Abstract
Studies have shown that maternal blood pressure level is associated with neonatal birthweight, but the results are not exactly consistent. As the most common hypertensive disorders during pregnancy, the mechanism of gestational hypertension and pre‐eclampsia that affect fetal growth remain unclear. Our objective was to examine the association of gestational hypertension and pre‐eclampsia with the risk of low birthweight (LBW) and small‐for‐gestational‐age (SGA). Data were obtained from the China–US Collaborative Project for Neural Tube Defects Prevention, a large population‐based cohort study. We selected participants who were registered in two southern provinces, had exact information on gestational blood pressure and pregnancy outcomes, and were not affected by chronic hypertension. Logistic regression was used to adjust for the effects of the main potential confounders, including age, body mass index, education, occupation, ethnicity, folic acid use, and parity. The overall incidences of LBW and SGA were 2.25% and 5.86%, respectively. The incidences of LBW/SGA were 3.58%/7.58% and 6.02%/10.67% for gestational hypertension and pre‐eclampsia group, relative to 2.11%/5.68% and 2.16%/5.74% for normal group. The adjusted odds ratios associated with gestational hypertension/pre‐eclampsia were 1.77 (95% CI: 1.63, 1.92)/3.01 (95% CI: 2.67, 3.40) for LBW and 1.40 (95% CI: 1.32, 1.48)/2.02 (95% CI: 1.84, 2.22) for SGA, respectively. The early onset of gestational hypertension/pre‐eclampsia appeared to be a relatively more detrimental exposure window for both LBW and SGA. Our results support an association between gestational hypertension or pre‐eclampsia and the increased risk of LBW and SGA.
Keywords: cohort study, gestational hypertension, low birthweight, pre‐eclampsia, small‐for‐gestational‐age
1. INTRODUCTION
Low birthweight (LBW) remains an important global public health problem, and it plays a vital role in determining the survival of newborns in a vulnerable condition. 1 LBW is associated with offspring's morbidity and mortality, growth disorders, and also chronic diseases in later life. 2 It is estimated that more than 20 million babies are born with LBW worldwide annually. 3 The global incidence of LBW has been reported as 15.5%, and Asia accounts for 75% of these infants. 4 , 5 LBW infants delivered at term usually exhibit inadequate growth for gestational age, whereas those delivered pre‐term include those with normal growth for gestational age and those with inadequate growth for gestational age. Abnormal blood pressure levels of pregnant women may be closely related to these adverse pregnancy outcomes. 6 , 7
Gestational hypertension and pre‐eclampsia are the most common hypertensive disorders of pregnancy, and they refer to women who develop novel hypertension after 20 weeks of gestation with/without proteinuria. 8 There have been conflicting results between gestational hypertension/pre‐eclampsia and poor fetal growth. 9 , 10 , 11 Many epidemiologic findings suggested that women with both gestational hypertension and pre‐eclampsia would contribute to a high occurrence of LBW or small‐for‐gestational‐age (SGA), 12 , 13 , 14 some studies even found women with pre‐eclampsia have a greater risk than gestational hypertension. 15 , 16 Xiong et al 17 investigated the effect of different types of hypertensive disorders of pregnancy on fetal growth with a retrospective cohort study in southern China. Their results showed that both pre‐eclampsia and gestational hypertension increased the risk of SGA after controlling for potential confounders. However, neither gestational hypertension nor pre‐eclampsia was associated with LBW. The different clinical manifestations, different spectrums of gestational hypertension/pre‐eclampsia, 18 the definition of LBW/SGA, 19 , 20 and whether important confounders have been accounted for 17 , 21 may all affect the consistency of diverse researches.
Therefore, we used the data from a large prospective population‐based cohort study to investigate whether gestational hypertension or pre‐eclampsia has any impact on the risk of LBW and SGA in China. In addition, we also assessed whether the impact of disease‐onset time (early‐ or late‐onset hypertensive disorders of pregnancy) on LBW and SGA is the same.
2. METHODS
2.1. Background and original cohort
The methods of the original study have been described previously. 22 , 23 Beginning in 1993, the Chinese Ministry of Health has conducted a public health campaign to prevent neural tube defects in 21 counties among women in two southern provinces (Zhejiang and Jiangsu) and one northern province (Hebei). During this campaign, all female residents who were planning to get married, or who became pregnant in the project counties, were registered in a pregnancy monitoring system. This system was used as the principal record of antenatal care and the source of demographic information. All women were advised to take a pill solely containing 400 μg of folic acid every day, starting at the time of registration on the pregnancy monitoring system, and continuing until completion of the first trimester of pregnancy. The pills were distributed at the time of registration if the woman agreed to take folic acid. At the end of each month, health care workers recorded the dates of all menstrual periods and how many pills remained in each bottle (if the subject was taking pills). All births at 20 complete gestational weeks, including live births, stillbirths and pregnancy terminations, and all structural congenital anomalies, regardless of gestational week, were recorded. The original cohort included 247 831 women who registered with the pregnancy monitoring system between October 1993 and September 1995 and who delivered by 31 December 1996. The project was approved by the institutional review boards of the US Centers for Disease Control and Prevention and the Peking University Health Science Center. Since rural women in the study area were mostly illiterate in the early 1990s, all women who took pills provided oral informed consent.
