Abstract
Dyslipidemia is an emerging disease in China, especially in the presence of hypertension and diabetes mellitus. We investigated the association of dyslipidemia with the use of antihypertensive and antidiabetic agents. The study participants (n = 2423) were hypertensive and diabetic patients enrolled in a China nationwide registry. Serum mean ± (SD, except for serum triglycerides, median [interquatile range]) concentrations were 1.38 (0.97‐2.02) mmol/L, 4.85 ± 1.12 mmol/L, 1.30 ± 0.36 mmol/L, and 2.89 ± 0.92 mmol/L for triglycerides and total, high‐density lipoprotein (HDL), and low‐density lipoprotein (LDL) cholesterol, respectively. The prevalence of dyslipidemia was 18.9%, 13.5%, 16.6%, and 37.7% for hypertriglyceridemia (serum triglycerides ≥2.3 mmol/L), hypercholesterolemia (total cholesterol ≥6.2 mmol/L or LDL cholesterol ≥4.1 mmol/L), low HDL cholesterol (HDL cholesterol <1.0 mmol/L), and any of the three lipid disorders, respectively. Treated (n = 1647), compared with untreated hypertensive patients (n = 303), had a significantly (P ≤ .0006) lower serum total, LDL, and HDL cholesterol, but similar serum triglycerides (P = .20). Treated (n = 1325), compared with untreated diabetic patients (n = 238), had a significantly (P ≤ .004) lower serum triglycerides, and total and LDL cholesterol, but similar serum HDL cholesterol (P = .81). After adjustment, the odds ratios (OR) were significant for hypercholesterolemia (OR 0.76, 95% confidence interval [CI] 0.58‐0.997, P = .048) and low HDL cholesterol (OR 1.56, CI 1.19‐2.03, P = .001) in treated versus untreated hypertension, and for low HDL cholesterol (OR 1.50, CI 1.18‐1.89, P = .0008) in treated versus untreated diabetes. In conclusion, the prevalence of dyslipidemia differed between treated and untreated hypertension and diabetes.
Keywords: cholesterol, diabetes mellitus, dyslipidemia, hypertension, treatment
The prevalence of dyslipidemia differed between treated and untreated hypertension and diabetes mellitus in favor of treatment, in spite of some compromise in HDL cholesterol with the use of some antihypertensive or antidiabetic medications.

1. INTRODUCTION
With the significant changes in lifestyle, there is an emerging epidemic of dyslipidemia in China. According to a series of national surveys in 2002, 2012, and 2015, serum lipid concentrations increased from 3.93 to 4.59 and to 4.63 mmol/L, respectively, for total cholesterol, from 1.12 to 1.41 and to 1.47 mmol/L, respectively, for triglycerides, and from 2.12 to 2.78 and to 2.87 mmol/L, respectively, for low‐density lipoprotein (LDL) cholesterol, and changed from 1.30 to 1.18 and to 1.26 mmol/L, respectively, for high‐density lipoprotein (HDL) cholesterol. 1 The corresponding changes in the prevalence of dyslipidemia were from 1.6% to 5.6% and to 5.8%, respectively, for hypercholesterolemia (serum total cholesterol ≥6.22 mmol/L or LDL cholesterol ≥4.14 mmol/L), from 5.7% to 13.6% and to 15.0%, respectively, for hypertriglyceridemia (triglycerides ≥2.26 mmol/L), and from 18.8% to 35.5% and to 24.9%, respectively, for low HDL cholesterol (<1.04 mmol/L). 1
Even worse is the concomitant presence of dyslipidemia with other cardiovascular risk factors, such as hypertension and diabetes mellitus. According to a national survey in an elderly (≥60 years) Chinese population sample, the prevalence of dyslipidemia (hypertriglyceridemia and hypercholesterolemia for either serum total or LDL cholesterol) was 17.3% in hypertensive patients (n = 13,000) and 15.2% in normotensive participants (n = 6967), and 23.2% in diabetic patients (n = 3911) and 12.6% in non‐diabetic participants (n = 16,062). 2 These diseases may share similar environmental, lifestyle, and genetic risk factors. However, the long‐term management strategies of one chronic disease may also increase the risk of another. It is known that thiazide diuretics increase blood glucose and lipids, 3 , 4 and β‐blockers also increase the risk of diabetes mellitus, especially when used in combination with high dose of thiazide diuretics. 5 It is also known that statins may increase the risk of diabetes mellitus. 6 We hypothesize that hypertensive and diabetic patients have high risk of dyslipidemia to some extent via the use of antihypertensive and antidiabetic agents.
