
| <5 years, n (%) | 50 (31%) | 67 (42%) | |
| Educational level | |||
| ≥college degree, n (%) | 85 (53%) | 100 (63%) | .152 |
| <college degree, n (%) | 75 (47%) | 60 (38%) | |
| Family history of hypertension, n (%) | 58 (36%) | 35 (22%) | .029 |
| Current antihypertensive drugs | |||
| ACEI, n (%) | 21 (13%) | 38 (24%) | .045 |
| ARB, n (%) | 61 (38%) | 85 (53%) | .033 |
| Beta‐blockers, n (%) | 10 (6%) | 16 (10%) | .297 |
| CCB, n (%) | 107 (67%) | 110 (69%) | .762 |
| Diuretic, n (%) | 43 (27%) | 35 (22%) | .411 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; DIU, diuretic; LVH, left ventricular hypertrophy; WHR, waist‐hip ratio.
TABLE 2.
Comparison of serological indexes in patients
| LVH (N = 100) | Non‐LVH (100) | P value | |
|---|---|---|---|
| RBC (10^12/L) | 4.55 ± 0.39 | 4.59 ± 0.29 | .293 |
| Hb (g/L) | 132.91 ± 15.22 | 136.56 ± 12.64 | .067 |
| HCT (L/L) | 41.77 ± 4.21 | 42.69 ± 3.16 | .082 |
| BUN (mmol/L) | 4.60 ± 1.19 | 4.81 ± 1.30 | .244 |
| CR (μmol/L) | 60.81 ± 9.63 | 59.96 ± 10.34 | .548 |
| TC (mmol/L) | 4.79 ± 0.80 | 4.50 ± 0.97 | .022 |
| TG (mmol/L) | 1.61 ± 0.99 | 2.31 ± 6.57 | .297 |
| LDL‐C (mmol/L) | 2.50 ± 0.61 | 2.64 ± 0.70 | .124 |
| mTOR (ng/mL) | 73.88 ± 16.71 | 68.44 ± 17.20 | .024 |
| PS6K (ng/mL) | 13.37 ± 2.33 | 12.57 ± 2.65 | .024 |
| AR (pg/mL) | 1532.03 ± 268.00 | 1581.09 ± 263.65 | .194 |
| PRL (ng/mL) | 8.01 ± 6.06 | 7.88 ± 3.99 | .863 |
| FSH (mIU/mL) | 61.53 ± 10.89 | 55.18 ± 10.87 | .147 |
| LH (mIU/mL) | 34.43 ± 54.44 | 27.02 ± 33.23 | .247 |
| E2 (pg/mL) | 29.02 ± 0.34 | 29.10 ± 0.05 | .840 |
| PRGE (ng/mL) | 0.22 ± 0.17 | 0.39 ± 0.99 | .099 |
| T (ng/dL) | 21.72 ± 6.19 | 21.02 ± 6.26 | .427 |
| FT (ng/dL) | 3.02 ± 0.76 | 2.57 ± 0.61 | .006 |
| BioT (ng/dL) | 70.52 ± 18.04 | 65.66 ± 14.74 | .038 |
| SHBG (nmol/L) | 115.60 ± 21.64 | 122.66 ± 20.57 | .019 |
| FAI | 0.19 ± 0.06 | 0.17 ± 0.05 | .020 |
Abbreviations: AR, androgen receptor; Bio T, bioavailable testosterone; BUN, blood urea nitrogen; CR, creatinine; E, estradiol; FSH, follicle‐stimulating hormone; FT, free testosterone; FAI, free androgen index; Hb, hemoglobin; HCT, hematocrit; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; LH, luteinizing hormone; mTOR, mammalian target of rapamycin; PRL, prolactin; PRGE, progesterone; PS6K, phosphorylated ribosome S6 protein kinase; RBC, red blood cell; SHBG, sex hormone–binding globulin; T, testosterone; TC, total cholesterol; TG, triglyceride.
TABLE 3.
