Abstract
Blood pressure (BP) variability may have its effect on the development of vascular disease. The authors aimed to examine the association between the visit‐to‐visit variability (VVV) of BP and arterial stiffness in Chinese adults. The authors included 1407 participants from a prospective cohort study of community residents who were ≥40 years, without a history of myocardial infarction or stroke, and with data at the baseline, the second and the third visits in 2008, 2009, and 2013. The VVV of BP was defined as the standard deviation (SD), the coefficient of variation (CV), the average successive variability (ASV), and the variability independent of the mean (VIM) in BP levels at the 3 visits. Arterial stiffness was measured by brachial‐ankle pulse wave velocity (ba‐PWV) at the 2nd and the 3rd visits. Levels of ba‐PWV change and the occurrence of an elevated ba‐PWV increased significantly in the highest tertile of VVV measures of systolic BP (SBP) and pulse pressure (PP) compared with the lowest tertile, respectively. The multivariable regression analysis revealed that VVV measures of SBP and PP were significantly associated with levels of ba‐PWV change and the risks of developing an elevated ba‐PWV. The odds ratios (ORs) and 95% confidence intervals (CIs) for the risk were 2.12 (1.57–3.12) and 1.92 (1.38–2.68) in participants with the highest versus the lowest tertile of SBP‐SD and PP‐SD, respectively. No significant association was found for diastolic BP variability measures. The increased long‐term variabilities of SBP and PP were associated with an increased risk of arterial stiffness.
Keywords: arterial stiffness, blood pressure variability, brachial‐ankle pulse wave velocity
1. INTRODUCTION
Blood pressure (BP) level is strongly associated with stroke, myocardial infarction (MI), and mortality. 1 , 2 , 3 Normal BP, defined as the mean of multiple BP measurements over a period of time, is the most important in the pathophysiology of vascular disease and the basis for recommending optimal BP targets. 1 , 4 However, average BP cannot fully capture BP‐related vascular risk, and changes in BP level have been shown to associate with cardiovascular (CV) events and mortality. 5 , 6 , 7 Although BP variability (BPV) is physiological, it may represent an adaptive humoral and neural response to environmental, behavioral, and emotional stimuli in daily life or may be increased or decreased by antihypertensive treatment. 8 Recently, increasing attention has been paid to the value of visit‐to‐visit BPV. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16
Visit‐to‐visit BPV, considered as a long‐term BPV, is an intraindividual variation in BP over different clinical visits. 17 Elevated BPV may reflect arterial stiffness and baroreceptor dysfunction, which may be associated with endothelial injury and atherosclerosis and may finally lead to cardiovascular events. 18 , 19 , 20 , 21
Previous studies have mostly focused on the visit‐to‐visit variability (VVV) of systolic BP (SBP) as the long‐term BPV and these studies were conducted in various patient populations such as patients with hypertension, 9 , 12 , 17 patients with diabetes, 10 , 22 , 23 , 24 and patients with a history of other CV disease (CVD) risk factors, 6 , 25 , 26 , 27 , 28 from which findings may not be generalizable to other populations. Moreover, these studies focused on the associations of BPV with clinical CVD events and all‐cause mortality. Few studies have investigated the associations of long‐term BPV with subclinical atherosclerosis in a general population. 29 Therefore, we used data from a community‐based cohort study with several times of BP measurements within years to examine the association between VVV of BP and arterial stiffness measured by brachial‐ankle pulse wave velocity (ba‐PWV) among Chinese adults aged ≥40 years.
2. METHODS
2.1. Study population
Study participants were from an ongoing prospective cohort study, the design of which has been published previously. 30 Briefly, participants were enrolled from a suburban community in Shanghai, China, and underwent 3 examination visits. At the baseline visit (June and July 2008), 10 185 community residents over 40 years or older participated in a screening examination. The fasting plasma glucose (FPG) levels were measured, and participants were divided into three groups accordingly 31 : normal glucose regulation (NGR), with FPG level less than 100 mg/dl and never having diabetes; impaired glucose regulation (IGR), with FPG level of 100 to 125 mg/dl and never having diabetes; and diabetes, with FPG level of 126 mg/dl or greater or a history of diabetes. In the 2nd visit (June through August 2009), participants were randomly selected from the three groups in a ratio of 1.0 (diabetes) to 1.2 (IGR) to 1.44 (NGR). We selected more people with lower blood glucose levels because they might have a lower participation rate than those with higher blood glucose levels. All the selected participants received a comprehensive examination including BP measurement and evaluation of arterial stiffness using ba‐PWV. In the 3rd visit (March through May 2013), participants who participated in the 2nd visit were invited to have re‐evaluations of BP and ba‐PWV. The participation rate in the 3rd visit of those seen at visit 2 was 71.9% (2883/4012).
For the current study, participants with a history of myocardial infarction or stroke prior to the baseline visit (n = 139), participants with missing data for BP at any of the 3 visits (n = 54), with missing data for ba‐PWV at the 2nd or the 3rd visit (n = 64), with antihypertensive medications at any of the 3 visits (n = 751), or with ba‐PWV level within the highest quartile (≥1618 cm/s) at the 2nd visit (n = 468) were excluded. Eventually, 1407 participants were included for the current analysis (Figure 1).
FIGURE 1.