We recently performed a China nationwide multicenter registry of treated patients with hypertension and diabetes mellitus, 7 which allows to test the abovementioned hypothesis according to the presence or absence of treated hypertension and diabetes mellitus. In the present analysis, we investigated the association of dyslipidemia with the use of antihypertensive and antidiabetic agents in patients with hypertension or diabetes mellitus.
2. METHODS
2.1. Study population
The study participants were hypertensive and diabetic patients enrolled in a China nationwide multicenter registry, carried out from June 2011 to March 2012. The study protocol of the registry had been described in detail previously. 8 Briefly, we registered consecutive patients with previously diagnosed hypertension from the departments of cardiovascular medicine and those with previously diagnosed diabetes mellitus from the departments of endocrine medicine. To be eligible for inclusion, a patient had to be at least 20 years of age and was able to participate in two clinic visits two to five days apart. At the first clinic visit, the study physicians administered a questionnaire to collect information on medical history, current smoking and alcohol intake, and use of medications. Blood pressure was measured. At the second clinic visit, blood pressure was measured for the second time. Venous blood samples were drawn after overnight fasting for measurements of plasma glucose, glycosylated hemoglobin A1c (HbA1c), and serum lipids. The ethics committees of all participating hospitals approved the study protocol. All study participants gave written informed consent.
Of the 2510 registered patients, 87 were excluded from the present analysis, because of extreme (n = 24) or missing lipid values (n = 63), leaving 2423 patients in the analysis.
2.2. Measurement of serum lipids and definition of dyslipidemia
Serum lipids were measured using the enzymatic method. Dyslipidemia was defined according to the 2016 Chinese guideline for the management of dyslipidemia in adults. 9 The diagnostic thresholds were ≥2.30 mmol/L of serum triglycerides concentration for hypertriglyceridemia, ≥6.20 mmol/L of serum total cholesterol concentration or ≥4.10 mmol/L of serum LDL cholesterol concentration for hypercholesterolemia, and <1.00 mmol/L of serum HDL cholesterol concentration for low HDL cholesterol. 9
2.3. Blood pressure, anthropometry, and blood glucose measurements
Blood pressure was measured at the first and second clinic visits. On each of the two occasions, three blood pressure readings were obtained consecutively with an interval of 30‐60 seconds in the sitting position after the study participants had rested for at least five minutes. A validated Omron HEM‐7201 automatic oscillometric blood pressure monitor (Omron Healthcare, Kyoto, Japan) was used in all participating hospitals. The six blood pressure readings on the two clinic visits were averaged for statistical analysis and for the definition of hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication).
Anthropometric measurements included body weight, body height, and waist and hip circumferences. Body mass index was calculated as the body weight in kilograms divided by the body height in meters squared.
In patients without previously diagnosed diabetes mellitus, an oral glucose tolerance test was performed, and diabetes mellitus was defined in the presence of a fasting plasma glucose ≥7.0 mmol/L or a 2‐h post‐loading plasma glucose ≥11.1 mmol/L. In patients with previously diabetes mellitus, plasma fasting glucose was measured. HbA1c was measured in all patients.
2.4. Statistical analysis
Statistical analysis was performed using the SAS software (version 9.4; SAS Institute, Cary, NC, USA). Means and proportions across the groups were compared by the analysis of variance (ANOVA) and Fisher's exact test, respectively. Continuous measurements with a skewed distribution were represented by median (interquartile range) and compared by the nonparametric test. Logistic regression analyses were performed to study the associations of interest, while adjusting for the confounding factors.
3. RESULTS
3.1. Clinical characteristics of patients
The 2423 study participants included 860 patients with hypertension alone, 473 patients with diabetes mellitus alone, and 1090 patients with both hypertension and diabetes mellitus. These three groups of patients were significantly different in all major characteristics, such as sex, age, body mass index, current smoking and alcohol intake, systolic and diastolic blood pressure, pulse rate, plasma fasting glucose, HbA1c, and the use of statins or other lipid‐lowering agents (P ≤.007; Table 1 ).
