Abstract
Faster pulse wave velocity (PWV) is known to be associated with the incidence of cardiovascular diseases (CVD). The aim of this study was to clarify the hypothesis that PWV may be associated with future CVD events even when its time‐dependent changes were adjusted. We also investigated a prognostic significance of cardio‐ankle vascular index, another index of arterial stiffness. Study participants included 8850 community residents. The repeated measures of the clinical parameters at 5.0 years after the baseline were available for 7249 of the participants. PWV was calculated using the arterial waveforms measured at the brachia and ankles (baPWV). The cardio‐ankle vascular index was calculated by estimated pulse transit time from aortic valve to tibial artery. During the 8.53 years follow‐up period, we observed 215 cases of CVD. The incidence rate increased linearly with baPWV quartiles (per 10 000 person‐years: Q1, 2.7; Q2, 12.6; Q3, 22.5; Q4, 76.2), and the highest quartile was identified as an independent determinant of incident CVD by conventional Cox proportional hazard analysis adjusted for known risk factors [hazard ratio (HR), 4.00; p = .007]. Per unit HR of baPWV (HR, 1.15; p < .001) remained significant in the time‐dependent Cox regression analysis including baPWV and other clinical values measured at 5‐year after the baseline as time‐varying variables (HR, 1.14; p < .001). The cardio‐ankle vascular index was also associated with CVD with similar manner though the associations were less clear than that of baPWV. baPWV is a good risk marker for the incidence of CVD.
Keywords: cardio‐ankle vascular index, cardiovascular disease, general population, longitudinal study, pulse wave velocity
1. INTRODUCTION
Arterial stiffness is known to be a risk factor for the incidence of cardiovascular diseases (CVD) in the general population. 1 , 2 Pulse wave velocity (PWV) between the carotid and femoral arteries (cfPWV) is a standard measure of arterial stiffness. Although several devices for cfPWV measurement have been developed, 3 the applicability of cfPWV, particularly in primary care and public health settings, is limited owing to the requirements of specific skills for pulse waveform measurement at the carotid and femoral arteries.
Pulse wave velocity between the brachium and ankle (baPWV) is another index of PWV, which can be measured automatically by attaching cuffs connecting to plethysmography at both the brachia and ankles. baPWV is calculated from the time interval between the wave fronts of the brachial and ankle arterial waveforms and the path length from the brachia to the ankle calculated from the body height. 4 It has been reported that baPWV was closely correlated with cfPWV 5 and was associated with the incidence of stroke in a sub‐analysis of a randomized controlled trial in hypertensive patients, 6 the incidence of CVD in the general population, 7 and all‐cause 8 , 9 and cause‐specific 8 mortality in populations of older adults 8 or community residents. 9 Furthermore, our recent individual‐level meta‐analysis including 14 700 Japanese community residents and individuals at risk for CVD 10 observed a significant association between baPWV and the incidence of CVD. However, it was uncertain whether baPWV predicts the onset of CVD when changes in baPWV and other covariates during the follow‐up period were considered in the analysis. Clarifying the hypothesis that baPWV may be associated with future CVD events even when time‐dependent changes in the clinical measures were adjusted may further support the significance of baPWV as a marker of cardiovascular disease risks 11 because a general population‐based study usually requires a long‐term follow‐up period to clarify a risk factor‐outcome relationship.
Given these backgrounds, we aimed to clarify longitudinal associations between baPWV and CVD events with consideration for changes in baPWV and other time‐varying covariates during the follow‐up period by analyzing a dataset of a general population‐based longitudinal study with follow‐up measurement at 5 years after the baseline. We also investigated the prognostic significance of the cardio‐ankle vascular index (CAVI), 12 another index of arterial stiffness that can be calculated from estimated pulse transit time from aortic valve to tibial artery. 13 , 14
2. METHODS
2.1. Study design and setting
We conducted a longitudinal analysis of a dataset of the Nagahama study, 12 , 15 , 16 an ongoing longitudinal study based on the community residents of Nagahama City, a largely sub‐urban city of 125 000 inhabitants (at 2010) located in central Japan. Mean age of the citizens was 44.7 years, and 24% of them were older (≥65 years) persons. Study participants in the Nagahama study, aged between 30 and 74 years and who were living independently without physical impairment or dysfunction, were recruited between 2008 and 2010. Among the baseline participants (n = 9764), which corresponded to approximately 14% of total residents at the age group, a total of 8289 individuals participated in a follow‐up investigation performed 5 years after the baseline evaluations (between 2013 and 2015). After excluding those who died (n = 147) or who left Nagahama City (n = 273), the follow‐up rate corresponds to 88.7%. In this study, we investigated the associations between baseline baPWV and CAVI and the incidence of CVD during the follow‐up period until December 2018 by Cox proportional hazard analysis adjusted for major covariates measured at the baseline investigation. We also performed a longitudinal association analysis including the clinical values measured at the follow‐up investigation as time‐dependent variables by time‐dependent Cox proportional hazard analysis.
