Abstract
Controversy exists about the association of choroidal thickness (CTh) with blood pressure (BP) values. There is some evidence suggesting that central hemodynamics changes are associated with microvascular disease. Our study was aimed to assess the relationships between CTh and clinic and 24‐h BP and between CTh and estimated 24‐h aortic pulse pressure (aPP), 24‐h aortic systolic BP (aSBP), and 24‐h aortic augmentation index (aAIx) in a group of hypertensive patients. We enrolled 158 hypertensive subjects (mean age 48 ± 13 years) all of which underwent evaluation of the choroidal district by Swept‐Source optical coherence tomography (SS‐OCT) and 24‐h BP monitoring, in order to measure peripheral BP and to estimate central hemodynamic parameters. Inverse significant correlations of clinic PP, 24‐h aPP, 24‐h aSBP, and 24‐h aAIx with thicknesses of central ring, inner ring, and outer ring of the choroid and its overall average were found. The strongest of these correlations was that relating 24‐h aPP with overall average choroidal thickness (r = −.531; P < .001). When we divided the study population in subjects with 24‐h aPP above and below the median value (35 mm Hg), CTh were thinner in subjects with higher values of 24‐aPP as compared to those with lower ones, even after adjustment for age, and other potential confounders. The relationships of CTh with 24‐h aPP remained significant also taking into account the effects of various covariates in linear multiple regression analyses. Our findings support the concept of a cross‐talk between macro‐ and microcirculation.
Keywords: aortic pressure, cardiovascular disease, central hemodynamics, choroidal thickness, hypertensive eye disease, microcirculation, optical coherence tomography
1. INTRODUCTION
Traditionally, the retina has been considered as the easiest accessible window to study the state of the systemic microcirculation, 1 even if the choroid is the most important vascular layer of the eye, which thinning may be due to microvascular damage, reflecting the local expression of a more generalized vascular injury. 2 , 3 , 4 Recent studies have associated changes in the thickness of the choroid with cardiovascular 2 , 3 and renal diseases. 4 , 5 , 6 Controversy exists about the association of CTh with blood pressure (BP) values. 4 , 5 , 6 , 7 , 8 , 9 , 10
The pulsatile nature of the central hemodynamics has a deleterious impact on vital organs. In a healthy cardiovascular system, the elastic properties of the large arteries ensure that pulsations in pressure and flow generated by cyclic left ventricular contraction are dampened, so that less pulsatile pressure and flow are delivered at the microvascular level. However, in response to aging, hypertension, and other disease states, arterial stiffening limits the buffering capacity of the elastic arteries, thus exposing the microvasculature to increased pulsatile stress. This is thought to be particularly pertinent to high flow/low resistance organs such as the brain and kidney, which may be sensitive to excess pressure and flow pulsatility, damaging capillary networks, reinforcing the concept of a cross‐talk between large and small arteries in hypertension. 11 , 12 , 13 , 14 , 15 , 16 . There is a substantial body of evidence that the aortic pulse pressure predicts future cardiovascular and total mortality risk in various patient populations. 17 , 18 , 19 The aortic pulse pressure is also a better predictive indicator than the brachial pulse pressure, 18 , 19 and clinical studies have associated its elevation with the development of macrovascular and microvascular damage.
However, little is known about the relationship of choroidal thickness (CTh) with peripheral and central measures of pulsatile hemodynamics. 4 , 10
The aim of our study was to assess the relationships of CTh with clinic and 24‐h peripheral BP values and of CTh with estimated measures of central hemodynamics, derived from 24‐h pulse wave analysis (PWA), in a group of hypertensive patients.
2. MATERIAL AND METHODS
The population of this cross‐sectional study was selected from the Caucasian hypertensive patients consecutively attending the Unit of Nephrology and Hypertension and European Society of Hypertension (ESH) Excellence center of the University of Palermo, between May 2016 and May 2018.
Written informed consent was obtained from all patients, after the nature of the study was explained to them. The study protocol followed the tenets of the Declaration of Helsinki and was approved by the local review board.
The exclusion criteria were:
Age <20 years and >70 years.
Known diabetes or fasting glycaemia >126 mg/dl.
Pregnancy.
Systemic or ocular diseases (glaucoma, uveitis, high myopia, age‐related macular degeneration, etc) and/or a history of ophthalmic surgery that may have affected the choroidal vascular network.
Nephroparenchymal, renovascular, malignant, or endocrine hypertension or obstructive sleep apnea syndrome.
Known proteinuria and hematuria.
Nephritic diseases and hereditary renal diseases.
Estimated GFR <15 ml/min/1.73 m2.
Heart failure.
Positive history or clinical signs of coronary artery disease.
Positive history or clinical signs of cerebrovascular disease.
Major non‐cardiovascular diseases.
All those conditions preventing from obtaining reliable automated BP measurements with the oscillometric technique (eg, atrial fibrillation, frequent ectopic beats or second or third‐degree atrioventricular blocks, and mid‐upper arm circumference <22 cm). Patients with an arm circumference larger than 32 cm were not excluded from the study, rather an appropriately sized cuff was used to obtain accurate BP measurements.