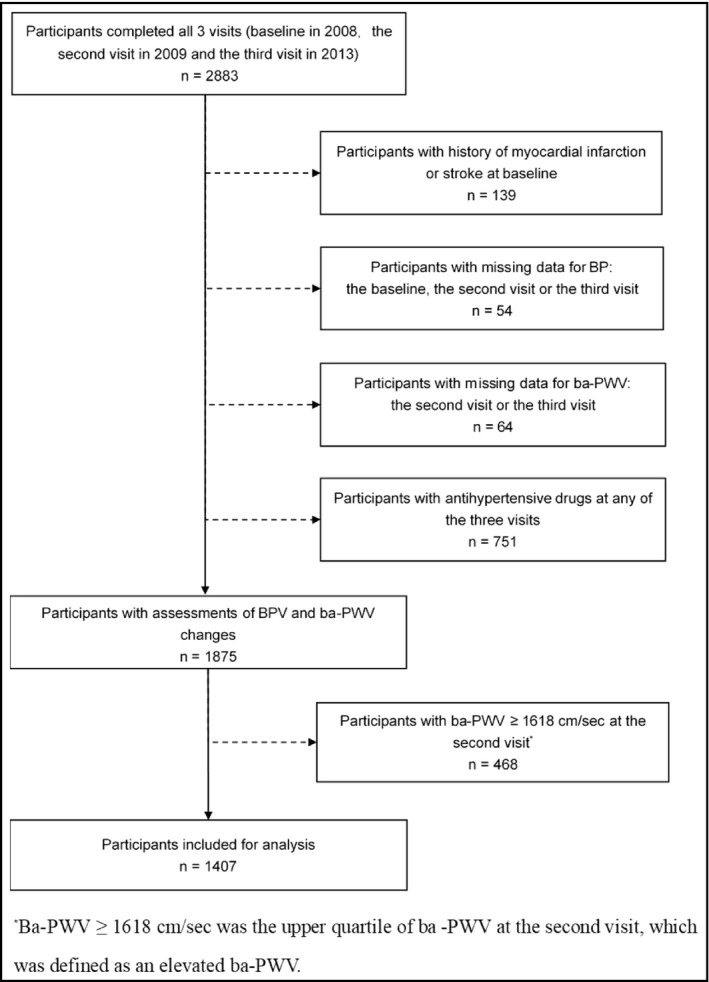
Flowchart of the study population
The study protocol was approved by the Institutional Review Board of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All study participants provided written informed consent.
2.2. Data collection
Detailed information such as demographic and lifestyle factors, medical history, and medication use was obtained through a standard questionnaire administered by trained physicians. The International Physical Activity Questionnaire was used to collect information on participants’ physical activities and being physically active was defined by the highest tertile of metabolic equivalent‐hours per week. 32 When measuring their height and weight, participants wore lightweight clothes without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured at the level of the umbilicus. BP and heart rate (HR) were measured three times consecutively with 1‐min intervals after resting for at least 5 min using an automated electronic device (OMRON Model HEM‐752, Omron Company). The mean value of the three measurements was used in analysis. Pulse pressure (PP) was defined as SBP‐diastolic BP (DBP). Participants were instructed to avoid alcohol, tea, coffee, and exercise 30 min before BP measurements.
Participants were asked to fast overnight for at least 10 h, and the venous blood samples were collected in the early morning. Plasma concentrations of fasting glucose, serum concentrations of triglycerides (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐c), and low‐density lipoprotein cholesterol (LDL‐c) were measured by an autoanalyzer (ADVIA‐1650 Chemistry System, Bayer).
2.3. Measures of visit‐to‐visit BPV
Using BP levels at each of the three visits, we calculated the VVV of BP using (1) the standard deviation (SD), (2) the coefficient of variation (CV), (3) the average successive variability (ASV) defined as the average absolute difference between successive values, and (4) the variability independent of the mean (VIM), which was calculated as 100*SD/meanβ, where β is the regression coefficient based on natural logarithm of SD on natural logarithm of mean. All these four metrics have been described in previous studies. 33 , 34 , 35
2.4. Measurement of ba‐PWV
Participants were required to take 15–30 min of rest before ba‐PWV examination. Ba‐PWV was measured using the Colin VP‐1000 (Model BP203RPEII, form PWV/ABI; OMRON Colin Medical Instruments) as reported previously. 36 Measured with cuffs placed on the upper arms and the ankles, pulse waves were obtained simultaneously from the brachial and tibial arteries. The greater value of the right and the left ba‐PWV was used for analysis.
An elevated ba‐PWV was defined as a ba‐PWV ≥ 1618 cm/s, which was the upper quartile of ba‐PWV at the 2nd visit. Ba‐PWV change was calculated by abstracting ba‐PWV at the 2nd visit from ba‐PWV at the 3rd visit. The ratio of ba‐PWV change was calculated by dividing ba‐PWV change by ba‐PWV level at the 2nd visit. The occurrence of an elevated ba‐PWV was defined as the proportion of elevated ba‐PWV (ba‐PWV ≥ 1618 cm/s) at the 3rd visit.
2.5. Statistical analysis
General characteristics were demonstrated in total participants and according to SBP‐SD tertiles. Continuous variables were presented as mean ± SD for normally distributed variables or medians (interquartile ranges) for the skewed variables. We log10‐transformed TG to achieve a normal distribution. All the categorical variables were presented as numbers and proportions. We used the ANOVA test to compare continuous variables and the chi‐square test to compare categorical variables.
Multiple linear regression models were used to explore the associations of each BPV measure (SD, CV, ASV, and VIM) with the ba‐PWV change and ratio of ba‐PWV change adjusted for covariates. Four models were used. Model 1 was adjusted for age and sex. Model 2 was further adjusted for education, current smoking, current drinking, and physical activity. Model 3 was further adjusted for baseline WC, DBP/SBP/‐ (for SBP variability/DBP variability/PP variability, respectively), FPG, log10TG, LDL‐c, and HR. Model 4 additionally accounted for average SBP/DBP/PP of the 3 visits (for SBP variability/DBP variability/PP variability, respectively) and ba‐PWV at the 2nd visit.
We also used multivariable logistic regression models to evaluate the associations of each BPV measure (SD, CV, ASV, and VIM) with elevated ba‐PWV. Tertiles of each BPV measure were used in the models with the lowest tertile as the reference. Similar models were used as the linear regression models except that model 4 did not adjust for ba‐PWV at the 2nd visit. Adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated.
Subgroup analyses were performed in participants defined by age, sex, BMI, smoking status, drinking status, diabetes status, and hypertension status. We assessed if there were interactions by adding interaction terms in the adjusted models.
All analyses were performed with the use of SPSS software version 22.0 (SPSS Inc). Significance tests were two‐tailed, with a p value < .05 considered as statistically significant.