2.2. Study participants
Among the baseline population of the Nagahama study, a total of 8850 participants were ultimately included in this study after excluding individuals who met the following exclusion criteria: history of stroke or myocardial infarction (n = 283) but not suspected cases of heart failure and angina pectoris, pacemaker implantation or taking hemodialysis therapy at baseline or follow‐up measurements (n = 14), pregnant women (n = 42), incomplete measurement (n = 34) or large bilateral differences (Mahalanobis’ distance > 5; n = 51) in baPWV or CAVI, lack of urinary albumin value (n = 429, including women during menstruation), suspected cases of peripheral arterial diseases (ankle‐brachial index ≤ 0.8; n = 15), and incomplete or widely deviating clinical values required for this study (n = 46). History of stroke and myocardial infarction was queried using a structured questionnaire. The clinical data on the follow‐up investigation were available for 7249 participants after applying the same exclusion criteria. All study procedures were approved by the ethics committee of Kyoto University Graduate School of Medicine and by the Nagahama Municipal Review Board. Written informed consents were obtained from all participants.
2.3. Measurement of arterial parameters required for baPWV and CAVI calculations
The arterial waveforms and blood pressure (BP) at both the brachia and ankles were measured in the supine position after a few minutes of rest (Vasera‐1500; Fukuda Denshi Co., Ltd.). In brief, cuffs were attached to the brachia and ankles, and the pulse volume waveforms at four extremities were simultaneously recorded using a plethysmography sensor connected to the cuffs. Measurements were recorded for 10 s under compression of 50 mmHg. BP at the right arm and ankle was measured continuously by a conventional cuff‐oscillometric method, followed by the left arm and ankle measurements.
2.4. Calculations of baPWV and CAVI
baPWV was calculated by the time interval between the wave fronts of the brachial and ankle arterial waveforms and the path length from the brachia to the ankle calculated based on the body height. 4 CAVI was calculated using the following formula, 13 where Ps is systolic BP, Pd is diastolic BP, ∆P is pulse pressure, ρ is blood density (fixed value), and PWV is pulse wave velocity calculated using the estimated path length from the origin of the aorta to the tibial artery at the ankle and the estimated pulse transit time from aortic valve to tibial artery:
After excluding cases with large bilateral differences in baPWV or CAVI, we calculated the means of right and left side measurements and used the mean value for the statistical analyses.
2.5. Assessment of CVD events
CVD events were defined as major adverse cardiac events including first‐ever myocardial infarction, patients who underwent coronary artery bypass grafting or angioplasty, 17 , 18 and stroke, including cerebral infarction, as well as intracerebral and subarachnoid hemorrhage. CVD events were decided by an independent physician who has an in‐depth knowledge of the epidemiological study of CVD using the following clinical information.
The clinical information required for the diagnosis of CVD was obtained from personal medical records stored in the three emergency care hospitals (Nagahama City Hospital, Nagahama Red Cross Hospital, and Nagahama City Kohoku Hospital) located in Nagahama City. Well‐trained nurses and laboratory technicians routinely visited these hospitals to find suspected cases of CVD and obtain the clinical records required for the diagnosis. When additional information from other hospitals was required for the diagnosis, we requested the hospitals to provide the information. Death records stored at the local healthcare center were also reviewed to identify individuals who suddenly died because of acute myocardial infarction or stroke.
Stroke was defined as the sudden onset of neurological symptoms lasting for at least 24 h, including death associated with neurological deficits, according to the World Health Organization's Monitoring of Trends and Determinants in Cardiovascular Disease (WHO‐MONICA) project. 19 The diagnosis was based on the clinical information at the time of onset (time, signs, and neurological symptoms), clinical observations (presence of atrial fibrillation and level of consciousness), and impairment of neurological function. Computed tomography and magnetic resonance imaging were also used to classify stroke subtypes. Among the study participants who were diagnosed with stroke, 99.2% had computed tomography or magnetic resonance images.
Major adverse cardiac events were diagnosed in accordance with the WHO‐MONICA project 19 with consideration for symptoms at the time of onset, electrocardiogram findings, blood markers (including creatine kinase), and coronary angiography findings. We considered patients classified as definitive or probable MI by the WHO‐MONICA criteria, as well as individuals who underwent coronary revascularization, as having had major adverse cardiac events.