2.1. Study design
In all patients, careful clinical history, physical examination, routine blood chemistry, urine examination, and 24‐hour ambulatory blood pressure monitoring (ABPM) were performed. Furthermore, an ophthalmologist carried out a complete eye examination.
Patients who reported smoking cigarettes regularly during the past year were considered current smokers.
Body weight, height, waist circumference, and office blood pressures were measured by a doctor.
2.2. Measurements
Clinic (office) BP was considered as the mean of three consecutive measurements obtained at 2‐minute intervals by an electronic oscillometric validated 20 device (WatchBP Office, Microlife AG, Widnau, Switzerland) after 5 min of rest in a sitting position.
A clinically validated and commercially available oscillometric brachial‐cuff based BPLab Vasotens device (BPLab GmbH, Schwalbach am Taunus) 21 was used to perform the 24‐h ABPM. Current ESH guidelines were followed for proper recording performance. 22 Briefly, the BP readings were performed automatically at 15‐min intervals during the day and at 20‐min intervals during night‐time resting. The time periods were standardized according to the time at which the patients rose and retired. The optimal adult cuff was wrapped around the nondominant arm, and patients were instructed to keep their arm extended and still and to avoid any movement during each automatic BP measurement. They were free to attend their usual daily activities during ABPM (avoiding strenuous exercise).
After fitting the device, patients were sent home and asked to resume normal life and to come back 24 h later for removal of the instrumentation.
The oscillometric BPLab device also allows measurements of ambulatory central hemodynamics by recording pulsatile pressure changes at the brachial artery level.
The ABPM waveforms were analyzed using BPLab Vasotens® technology (Petr Telegin), which allows calculation of central pulse wave parameters from the peripheral pulse wave. In measuring BP, the pressure pulsations in the cuff are registered during a step‐by‐step deflation. The sampling rate of the device is 100 Hz. After digitalization, the signal processing is performed using a proprietary mathematical algorithm, which is based on a generalized transfer function that uses a modification of a certain frequency range. The amplitude of all signals recorded when the pressure in the cuff exceeded the systolic BP was used for waveform averaging. 23
The accuracy of the BPLab device for the assessment of vascular indices has been validated in studies against a non‐invasive gold standard. 23 , 24
A detailed description of the methodology may be found elsewhere. 23 , 24 , 25
Routine biochemical parameter determination was performed with standard techniques using an autoanalyzer. Low‐density lipoprotein cholesterol was calculated by the Friedewald formula.
Estimated GFR (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation. 26
Ophthalmologists, not aware of clinical and biohumoral data of the patient, performed in all patients a complete eye examination, including the best corrected visual acuity with Early Treatment Diabetic Retinopathy Study (ETDRS) charts, intraocular pressure measurement, anterior segment, and dilated fundus evaluation.
The choroidal district was evaluated morphologically by Swept‐Source (SS) optical coherence tomography (OCT) (DRI Triton, Topcon Inc, Japan), always in the morning, during the same temporal interval (10‐12 AM). All scans were performed by a single operator. The right eye was examined first. A standardized protocol was followed for all scans, as previously described. 5 , 6 , 27 Poor quality scans were rejected or repeated.
The scans were then read and analyzed by two specialized ophthalmologists.
Because not significant differences were noted between the two eyes, only one eye of each subject was randomly selected for analysis, by using a random number generator. If the image quality of the selected eye was deemed insufficient, the contralateral eye was chosen for analyses.
2.3. Quantitative analysis
The choroidal thickness (from the outer surface of the RPE, to the choroidal scleral interface, CSI) was automatically calculated by the OCT mapping software. OCT measurements were performed according to the ETDRS protocol. 28 The ETDRS map divides the macula into 9 subfields. The circular grid is centered over the fovea and consists of 3 concentric rings of diameters 1, 3, and 6 mm, respectively. The inner and outer rings are further divided into quadrants: temporal, nasal, superior, and inferior (Figure 1).
FIGURE 1.
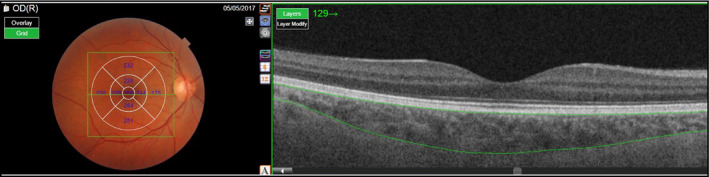
The chorioretinal structures en face (left images) and as a cross‐section (right images). The Early Treatment Diabetic Retinopathy Study map divides the macula into 9 subfields. A circular grid is centered over the fovea and consists of 3 concentric rings of diameters 1, 3, and 6 mm, respectively. The inner and outer rings are further divided into quadrants: temporal, nasal, superior, and inferior
Measurements were performed for each of the nine regions of the ETDRS grid, for both the retina and the choroid. Furthermore, we calculated the average of the individuals values of the four quadrants separately for the inner and the outer ring. The average of all the nine regions of the ETRDS grid (including the inner, the outer, and the central rings) was also calculated.