TABLE 1.
Characteristics of patients with hypertension, diabetes mellitus, or both
| Variable | Overall (n = 2423) | Hypertension alone (n = 860) | Diabetes mellitus alone (n = 473) | Both hypertension and diabetes mellitus (n = 1090) | P value (ANOVA) |
|---|---|---|---|---|---|
| Men, % (n) | 47.1 (1140) | 46.2 (397) | 54.1 (256) | 44.7 (487) | .002 |
| Age, years | 58.4 ± 11.6 | 57.5 ± 11.8 | 53.3 ± 11.8 | 61.2 ± 10.4 | <.0001 |
| Body mass index, kg/m2 | 25.3 ± 3.4 | 25.1 ± 3.2 | 24.4 ± 3.1 | 25.8 ± 3.5 | <.0001 |
| Current smoking, % (n) | 17.3 (419) | 16.5 (142) | 23.9 (113) | 15.1 (164) | <.0001 |
| Alcohol intake, % (n) | 16.6 (403) | 17.9 (154) | 20.1 (95) | 14.1 (154) | .007 |
| Systolic blood pressure, mmHg | 137.1 ± 17.6 | 140.6 ± 16.7 | 121.7 ± 10.4 | 140.9 ± 17.1 | <.0001 |
| Diastolic blood pressure, mmHg | 80.2 ± 11.5 | 83.7 ± 12.1 | 74.2 ± 7.8 | 80.2 ± 11.3 | <.0001 |
| Heart rate, beats/min | 73.6 ± 12.9 | 72.4 ± 12.8 | 74.7 ± 12.5 | 74.1 ± 13.2 | .0005 |
| Plasma fasting glucose, mmol/L | 6.2 (5.4‐7.9) | 5.3 (5.0‐5.8) | 7.6 (6.3‐9.8) | 7.2 (6.1‐8.8) | <.0001 |
| Plasma glycosylated hemoglobin A1c, % | 6.3 (5.8‐7.5) | 5.7 (5.5‐6.1) | 7.0 (6.3‐8.3) | 6.9 (6.2‐8.0) | <.0001 |
| Use of statins, % (n) | 20.2 (489) | 19.2 (165) | 10.6 (50) | 25.1 (274) | <.0001 |
| Use of other lipid‐lowering agents, % (n) | 3.0 (72) | 2.0 (17) | 5.7 (27) | 2.6 (28) | .0004 |
Values are arithmetic mean ±standard deviation, median (interquartile range) or percentage of subjects (number), unless otherwise indicated.
Overall, serum mean ± (SD, except for serum triglycerides, median [interquatile range]) concentrations were 1.38 (0.97‐2.02) mmol/L, 4.85 ± 1.12 mmol/L, 1.30 ± 0.36 mmol/L, and 2.89 ± 0.92 mmol/L for triglycerides and total, HDL, and LDL cholesterol, respectively. The overall prevalence of dyslipidemia was 18.9%, 13.5%, 16.6%, and 37.7% for hypertriglyceridemia, hypercholesterolemia (total or LDL cholesterol), low HDL cholesterol, and any of the three lipid disorders, respectively.
3.2. Prevalence of dyslipidemia according to antihypertensive and antidiabetic treatments
Table 2 shows the lipid characteristics of patients according to the treatment status of hypertension and diabetes mellitus. Treated, compared with untreated, patients with hypertension had similar serum triglycerides (P =.20) but a significantly lower concentration of serum total, LDL, and HDL cholesterol (P ≤.0006), and accordingly had a similar prevalence of hypertriglyceridemia (P =.79) but a significantly lower prevalence of hypercholesterolemia (P =.03) and higher prevalence of low HDL cholesterol (P =.003). Treated, compared with untreated, patients with diabetes mellitus had similar serum HDL cholesterol (P =.81), but a significantly lower serum triglycerides and total and LDL cholesterol (P ≤.004), and had a lower prevalence of hypertriglyceridemia (P =.004). The use of statins and any other lipid‐lowering agents was significantly (P <.0001) higher in patients on antihypertensive (21.5% and 2.0%, respectively), antidiabetic (12.0% and 4.8%, respectively), or both treatments (29.9% and 3.4%, respectively) than untreated patients (1.92% and 0.48%, respectively; Figure 1).