Comparison of 24‐hour ambulatory blood pressure in patients
| LVH (N = 160) | Non‐LVH (N = 160) | P value | |
|---|---|---|---|
| 24h‐mean SBP (mmHg) | 142.24 ± 11.30 | 138.76 ± 11.15 | .030 |
| 24h‐mean DBP (mmHg) | 86.55 ± 9.20 | 82.90 ± 9.42 | .006 |
| 24 h load of SBP (%) | 72.98 ± 20.04 | 67.09 ± 21.11 | .045 |
| 24 h load of DBP (%) | 61.82 ± 25.42 | 54.47 ± 25.10 | .041 |
| 24 h CV of SBP (%) | 11.63 ± 2.87 | 12.93 ± 3.30 | .003 |
| 24 h CV of DBP (%) | 12.96 ± 3.05 | 14.10 ± 3.33 | .012 |
| The nocturnal BP decline rate—SBP (n, %) | .038 | ||
| Dipper pattern | 35 (22%) | 86 (54%) | |
| Non‐dipper pattern | 51 (32%) | 29 (18%) | |
| Reverse‐dipper pattern | 74 (46%) | 45 (28%) | |
| The nocturnal BP decline rate—DBP (n, %) | .041 | ||
| Dipper pattern | 32 (20%) | 50 (31%) | |
| Non‐dipper pattern | 51 (32%) | 29 (18%) | |
| Reverse‐dipper pattern | 77 (48%) | 81 (51%) | |
| Arterial stiffness index | 0.59 ± 0.13 | 0.56 ± 0.13 | .041 |
Abbreviations: CV, coefficient of variation; LVH, left ventricular hypertrophy; DBP, diastolic blood pressure; SBP, systolic blood pressure.
3.3. Multivariable analysis of LVH in postmenopausal hypertensive patients
After correcting for confounding factors by various models, multivariable logistic regression analysis showed that FAI and SHBG were the influencing factors of LVH in postmenopausal women with hypertension. Patients with one‐unit increase in serum SHBG levels were 5.2% less likely to develop LVH than those without elevated SHBG (OR:0.950, 95% CI 0.922‐1.578). Postmenopausal hypertensive patients with one‐unit increase in FAI level were 108.2% more likely to have LVH than those without elevated FAI (OR:2.082, 95% CI 1.067‐4.064) (Table 4). Multiple linear regression was used to further explore the correlation between FAI, SHBG, and LVMI. After adjusting for confounding factors, it was found that LVMI increased by 61.82g/m2 for every 1 unit increase in FAI. In addition, SHBG decreased by 1 nmol/l, and LVMI increased by 0.177g/m2 (Table 5, Figure 2).
TABLE 4.
Multivariable analysis of postmenopausal hypertensive women with LVH
| Parameter | Model1 | Model2 | Model3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | P | OR (95% CI) | β | P | OR (95% CI) | β | P | OR (95% CI) | |
| FT | 7.577 | .569 | 1.952 (0.000, 4.014) | 9.694 | .732 | 2.247 (0.000, 9.504) | 9.556 | .717 | 1.936 (0.000, 3.226) |
| BioT | −0.187 | .740 | 0.829 (0.273, 2.514) | −0.280 | .817 | 0.744 (0.081, 6.821) | −0.280 | .803 | 0.826 (0.274, 2.484) |
| FAI | 1.537 | .000 | 4.654 (3.098, 6.975) | 1.101 | .000 | 3.006 (1.930, 4.681) | 0.733 | .001 | 2.082 (1.067, 4.064) |
| SHBG | −0.059 | .000 | 0.943 (0.917, 0.970) | −0.055 | .000 | 0.950 (0.924, 0.978) | −0.051 | .001 | 0.948 (0.920, 0.976) |
| mTOR | 0.012 | .232 | 1.013 (0.992, 1.033) | 0.009 | .416 | 1.013 (0.991, 1.035) | 0.014 | .237 | 1.015 (0.992, 1.037) |
| PS6K1 | 0.096 | .160 | 1.101 (0.963, 1.259) | 0.112 | .114 | 1.108 (0.959, 1.280) | 0.095 | .220 | 1.113 (0.959, 1.292) |
Model 1: no adjustment; Model 2: adjusted for the course of hypertension, family history of hypertension, antihypertensive drugs, and serological indexes (TC); Model 3: Model 1+ Model 2+ the 24‐hour mean SBP+24‐hour mean DBP.
Abbreviations: Bio T, bioavailable testosterone; DBP, diastolic blood pressure; FT, free testosterone; FAI, free androgen index; SBP, systolic blood pressure; SHBG, sex hormone–binding globulin; mTOR, mammalian target of rapamycin; PS6K, phosphorylated ribosome S6 protein kinase.