2.4. Statistical analysis
Normal distribution of the continuous variables was assessed using the Kolmogorov–Smirnov test, and the assumption of satisfactory Gaussian distribution was met for all of the examined parameters, except for serum triglycerides, and for aortic augmentation index (aAix) which showed a positively skewed distribution. This variable was given as median and interquartile range (IQR) and mathematically transformed to better satisfy distributional assumptions before parametric tests were used. All the other normal distributed continuous variables were expressed as mean ± standard deviation.
Categorical variables were expressed as percentage values.
The overall study population was divided in two groups based on the median value (35 mmHg) of 24‐h aortic pulse pressure.
Differences between groups were evaluated using the independent‐sample Student t test for continuous variables and the χ2 test, with the Yates correction (or Fisher exact test when appropriate), for the categorical variables. Adjustment for age, estimated GFR, (Log) triglycerides, serum glucose, and HDL cholesterol (Figure 2) was carried out by analysis of covariance.
FIGURE 2.
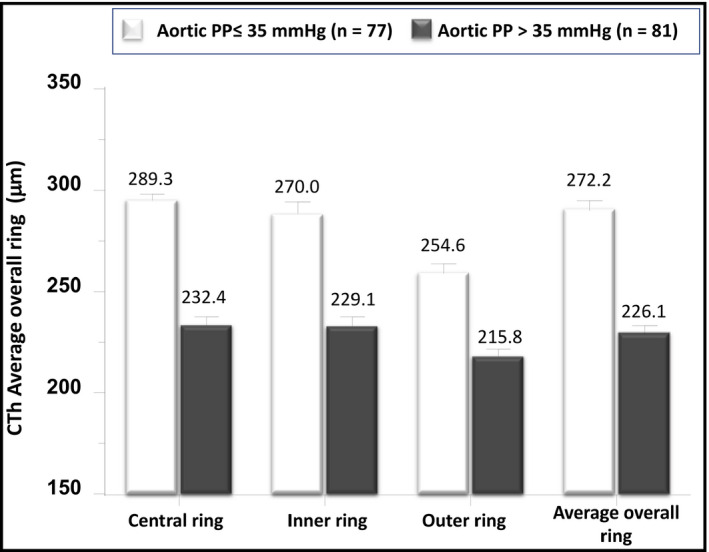
Differences in choroidal thicknesses between hypertensive subjects with 24‐h aortic estimated aortic pulse pressure (aPP) PWV above and below the median value (35 mmHg). The values are given as the mean ± SEM and are adjusted, by ANCOVA, for age, estimated GFR, (Log) triglycerides, serum glucose and HDL cholesterol
Simple regression analyses and Pearson correlation coefficients were used to test the relationships between choroidal measures and the other variables. In order to assess the independent relationships between the examined variables, stepwise multiple regression models were built considering initially each choroidal parameter as outcome variable and 24‐h peripheral or central pulse pressure, separately from each other, as regressors, along with age, sex, smoking habit, antihypertensive therapy (yes/no), mean 24‐h (or clinic) blood pressure, serum glucose, eGFR, HDL cholesterol, (log) triglycerides. Subsequently, we built other multivariate models in which 24‐h brachial PP, or clinic PP, or 24‐h central or peripheral systolic BP, or 24‐h aortic augmentation index were forced, separately from each other, into the same previous multivariate models including also 24‐h aortic PP.
In all multiple regression analyses, a backward stepwise procedure was used, with α = 0.15 as the cutoff for entry or removal of variables. Collinearity was assessed by calculating the variance inflation factor (VIF): Variables with VIF ≥ 2 were excluded from the models.
The null hypothesis was rejected at a two‐tailed P < .05.
The statistical analyses were performed using the IBM SPSS Statistics software package, version 22 for Macintosh (SPSS).
3. RESULTS
A total of 155 subjects were included in this analysis. Table 1 gives the general characteristics of the overall study population and of the two groups divided on the basis of a 24‐h aPP above or below the median value, that is 35 mmHg.
TABLE 1.