TABLE 2.
Lipid profile according to the treatment status of hypertension and diabetes mellitus
| Variable | Hypertension | Diabetes mellitus | ||||
|---|---|---|---|---|---|---|
| Treated (n = 1647) | Untreated (n = 303) | P value | Treated (n = 1325) | Untreated (n = 238) | P value | |
| Serum triglycerides, mmol/L | ||||||
| Median (interquartile range) | 1.42 (1.01‐2.03) | 1.35 (0.93‐1.99) | .20 | 1.37 (0.93‐2.02) | 1.52 (1.04‐2.36) | .004 |
| ≥2.3 mmol/L, % (n) | 19.1 (315) | 18.5 (56) | .79 | 18.7 (248) | 26.9 (64) | .004 |
| Serum HDL cholesterol, mmol/L | ||||||
| Mean ±SD | 1.29 ± 0.35 | 1.37 ± 0.41 | .0005 | 1.30 ± 0.38 | 1.29 ± 0.32 | .81 |
| <1.0 mmol/L, % (n) | 17.8 (293) | 10.9 (33) | .003 | 18.4 (244) | 13.5 (32) | .06 |
| Serum cholesterol, mmol/L | ||||||
| Total, Mean ±SD | 4.79 ± 1.08 | 5.04 ± 1.13 | .0004 | 4.82 ± 1.18 | 5.06 ± 1.04 | <.0001 |
| LDL, Mean ±SD | 2.86 ± 0.91 | 3.06 ± 0.95 | .0006 | 2.85 ± 0.93 | 3.04 ± 0.93 | .0005 |
| Hypercholesterolemia (≥6.2 mmol/L [total] or ≥4.1 mmol/L [LDL]), % (n) | 12.6 (207) | 17.2 (52) | .03 | 12.5 (165) | 16.8 (40) | .07 |
| Any dyslipidemia, % (n) | ||||||
| Any above lipid abnormality, % (n) | 38.6 (636) | 35.0 (106) | .23 | 37.7 (499) | 42.0 (100) | .20 |
| Use of lipid‐lowering agents, % (n) | 28.0 (461) | 7.6 (23) | <.0001 | 26.2 (347) | 13.5 (32) | <.0001 |
Values are arithmetic mean ±standard deviation, median (interquartile range) or percentage of subjects (number), unless otherwise indicated.
Abbreviations: HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
FIGURE 1.
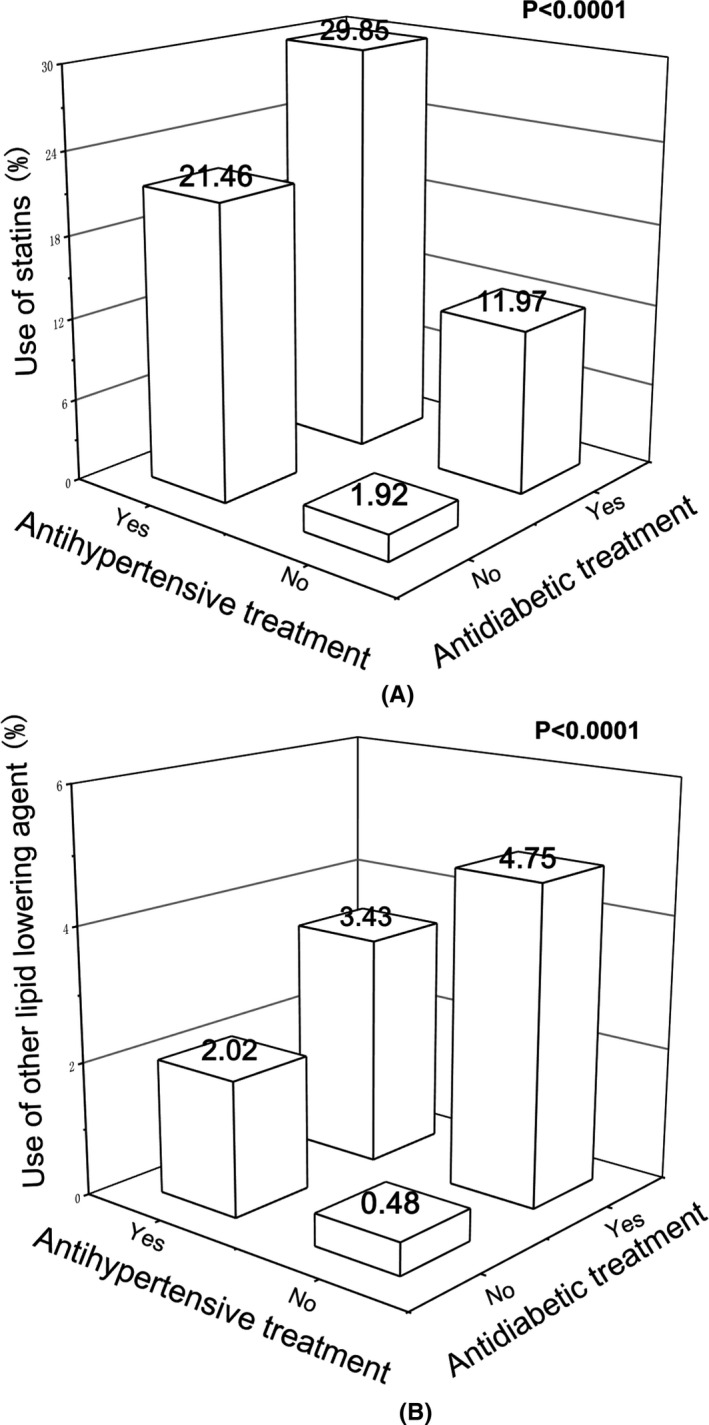
Percentage of patients on statins (a) or other lipid‐lowering agents (b) according to treatments. Percentage is given for each category at the top of the bar. P values are given for the comparisons between patients on antihypertensive or antidiabetic treatment and those without any treatment, and between patients on both antihypertensive and antidiabetic treatments and those with one or no treatment