TABLE 5.
Correlation between LVMI and FAI, SHBG in postmenopausal hypertension
| Regression coefficient and 95% CI | Standard Error | t | P value | |
|---|---|---|---|---|
| SHBG | ||||
| Model 1 | −0.255 (−0.424, −0.086) | 0.086 | −2.970 | .003 |
| Model 2 | −0.192 (−0.025, −0.395) | 0.085 | −2.269 | .024 |
| Model 3 | −0.177 (−0.009, −0.346) | 0.085 | −2.082 | .039 |
| FAI | ||||
| Model 1 | 65.424(3.336, 127.512) | 31.485 | 2.097 | .031 |
| Model 2 | 65.263(3.604, 126.921) | 31.266 | 2.078 | .038 |
| Model 3 | 61.820(2.386, 124.008) | 31.528 | 1.961 | .039 |
Model 1: no adjustment; Model 2: adjusted for the course of hypertension, family history of hypertension, antihypertensive drug, and serological indexes; Model 3: Model 1+ Model 2+ the 24‐hour ambulatory blood pressure.
Abbreviations: FAI, free androgen index; LVMI, left ventricular mass index; SHBG, sex hormone–binding globulin.
FIGURE 2.
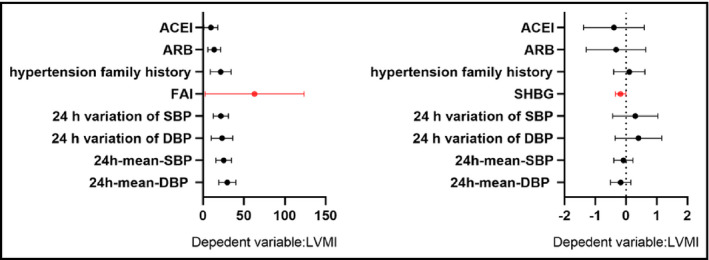
Multiple regression analyses with FAI and SHBG
3.4. Multivariable subgroup analysis of BP regulation in postmenopausal hypertensive patients with LVH
According to the subgroup analysis in Appendix [Link], [Link], the following results can be obtained: (1) compared with the uncontrolled BP group, patients in the controlled BP group had a lower risk of LVH for every additional unit of SHBG (OR 0.987 VS 0.844); (2) the risk of LVH for each additional unit of FAI in the uncontrolled BP group was higher than that in the controlled BP group (OR 4.054 VS 3.913).
4. DISCUSSION
To the best of our knowledge, this is the first cross‐sectional study of androgen involvement in postmenopausal hypertensive with LVH. The results of this study demonstrated that the occurrence of LVH was positively correlated with FAI and negatively correlated with SHBG in postmenopausal women with hypertension. FAI and SHBG may be the influencing factors of LVH in postmenopausal women with hypertension. In addition, the possibility of LVH in postmenopausal hypertensive patients was relatively greater for each unit increase in FAI or decrease in SHBG.
The index TT is often used clinically to evaluate androgen levels. However, only FT and a portion of it that binds to ALB with a nonspecific low affinity are actually bioavailable testosterone. 17 Therefore, many experts point out that TT alone is not a reliable diagnostic criterion for androgen levels. Based on excluding the influence of testosterone content combined with SHBG, FAI is superior to the method of simply measuring the TT concentration in evaluating hyperandrogenemia. 18 , 19 In a cohort study, Georgiopoulos et al found that an increase in baseline FAI was significantly associated with new‐onset hypertension. 20 Another study reported that higher FAI was associated with subclinical atherosclerosis in healthy postmenopausal women. 7 After correcting for confounding factors through multiple models, this study also found that FAI was an independent influencing factor for LVH in postmenopausal women with hypertension.
SHBG is a glycoprotein produced by the liver. 21 On the one hand, it can specifically bind to sex hormones and participate in their transport. 22 On the other hand, it can regulate the concentration of biologically active sex hormones in the blood. SHBG has high binding power with testosterone. 23
The results of this study showed that the serum SHBG level of postmenopausal women with hypertension was lower than that in LVH group. The possible reasons for the above phenomenon are (1) the abnormal gonadotropin‐releasing hormone frequency and amplitude of hypothalamus release may lead to increased luteinizing hormone (LH) release. LH can directly act on ovarian follicular membrane cells to increase the activity of intracellular P450C17α, so that ovarian follicular membrane cells produce excessive androgen. The increase in androgen in the liver can inhibit the synthesis of SHBG, resulting in the decrease in serum SHBG level; (2) the increased SHBG specifically binds to excessive free androgens in the body, resulting in a decrease in the bioavailability of androgens, which indirectly exerts a myocardial protective effect.