Demographic, anthropometric and clinical characteristics of the whole population and of the groups divided on the basis of the median value (35 mmHg) of 24 h aortic pulse pressures (aPP)
| Overall study population (n = 158) |
Subjects with 24‐h aPP ≤35 mmHg (n = 77) |
Subjects with 24‐h aPP >35 mmHg (n = 81) |
p | |
|---|---|---|---|---|
| Age (years) | 47.7 ± 12.8 | 44.9 ± 11.0 | 50.3 ± 13.7 | .01 |
| Sex, Males (%) | 72.2 | 74.0 | 70.4 | .61 |
| Smokers (%) | 22.8 | 24.7 | 21.0 | .37 |
| Patients treated with antihypertensive drugs (%) | 66.5 | 64.9 | 67.9 | .69 |
| Body mass index (kg/m2) | 27.8 ± 4.3 | 27.8 ± 4.4 | 28.02 ± 4.3 | .73 |
| Waist circumference (cm) | 96.1 ± 11.6 | 95.4 ± 12.2 | 96.7 ± 10.9 | .49 |
| Estimated GFR (ml/min/1.73 m2) | 85.5 ± 24.2 | 89.6 ± 20.7 | 81.5 ± 26.7 | .03 |
| Total cholesterol (mg/dl) | 192.3 ± 30.0 | 195.1 ± 28.8 | 195.1 ± 32.1 | .27 |
| HDL cholesterol (mg/dl) | 50.0 ± 12.9 | 48.8 ± 11.8 | 51.2 ± 13.9 | .24 |
| Serum Triglycerides (mg/dl) | 108.7 (81.6‐144.3) | 109.2 (78.8‐143) | 106.2 (81.5‐145) | .99 |
| LDL cholesterol (mg/dl) | 118.7 ± 29.0 | 122.7 ± 27.8 | 114.9 ± 29.8 | .09 |
| Serum glucose (mg/dl) | 95.4 ± 14.8 | 94.9 ± 16.2 | 96.0 ± 13.4 | .63 |
The group of patients with 24‐h aortic PP > 35 m/s were older and had lower estimated GFR values when compared to the counterparts with 24‐h aortic PP ≤ 35 m/s. Sixty‐six percent of patients were pharmacologically treated for hypertension. The distribution of these subjects was not significantly different between the two groups and only a slightly greater percentage of subjects treated with centrally acting antiadrenergic drugs and allopurinol was observed in the group with highest 24‐h aortic PP (Table S1).
The patients treated with antihypertensive medications were older (P < .001), had lower estimated GFR values (P = .004), and thinner thicknesses of the inner (P = .02) and outer (P = .03) choroidal rings. These two latter differences lost statistical significance after adjustment for age (both P > .44).
Clinic, 24‐h brachial and central PP, 24‐h peripheral and central systolic BP were higher and 24‐h aortic diastolic BP and mean BP were lower in the groups with greater values of ambulatory aortic PP when compared to the counterparts with lower ones (Table 2). All the CTh measurements examined were thinner in the subjects with higher 24‐h aortic PP than in those with lower aPP (Table 3). The differences regarding CTh thicknesses remained significant even after adjustment, by ANCOVA for age, estimated GFR, (Log) Triglycerides, Serum glucose and HDL cholesterol (Figure 2).
TABLE 2.
Main hemodynamic data of the overall population and of the groups with 24‐h aPP above and below 35 mmHg
| Overall study population (n = 158) |
Subjects with 24‐h aPP ≤35 mmHg (n = 77) |
Subjects with 24‐h aPP >35 mmHg (n = 81) |
p | |
|---|---|---|---|---|
| Peripheral parameters | ||||
| Clinic systolic BP (mmHg) | 139 ± 13.5 | 137 ± 12.0 | 141 ± 14.5 | .09 |
| Clinic diastolic BP(mmHg) | 86 ± 9.5 | 87 ± 9.7 | 85 ± 9.2 | .26 |
| Clinic pulse pressure (mmHg) | 53 ± 10.9 | 50 ± 9.8 | 56 ± 11.3 | <.001 |
| Clinic mean BP (mmHg) | 104 ± 9.7 | 104 ± 9.5 | 104 ± 9.9 | .95 |
| Clinic heart rate (bpm) | 73 ± 10.9 | 74 ± 10.9 | 73 ± 10.9 | .7 |
| 24‐h systolic BP (mmHg) | 130 ± 12.9 | 127 ± 11.7 | 133 ± 13.5 | <.001 |
| 24‐h diastolic BP (mmHg) | 82 ± 8.9 | 84 ± 8.3 | 80 ± 9.4 | .004 |
| 24‐h pulse pressure (mmHg) | 47 ± 10.4 | 43 ± 8.5 | 53 ± 11.4 | <.001 |
| 24‐h mean BP (mmHg) | 98 ± 9.2 | 98 ± 8.1 | 98 ± 10.3 | .99 |
| 24‐h heart rate (bpm) | 72 ± 10.5 | 72 ± 11.3 | 72 ± 9.7 | .99 |
| Central measures | ||||
| 24‐h aortic systolic BP (mmHg) | 120 ± 12.6 | 113 ± 11.0 | 127 ± 12.8 | <.001 |
| 24‐h aortic diastolic BP (mmHg) | 83 ± 13.6 | 85 ± 10.6 | 79 ± 13.8 | .003 |
| 24‐h aortic pulse pressure (mmHg) | 37 ± 13.6 | 28 ± 5.0 | 48 ± 10 | <.001 |
| 24‐h aortic mean BP (mmHg) | 95 ± 12 | 94 ± 12.7 | 95 ± 10.8 | .594 |
| 24‐h aortic augmentation index (%) | 11.84 (2‐22.4) | 2.3 (−3.03‐8.90) | 21 (12.4‐29.8) | <.001 |
TABLE 3.