2.6. Basic clinical characteristics
Smoking habits and medication use were determined using a structured self‐administered questionnaire. Brachial BP was measured twice using a cuff‐oscillometric device (HEM‐9000AI; Omron Healthcare) after a few minutes of rest in the sitting position. The mean of the two readings was used as a representative value. Hypertension was defined as either systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or taking antihypertensive drugs. Diabetes was defined as either fasting (≥6 h after last meal) glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, hemoglobin A1c ≥6.5%, or using hypoglycemic drugs. Dyslipidemia was defined as either high‐density lipoprotein cholesterol of <40 mg/dl, low‐density lipoprotein cholesterol ≥140 mg/dl, or taking lipid‐lowering drugs. The estimated glomerular filtration rate was calculated using the following formula: 194 × creatinine−1.094 × age−0.287 (×0.739 if female). 20 Chronic kidney disease was defined as estimated glomerular filtration rate of <60 ml/min/1.73 m2 or albuminuria (urinary albumin ≥30 mg per day).
2.7. Statistical analysis
Values are expressed as mean ± standard deviation or frequency. Group differences in numeric variables were assessed by analysis of variance and frequency differences by a chi‐squared test. The incidence rate was calculated as per 10 000 person‐years. Cox proportional hazard analysis was used to identify factors independently associated with the incidence of CVD. Penalized cubic splines were applied to clarify the relationship between the arterial stiffness parameters and its cardiovascular disease risks. In the longitudinal association analysis including clinical values measured at the follow‐up investigation as time‐varying variables, a time‐dependent Cox proportional hazard model was applied for the analysis. In this analysis, each participant's follow‐up time was divided into two time windows (initial five years and the later follow‐up period), and the weighted average of all the time window‐specific results was calculated. 11 In cases in which those follow‐up measurements were not available, only the baseline measurements were included in the model. The proportional hazards assumptions were verified with a Schoenfeld residual test. Statistical analyses were performed using JMP 15.1.0 software (SAS Institute), and Cox proportional hazard analysis was assessed using R 4.0.0 software (The R Foundation) with the survival package. A p value of less than 0.05 was considered to indicate statistical significance.
3. RESULTS
The clinical characteristics of study participants at the baseline and follow‐up investigations are summarized in Table 1. There was a strong correlation between baseline baPWV and CAVI (r = 0.804; p <.001), as well as between baseline and follow‐up measurements (5.0 ± 0.4 years after the baseline) of baPWV (r = 0.832; p <.001) and CAVI (r = 0.820; p <.001). In addition to the well‐known risk factors for CVD, we included B‐type natriuretic peptide (BNP) in the analysis as a marker of potential cardiac functional decline. At the baseline, 48 participants showed BNP of more than 100 pg/ml, which is a conventional cutoff value of heart failure in a population‐based study.
TABLE 1.
Clinical characteristics of study participants
| Baseline (n = 8850) | Follow‐up (n = 7249) | |
|---|---|---|
| Age (years) | 53.9 ± 13.1 | 59.8 ± 12.6 |
| Male sex (%) | 33.5 | 33.5 |
| BMI (kg/m2) | 22.3 ± 3.3 | 22.2 ± 3.3 |
| Smoking habit (current/past/never, %) | 14.7/19.8/65.5 | 10.4/21.0/68.5 |
| Blood pressure | ||
| Systolic (mmHg) | 124 ± 18 | 125 ± 18 |
| Diastolic (mmHg) | 76 ± 11 | 72 ± 11 |
| Heart rate (beats/min) | 69 ± 10 | 68 ± 10 |
| Antihypertensive medication (%) | 16.3 | 25.5 |
| Hypertension (%) | 30.6 | 37.5 |
| Glycemic markers | ||
| Glucose (mg/dl) | 90 ± 15 | 88 ± 15 |
| HbA1c (%) | 5.5 ± 0.5 | 5.6 ± 0.5 |
| Hypoglycemic medication (%) | 2.7 | 5.0 |
| Diabetes (%) | 4.1 | 3.9 |
| Lipid markers | ||
| HDL cholesterol (mg/dl) | 65 ± 17 | 67 ± 17 |
| LDL cholesterol (mg/dl) | 124 ± 31 | 119 ± 29 |
| Lipid‐lowering medication (%) | 11.6 | 21.7 |
| Dyslipidemia (%) | 41.2 | 43.1 |
| Renal function | ||
| Creatinine (mg/dl) | 0.7 ± 0.2 | 0.7 ± 0.2 |
| eGFR (ml/min/1.73 m2) | 79.2 ± 15.6 | 76.5 ± 14.6 |
| Urinary albumin (mg/day) | 19 ± 81 | 18 ± 76 |
| Albuminuria (%) | 8.8 | 8.2 |
| CKD (%) | 16.0 | 17.0 |
| BNP (pg/ml) | 17.0 ± 19.3 | 21.9 ± 22.2 |
| baPWV (cm/s) | 1,265 ± 216 | 1,313 ± 239 |
| CAVI | 7.40 ± 1.09 | 7.91 ± 1.15 |
Values are mean ± standard deviation or frequency. Hypertension was defined as either systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg or taking antihypertensive drugs. Diabetes was defined as either fasting (≥6 h after last meal) glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, hemoglobin A1c (HbA1c) ≥6.5%, or using hypoglycemic drugs. Dyslipidemia was defined as either high‐density lipoprotein (HDL) cholesterol <40 mg/dl, low‐density lipoprotein (LDL) cholesterol ≥140 mg/dl, or taking lipid‐lowering drugs. Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, or albuminuria (urinary albumin ≥30 mg/day).