However, it is worth noting that there is no research on the mechanism of androgen‐mediated myocardial hypertrophy in postmenopausal hypertension. The possible mechanisms are as follows: (1) androgen activates the renin‐angiotensin system, thereby increasing local myocardial ACE expression to promote myocardial hypertrophy and collagen production 24 ; (2) androgen increases the ratio of myosin heavy chain to a melanocyte‐stimulating hormone, thus accelerating myocardial remodeling 25 ; and (3) previous studies have shown that mTOR plays a key regulatory role in protein synthesis, cell proliferation, and metabolism. 26 , 27 Some recent animal studies on mTOR inactivation have shown that mTOR plays a key role in regulating the physiological and pathological processes of the cardiovascular system. 28 , 29 The results of this study demonstrated that postmenopausal hypertensive patients with myocardial hypertrophy had higher serum mTOR levels as compared with the patient in non‐LVH group. At present, there is no relevant study on the relationship between mTOR/PS6K signaling pathway and hypertension target organ damage caused by high level of free androgen in postmenopausal women. Based on the current research status and preliminary experimental studies, our team will further explore its mechanism in order to find a unique site for target organ protection in postmenopausal hypertensive patients.
There are several limitations in this study. First, since this study used the cross‐sectional design method, the authors could only get the correlation between influence factors and LVH risk of postmenopausal hypertension. The underlying pathological mechanism needs further study and elaboration. Second, the sample size of the study population is relatively small and may not be representative enough. Third, limited by the design of the study and measurement‐related bias cannot be eliminated.
5. CONCLUSION
In conclusion, our study showed that the occurrence of LVH was positively correlated with FAI and negatively correlated with SHBG in postmenopausal women with hypertension. FAI and SHBG may be independent predictors of LVH in postmenopausal hypertension. Early detection of FAI and SHBG levels has certain clinical significance in evaluating the incidence of LVH postmenopausal women with hypertension.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
All authors fulfill the criteria for authorship. Jing Yu and Jian‐Shu Chen conceived and designed the research. Jian‐Shu Chen, Ying Pei, Ning‐yin Li, and Jun‐chen Han acquired the data. Jian‐Shu Chen and Qiong‐ying Wang drafted the manuscript and made critical revision of the manuscript for key intellectual content. All authors read and approved the final manuscript.
Supporting information
Appendix S1
Appendix S2
Jianshu C, Qiongying W, Ying P, Ningyin L, junchen H, Jing Y. Association of free androgen index and sex hormone–binding globulin and left ventricular hypertrophy in postmenopausal hypertensive women. J Clin Hypertens. 2021;23:1413–1419. 10.1111/jch.14301
Funding information
This study was supported by the National Natural Science Foundation of China (NSFC 81670385); Gansu Province Health Research Project (GSWSKY2017‐02); and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2017‐QN09)
REFERENCES
- 1. Cuspidi C, Facchetti R, Quarti‐Trevano F, et al. Incident Left Ventricular Hypertrophy in Masked Hypertension. Hypertension. 2019;74(1):56‐62. [DOI] [PubMed] [Google Scholar]
- 2. Yildiz M, Oktay AA, Stewart MH, et al. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. 2020;63(1):10‐21. [DOI] [PubMed] [Google Scholar]
- 3. Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol. 2017;237:60‐63. [DOI] [PubMed] [Google Scholar]
- 4. Luczak ED, Leinwand LA. Sex‐based cardiac physiology. Annu Rev Physiol. 2009;71:1‐18. [DOI] [PubMed] [Google Scholar]
- 5. Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post‐menopausal women. J Am Coll Cardiol. 2018;71(22):2555‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis GK, Intapad S, Newsome AD, et al. Androgen receptor blockade differentially regulates blood pressure in growth‐restricted versus ovarian deficient rats. Hypertension. 2019;74(4):975‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lambrinoudaki I, Georgiopoulos GA, Athanasouli F, et al. Free androgen index as a determinant of arterial stiffness in menopause: a mediation analysis. Menopause. 2017;24(6):635‐644. [DOI] [PubMed] [Google Scholar]