Choroidal thickness measurements of the overall population and in the groups with 24h aPP above and below 35 mmHg
| Overall study population (n = 158) |
Subjects with 24 h aPP ≤35 mmHg (n = 77) |
Subjects with 24 h aPP >35 mmHg (n = 81) |
p | |
|---|---|---|---|---|
| Choroidal thickness (μm) | ||||
| Average overall ring | 250.4 ± 62.0 | 278.9 ± 51.2 | 223.5 ± 59.5 | <.001 |
| Central ring | 263.2 ± 73.2 | 297.2 ± 61.6 | 230.9 ± 68.9 | <.001 |
| Inner ring | 251.9 ± 65.6 | 278.1 ± 59.8 | 227.4 ± 61.5 | <.001 |
| Outer ring | 235.9 ± 59.15 | 260.6 ± 48.2 | 212.6 ± 59.4 | <.001 |
Close correlations of all the assessed CTh measures with measures of central hemodynamics were found (Table S2). Particularly strong was the correlation of 24‐h aPP with CTh average overall ring that is shown in Figure 3, where the regression line and the regression equation are also reported. The independent associations of 24 h aortic PP with CTh measurements were confirmed in various multiple stepwise linear regression models (Table 4), in which CTh measurements were included, separately from each other, as outcome variables and 24 h aortic PP were regarded as independent variables, along with age, sex, smoking habit, antihypertensive therapy (yes/no), mean 24‐h aortic BP, serum glucose, eGFR, HDL cholesterol, (log) triglycerides. When in the same multiple regression models, 24 h aortic PP was replaced by 24 h brachial PP, also this hemodynamic parameter showed a significant, but less strong, association with choroidal thickness. However, when 24‐h brachial PP, or clinic PP, or 24 h central or peripheral systolic BP, or 24‐h aortic augmentation index were forced, separately from each other, into the same previous multivariate models including also 24 h aortic PP, only this latter remained significantly associated with choroidal thicknesses.
FIGURE 3.
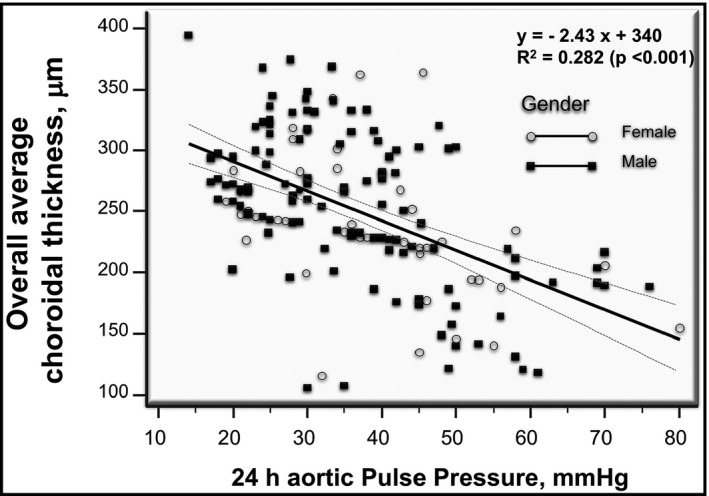
Scattergram showing the negative relationship between 24‐h aortic pulse pressure and the average global choroidal thickness in men (dark squares) and women (gray circles) of the overall study population. The calculated regression line is also shown. Broken hyperbolic lines represent the 95% confidence bands around the regression line
TABLE 4.