3. SELECTION OF STUDY SUBJECTS
We selected the participants who were registered in two southern provinces (Jiangsu Province and Zhejiang Province). These two neighboring provinces had detailed records about hypertensive disorders of pregnancy in their pregnancy monitoring system. Of 215 871 women from the selected provinces, we excluded: 3247 (1.50%) with outcomes of major external birth defects, neonatal death or stillbirth; 2155 (1.00%) for whom gestational age was unknown, <28 weeks, or >45 weeks 24 ; 8749 (4.05%) with multifetal gestation; 5711 (2.65%) women whose gestational hypertension diagnosis was unknown; and 8525 (3.95%) with unknown or outlier infant birthweight. After these exclusions, 201 484 participants (93.34% of the targeted population) were included in the final analysis. Information regarding formation of the target recruitment population, and derivation of the population used in the final analysis, is shown in Figure 1.
FIGURE 1.
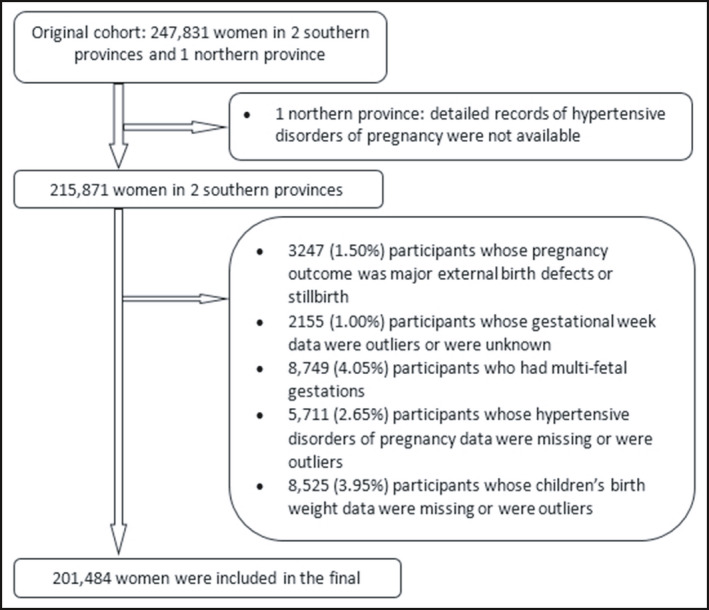
Flowchart of participants
3.1. Diagnosis of gestational hypertension and pre‐eclampsia
Appropriate cuff bladder size was determined at each visit based on arm circumference. Blood pressure was measured in the right arm with a mercury sphygmomanometer and was observed on two or more consecutive occasions with an interval of ≥6 h. Gestational hypertension was defined as an absolute blood pressure ≥140/90 mm Hg after 20 weeks of gestation, or as a blood pressure increment of ≥30/15 mm Hg after 20 weeks of gestation as compared with the first trimester. 25 Pre‐eclampsia (including eclampsia) was defined as a blood pressure of ≥140/90 mm Hg or a blood pressure increment of 30/15 mm Hg after 20 weeks of gestation, with concurrent proteinuria (a single random urine specimen containing at least 1+ protein by dipstick test) after 20 weeks of gestation.
3.2. Statistical analysis
We compared the mean age and body mass index (BMI), and distributions of folic acid use, parity, ethnic origin, education, and occupation between gestational hypertension and non‐gestational hypertension subjects. Missing values of occupation and education were imputed using multiple imputation method (five repetitions). For the basic characteristics of different groups, we use Student's t test for quantitative variables and the chi‐square test for categorical variables. We calculated the incidences of LBW or SGA in different characteristic groups, respectively. Logistic regression models were used to calculate odds ratios (ORs) after adjusting for the main underlying confounders such as maternal age (continuous), BMI (continuous), education, occupation, parity, ethnicity, folic acid use, and anemia during pregnancy. The method we used was backward in multivariate logistic regression. Considering the different heritability, clinical manifestations, and prognosis of early‐ and late‐onset gestational hypertension and pre‐eclampsia, 8 , 26 we classified gestational hypertension and pre‐eclampsia into two types: early‐onset (onset at <28th week of gestation) and late‐onset (onset at ≥28th week of gestation), and then compared the distribution of LBW and SGA according to different onset time of gestational hypertension and pre‐eclampsia. The mean BMI was used if individual data were missing. All data were analyzed using SPSS for Windows software (ver. 20.0; SPSS Inc). Statistical significance was defined as two‐sided p < .05.