3. RESULTS
3.1. Clinical characteristics of the study population
The baseline demographic and clinical characteristics of the participants across tertiles of SBP‐SD are presented in Table 1. Of the 1407 participants included in the study, mean age was 54.7 ± 8.0 years and 36.3% (n = 511) were men. Participants in the highest tertile of SBP‐SD were more likely to be older. They had higher levels of baseline WC, PP, FPG, LDL‐c, average SBP, DBP, and PP and a lower level of baseline HR.
TABLE 1.
Baseline characteristics of study participants by tertiles of SBP‐SD
| Characteristics | Total | T1 (<6.17 mmHg) | T2 (6.17–10.60 mmHg) | T3 (>10.60 mmHg) | p value |
|---|---|---|---|---|---|
| Participants, n | 1407 | 468 | 469 | 470 | / |
| SBP‐SD, mmHg | 8.26 (5.21–12.03) | 4.16 (2.81–5.21) | 8.26 (7.20–9.49) | 14.02 (12.03–16.85) | / |
| Age, years | 54.7 ± 8.0 | 53.4 ± 7.4 | 54.2 ± 7.9 | 56.4 ± 8.3 | <.001 |
| Men, n (%) | 511 (36.3) | 178 (38.0) | 166 (35.5) | 166 (35.3) | .637 |
| Body mass index, kg/m2 | 24.8 ± 3.5 | 24.7 ± 3.4 | 24.7 ± 3.6 | 25.1 ± 3.6 | .083 |
| Waist circumference, cm | 83.1 ± 9.7 | 82.7 ± 9.8 | 82.6 ± 9.8 | 84.1 ± 9.4 | .020 |
| High school education or above, n (%) | 433 (30.8) | 155 (33.1) | 147 (31.3) | 131 (27.9) | .239 |
| Life style factors, n (%) | |||||
| Current smoking | 363 (25.8) | 108 (23.1) | 127 (27.1) | 127 (27.0) | .256 |
| Current drinking | 233 (16.6) | 75 (16.0) | 83 (17.7) | 75 (16.0) | .719 |
| Physically active | 462 (32.8) | 148 (31.6) | 161 (34.4) | 152 (32.3) | .612 |
| Heart rate, beats per minute | 76.3 ± 10.1 | 77.2 ± 10.1 | 76.5 ± 9.9 | 75.3 ± 10.4 | .016 |
| Blood pressure, mmHg | |||||
| Systolic blood pressure | 123.1 ± 16.2 | 122.2 ± 14.7 | 123.1 ± 15.2 | 124.1 ± 18.5 | .181 |
| Diastolic blood pressure | 76.6 ± 9.4 | 76.8 ± 9.0 | 76.6 ± 9.3 | 76.6 ± 9.9 | .915 |
| Pulse pressure | 46.5 ± 11.9 | 45.4 ± 10.3 | 46.5 ± 11.5 | 47.6 ± 13.5 | .020 |
| Average blood pressure*, mmHg | |||||
| Systolic blood pressure | 127.3 ± 14.5 | 123.2 ± 14.0 | 126.8 ± 13.3 | 131.8 ± 14.7 | <.001 |
| Diastolic blood pressure | 76.4 ± 8.2 | 75.6 ± 8.3 | 76.1 ± 8.1 | 77.5 ± 8.0 | .001 |
| Pulse pressure | 50.9 ± 11.3 | 47.7 ± 10.2 | 50.7 ± 10.7 | 54.3 ± 12.0 | <.001 |
| Fasting plasma glucose, mg/dl | 98.9 ± 31.0 | 96.0 ± 24.5 | 98.6 ± 31.9 | 102.0 ± 35.5 | .011 |
| Lipid profile, mg/dl | |||||
| Triglycerides | 117.0 (82.4–171.9) | 120.1 (79.7–172.5) | 110.75 (81.51–162.14) | 118.3 (85.06–181.63) | .202 |
| Total cholesterol | 196.5 ± 36.1 | 194.1 ± 33.9 | 196.9 ± 39.3 | 198.5 ± 34.9 | .172 |
| High‐density lipoprotein cholesterol | 54.5 ± 11.5 | 54.5 ± 11.8 | 55.1 ± 11.6 | 53.9 ± 11.2 | .244 |
| Low‐density lipoprotein cholesterol | 94.0 ± 25.6 | 92.8 ± 25.1 | 92.8 ± 26.3 | 96.5 ± 25.3 | .033 |
| Diabetes, n (%) | 243 (17.3) | 63 (13.5) | 86 (18.3) | 63 (13.5) | .023 |
| Hypertension, n (%) | 366 (26.0) | 113 (24.1) | 112 (23.9) | 141 (30.0) | .054 |
| Ba‐PWV at the second visit, cm/s | 1300 ± 168 | 1271 ± 172 | 1291 ± 171 | 1338 ± 155 | <.001 |
| Ba‐PWV at the third visit, cm/s | 1556 ± 262 | 1477 ± 236 | 1543 ± 241 | 1649 ± 278 | <.001 |
| Ba‐PWV change a , cm/s | 256 ± 204 | 206 ± 181 | 251 ± 186 | 311 ± 228 | <.001 |
| Ratio of ba‐PWV change b , % | 20.1 ± 15.9 | 16.8 ± 15.0 | 20.0 ± 15.0 | 23.6 ± 17.0 | <.001 |
| Elevated ba‐PWV c , n (%) | 501 (35.6) | 109 (23.3) | 151 (32.3) | 240 (51.1) | <.001 |
Data are baseline characteristics of study participants unless indicated otherwise.
Data are mean ± SD or median (quartile 1‐quartile 3) for continuous variables and number (percentage) for categorical variables.
Abbreviations: ba‐PWV, brachial‐ankle pulse wave velocity; SBP, systolic blood pressure; SD, standard deviation; T, tertial.
Ba‐PWV change was calculated by abstracting ba‐PWV at the 2nd visit from ba‐PWV at the 3rd visit.