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; BNP, B‐type natriuretic peptide; CAVI, cardio‐ankle vascular index; HbA1c, hemoglobin A1c.
During the follow‐up period (mean duration, 8.53 years), we observed 215 incident cases of CVD (stroke, n = 96; MI, n = 115; both, n = 4). The incidence rate was increased with the quartiles of baPWV (Q1, <1102 cm/s; Q2, <1226 cm/s; Q3, <1395 cm/s; and Q4, ≥1395 cm/s) or CAVI (Q1, <6.59; Q2, <7.33; Q3, <8.14; and Q4, ≥8.14) (Table 2). The highest quartiles of baPWV and CAVI shared 122 CVD cases. The C‐statistics of baPWV and CAVI (continuous variables) calculated by Cox regression analysis without any adjustment were 0.772 and 0.755, respectively.
TABLE 2.
Incidence rate of CVD by the quartiles of baseline baPWV or CAVI
| Baseline baPWV quartiles | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| baPWV (cm/s) | 1,028 ± 54 | 1,163 ± 36 | 1,303 ± 48 | 1,563 ± 152 |
| Number of participants | 2201 | 2217 | 2209 | 2223 |
| Total participants | ||||
| Person‐years | 18 660 | 19 000 | 19 075 | 18 765 |
| Number of CVD | 5 | 24 | 43 | 143 |
| Incidence rate | 2.7 | 12.6 | 22.5 | 76.2 |
| Men | ||||
| Person‐years | 3951 | 6850 | 6903 | 7220 |
| Number of CVD | 2 | 14 | 24 | 88 |
| Incidence rate | 5.1 | 20.4 | 34.8 | 121.9 |
| Women | ||||
| Person‐years | 14 709 | 12 150 | 12 172 | 11 544 |
| Number of CVD | 3 | 10 | 19 | 55 |
| Incidence rate | 2.0 | 8.2 | 15.6 | 47.6 |
| Baseline CAVI quartiles | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| CAVI | 6.06 ± 0.43 | 6.97 ± 0.21 | 7.72 ± 0.23 | 8.86 ± 0.58 |
| Number of participants | 2187 | 2230 | 2228 | 2205 |
| Total participants | ||||
| Person‐years | 18 710 | 19 082 | 19 193 | 18 514 |
| Number of CVD | 10 | 23 | 43 | 139 |
| Incidence rate | 5.3 | 12.1 | 22.4 | 75.1 |
| Men | ||||
| Person‐years | 3966 | 5529 | 6392 | 9038 |
| Number of CVD | 4 | 9 | 17 | 98 |
| Incidence rate | 10.1 | 16.3 | 26.6 | 108.4 |
| Women | ||||
| Person‐years | 14 744 | 13 553 | 12 802 | 9476 |
| Number of CVD | 6 | 14 | 26 | 41 |
| Incidence rate | 4.1 | 10.3 | 20.3 | 43.3 |
Cardiovascular disease (CVD) includes symptomatic stroke, myocardial infarction, or percutaneous coronary intervention. The incidence rate is shown per 10 000 person‐years.
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CAVI, cardio‐ankle vascular index.
There were considerable differences in the baseline clinical characteristics among the quartiles of baPWV (Table S1) and CAVI (Table S2). However, Cox regression analysis adjusted for these factors identified the highest quartiles of baPWV as an independent risk factor for CVD (Table 3, Model 1). Similar results were observed in the analysis excluding participants who developed CVD within 1 year after the baseline investigation (n = 24) [hazard ratio (HR), 3.30; 95% confidence interval (CI), 1.19–9.20; p =.022], as well as in the analysis including baPWV as a continuous variable (Table 3, Model 2). The hazard ratio of baPWV in the sex separated analysis was as follows: men, HR = 1.14 (95% CI, 1.04–1.25), p =.005; women, HR = 1.17 (95% CI, 1.04–1.32), p =.013, while that in the age‐stratified analysis was follows: <60 years old, HR = 1.25 (95% CI, 1.02–1.54), p = .035; ≥60 years old, HR = 1.15 (95% CI, 1.06–1.25), p = .001). Penalized cubic splines indicated linear correlations between baseline baPWV and HR for incident CVD (Figure 1). CAVI was also independently associated with CVD when it was included in the model as a continuous variable (Table 3, Model 2), particularly in men [HR = 1.32 (95% CI, 1.08–1.62), p = .008] but not in women [HR = 1.16 (95% CI, 0.88–1.53), p = .290], as well as in the older population [≥60 years old, HR = 1.23 (95% CI, 1.03–1.47), p = .025; <60 years old, HR = 1.44 (95% CI, 0.95–2.18), p = .089]. The notably higher incident rate of the fourth quartiles of CAVI in men (Table 2) might be a reason for the different association by sex. In contrast, no significant association was observed in the analysis including CAVI as quartiles (Table 3, Model 1), probably due to nonlinear correlations between baseline CAVI and HR for CVD (Figure 1). Although BP was used for the calculation of CAVI, we further adjusted mean BP in the Cox regression model due to the substantial differences in mean BP among the quartiles of CAVI (Table S2).