4. RESULTS
The final study population included 201 484 women. Table 1 summarized the demographic and reproductive characteristics of those patients with gestational hypertension. Nearly, all participants were of the Han ethnicity. 19 267 (9.56%) had gestational hypertension and 4901 (2.43%) had pre‐eclampsia, respectively. Compared with the non‐gestational hypertension group, women with gestational hypertension tended to be older, of greater body sizes, to be primiparous, more likely occurring anemia, less educated, and were more likely to be factory workers.
TABLE 1.
Characteristics of women who enrolled in the pregnancy monitoring system according to gestational hypertension, China, 1993 to 1996
| Characteristics | Gestational hypertension group (n = 19 267) | Non‐gestational hypertension group (n = 182 217) | p | ||
|---|---|---|---|---|---|
| n a | % b | n a | % b | ||
|
Age at pregnancy (years, mean [SD]) |
25.03 (3.35) | 24.86 (3.19) | <.001 | ||
|
Body mass index (kg/m2, mean [SD]) |
20.78 (2.25) | 20.50 (2.09) | <.001 | ||
| Primiparous | 16 393 | 85.08 | 151 015 | 82.88 | <.001 |
| Han ethnic group | 19 123 | 99.25 | 180 887 | 99.27 | .786 |
| Folic acid use | 10 313 | 53.53 | 95 246 | 52.27 | .001 |
| Anemia during pregnancy | 12 714 | 80.45 | 116 494 | 79.56 | .009 |
| Education | |||||
| High school or higher | 2019 | 10.48 | 20 126 | 11.05 | .055 |
| Junior high school | 11 490 | 59.64 | 108 184 | 59.37 | |
| Primary school or lower | 5758 | 29.89 | 53 907 | 29.58 | |
| Occupation | |||||
| Farmer | 11 039 | 57.29 | 108 008 | 59.27 | <.001 |
| Factory worker | 5477 | 28.43 | 49 702 | 27.28 | |
| Other | 2751 | 14.28 | 24 507 | 13.45 | |
Abbreviation: SD, standard deviation.
Values for some characteristics may not be equal to total numbers of gestational hypertension or non‐gestational hypertension groups because of missing values.
Values for some characteristics may not be equal to 100 because of rounding.
The incidences of LBW and SGA and associations with different characteristics were shown in Table 2. The incidences of LBW/SGA were 3.58%/7.58% and 6.02%/10.67% for women with gestational hypertension and pre‐eclampsia, relative to 2.11%/5.68% and 2.16%/5.74% for women with normal blood pressure group. Compared as women with normal blood pressure group, the ORs of LBW/SGA regard to those with gestational hypertension and pre‐eclampsia were 1.72 (95% CI: 1.58, 1.87)/1.36 (95% CI: 1.29, 1.44) and 2.90 (95% CI: 2.57, 3.28)/1.96 (95% CI: 1.79, 2.15). Younger age, less body mass index, lower education, factory worker, being primiparous, non‐Han ethnicity, and non‐folic acid use were associated with elevated risk of LBW or SGA. The results before imputation for missing data of education and occupation were close to the results above.
TABLE 2.
Incidence and crude ORs of low birthweight (LBW) and small‐for‐gestational‐age (SGA) according to gestational hypertension, pre‐eclampsia, and other women's characteristics, China, 1993 to 1996
| Characteristics | No. | LBW | SGA | ||||
|---|---|---|---|---|---|---|---|
| Incidence (%) | OR | 95% CI | Incidence (%) | OR | 95% CI | ||
| Age, year | |||||||
| <20 | 922 | 4.66 | 2.03 | 1.49, 2.77 | 8.35 | 1.36 | 1.08, 1.73 |
| 20–25 | 115 995 | 2.35 | 1 | — | 6.26 | 1 | — |
| 25–30 | 60 323 | 2.24 | 0.95 | 0.89, 1.02 | 5.65 | 0.90 | 0.86, 0.94 |
| ≥30 | 24 244 | 1.74 | 0.74 | 0.67, 0.82 | 4.36 | 0.68 | 0.64, 0.73 |
| Body mass index, kg/m2 | |||||||
| <18.5 | 28 695 | 2.94 | 1.37 | 1.27, 1.48 | 8.10 | 1.49 | 1.43, 1.57 |
| 18.5–23.9 | 161 069 | 2.17 | 1 | — | 5.57 | 1 | — |
| 24–27.9 | 10 765 | 1.71 | 0.78 | 0.68, 0.91 | 4.37 | 0.77 | 0.70, 0.85 |
| ≥28 | 955 | 1.88 | 0.87 | 0.54, 1.38 | 3.77 | 0.66 | 0.48, 0.93 |
| Education | |||||||