Ratio of ba‐PWV change was calculated by dividing ba‐PWV change by ba‐PWV at the 2nd visit.
Elevated ba‐PWV was defined as ba‐PWV ≥ 1618 cm/s, which was the upper quartile of ba‐PWV at the 2nd visit.
Average blood pressure was the mean level of blood pressure of the 3 visits.
3.2. Visit‐to‐visit BPV and ba‐PWV
The ba‐PWV change, the ratio of ba‐PWV change and the occurrence of an elevated ba‐PWV across the SBP, DBP and PP VVV tertiles are shown in Figure 2 and Supplemental Figures S1–S3. They increased significantly across the tertiles of SBP and PP VVV measures. Less evident increase was found across the tertiles of DBP VVV measures.
FIGURE 2.
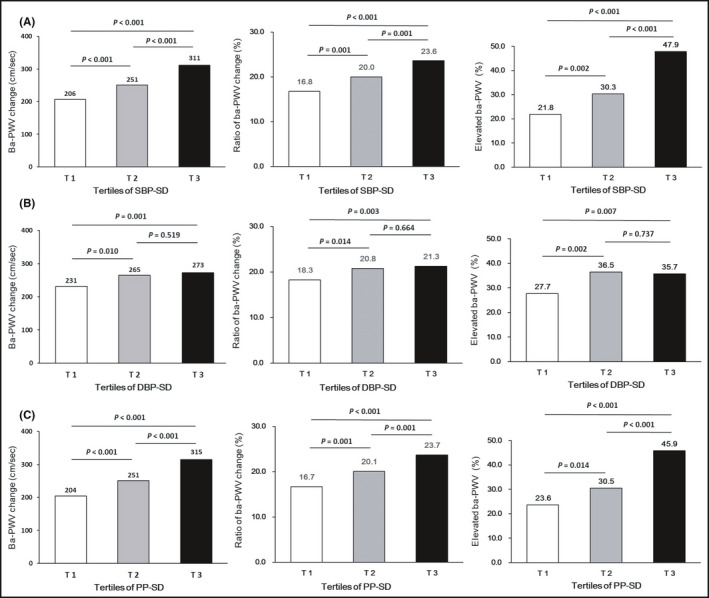
(A) Ba‐PWV changes and the occurrence of an elevated ba‐PWV according to tertiles of SBP‐SD. (B) Ba‐PWV changes and the occurrence of an elevated ba‐PWV according to tertiles of DBP‐SD. (C) Ba‐PWV changes and the occurrence of an elevated ba‐PWV according to tertiles of PP‐SD
The multiple linear regression analysis showed that measures of SBP and PP VVV were significantly and independently associated with the ba‐PWV change and the ratio of ba‐PWV change even after full adjustment for confounders. The associations for DBP VVV measures were not significant or borderline significant (Table 2 and Supplemental Table S1).
TABLE 2.
Linear regression analysis of SBP‐SD, DBP‐SD, and PP‐SD associated with the changes of ba‐PWV
| Measures of variability | Changes of ba‐PWV | ||||
|---|---|---|---|---|---|
| Ba‐PWV change (cm/s) | Ratio of ba‐PWV change (%) | ||||
| β ± SE | p value | β ± SE | p value | ||
| SBP‐SD | Model 1 | 7.579 ± 0.903 | <.001 | 0.519 ± 0.075 | <.001 |
| Model 2 | 7.637 ± 0.942 | <.001 | 0.523 ± 0.076 | <.001 | |
| a Model 3 | 7.307 ± 0.935 | <.001 | 0.505 ± 0.067 | <.001 | |
| d Model 4 | 5.667 ± 0.930 | <.001 | 0.424 ± 0.072 | <.001 | |
| DBP‐SD | Model 1 | 5.990 ± 1.787 | .001 | 0.418 ± 0.143 | .003 |
| Model 2 | 6.166 ± 1.791 | .015 | 0.430 ± 0.143 | .018 | |
| b Model 3 | 4.260 ± 1.784 | .017 | 0.341 ± 0.144 | .018 | |
| e Model 4 | 3.203 ± 1.733 | .065 | 0.229 ± 0.134 | .087 | |
| PP‐SD | Model 1 | 8.574 ± 1.121 | <.001 | 0.584 ± 0.090 | <.001 |
| Model 2 | 8.590 ± 1.123 | <.001 | 0.585 ± 0.090 | <.001 | |
| c Model 3 | 8.046 ± 1.131 | <.001 | 0.554 ± 0.091 | <.001 | |
| f Model 4 | 6.159 ± 1.141 | <.001 | 0.461 ± 0.088 | <.001 | |
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for education, current smoking, current drinking, and physical activity.
Abbreviations: ba‐PWV, brachial‐ankle pulse wave velocity; DBP, diastolic pressure; FPG, fasting plasma glucose; HR, heart rate; LDL‐c, low‐density lipoprotein cholesterol; log10TG, log10‐transformed triglycerides; PP, pulse pressure; SBP, systolic blood pressure; SD, the standard deviation; SE, standard error; VVV, visit‐to‐visit variability; WC, waist circumference; β, regression coefficient.
Model 3 was additionally adjusted for baseline WC, DBP, FPG, log10TG, LDL‐c, and HR.
Model 3 was additionally adjusted for baseline WC, SBP, FPG, log10TG, LDL‐c, and HR.
Model 3 was additionally adjusted for baseline WC, FPG, log10TG, LDL‐c, and HR.
Model 4 was additionally adjusted for average SBP and ba‐PWV at the 2nd visit.
Model 4 was additionally adjusted for average DBP and ba‐PWV at the 2nd visit.
Model 4 was additionally adjusted for average PP and ba‐PWV at the 2nd visit.