TABLE 3.
Cox regression analysis for incident CVD
| Cox regression model | Time‐dependent Cox regression model | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| baPWV | 1st quartile | reference | |||||
| 2nd quartile | 2.02 (0.75 − 5.41) | .162 | |||||
| 3rd quartile | 1.95 (0.73 − 5.25) | .184 | |||||
| 4th quartile | 4.00 (1.46 − 10.98) | .007 | |||||
| Per 1 m/s | 1.15 (1.07 − 1.24) | <.001 | 1.14 (1.06 − 1.23) | <.001 | |||
| CAVI | 1st quartile | reference | |||||
| 2nd quartile | 0.98 (0.46 − 2.12) | .966 | |||||
| 3rd quartile | 0.97 (0.46 − 2.05) | .936 | |||||
| 4th quartile | 1.70 (0.80 − 3.63) | .167 | |||||
| Per 1 unit | 1.26 (1.07 − 1.48) | .006 | 1.23 (1.04 − 1.44) | .013 | |||
Adjusted factors in all Models were age, sex, body mass index, current smoking, mean blood pressure, heart rate, hemoglobin A1c, low‐density lipoprotein cholesterol, estimated glomerular filtration rate, urinary albumin (≥30 mg/day), and B‐type natriuretic peptide (≥100 pg/ml). Overall p values of each model for Schoenfeld residuals test were as follows: baPWV: Model 1, 0.450; Model 2, 0.352; Model 3, 0.169 and CAVI: Model 1, 0.346; Model 2, 0.518; Model 3, 0.240. Full results of the regression analysis are shown in Tables S3 and S4.
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CAVI, cardio‐ankle vascular index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
FIGURE 1.
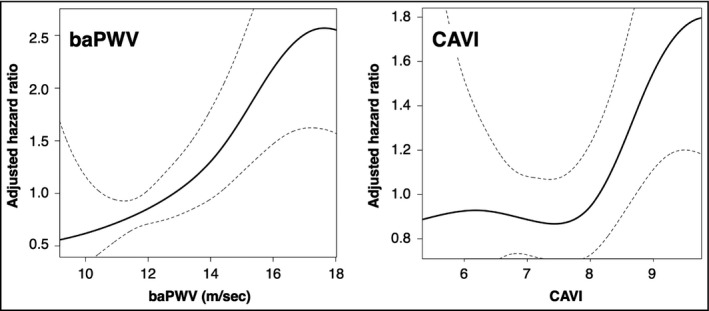
Penalized cubic splines of the association between the arterial stiffness parameters and the hazard ratio for CVD events. Solid line, hazard ratio; dashed line, 95% confidence interval. Adjusted factors are age, sex, body mass index, smoking, mean blood pressure, heart rate, hemoglobin A1c, low‐density lipoprotein cholesterol, estimated glomerular filtration rate, urinary albumin (≥30 mg/day), and B‐type natriuretic peptide (≥100 pg/ml). baPWV, brachial‐ankle pulse wave velocity; CAVI, cardio‐ankle vascular index
The per unit HR of baPWV and CAVI did not change substantially when clinical values measured at the follow‐up investigation were considered in the time‐dependent Cox regression model as time‐varying variables (Table 3, Model 3). The full results of the regression analyses are summarized in Tables S3 and S4.