| High school or higher | 22 145 | 1.83 | 1 | — | 4.75 | 1 | — |
| Junior high school | 119 674 | 2.19 | 1.20 | 1.08, 1.33 | 5.70 | 1.21 | 1.13, 1.30 |
| Primary school or lower | 59 665 | 2.55 | 1.40 | 1.26, 1.57 | 6.60 | 1.42 | 1.32, 1.52 |
| Occupation | |||||||
| Farmer | 119 047 | 2.24 | 1 | — | 5.86 | 1 | — |
| Factory worker | 55 179 | 2.41 | 1.08 | 1.01, 1.15 | 6.29 | 1.08 | 1.03, 1.12 |
| Other | 27 258 | 2.02 | 0.90 | 0.82, 0.99 | 5.01 | 0.85 | 0.80, 0.90 |
| Parity | |||||||
| Multiparous | 34 076 | 1.92 | 1 | — | 4.21 | 1 | — |
| Primiparous | 167 408 | 2.32 | 1.21 | 1.12, 1.32 | 6.20 | 1.50 | 1.42, 1.59 |
| Ethnicity | |||||||
| Han | 200 010 | 2.24 | 1 | — | 5.85 | 1 | — |
| Other | 1474 | 3.53 | 1.59 | 1.21, 2.10 | 7.46 | 1.30 | 1.07, 1.58 |
| Folic acid use | |||||||
| None | 95 925 | 2.37 | 1 | — | 5.80 | 1 | — |
| Use | 105 559 | 2.14 | 0.90 | 0.85, 0.96 | 5.92 | 1.02 | 0.99, 1.06 |
| Anemia during pregnancy | |||||||
| No | 33 016 | 2.19 | 1 | — | 5.96 | 1 | — |
| Yes | 129 208 | 2.16 | 0.98 | 0.91, 1.07 | 5.79 | 0.97 | 0.92, 1.02 |
| Gestational Hypertension | |||||||
| No | 182 217 | 2.11 | 1 | — | 5.68 | 1 | — |
| Yes | 19 267 | 3.58 | 1.72 | 1.58, 1.87 | 7.58 | 1.36 | 1.29, 1.44 |
| Pre‐eclampsia | |||||||
| No | 196 583 | 2.16 | 1 | — | 5.74 | 1 | — |
| Yes | 4901 | 6.02 | 2.90 | 2.57, 3.28 | 10.67 | 1.96 | 1.79, 2.15 |
Abbreviation: CI, confidence interval; and OR, odds ratio.
After adjustment for the effects of these major confounding factors, gestational hypertension and pre‐eclampsia were still associated with increased risks of LBW/SGA with the ORs were 1.77 (95% CI: 1.63, 1.92)/1.40 (95% CI: 1.32, 1.48) and 3.01 (95% CI: 2.67, 3.40)/2.02 (95% CI: 1.84, 2.22), respectively (Table 3). We further compared the distribution of LBW and SGA cases according to different disease‐onset time in Table 4. Although statistically significant in both early‐ and late‐onset patients, the associations between gestational hypertension/pre‐eclampsia with LBW and SGA appear stronger in early‐onset pregnancies than in late‐onset pregnancies. Considering that the history of pregnancy, delivery and other characteristics in multiparous population were substantially different from that in primiparous population, sensitivity analysis was performed to avoid the potential effect of parity on LBW and SGA. After the exclusion of 34 076 (16.9%) multiparous women, the remaining 167 408 primiparous women were included in the sensitivity analysis. The results of sensitivity analysis were similar to the above results.
TABLE 3.
The association of gestational hypertension and preeclampsia with low birthweight (LBW) and small‐for‐gestational‐age (SGA) in multivariate logistic regression, China, 1993 to 1996
| Risk factor | LBW | SGA | ||
|---|---|---|---|---|
| Adjusted OR a | 95% CI | Adjusted OR a | 95% CI | |
| Age (continuous) | 1.04 | 1.02, 1.05 | 1.02 | 1.01, 1.03 |
| Body mass index (continuous) | 1.10 | 1.09, 1.12 | 1.11 | 1.10, 1.12 |
| Factory worker | 1.09 | 1.02, 1.17 | 1.07 | 1.02, 1.11 |
| Other occupation | 0.97 | 0.88, 1.08 | 0.88 | 0.82, 0.94 |
| Junior high school | 1.20 | 1.08, 1.35 | 1.20 | 1.12, 1.29 |
| Primary school or lower | 1.50 | 1.33, 1.70 | 1.53 | 1.41, 1.65 |
| Primiparous | 1.06 | 0.95, 1.18 | 1.39 | 1.29, 1.49 |
| Other race | 1.59 | 1.20, 2.10 | 1.29 | 1.06, 1.57 |
| Folic acid use | 0.83 | 0.78, 0.88 | 0.92 | 0.88, 0.95 |
| Gestational hypertension | ||||
| No | 1 | — | 1 | — |
| Yes | 1.77 | 1.63, 1.92 | 1.40 | 1.32, 1.48 |
| Pre‐eclampsia | ||||
| No | 1 | — | 1 | — |
| Yes | 3.01 | 2.67, 3.40 | 2.02 | 1.84, 2.22 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for maternal age (continuous), BMI (continuous), education, occupation, parity, ethnicity, folic acid use, and anemia during pregnancy.