Standardized multiple regression coefficients (β) relating 24‐h aortic pulse pressure (A), or 24‐h brachial pulse pressure (B) with choroidal thickness, calculated after adjustment for various potential confounding factors. Only variables that entered the final model are reported. See text for further explanations
| (A) Outcome variables | CTh central ring (R 2: .337) | CTh inner ring (R 2: .306) | CTh outer ring (R 2: .352) | CTh average overall ring (R 2: .378) | ||||
|---|---|---|---|---|---|---|---|---|
| Independent variables | β | p | β | p | β | p | β | p |
| 24‐h aortic pulse pressure | −0.452 | <.001 | −0.38 | <.001 | −0.413 | <.001 | −0.441 | <.001 |
| Age | −0.260 | <.001 | −0.321 | <.001 | −0.336 | <.001 | −0.324 | <.001 |
| (B) Outcome variables | CTh central ring (R 2: .206) | CTh inner ring (R 2: .237) | CTh outer ring (R 2: .262) | CTh average overall ring (R 2: .260) | ||||
|---|---|---|---|---|---|---|---|---|
| Independent variables | β | p | β | p | β | p | β | p |
| 24‐h brachial pulse pressure | −0.244 | .001 | −0.243 | .001 | −0.247 | .001 | −0.252 | <.001 |
| Age | −0.336 | <.001 | −0.376 | <.001 | −0.401 | <.001 | −0.395 | <.001 |
4. DISCUSSION
Alterations in microvascular structure and function contribute to the development and progression of hypertension, diabetes, CKD, and CV diseases. 29 , 30 Notably, such changes precede the development of end‐organ damage and appear modifiable. The use of retinal examination as a method to evaluate microcirculation has been traditionally proposed due to its unique capability to view easily the vasculature of the posterior segment in vivo and in a non‐invasive manner, thus providing a snapshot of vascular health. 1 However, the choroid is the most vascularized layer of the eye with a very high perfusion rate. Per unit weight, the choroid has the highest blood flow of any tissue in the body, 31 even greater than that of the brain and the kidney. These latter organs are continually perfused at high volume flow throughout systole and diastole so that pulsations are transmitted through the capillary network up to the venous efflux. Such fluctuations, measurable as central pulse pressure, increase if the elastic properties of the aorta are impaired. Indeed, aortic stiffness increases the delivery of pulsatile energy into the microcirculation, especially in those vascular districts more vulnerable for their low impedance. Exposure of renal and cerebral small vessels to highly pulsatile pressure and flow can lead to constriction of resistance vessels and subsequently to structural damage. 11 , 12 , 13 , 14 , 15 , 16 The chorioretinal microcirculation shares similar embryological origin, anatomic features, and physiological properties with renal and cerebral microvasculature. 32 It is therefore conceivable that the choroid may be exposed, and damaged by increased flow pulsatility like the brain and the kidney. The results of our study, showing that choroidal thickness is reduced in hypertensive patients with increased indices of pulsatile hemodynamics, seem to support this hypothesis and are in agreement with a mounting evidence suggesting that choroidal thickness changes may be a non‐invasive biomarker of CV damage. 2 , 3 , 4 , 31 Indeed, we found a close inverse association of the thickness of various regions of the choroid, as well as of its overall thickness, with increased aortic augmentation index and with elevated clinic and 24‐h peripheral PP and above all with central PP.
It is reasonable that morphological alterations of the choroid could represent the local epiphenomenon of a systemic vascular injury not less than the retinal ones, and the evidence that choroid may be directly damaged by acute and chronic severe elevation of BP seems to confirm this hypothesis. 31 , 33
The structural evaluation of the choroid in vivo was for a long time limited by its deep location. The introduction in last years of advanced techniques of OCT, providing high‐resolution, cross‐sectional imaging of the eye with near histological detail, has greatly increased our understanding of the choroid. 34 This allowed us to recognize that alterations of its microvasculature can be indicative of systemic diseases that affect blood vessels of both the macro‐ and microcirculation. 2 , 33 In particular, in recent years it has been observed that changes in the thickness of the choroid are associated with CVaand renal abnormalities. 4 , 5 , 6 , 27
The independent prognostic value of vascular biomarkers obtained through pulse wave analysis (PWA), such as central BP, and augmentation index (AIx), in resting conditions, has been documented in a variety of patient groups and populations, even if more evidence is needed before recommending their routine clinical use. 11 , 13
The most widely adopted methods for evaluating pulse waveforms are those based on applanation tonometry and transfer functions. 11 , 13 In last years, instruments, using an oscillometric technique, and a procedure that takes only 2‐3 min, allow estimation of aortic PWA indices by using a simple upper arm cuff. 23 , 24 Some of these devices, like that used in our study, have versions developed for 24‐h BP and 24 h PWA monitoring 23 , 24 and provide PWA values very close to those recorded by widely accepted and clinically validated devices. 24 Such techniques are affordable and have made the assessment largely operator‐independent and allowed to extend the evaluation of arterial functional state to ambulatory conditions. These new methods may provide an opportunity for the spread of central PWA measurements and to further refine the CV risk stratification. As it is well known that organ damage is more closely related to ambulatory than to office BP, routine measurements of ambulatory central hemodynamics may have some potential for a more affordable and detailed assessment of vascular health in daily‐life conditions.
Our findings seem also to corroborate the concept of a cross‐talk between macro‐ and microcirculation, 12 that is also supported by many evidences of an association of measures of central hemodynamics with microvascular changes in the heart, 12 the kidney, 4 , 5 the brain, 13 and the retina. 12
Our results are also in agreement with the study of Balmforth and colleagues, 4 that in a subgroup of 20 nondiabetic subjects with chronic kidney diseases found inverse significant correlations between CTh and aortic stiffness, measured by a tonometric method. Remarkably, in the same investigation significant relationships were found of choroidal thinning with endothelin‐1, asymmetric dimethylarginine and interleukin‐6 serum levels, 4 all markers of endothelial dysfunction and vascular inflammation, that are also closely linked to aortic stiffness.