4. DISCUSSION
In this longitudinal study in a large general population with follow‐up measurements at 5 years after baseline, we showed that high baPWV and CAVI were associated with future CVD events in either analysis including the arterial stiffness indices as a fixed baseline risk factor or a time‐varying risk factor. These associations were independent of conventional risk factors, suggesting that the stiffness index calculated from a transient time of arterial waveform is a good prognostic marker for incident CVD.
baPWV was calculated using arterial waveforms measured at the brachial and tibial arteries. The concept to assess arterial stiffness by pulse wave velocity calculated from its arrival time differences between brachia and tibial arteries was developed in Japan. 4 Therefore, baPWV has been widely used in clinical and epidemiological studies in Japan and other East Asian countries, whereas its use in European studies was limited. 21 Because the stiffness of large elastic arteries is recognized as physiologically important for cardiovascular frailty, baPWV partly reflecting peripheral arterial stiffness might not be considered as a standard measure for arterial stiffness. However, given the results of the present study and other studies that reported a close correlation between baPWV and invasively measured aortic PWV 4 or non‐invasively measured cfPWV, 5 as well as between exercise‐induced decreases in baPWV and cfPWV, 22 baPWV may be useful as a risk marker for CVD, though baPWV is, in general, slightly faster than cfPWV. 5 Easy and automatic measurement without the requirement of specific skills for examiners is an advantage of baPWV. baPWV has higher repeatability than cfPWV in its measurement, 23 which is also its strength. A cross‐sectional study in the Atherosclerosis Risk in Communities study population 24 reported that PWV at the leg artery (femoral‐ankle PWV) had distinct features, namely the femoral‐ankle PWV showed a smaller age‐related change and weak associations with cardiovascular risk factors, which might be a reason for the weaker associations of baPWV with diabetes 25 and urinary albumin value than cfPWV. 26 On the other hand, exercise intervention was reported to decrease carotid‐to‐radial PWV and femoral‐to‐dorsalis pedis PWV but not cfPWV, 27 suggesting the involvement of PWV at muscular arteries in the favorable effects of exercise intervention on cardiovascular disease prevention. Studies investigating the segmental differences in PWV in relation to the CVD events may further clarify the prognostic significance of baPWV.
baPWV and CAVI showed significant association with CVD in either analysis using the traditional Cox model or time‐dependent Cox model. Basically, result of the traditional Cox regression analysis indicates the relative risks of baseline risk factors related to the entire follow‐up period in a study, whereas that of a time‐dependent analysis indicates the weighted average of time window‐specific risk ratios. 11 Results of the time‐dependent analysis thus indicated relatively short‐time effects of the risk factors. The HRs of baPWV and CAVI calculated by the time‐dependent analyses did not differ substantially with those obtained by the traditional analysis, suggesting that the prognostic significance of these stiffness indices might not be varied by follow‐up period.
baPWV and CAVI showed similar associations with the incidence of CVD when they were included in the regression model as a continuous variable. However, in the analysis considering the lowest quartile as a reference, any higher quartiles of CAVI did not identify as a significant risk factor for incident CVD. Because the highest quartile of baPWV and CAVI shared a large number of CVD cases, differences in the results of the association analysis might be owing to the higher incidence rate in the lowest quartile of CAVI. Nonlinear association in the penalized cubic splines between CAVI and HR for incident CVD supports this consideration. We previously reported that obese individuals had a lower value of CAVI, and baseline body mass index was inversely associated with 5‐year changes in CAVI, possibly because of using a fixed ρ value in the calculation of CAVI. 12 Although several studies 28 , 29 reported an inverse association between BMI and baPWV, the degree was more substantial for CAVI when the results were compared with that in our previous study. 12 A complication in its calculation and a consequent inverse association between body size and CAVI might be a reason for the higher incidence rate in the lowest quartile of CAVI. Furthermore, CAVI was strongly associated with BP though it was advocated as a BP independent index, 13 , 14 suggesting that the superiority of CAVI to baPWV regarding the BP independency was limited. Another distinction of CAVI was without using heart‐to‐brachial PWV, PWV at the muscular artery, in its calculation. However, correlations between CAVI and cfPWV were weaker than those between baPWV and cfPWV, 24 while another study reported similar associations among them. 30 Given a result of a previous study in a healthy employee‐based population, 31 namely the association between CAVI and new onset of hypertension or retinopathy was less clear than baPWV, a simple calculable baPWV may be better suited for CVD risk assessment in primary care and public health practice.
An opposite result has recently been reported from a longitudinal study in older adults (mean age: 75 years), 32 that is, cfPWV but not baPWV and CAVI was associated with CVD events, particularly with the incidence of heart failure. A possible reason for the discrepancy may be a difference in the mean age of the study population. Although, in our study population, the association between baPWV and CVD did not differ between older (≥60 years) and younger sub‐populations, we did not include individuals aged ≥75 years at baseline. Another reason might be differences in the outcome definition. We did not consider heart failure as an outcome because accurate identification of heart failure in a general population is difficult.