TABLE 4.
The early onset and late onset of gestational hypertension and pre‐eclampsia with risk of low birthweight (LBW) and small‐for‐gestational‐age (SGA)
| Different onset times | LBW | SGA | |||||
|---|---|---|---|---|---|---|---|
| No. | Incidence (%) | Crude OR (95% CI) | Adjusted OR (95% CI) a | Incidence (%) | Crude OR (95% CI) | Adjusted OR (95% CI) a | |
| Gestational hypertension | |||||||
| No b | 182 217 | 2.11 | 1 | 1 | 5.68 | 1 | 1 |
| Early‐onset | 833 | 5.28 | 2.58 (1.90–3.50) | 2.71 (1.99–3.68) | 8.16 | 1.48 (1.15–1.89) | 1.54 (1.20–1.98) |
| Late‐onset | 18 434 | 3.50 | 1.68 (1.54–1.83) | 1.73 (1.59–1.88) | 7.55 | 1.36 (1.28–1.44) | 1.39 (1.31–1.47) |
| Pre‐eclampsia | |||||||
| No b | 196 583 | 2.16 | 1 | 1 | 5.74 | 1 | 1 |
| Early‐onset | 89 | 17.98 | 9.93 (5.78–17.07) | 10.55 (6.12–18.20) | 16.85 | 3.33 (1.91–5.80) | 3.54 (2.02–6.19) |
| Late‐onset | 4812 | 5.80 | 2.79 (2.46–3.16) | 2.89 (2.55–3.28) | 10.56 | 1.94 (1.77–2.13) | 1.99 (1.81–2.19) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for maternal age (continuous), BMI (continuous), education, occupation, parity, ethnicity, folic acid use, and anemia during pregnancy.
Reference group.
5. DISCUSSION
In this large population‐based prospective cohort study, we explored the association of gestational hypertension and pre‐eclampsia with the risk of LBW and SGA. We found that both gestational hypertension and pre‐eclampsia increased infants in terms of LBW and SGA status. The effects were evident in women diagnosed with both early and late onset of gestational hypertension as well as pre‐eclampsia. We also found a stronger risk increase in terms of LBW and SGA status in early‐onset cases.
Our result is consistent with many similar findings in epidemiologic studies. 14 , 20 , 27 Jaddoe et al 14 reported that infant birthweight would reduce 16.9 and 50.6 g for per one‐SD increment in systolic blood pressure and diastolic blood pressure at mean gestation of 30.2 weeks (range 28.4–32.9 weeks). In a prospective early life cohort study of 1162 subjects, Pan et al 12 also found higher second‐trimester blood pressure (median gestation of 27 weeks) was associated with smaller offspring size at birth, particularly in Chinese women. However, some other studies found that only severe gestational hypertension or pre‐eclampsia could lead to an increase in the incidence of LBW or SGA. 18 , 20 , 28 A retrospective cohort study found a U‐shaped association between diastolic blood pressure and infant birthweight after 34 weeks of gestation. 29 The conflicting findings of gestational hypertension or pre‐eclampsia as a predictor of LBW may be explained by the differences in the characteristics of study populations, the types of study design and the outcome definition used. Hypertensive disorders of pregnancy, especially gestational hypertension and pre‐eclampsia, may play an important role in infants born with LBW and SGA. Those findings gave us clues that management of elevated blood pressure levels in pregnancy is essential in the prevention of LBW and SGA status.