It is important to note that, with the exception of 24 h central systolic BP, pulse pressure was the only BP component independently related to CTh. Regarding the association of blood pressures with CTh, previous studies yielded conflicting results. In the above‐mentioned study of Balmforth and colleagues, 4 no relationship was detected between systolic and diastolic BP and chorioretinal thicknesses, and the latter were not different when comparing hypertensive subjects to normotensive controls. 4 Contrariwise, Akay and colleagues 7 and Masis and colleagues 8 reported that the choroid was thinner in hypertensive patients compared to nonhypertensive ones. It is noteworthy that in both these investigations important confounding factors such as renal function were not taken into account. More recently, Gök and colleagues, 9 in 116 hypertensive patients who underwent 24‐h ABPM and OCT, did not find any significant relationship of choroidal thickness with the average of BP readings recorded over 24 h. In contrast, weak correlations between BP and CTh were observed in the study of Dagel and colleagues, 10 but no multivariate analyses were performed. However, in none of these studies information about pulse pressure were provided.
The relationships that we found between CTh measures and aortic PP were independent of many potential confounding factors, such as particularly age and estimated GFR that in our previous studies were the strongest correlates of CTh. 5 , 6 It was also independent of serum glucose that, even if diabetic patients were not included in our study, disclosed a negative correlation with CTh.
The cross‐sectional design of our study precludes conclusions regarding temporal causal relationships between CTh and aortic PP. Among the hypotheses that may be formulated, the most reasonable seems to be that previously discussed, that CTh thinning may be caused by the excessive pulsatile barotrauma due to increased vascular stiffness, that limits the buffering capacity of elastic arteries. On the other hands, we cannot exclude the possibility that CTh thinning and changes in central hemodynamics are not causally related, but both may be the consequence of common factors able to exert their detrimental effects independently on the micro‐ and macrocirculation. Moreover, CTh thinning may be the marker of a more generalized microcirculation rarefaction. This may increase peripheral resistances, leading to a greater magnitude of wave reflection and therefore to a worsening of central hemodynamics.
In conclusion, our study, by using an innovative imaging technique such as SS‐OCT, documented a significant association between increased pulsatile hemodynamics, a well‐known index of early vascular aging and predictor of CV risk, and reduction of choroidal thickness in patients with primary hypertension.
Our findings support the concept of a cross‐talk between macro‐ and microcirculation.
Further studies, with a different experimental design, are needed to explain the pathophysiological reasons of our results and to understand whether the information obtained about choroidal thickness with SS‐OCT may have cardiovascular prognostic implications and clinical usefulness.
CONFLICT OF INTEREST
None.
AUTHORS CONTRIBUTIONS
Giuseppe Mulè, Maria Vadalà, Salvatore Cillino and Santina Cottone involved in study conception and design. Nicola Sinatra, Ettore Mancia, Alessandra Sorce, Giulio Geraci, Caterina Carollo, Katia Montalbano, Massimo Castellucci, Giulia Guarrasi involved in acquisition of data. Giuseppe Mulè, Maria Vadalà, Alessandra Sorce, Giulio Geraci, Massimo Castellucci involved in analysis and interpretation of data. Giuseppe Mulè, Maria Vadalà and Alessandra Sorce involved in drafting of manuscript. Giuseppe Mulè, Maria Vadalà, Salvatore Cillino, Santina Cottone, Alessandra Sorce, Giulio Geraci, Caterina Carollo involved in critical revision.
Supporting information
Table S1
Table S2
Mulè G, Vadalà M, Sinatra N, et al. Relationship of choroidal thickness with pulsatile hemodynamics in essential hypertensive patients. J Clin Hypertens. 2021;23:1030–1038. 10.1111/jch.14196
Giuseppe Mulè and Maria Vadalà contributed equally to this work.
REFERENCES
- 1. Mulè G, Vadalà M, Geraci G, Cottone S. Retinal vascular imaging in cardiovascular medicine: New tools for an old examination. Atherosclerosis. 2018;268(1):188‐190. [DOI] [PubMed] [Google Scholar]
- 2. Yeung SC, You Y, Howe KL, Yan P. Choroidal thickness in patients with cardiovascular disease: a review. Surv Ophthalmol. 2020;65(4):473‐486. 10.1016/j.survophthal.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad M, Kaszubski PA, Cobbs L, Reynolds H, Smith RT. Choroidal thickness in patients with coronary artery disease. PLoS One. 2017;12(6):e0175691. 10.1371/journal.pone.0175691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balmforth C, van Bragt JJMH, Ruijs T, et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight. 2016;1(20):1‐13. 10.1172/jci.insight.89173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulè G, Vadalà M, La Blasca T, et al. Association between early‐stage chronic kidney disease and reduced choroidal thickness in essential hypertensive patients. Hypertens Res. 2019;42(7):990‐1000. 10.1038/s41440-018-0195-1 [DOI] [PubMed] [Google Scholar]