We further analyzed the associations between visit‐to‐visit BPV and the occurrence of an elevated ba‐PWV in multivariable logistic regression models. As shown in Table 3 and Supplemental Table S2, when visit‐to‐visit BPV was examined as a continuous variable in the fully adjusted model (model 4), higher VVV of SBP was significantly associated with an increased risk of developing elevated ba‐PWV. Greater VVV of PP was also significantly associated with an increased occurrence of elevated ba‐PWV, while no significant association was found for VVV of DBP and the occurrence of elevated ba‐PWV. Participants with the highest tertile of SBP‐SD had a 1.22‐fold increased risk of developing elevated ba‐PWV compared with participants with the lowest tertile of SBP‐SD after full adjustment (model 4; OR 2.22 [95% CI 1.57–3.12]). The highest tertile of PP‐SD conferred an 92% increased risk of developing elevated ba‐PWV compared with the lowest tertile of PP‐SD (OR 1.92 [95% CI 1.38–2.68]). However, no significant increase in the occurrence of an elevated ba‐PWV was found in participants with the highest tertile of DBP‐SD compared with those with the lowest tertile of DBP‐SD (OR 1.11 [95% CI 0.81–1.53]) (Table 4). Similar results were observed for other measures of VVV (Supplemental Table S3). In addition, we have conducted a sensitivity analysis using the upper quintile of ba‐PWV at the 2nd visit (ba‐PWV ≥ 1672 cm/s) as the cut‐point of elevated ba‐PWV and re‐did the multivariable logistic regression analysis. Similar findings were observed (Supplemental Tables S4 and S5).
TABLE 3.
Logistic regression analysis of SBP‐SD, DBP‐SD, and PP‐SD as continuous variables and the development of an elevated ba‐PWV
| Measures of variability | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| SBP‐SD | 1.09 (1.07–1.12) | <.001 | 1.09 (1.07–1.12) | <.001 | 1.10 (1.07–1.13) a | <.001 | 1.06 (1.03–1.09) d | <.001 |
| DBP‐SD | 1.06 (1.02–1.10) | <.001 | 1.06 (1.02–1.11) | .004 | 1.03 (0.99–1.08) b | .193 | 1.03 (0.98–1.07) e | .275 |
| PP‐SD | 1.11 (1.08–1.15) | <.001 | 1.11 (1.08–1.15) | <.001 | 1.11 (1.08–1.14) c | <.001 | 1.07 (1.03–1.10) f | <.001 |
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for education, current smoking, current drinking, and physical activity.
Abbreviations: ba‐PWV, brachial‐ankle pulse wave velocity; CI, confidence internal; DBP, diastolic pressure; FPG, fasting plasma glucose; HR, heart rate; LDL‐c, low‐density lipoprotein cholesterol; log10TG, log10‐transformed triglycerides; OR, odds ratio; PP, pulse pressure; SBP, systolic blood pressure; SD, the standard deviation; VVV, visit‐to‐visit variability; WC, waist circumference.
Model 3 was additionally adjusted for baseline WC, DBP, FPG, log10TG, LDL‐c, and HR.
Model 3 was additionally adjusted for baseline WC, SBP, FPG, log10TG, LDL‐c, and HR.
Model 3 was additionally adjusted for baseline WC, FPG, log10TG, LDL‐c, and HR.
Model 4 was additionally adjusted for average SBP.
Model 4 was additionally adjusted for average DBP.
Model 4 was additionally adjusted for average PP.
TABLE 4.
Logistic regression analysis of VVV in SBP‐SD, DBP‐SD, and PP‐SD as categorical variables and the development of an elevated ba‐PWV
| Measures of variability | OR (95% CI) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| SBP‐SD | ||||
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.52 (1.12–2.07) | 1.53 (1.12–2.09) | 1.62 (1.17–2.24) a | 1.36 (0.96–1.91) d |
| T3 | 2.97 (2.20–4.01) | 2.98 (2.21–4.03) | 3.27 (2.37–4.50) a | 2.22 (1.57–3.12) d |
| DBP‐SD | ||||
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.53 (1.15–2.05) | 1.54 (1.15–2.06) | 1.49 (1.09–2.03) b | 1.51 (1.10–2.06) e |
| T3 | 1.39 (1.04–1.86) | 1.40 (1.04–1.88) | 1.11 (0.81–1.53) b | 1.11 (0.81–1.53) e |
| PP‐SD | ||||
| T1 | Reference | Reference | Reference | Reference |
| T2 | 1.49 (1.09–2.03) | 1.50 (1.10–2.04) | 1.50 (1.09–2.06) c | 1.54 (1.10–2.14) f |
| T3 | 2.88 (2.13–3.89) | 2.90 (2.14–3.92) | 2.80 (2.05–3.82) c | 1.92 (1.38–2.68) f |
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for education, current smoking, current drinking, and physical activity.
Abbreviations: ba‐PWV, brachial‐ankle pulse wave velocity; CI, confidence internal; DBP, diastolic pressure; FPG, fasting plasma glucose; HR, heart rate; LDL‐c, low‐density lipoprotein cholesterol; log10TG, log10‐transformed triglycerides; OR, odds ratio; PP, pulse pressure; SBP, systolic blood pressure; SD, the standard deviation; VVV, visit‐to‐visit variability.
Model 3 was additionally adjusted for baseline WC, DBP, FPG, log10TG, LDL‐c, and HR.
Model 3 was additionally adjusted for baseline WC, SBP, FPG, log10TG, LDL‐c, and HR.
Model 3 was additionally adjusted for baseline WC, FPG, log10TG, LDL‐c, and HR.
Model 4 was additionally adjusted for average SBP.
Model 4 was additionally adjusted for average DBP.
Model 4 was additionally adjusted for average PP.
Results for subgroup analysis are shown in Figure 3 and Supplemental Figures S4–S6. Sex, diabetes status, and hypertension status all significantly interacted with SBP VVV measures in association with the risk of developing an elevated ba‐PWV, whereas hypertension status had significant interactions with DBP VVV measures and PP VVV measures in association with arterial stiffness (all p for interaction < .05). BP VVV measures were associated with the development of an elevated ba‐PWV more closely in participants without hypertension compared with those with hypertension.
FIGURE 3.