We also found stronger effect among pre‐eclampsia pregnancies than gestational hypertension pregnancies. Our results were comparable to the study by Jaddoe et al 14 Changes in diastolic blood pressure during pregnancy are more closely related to fetal growth than systolic blood pressure. 9 , 10 , 29 Higher diastolic blood pressure level was also considered as a risk factor for pre‐eclampsia. In non‐hypertensive pregnant women, blood pressure, especially diastolic blood pressure, steadily decreases during the second trimester of pregnancy and then rises again until delivery. But in women with pre‐eclampsia, this intermediate drop in blood pressure would not occur. In contrast, blood pressure stabilized during the first half of pregnancy and then continued to increase until delivery. 30 As a result, women with pre‐eclampsia were more susceptible to increased diastolic blood pressure and are at greater risk of giving birth to infants with LBW or SGA. Other studies have found that uterine placental vascular disease is also a risk factor for pre‐eclampsia. 31 Poor remodeling of the spiral arteries would lead to decreased oxygen and nutrients delivery to the developing fetus, resulting in fetal growth restriction. The hypoxia placenta also released anti‐angiogenic factors to the mother in turn, causing the mother's inflammatory response (including endothelial dysfunction and elevated blood pressure, et al), which may be the cause of pre‐eclampsia. 27 These studies also suggested that pre‐eclampsia and gestational hypertension may have different effect mechanisms on fetal growth and development. Women with different severe spectrum of pregnancy hypertensive disorders would have different physiological status. Our study further compared the effects among pre‐eclampsia pregnancies and gestational hypertension pregnancies. For women with pre‐eclampsia, more attention should be paid to the intrauterine growth and development of the fetus.
Our results also suggested that the earlier hypertensive disorders of pregnancy occurs, the more likely it would affect fetal intrauterine growth and development. Few literatures have compared the effects of gestational hypertension and pre‐eclampsia occurring at different time on adverse pregnancy outcomes. A recent case‐control study found that compared with the late‐onset pre‐eclampsia group (>34 weeks), the early‐onset pre‐eclampsia group had lower average birthweight of infants and a higher incidence of other adverse pregnancy outcomes, such as preterm birth. 32 Jelin et al 33 also found that the rate of SGA in newborns of women with early‐onset pre‐eclampsia was three times higher than that of women without pre‐eclampsia (18% vs 6%). This is consistent with the results of our study. The Avon longitudinal study of parents and children suggested the fetal growth would be more restricted with the blood pressure increased from the 18 weeks of gestation. 27 The increase of diastolic blood pressure in the second trimester (18–30 weeks) had a significantly higher impact on LBW and SGA than that in the third trimester (30–36 weeks), and this was true even in pregnant women whose blood pressure did not cross the threshold for hypertensive disorders of pregnancy. Birthweight is mainly determined by the duration of intrauterine growth, so a longer abnormal physiological status would cause more LBW and SGA cases. Together with above findings, we thought that early or late onset of hypertensive disorders of pregnancy may result from distinct mechanisms and the effect should be observed separately.
The most common pathophysiologic mechanism of LBW and SGA status is decreased uteroplacental blood perfusion. Higher blood pressure during pregnancy is characterized by both endothelial dysfunction and decreased placental perfusion. 34 Uteroplacental vasculopathy, including histopathologic lesions and intravascular coagulation, is associated with fetal growth restriction. 35 There are also animal models suggesting that hypertension during pregnancy is associated with inadequate perfusion of placental blood flow. 36 , 37 Increased blood pressure may also affect the development of the placental villous tree and lead to the decline of placental function, thus resulting in reduced fetal growth and lower birthweight. 38
There were several strengths in our study. The work was a population‐based, prospective cohort study. We collected data on exposure and outcomes, thereby minimizing the risks of selection and recall bias. Hypertension was diagnosed by measuring blood pressure directly, thus minimizing the likelihood of misclassification bias. In addition, we evaluated LBW and SGA while excluding congenital malformations and stillbirths, thereby reducing information bias. The environment, climate and living habits are similar in both regions, and the population is ethnically homogenous with more than 99% of our population being Han Chinese. The sample size was large enough for us to detect both overall and subgroup effects. Detailed clinical records enabled us to examine associations among more subtypes of LBW, and different gestational ages of gestational hypertensive disorders.
Limitations should also be acknowledged in the interpretation of our findings. This study was based on existing data, some unmeasured confounding factors, such as maternal smoking and drinking, could not be accounted for. However, smoking and drinking were both rare in Chinese women at the time of our study, especially among reproductive‐age women living in rural regions. A survey conducted in 1996 on the national prevalence of smoking in China showed that the smoking prevalence among women aged 20–29 years was <2%. 39 Another limitation in our study pertained the population selection. The original study cohort included two southern provinces and one northern province. Our current analysis did not include the northern province because detailed clinical records of hypertensive diagnosis during pregnancy were not available. Finally, we calculated gestational age based on menstrual dates, which could potentially lead to misclassification of SGA outcomes, resulting in an underestimation of risk.
Overall, this study provided a comprehensive insight into the impact of different conditions of hypertension in pregnancy (gestational hypertension and pre‐eclampsia, respectively) on LBW and SGA, and further compared whether the effects vary on different disease‐onset time. Our results confirm that gestational hypertension and pre‐eclampsia could both significantly increase the risk of LBW and SGA, and this effect was even more obvious in early‐onset groups. Pre‐eclampsia has a greater influence than gestational hypertension on the etiology of LBW and SGA status. Our findings may contribute data important with respect to the modification of public health policies for prevention of LBW. We also suggest that hypertensive disorders of pregnancy represents a major public health challenge, such that the Chinese government should take prophylactic measures to increase awareness of the health risks associated disorders of pregnancy represents.