- 8. Sherman SB, Sarsour N, Salehi M, et al. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9(5):400‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu K, Shen C, Chen X. Expression of androgen receptor in coronary artery in the cases of sudden coronary death. Int J Clin Exp Pathol. 2015;8(4):3742‐3747. [PMC free article] [PubMed] [Google Scholar]
- 10. Loh SY, Salleh N. Influence of testosterone on mean arterial pressure: A physiological study in male and female normotensive WKY and hypertensive SHR rats. Physiol Int. 2017;104(1):25‐34. [DOI] [PubMed] [Google Scholar]
- 11. Davis DD, Ruiz AL, Yanes LL, et al. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension. 2012;59(3):726‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 13. Decaroli MC, Rochira V. Aging and sex hormones in males. Virulence. 2017;8(5):545‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang K, Wang Y, Ding Y, et al. Valsartan chronotherapy reverts the non‐dipper pattern and improves blood pressure control through mediation of circadian rhythms of the renin‐angiotensin system in spontaneous hypertension rats. Chronobiol Int. 2019;36(8):1058‐1071. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American society of echocardiography. J Am Soc Echocardiogr. 2019;32(1):1‐64. [DOI] [PubMed] [Google Scholar]
- 16. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450‐458. [DOI] [PubMed] [Google Scholar]
- 17. Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male. 2016;19(2):85‐89. [DOI] [PubMed] [Google Scholar]
- 18. Torkler S, Wallaschofski H, Baumeister SE, et al. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male. 2011;14(3):176‐182. [DOI] [PubMed] [Google Scholar]
- 19. Georgiopoulos G, Kontogiannis C, Lambrinoudaki I, et al. Free Androgen Index as a Biomarker of Increased Cardiovascular Risk in Postmenopausal Women. J Am Coll Cardiol. 2018;72(16):1986. [DOI] [PubMed] [Google Scholar]
- 20. Georgiopoulos GA, Lambrinoudaki I, Athanasouli F, et al. Free androgen index as a predictor of blood pressure progression and accelerated vascular aging in menopause. Atherosclerosis. 2016;247:177‐183. [DOI] [PubMed] [Google Scholar]
- 21. Glisic M, Rojas LZ, Asllanaj E, et al. Sex steroids, sex hormone‐binding globulin and levels of N‐terminal pro‐brain natriuretic peptide in postmenopausal women. Int J Cardiol. 2018;261:189‐195. [DOI] [PubMed] [Google Scholar]
- 22. Wang A, Arver S, Boman K, et al. Testosterone, sex hormone‐binding globulin and risk of cardiovascular events: A report from the Outcome Reduction with an Initial Glargine Intervention trial. Eur J Prev Cardiol. 2019;26(8):847‐854. [DOI] [PubMed] [Google Scholar]
- 23. Keevil BG, Adaway J, Fiers T, et al. The free androgen index is inaccurate in women when the SHBG concentration is low. Clin Endocrinol (Oxf). 2018;88(5):706‐710. [DOI] [PubMed] [Google Scholar]
- 24. Mishra JS, More AS, Gopalakrishnan K, Kumar S. Testosterone plays a permissive role in angiotensin II‐induced hypertension and cardiac hypertrophy in male rats. Biol Reprod. 2019;100(1):139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Kishimoto I, Saito Y, et al. Androgen contributes to gender‐related cardiac hypertrophy and fibrosis in mice lacking the gene encoding guanylyl cyclase‐A. Endocrinology. 2004;145(2):951‐958. [DOI] [PubMed] [Google Scholar]
- 26. Wong MH, Xue A, Baxter R, et al. Upstream and downstream Co‐inhibition of mitogen‐activated protein kinase and PI3K/Akt/m TOR pathways in pancreatic ductal adenocarcinoma. Neoplasia. 2016;18(7):425‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang SA, Pacold ME, Cervantes CL, et al. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341(6144):1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E‐BP1 inhibition in mice. J Clin Invest. 2010;120(8):2805‐2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altamirano F, Oyarce C, Silva P, et al. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol. 2009;202(2):299‐307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