- 6. Vadalà M, Castellucci M, Guarrasi G, Terrasi M, La Blasca T, Mulè G. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefe's Arch Clin Exp Ophthalmol. 2019;257(8):1687‐1698. 10.1007/s00417-019-04358-3 [DOI] [PubMed] [Google Scholar]
- 7. Akay F, Gundogan FC, Yolcu U, Toyran S, Uzun S. Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol. 2016;26(2):152‐157. 10.5301/ejo.5000675 [DOI] [PubMed] [Google Scholar]
- 8. Masis M, Hernandez E, Wu L. Choroidal thickness in patients with systemic hypertension. Invest Ophthalmol Vis Sci. 2011;52(14):5296. [Google Scholar]
- 9. Gök M, Karabas VL, Emre E, Aksar AT, Aslan MS, Ural D. Evaluation of choroidal thickness via enhanced depth‐imaging optical coherence tomography in patients with systemic hypertension. Indian J Ophthalmol. 2015;63(3):239‐243. 10.4103/0301-4738.156928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dagel T, Afsar B, Sag AA, et al. Noninvasive optical coherence tomography imaging correlates with anatomic and physiologic end‐organ changes in healthy normotensives with systemic blood pressure variability. Blood Pressure Monitor. 2020;25(2):89‐94. [DOI] [PubMed] [Google Scholar]
- 11. Safar ME, Levy BI. Studies on arterial stiffness and wave reflections in hypertension. Am J Hypertens. 2015;28(1):1‐6. 10.1093/ajh/hpu155 [DOI] [PubMed] [Google Scholar]
- 12. Safar ME, Struijker‐Boudier HA. Cross‐talk between macro‐ and microcirculation. Acta Physiol. 2010;198(4):417‐430. 10.1111/j.1748-1716.2009.02073.x [DOI] [PubMed] [Google Scholar]
- 13. Cheng HM, Chuang SY, Wang TD, et al. Central blood pressure for the diagnosis of hypertension‐is it a practical clinical tool in current practice? J Clin Hypertens (Greenwich). 2020;22:391‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao W, Wen Y, Ye P, et al. Noninvasive central pulse pressure is an independent determinant of renal function. J Clin Hypertens (Greenwich). 2020;22(2):234‐242. 10.1111/jch.13792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geraci G, Mule G, Costanza G, Mogavero M, Geraci C, Cottone S. Relationship between carotid atherosclerosis and pulse pressure with renal hemodynamics in hypertensive patients. Am J Hypertens. 2016;29:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839‐846. [DOI] [PubMed] [Google Scholar]
- 17. Jankowski P, Kawecka‐Jaszcz K, Czarnecka D, et al. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848‐855. [DOI] [PubMed] [Google Scholar]
- 18. Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end‐stage renal disease. Hypertension. 2002;39:735‐738. [DOI] [PubMed] [Google Scholar]
- 19. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197‐203. [DOI] [PubMed] [Google Scholar]
- 20. Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit. 2008;13(5):299‐303. 10.1097/MBP.0b013e3283057af6 [DOI] [PubMed] [Google Scholar]
- 21. Koudryavtcev SA, Lazarev VM. Validation of the BPLab1 24‐h blood pressure monitoring system according to the European standard BS EN 1060–4:2004 and British Hypertension Society protocol. Med Devices. 2011;4:193‐196. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Parati G, Stergiou G, O'Brien E, et al. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32(7):1359‐1366. [DOI] [PubMed] [Google Scholar]
- 23. Kotovskaya YV, Kobalava ZD, Orlov AV. Validation of the integration of technology that measures additional ‘vascular’ indices into an ambulatory blood pressure monitoring system. Med Devices. 2014;7:91‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ageenkova OA, Purygina MA. Central aortic blood pressure, augmentation index, and reflected wave transit time: reproducibility and repeatability of data obtained by oscillometry. Vasc Health Risk Manag. 2011;7:649‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Omboni S, Posokhov I, Parati G, et al. Ambulatory blood pressure and arterial stiffness web‐based telemonitoring in patients at cardiovascular risk. First results of the VASOTENS (Vascular health ASsessment Of The hypertENSive patients) Registry. J Clin Hypertens. 2019;2(21):1155‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH, et al. CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geraci G, Zammuto MM, Vadalà M, et al. Choroidal thickness is associated with renal hemodynamics in essential hypertension. J Clin Hypertens. 2020;22(2):245‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796‐1806. [PubMed] [Google Scholar]
- 29. Izzard AS, Rizzoni D, Agabiti‐Rosei E, Heagerty AM. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23(2):247‐250. 10.1097/00004872-200502000-00002 [DOI] [PubMed] [Google Scholar]
- 30. Rizzoni D, Agabiti‐Rosei C, Agabiti‐Rosei E. Hemodynamic consequences of changes in microvascular structure. Am J Hypertens. 2017;30(10):939‐946. 10.1093/ajh/hpx032 [DOI] [PubMed] [Google Scholar]
- 31. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144‐168. 10.1016/j.preteyeres.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farrah TE, Dhillon B, Keane PA, Webb DJ, Dhaun N. The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int. 2020;98:323‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan KA, Gupta P, Agarwal A, et al. State of science: choroidal thickness and systemic health. Surv Ophthalmol. 2016;61:566‐581. 10.1016/j.survophthal.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 34. Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130‐155. 10.1016/j.preteyeres.2015.10.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