CONFLICT OF INTEREST
All authors read and approved the final manuscript and none of conflict of interest or competing interest consists.
AUTHOR CONTRIBUTIONS
Yingying Liu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Yingying Liu, Nan Li, and Rongwei Ye. Acquisition of data: Yingying Liu, Zhiwen Li, Nan Li, and Rongwei Ye. Analysis and interpretation of data: Yingying Liu, Nan Li, Hang An, Le Zhang, Hongtian Li, and Yali Zhang. Drafting of the manuscript: Yingying Liu. Critical revision of the manuscript for important intellectual content: Yingying Liu, Nan Li, Rongwei Ye, and Zhiwen Li. Statistical analysis: Yingying Liu and Nan Li, and Hang An. Obtained funding: Nan Li, Zhiwen Li, and Rongwei Ye. Administrative, technical, and material support: Nan Li, Hang An, Le Zhang, Zhiwen Li, and Rongwei Ye.
ACKNOWLEDGEMENTS
The authors thank all of the volunteers and staff involved in this research.
Liu Y, Li N, An H, et al. Impact of gestational hypertension and preeclampsia on low birthweight and small‐for‐gestational‐age infants in China: A large prospective cohort study. J Clin Hypertens. 2021;23:835–842. 10.1111/jch.14176
Funding information
Nan Li was supported by Natural Science Foundation of Beijing Municipality (7194285), the National Natural Science Foundation of China (81373014), the startup funding from the “Incubation” Program of China and Peking University Health Science Center (No. BMU2017YB003) and Young Elite Scientist Sponsorship Program by CAST (YESS) (2018QNRC001). The original project was supported by a cooperative agreement between the US Centers for Disease Control and Prevention and Peking University (Grant No. U01 DD000293).
Contributor Information
Nan Li, Email: linan01@pku.edu.cn.
Rongwei Ye, Email: yerw@bjmu.edu.cn.
REFERENCES
- 1. Lake EA, Olana Fite R. Low birth weight and its associated factors among newborns delivered at Wolaita Sodo University Teaching and Referral Hospital, Southern Ethiopia, 2018. Int J Pediatr. 2019;2019:4628301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tchamo ME, Prista A, Leandro CG. Low birth weight, very low birth weight and extremely low birth weight in African children aged between 0 and 5 years old: a systematic review. J Dev Orig Health Dis. 2016;7(4):408‐415. [DOI] [PubMed] [Google Scholar]
- 3. UNICEF, WHO . Low Birthweight: Country, Regional and GLOBAL estimates. New York, NY: UNICEF; 2004. [Google Scholar]
- 4. Silveira MF, Victora CG, Horta BL, et al. Low birthweight and preterm birth: trends and inequalities in four population‐based birth cohorts in Pelotas, Brazil, 1982–2015. Int J Epidemiol. 2019;48:i46‐i53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhaskar RK, Deo KK, Neupane U, et al. A case control study on risk factors associated with low birth weight babies in Eastern Nepal. Int J Pediatr. 2015;2015:807373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li N, Li Z, Ye R, et al. Preconception blood pressure and risk of low birth weight and small for gestational age: a large cohort study in China. Hypertension. 2016;68(4):873‐879. [DOI] [PubMed] [Google Scholar]
- 7. Li N, Li Z, Ye R, et al. Preconception blood pressure and risk of preterm birth: a large cohort study in China. J Hypertens. 2016;34(11):2243‐2247. [DOI] [PubMed] [Google Scholar]
- 8. Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. 2013;61(4):873‐879. [DOI] [PubMed] [Google Scholar]
- 9. Waugh J, Perry IJ, Halligan AW, et al. Birth weight and 24‐hour ambulatory blood pressure in nonproteinuric hypertensive pregnancy. Am J Obstet Gynecol. 2000;183(3):633‐637. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet Gynecol. 2001;97(2):261‐267. [DOI] [PubMed] [Google Scholar]
- 11. Wu Y, Ma Y, Wu K, et al. Blood pressure in early and mid‐pregnancy and the risk of small‐for‐gestational‐age birth: findings of a large cohort study in China. J Hum Hypertens. 2019;33(6):475‐481. [DOI] [PubMed] [Google Scholar]
- 12. Lim WY, Lee YS, Tan CS, et al. The association between maternal blood pressures and offspring size at birth in Southeast Asian women. BMC Pregnancy Childbirth. 2014;14:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yadav H, Lee N. Maternal factors in predicting low birth weight babies. Med J Malaysia. 2013;68(1):44‐47. [PubMed] [Google Scholar]