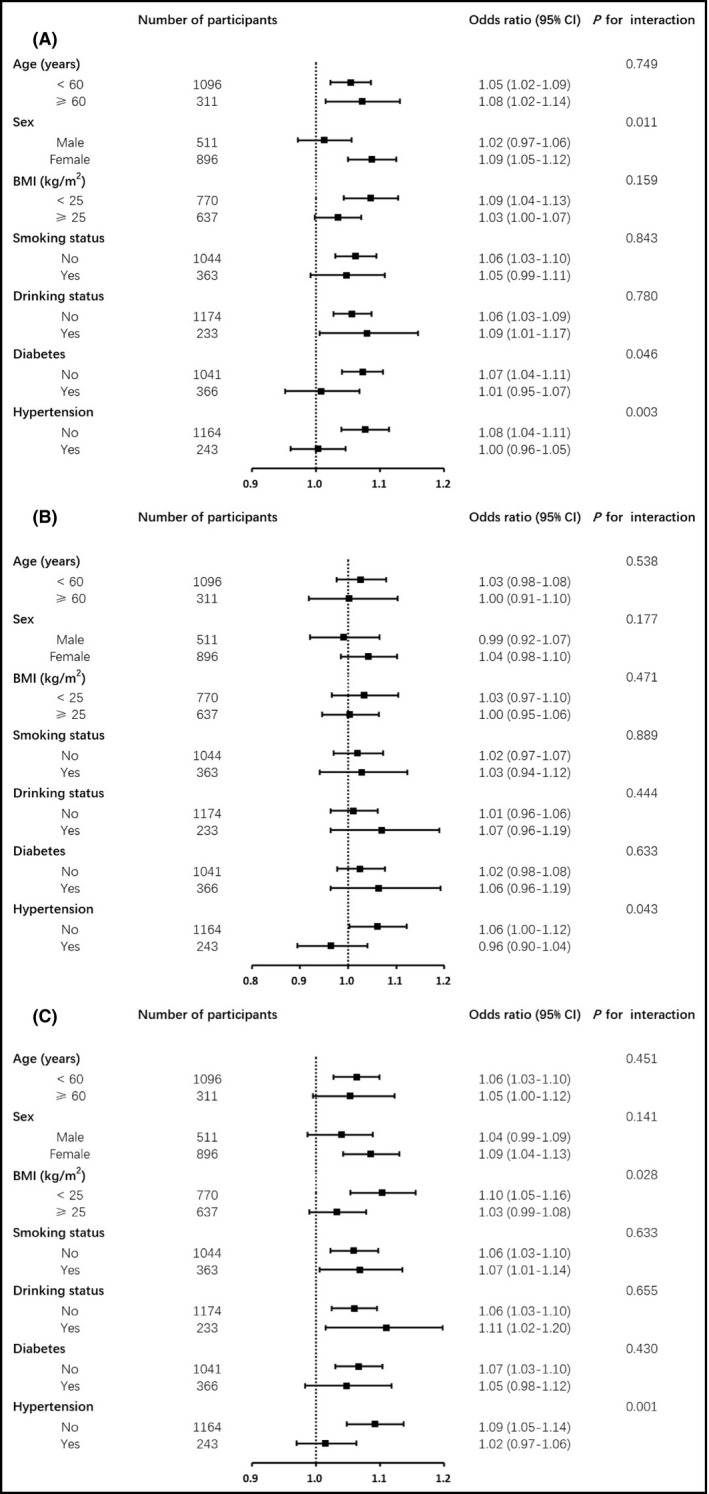
(A) Association of SBP‐SD with elevated ba‐PWV in different subgroups of participants. (B) Association of DBP‐SD with elevated ba‐PWV in different subgroups of participants. (C) Association of PP‐SD with elevated ba‐PWV in different subgroups of participants. All models are adjusted for potential confounding factors including age, sex, education, current smoking, current drinking, physical activity, baseline WC, DBP/SBP/‐, FPG, log10TG, LDL‐c, HR, and average SBP/DBP/PP
4. DISCUSSION
In the current study, we observed a significant association between the increased VVV of SBP or PP assessed by four indicators (SD, CV, ASV, and VIM) and increased arterial stiffness measured by ba‐PWV. The direction and the magnitude of these associations were roughly consistent across measures of SBP or PP variability, and they remained significant even after adjustment for average SBP or average PP, suggesting that the long‐term SBP and PP variabilities may play important roles in the subclinical stage of atherosclerosis in Chinese community adults.
Studies investigating the relationship between visit‐to‐visit BPV and ba‐PWV are rare. Recently, several studies have examined the long‐term BPV and found that not only average SBP but also SBP variability is an independent risk factor for atherosclerosis and organ damage in patients with hypertension or other cardiovascular risk factors. 17 , 28 , 37 , 38 Unlike previous studies, our study focused on the association of long‐term BPV with subclinical atherosclerosis in the community adults. It should be noted that the study population was a selected sample from the general population because we randomly selected participants with different glycemic status at a 1:1.2:1.44 ratio at the 2nd visit, leading to more participants with diabetes or IGR in the selected sample than the general population. The proportion of the population with impaired glucose regulation at baseline increased, which could make the overall baseline FPG level higher than that of the general population. Considering the effect of higher blood glucose itself on blood vessels, the overall ba‐PWV may also be higher than that of the general population. Therefore, we have adjusted both the baseline FPG and ba‐PWV at the 2nd visit in the regression analysis. Our findings suggested that the long‐term SBP and PP variabilities may play important roles in the development of atherosclerosis at an early and subclinical stage, before a clinical CVD event occurred. This has important clinical and public health implications. Regular monitoring of BP levels is recommended and besides BP levels per se, an evaluation of BPV should also be considered in early prevention of subclinical atherosclerosis.