Our previous meta‐analysis indicated approximately 16 m/s as the best cutoff value of baPWV in the assessment of CVD risk. 10 In the meta‐analysis, baPWV was calculated from the arterial waveforms measured using BP‐203RPE II form PWV/ABI (Omron healthcare Co., Ltd.), whereas this study used the Vasera‐1500 for the measurement. Although, in this study, the path length between the brachia and ankle required for baPWV calculation was estimated using the same equation with the meta‐analysis, the mean baPWV value was substantially different between the studies, owing to differences in the algorithm for wave front detection of the arterial waveform between the devices. However, because we calculated both baPWV and CAVI from the same arterial waveform measures obtained using the same device, our study setting comparing prognostic significance of baPWV and CAVI might not be inadequate. Furthermore, although we observed a significant association between the highest quartile of baPWV and the incidence of CVD, the cutoff value of baPWV could not be simply compared with other studies using a different device to measure the arterial waveforms.
In summary, the arterial stiffness assessed by transient time of arterial waveforms, particularly baPWV, was found to be an independent risk factor for future CVD events in a general population. Results of the present study strengthen the prognostic significance of baPWV by clarifying that the association remained significant when time‐dependent changes in baPWV and other conventional risk factors were considered in the analysis.
CONFLICT OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTION
Yasuharu Tabara involved in study conception, data acquisition, data analysis, writing and revising the manuscript. Kazuya Setoh and Takahisa Kawaguchi involved in data acquisition and curation. Takeo Nakayama and Fumihiko Matsuda involved in review and revising the manuscript and supervising the study.
Supporting information
Table S1‐S4
ACKNOWLEDGEMENT
We are extremely grateful to the Nagahama City Office and the nonprofit organization Zeroji Club for their help in performing the Nagahama study. We also thank the editors of Crimson Interactive Pvt. Ltd. for their help in the preparation of this manuscript.
Yasuharu T, Setoh K, Kawaguchi T, Nakayama T, Matsuda F; the Nagahama study group . Brachial‐ankle pulse wave velocity and cardio‐ankle vascular index are associated with future cardiovascular events in a general population: The Nagahama Study. J Clin Hypertens. 2021;23:1390–1398. 10.1111/jch.14294
Funding information
The work was supported by a university grant, Center of Innovation Program, Global University Project, and a Grant‐in‐Aid for Scientific Research (25293141, 26670313, 26293198, 17H04182, 17H04126, 17H04123, 18K18450) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Practical Research Project for Rare/Intractable Diseases (ek0109070, ek0109283, ek0109196, ek0109348), Research and Development Grants for Dementia (dk0207006, dk0207027), Program for an Integrated Database of Clinical and Genomic Information (kk0205008), Practical Research Project for Lifestyle‐related Diseases including Cardiovascular Diseases and Diabetes Mellitus (ek0210066, ek0210096, ek0210116), Research Program for Health Behavior Modification by Utilizing IoT (le0110005, le0110013), and Research and Development Grants for Longevity Science (dk0110040) from the Japan Agency for Medical Research and Development (AMED), Takeda Medical Research Foundation.
References
- 1. Townsend RR, Wilkinson IB, Schiffrin EL, et al. American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698‐722. 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maruhashi T, Soga J, Fujimura N, et al. Increased arterial stiffness and cardiovascular risk prediction in controlled hypertensive patients with coronary artery disease: post hoc analysis of FMD‐J (Flow‐mediated Dilation Japan) Study A. Hypertens Res. 2020;43:781‐790. 10.1038/s41440-020-0420-6 [DOI] [PubMed] [Google Scholar]
- 3. Milan A, Zocaro G, Leone D, et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. 2019;37:1547‐1557. 10.1097/HJH.0000000000002081 [DOI] [PubMed] [Google Scholar]
- 4. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359‐364. 10.1291/hypres.25.359 [DOI] [PubMed] [Google Scholar]
- 5. Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022‐2027. 10.1097/HJH.0b013e32832e94e7 [DOI] [PubMed] [Google Scholar]
- 6. Song Y, Xu B, Xu R, et al. Independent and joint effect of brachial‐ankle pulse wave velocity and blood pressure control on incident stroke in hypertensive adults. Hypertension. 2016;68:46‐53. 10.1161/HYPERTENSIONAHA.115.07023 [DOI] [PubMed] [Google Scholar]
- 7. Takashima N, Turin TC, Matsui K, et al. The relationship of brachial‐ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28:323‐327. 10.1038/jhh.2013.103 [DOI] [PubMed] [Google Scholar]
- 8. Sheng CS, Li Y, Li LH, et al. Brachial‐ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64:1124‐1130. 10.1161/HYPERTENSIONAHA.114.04063 [DOI] [PubMed] [Google Scholar]