- 14. Bakker R, Steegers EA, Hofman A, Jaddoe VW. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol. 2011;174(7):797‐806. [DOI] [PubMed] [Google Scholar]
- 15. Xiong X, Demianczuk NN, Buekens P, Saunders LD. Association of preeclampsia with high birth weight for age. Am J Obstet Gynecol. 2000;183(1):148‐155. [DOI] [PubMed] [Google Scholar]
- 16. Eskild A, Romundstad PR, Vatten LJ. Placental weight and birthweight: does the association differ between pregnancies with and without preeclampsia? Am J Obstet Gynecol. 2009;201(6):595.e591‐e595. [DOI] [PubMed] [Google Scholar]
- 17. Xiong X, Fraser WD. Impact of pregnancy‐induced hypertension on birthweight by gestational age. Paediatr Perinat Epidemiol. 2004;18(3):186‐191. [DOI] [PubMed] [Google Scholar]
- 18. Ye RW, Li HT, Ma R, Ren AG, Liu JM. Prospective cohort study of pregnancy‐induced hypertension and risk of preterm delivery and low birth weight. Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44(1):70‐74. [PubMed] [Google Scholar]
- 19. Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155(3):203‐209. [DOI] [PubMed] [Google Scholar]
- 20. Lau TK, Pang MW, Sahota DS, Leung TN. Impact of hypertensive disorders of pregnancy at term on infant birth weight. Acta Obstet Gynecol Scand. 2005;84(9):875‐877. [DOI] [PubMed] [Google Scholar]
- 21. Kidanto HL, Mogren I, Massawe SN, Lindmark G, Nystrom L. Criteria‐based audit on management of eclampsia patients at a tertiary hospital in Dar es Salaam, Tanzania. BMC Pregnancy Childbirth. 2009;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berry RJ, Li Z, Erickson JD, et al. Prevention of neural‐tube defects with folic acid in China. China‐U.S. Collaborative project for neural tube defect prevention. N Engl J Med. 1999;341(20):1485‐1490. [DOI] [PubMed] [Google Scholar]
- 23. Gindler J, Li Z, Berry RJ, et al. Folic acid supplements during pregnancy and risk of miscarriage. Lancet. 2001;358(9284):796‐800. [DOI] [PubMed] [Google Scholar]
- 24. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163‐168. [DOI] [PubMed] [Google Scholar]
- 25. Group CNGHW . National epidemiological investigation of gestational hypertension (in Chinese). Chin J Gynecol Obstet. 1991;26:67‐70. [Google Scholar]
- 26. Hernandez‐Diaz S, Werler MM, Louik C, Mitchell AA. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am J Epidemiol. 2002;156(9):806‐812. [DOI] [PubMed] [Google Scholar]
- 27. Macdonald‐Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension. 2014;64(1):36‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66‐71. [DOI] [PubMed] [Google Scholar]
- 29. Steer PJ, Little MP, Kold‐Jensen T, Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study. BMJ. 2004;329(7478):1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hermida RC, Ayala DE, Iglesias M. Predictable blood pressure variability in healthy and complicated pregnancies. Hypertension. 2001;38(3 Pt 2):736‐741. [DOI] [PubMed] [Google Scholar]
- 31. Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):136‐145. [DOI] [PubMed] [Google Scholar]
- 32. Gui J, Xu W, Zhang J. Association between thyroid dysfunction and perinatal outcomes in women with gestational hypertension: a retrospective study. BMC Pregnancy Childbirth. 2020;20(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jelin AC, Cheng YW, Shaffer BL, Kaimal AJ, Little SE, Caughey AB. Early‐onset preeclampsia and neonatal outcomes. J Matern Fetal Neonatal Med. 2010;23(5):389‐392. [DOI] [PubMed] [Google Scholar]
- 34. Everett TR, Lees CC. Beyond the placental bed: placental and systemic determinants of the uterine artery Doppler waveform. Placenta. 2012;33(11):893‐901. [DOI] [PubMed] [Google Scholar]
- 35. Van Le L, Thorp JM. Placental vascular compromise: Unifying the etiologic pathways of perinatal compromise. Curr Probl Obstet Gynecol Fertil. 2001;24(6):201‐220. [Google Scholar]
- 36. Casper FW, Seufert RJ. Atrial natriuretic peptide (ANP) in preeclampsia‐like syndrome in a rat model. Exp Clin Endocrinol Diabetes. 1995;103(5):292‐296. [DOI] [PubMed] [Google Scholar]
- 37. Makris A, Thornton C, Thompson J, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT‐1. Kidney Int. 2007;71(10):977‐984. [DOI] [PubMed] [Google Scholar]
- 38. Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613‐621. [DOI] [PubMed] [Google Scholar]
- 39. Yang G, Fan L, Tan J, et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282(13):1247‐1253. [DOI] [PubMed] [Google Scholar]