The mechanism connecting visit‐to‐visit BPV with vascular damage remains uncertain. Kikuya et al. suggested that increased BPV in elderly and hypertensive patients may be partly due to the increased stiffness and decreased compliance of the large elastic artery caused by aging and hypertension, leading to the decreased function of the pressure reflex. 39 Their study also suggested that disturbed baroreflex function was associated with overpressuring responses to mental and physical stimuli and regulates orthostatic hypotension, postprandial hypotension, and other conditions that lead to increased BPV. Another study suggested that the adverse effects of an increased BPV possibly relate to a greater traumatic effect of wider BP swings on the vessel wall, promoting early target‐organ damage. 40 Eto et al. suggested that, independent of average BP, the increase of BPV may contribute to the formation of atherosclerosis in animal models by inhibiting the production of nitric oxide and damaging endothelial function and enhancing the formation of neointima. 41
Findings from the current study also revealed that compared to individuals with hypertension, individuals without hypertension were particularly susceptible to the impact of long‐term BPV. One possible explanation is that individuals without hypertension, often with fewer vascular risk factors, are more sensitive to BPV and that, with hypertension, perhaps other risk factors might overshadow the negative influence of BPV. Another possible explanation is that individuals without hypertension, often with lower blood pressure, are more vulnerable to blood pressure variability 42 and that, with higher blood pressure, the contribution of variability is less pronounced. 1 , 17
To the best of our knowledge, studies on the association between visit‐to‐visit BPV and arterial stiffness in the community adults are rare. In addition, because antihypertensive drugs could delay the development of atherosclerosis 43 and interfere with BPV, 44 we excluded individuals with antihypertensive drugs at any of the 3 visits in the current analysis. The current study has several limitations. The long‐term BPV was calculated using BP levels at the 3 visits, and ba‐PWV was assessed at the 2nd and the 3rd visits. Because BP varies a great deal even in a short period of time, using BP measurements in 1‐day time to represent BP levels around one visit could be inaccurate to some extent. Multiple BP measurements over several days around each visit could have provided a more accurate evaluation of the long‐term BP variability. A ba‐PWV measurement at the baseline visit and a prospective analysis with the assessment of ba‐PWV changes after the 3rd visit are needed to better elucidate the potential causal relationship between BPV and arterial stiffness. In addition, the study participants were middle‐aged and elderly community residents recruited from suburban Shanghai; therefore, findings from the current study may not be generalizable to people within other age groups or with different socioeconomic or lifestyle background.
5. CONCLUSIONS
Our findings suggest that a greater visit‐to‐visit BPV is significantly associated with an increased arterial stiffness, above and beyond the effect of mean BP in the community adults. These findings add to the growing body of evidence on the prognostic value of long‐term BPV and highlight the importance of stable BP control in a long term. More prospective studies are needed to further demonstrate the importance of the long‐term BPV in the development of early cardiovascular diseases in diverse populations.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
YZ, LB, YX, and YC had access to all data and take responsibility for its integrity and analysis. YZ and YX conceived the hypotheses and analyses. YZ drafted the paper. YZ and LB provided statistical analysis. YZ, LB, and JZ collected the data. YX and YC revised the manuscript. ML, TW, MX, JL, SW, YB, WW, and GN refined interpretation and the final manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the fieldworkers for their contribution to the study and the participants for their cooperation.
Zhang Y, Bie L, Li M, et al. Visit‑to‑visit blood pressure variability is associated with arterial stiffness in Chinese adults: A prospective analysis. J Clin Hypertens. 2021;23:802–812. 10.1111/jch.14166
Yuwen Zhang and Lizhan Bie contributed equally to this work.
Funding information
This work was supported by the grants from the National Natural Science Foundation of China (81870560, 81870604, and 8151128019), the National Key R&D Program of China (2017YFC1310700, 2016YFC1304904, and 2016YFC1305600), the Shanghai Municipal Government (18411951800), the Shanghai Shenkang Hospital Development Center (SHDC12019101), the Shanghai Jiaotong University School of Medicine (DLY201801), and the Ruijin Hospital (2018CR002) and the Innovative Research Team of High‐level Local Universities in Shanghai.
[Correction added on January 30, 2021, after first online publication: The statement “Yuhong Chen and Yu Xu contributed equally to this work” has been changed to “Yuwen Zhang and Lizhan Bie contributed equally to this work”.]
Contributor Information
Yuhong Chen, Email: chenyh70@126.com.
Yu Xu, Email: chenyh70@126.com, Email: jane.yuxu@gmail.com.
REFERENCES
- 1. Lewington SCR, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903‐1913. [DOI] [PubMed] [Google Scholar]
- 2. Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161(9):1183‐1192. [DOI] [PubMed] [Google Scholar]
- 3. Staessen J. Risks of untreated and treated isolated systolic hypertension in the elderly: meta analysis of outcome trial. Lancet. 2000;355(9207):865‐872. [DOI] [PubMed] [Google Scholar]
- 4. Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Circulation. 2003;107(24):2993‐2994. 10.1161/01.Cir.0000080481.62058.03 [DOI] [PubMed] [Google Scholar]
- 5. Gao S, Hendrie HC, Wang C, et al. Redefined blood pressure variability measure and its association with mortality in elderly primary care patients. Hypertension. 2014;64(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poortvliet RKE, Ford I, Lloyd SM, et al. Blood pressure variability and cardiovascular risk in the PROspective study of pravastatin in the elderly at risk (PROSPER). PLoS One. 2012;7(12):e52438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimbo D, Newman JD, Aragaki AK, et al. Association between annual visit‐to‐visit blood pressure variability and stroke in postmenopausal women: data from the women's health initiative. Hypertension. 2012;60(3):625‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell’Oro R, Mancia G. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012;14(4):333‐338. [DOI] [PubMed] [Google Scholar]
- 9. Hastie CEJP, Coleman H, McCallum L, et al. Long‐term and ultra long‐term blood pressure variability during follow‐up and mortality in 14 522 patients with hypertension. Hypertension. 2013;62(4):698‐705. [DOI] [PubMed] [Google Scholar]