- 9. Turin TC, Kita Y, Rumana N, et al. Brachial‐ankle pulse wave velocity predicts all‐cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res. 2010;33:922‐925. 10.1038/hr.2010.103 [DOI] [PubMed] [Google Scholar]
- 10. Ohkuma T, Ninomiya T, Tomiyama H, et al. Yamashina A; Collaborative Group for J‐BAVEL. Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69:1045‐1052. 10.1161/HYPERTENSIONAHA.117.09097 [DOI] [PubMed] [Google Scholar]
- 11. Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time‐dependent effects and time‐varying risk factors. Kidney Int. 2008;74:994‐997. 10.1038/ki.2008.328 [DOI] [PubMed] [Google Scholar]
- 12. Tabara Y, Setoh K, Kawaguchi T, et al. Factors affecting longitudinal changes in cardio‐ankle vascular index in a large general population: the Nagahama study. J Hypertens. 2018;36:1147‐1153. 10.1097/HJH.0000000000001672 [DOI] [PubMed] [Google Scholar]
- 13. Shirai K, Hiruta N, Song M, et al. Cardio‐ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18:924‐938. 10.5551/jat.7716 [DOI] [PubMed] [Google Scholar]
- 14. Saiki A, Sato Y, Watanabe R, et al. The role of a novel arterial stiffness parameter, cardio‐ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb. 2016;23:155‐168. 10.5551/jat.32797 [DOI] [PubMed] [Google Scholar]
- 15. Kawashima‐Kumagai K, Tabara Y, Yamashiro K, et al. Nagahama Study group. Association of retinal vessel calibers and longitudinal changes in arterial stiffness: the Nagahama study. J Hypertens. 2018;36:587‐593. 10.1097/HJH.0000000000001602 [DOI] [PubMed] [Google Scholar]
- 16. Higo Y, Nagashima S, Tabara Y, et al. Nagahama study group. Association of the spot urine sodium‐to‐potassium ratio with blood pressure is independent of urinary Na and K levels: The Nagahama study. Hypertens Res. 2019;42:1624‐1630. 10.1038/s41440-019-0276-9 [DOI] [PubMed] [Google Scholar]
- 17. Nishimura K, Okamura T, Watanabe M, et al. Predicting coronary heart disease using risk factor categories for a Japanese yrban population, and comparison with the Framingham risk score: The Suita Study. J Atheroscler Thromb. 2014;21:784‐798. 10.5551/jat.19356 [DOI] [PubMed] [Google Scholar]
- 18. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611‐619. 10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 19. WHO Monica Project . MONICA Manual. (1998–1999). http://www.ktl.fi/publications/monica/manual/index.htm, URN:NBN:fi‐fe19981146. (Accessed on 29 October 2019).
- 20. Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 21. Sugawara J, Tanaka H. Brachial‐ankle pulse wave velocity: Myths, misconceptions, and realities. Pulse. 2015;3:106‐113. 10.1159/000430771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugawara J, Hayashi K, Yokoi T, et al. Brachial‐ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401‐406. 10.1038/sj.jhh.1001838 [DOI] [PubMed] [Google Scholar]
- 23. Meyer ML, Tanaka H, Palta P, et al. Repeatability of central and peripheral pulse wave velocity measures: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2016;29:470‐475. 10.1093/ajh/hpv127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer ML, Tanaka H, Palta P, et al. Correlates of segmental pulse wave velocity in older adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2016;29:114‐1122. 10.1093/ajh/hpv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loehr LR, Meyer ML, Poon AK, et al. Prediabetes and diabetes are associated with arterial stiffness in older adults: The ARIC Study. Am J Hypertens. 2016;29:1038‐1045. 10.1093/ajh/hpw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim ED, Tanaka H, Ballew SH, et al. Associations between kidney disease measures and regional pulse wave velocity in a large community‐based cohort: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2018;72:682‐690. 10.1053/j.ajkd.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 27. Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. 2013;26:1093‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomiyama H, Arai T, Koji Y, et al. The relationship between high‐sensitive C‐reactive protein and pulse wave velocity in healthy Japanese men. Atherosclerosis. 2004;174:373‐377. 10.1016/j.atherosclerosis.2004.01.032 [DOI] [PubMed] [Google Scholar]
- 29. Yang H, Zhao J, Deng X, et al. Pulse wave velocity is decreased with obesity in an elderly Chinese population. J Clin Hypertens. 2019;21:1379‐1385. 10.1111/jch.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim J, Pearman M, Park W, Alkatan M, Tanaka H. Interrelationships among various measures of central artery stiffness. Am J Hypertens. 2016;29:1024‐1028. 10.1093/ajh/hpw045 [DOI] [PubMed] [Google Scholar]
- 31. Tomiyama H, Ohkuma T, Ninomiya T, et al. Brachial‐ankle pulse wave velocity versus its stiffness index β‐transformed value as risk marker for cardiovascular disease. J Am Heart Assoc. 2019;8:e013004. 10.1161/JAHA.119.013004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim ED, Ballew SH, Tanaka H, Heiss G, Coresh J, Matsushita K. Short‐term prognostic impact of arterial stiffness in older adults without prevalent cardiovascular disease. Hypertension. 2019;74:1373‐1382. 10.1161/HYPERTENSIONAHA.119.13496 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4