- 10. Hsieh YTTS, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit‐to‐visit variability in blood pressure strongly predicts all‐cause mortality in patients with type 2 diabetes: a 5·5‐year prospective analysis. Eur J Clin Invest. 2012;42(3):245‐253. [DOI] [PubMed] [Google Scholar]
- 11. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit‐to‐visit variability in systolic blood pressure and all‐cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57(2):160‐166. [DOI] [PubMed] [Google Scholar]
- 12. Muntner P, Whittle J, Lynch AI, et al. Visit‐to‐visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality. Ann Intern Med. 2015;163(5):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;9(354):i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suchy‐Dicey AM, Wallace ER, Elkind MS, et al. Blood pressure variability and the risk of all‐cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26(10):1210‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tai C, Sun Y, Dai N, et al. Prognostic significance of visit‐to‐visit systolic blood pressure variability: a meta‐analysis of 77,299 patients. J Clin Hypertens. 2015;17(2):107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Shi X, Ma C, et al. Visit‐to‐visit blood pressure variability is a risk factor for all‐cause mortality and cardiovascular disease. J Hypertens. 2017;35(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 17. Pm R. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895‐905. [DOI] [PubMed] [Google Scholar]
- 18. Parati G, Ochoa JE, Lombardi C, Bilo G. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep. 2015;17(4):23. [DOI] [PubMed] [Google Scholar]
- 19. Brickman AMRC, Luchsinger JA. Long‐term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;2010(67):564‐569. 10.1016/j.ycar.2011.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit‐to‐visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. 2012;30(8):1556‐1563. [DOI] [PubMed] [Google Scholar]
- 21. Shimbo D, Shea S, McClelland RL, et al. Associations of aortic distensibility and arterial elasticity with long‐term visit‐to‐visit blood pressure variability: the multi‐ethnic study of atherosclerosis (MESA). Am J Hypertens. 2013;26(7):896‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kilpatrick ES, Rigby AS, Atkin SL. The role of blood pressure variability in the development of nephropathy in type 1 diabetes. Diabetes Care. 2010;33(11):2442‐2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, Jin C, Vaidya A, et al. Longitudinal patterns of blood pressure, incident cardiovascular events, and all‐cause mortality in normotensive diabetic peoplenovelty and significance. Hypertension. 2016;68(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu ZB, Li D, Chen XY, et al. Association of visit‐to‐visit variability of blood pressure with cardiovascular disease among type 2 diabetes mellitus patients: a cohort study. Diabetes Metab J. 2019;43(3):350‐367. 10.4093/dmj.2018.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau K‐K, Wong Y‐K, Teo K‐C, et al. Long‐term prognostic implications of visit‐to‐visit blood pressure variability in patients with ischemic stroke. Am J Hypertens. 2014;27(12):1486‐1494. [DOI] [PubMed] [Google Scholar]
- 26. Mezue K, Goyal A, Pressman GS, Horrow JC, Rangaswami J. Blood pressure variability predicts adverse events and cardiovascular outcomes in chronic kidney disease: a post‐hoc analysis of the SPRINT trial. Am J Hypertens. 2018;31(1):48‐52. 10.1093/ajh/hpx128 [DOI] [PubMed] [Google Scholar]
- 27. Mehlum MH, Liestol K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39(24):2243‐2251. 10.1093/eurheartj/ehx760 [DOI] [PubMed] [Google Scholar]
- 28. Nagai M, Dote K, Kato M, et al. Visit‐to‐visit blood pressure variability, average BP level and carotid arterial stiffness in the elderly: a prospective study. J Hum Hypertens. 2017;31(4):292‐298. 10.1038/jhh.2016.77 [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Yang Y, Wang A, et al. Association of long‐term blood pressure variability and brachial‐ankle pulse wave velocity: a retrospective study from the APAC cohort. Sci Rep. 2016;6:21303. 10.1038/srep21303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ning G, Bi Y, Wang T, et al. Relationship of urinary Bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults. Ann Intern Med. 2011;155(6):368. [DOI] [PubMed] [Google Scholar]
- 31. Wang T, Lu J, Xu Y, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: a population‐based study. Diabetes Care. 2013;36(7):1974‐1980. 10.2337/dc12-1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee P, Macfarlane D, Lam T, Stewart S. Validity of the international physical activity questionnaire short form (IPAQ‐SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul M, Jeff W, Lynch AI, et al. Visit‐to‐visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163(5):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bangalore S, Fayyad R, Laskey R, Demicco DA, Messerli FH, Waters DD. Body‐weight fluctuations and outcomes in coronary disease. N Engl J Med. 2017;376(14):1332‐1340. [DOI] [PubMed] [Google Scholar]
- 35. Echouffo‐Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit‐to‐visit glycemic variability and risks of cardiovascular events and all‐cause mortality: the ALLHAT study. Diabetes Care. 2019;42(3):486‐493. 10.2337/dc18-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin L, Peng K, Du R, et al. High glomerular filtration rate is associated with arterial stiffness in Chinese population. J Hypertens. 2017;35(2):385‐391. 10.1097/HJH.0000000000001158 [DOI] [PubMed] [Google Scholar]
- 37. Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target‐organ damage in hypertensive subjects. Hypertension. 2007;50(2):325‐332. [DOI] [PubMed] [Google Scholar]
- 38. Okada H, Fukui M, Tanaka M, et al. Visit‐to‐visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220(1):155‐159. [DOI] [PubMed] [Google Scholar]
- 39. Kikuya MHA, Ohokubo T, Tsuji I, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36(5):901‐906. [DOI] [PubMed] [Google Scholar]
- 40. Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet. 2003;362(9391):1211‐1224. [DOI] [PubMed] [Google Scholar]
- 41. Masato E, Kenji T, Masahiro A, et al. Reduced endothelial vasomotor function and enhanced neointimal formation after vascular injury in a rat model of blood pressure lability. Hypertens Res. 2003;26(12):991‐998. [DOI] [PubMed] [Google Scholar]
- 42. Rothwell PM. Limitations of the usual blood‐pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938‐948. [DOI] [PubMed] [Google Scholar]
- 43. Wu AYT, Low LP. Managing vascular risk in hypertension with a focus on microalbuminuria: attitude and practices. Singapore Med J. 2009;50(10):976‐981. [PubMed] [Google Scholar]
- 44. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375(9718):906‐915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