3.3. Adjusted analyses on the association between dyslipidemia and antihypertensive and antidiabetic treatments
After adjustment for age, sex, body mass index, current smoking, and alcohol intake, the results were confirmatory (Table 3 ). The adjusted odds ratios for treated versus untreated hypertension were 1.56 (95% confidence interval [CI] 1.19‐2.03, P =.001) for low HDL cholesterol, 0.76 (95% CI 0.58‐0.997, P =.048) for hypercholesterolemia, 1.23 (95% CI 1.01‐1.50, P =.04) for any dyslipidemia, 3.04 (95% CI 2.28‐4.04, P <.0001) for the use of statins, and 2.53 (95% CI 1.96‐3.26, P <.0001) for the use of any lipid‐lowering agents. The adjusted odds ratios for treated versus untreated diabetes mellitus were 1.50 (95% CI 1.18‐1.89, P =.0008) for low HDL cholesterol, 1.58 (95% CI 1.27‐1.96, P <.0001) for the use of statins and 1.72 (95% CI 1.40‐2.12, P <.0001) for the use of any lipid‐lowering agents.
TABLE 3.
Dyslipidemia in relation to antihypertensive or antidiabetic treatment
| Dyslipidemia | Treated vs. untreated hypertension | Treated vs. untreated diabetes mellitus | ||
|---|---|---|---|---|
| Odds ratio (95% CI) a | P value | Odds ratio (95% CI) a | P value | |
| Hypertriglyceridemia | 1.20 (0.94‐1.54) | .14 | 1.07 (0.86‐1.33) | .57 |
| Low serum HDL cholesterol | 1.56 (1.19‐2.03) | .001 | 1.50 (1.18‐1.89) | .0008 |
| Total or LDL hypercholesterolemia | 0.76 (0.58‐0.997) | .048 | 0.79 (0.62‐1.02) | .07 |
| Any above lipid abnormality | 1.23 (1.01‐1.50) | .04 | 1.08 (0.91‐1.29) | .40 |
| Use of lipid‐lowering agents | 2.53 (1.96‐3.26) | <.0001 | 1.72 (1.40‐2.12) | <.0001 |
Abbreviations: HDL, indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
Adjusted for sex, age, body mass index, current smoking and alcohol intake. CI indicates confidence interval.
Further adjusted analyses on the use of various antihypertensive and antidiabetic agents showed significant associations for low HDL cholesterol (P ≤.001) but not the other dyslipidemia (P ≥.57). Indeed, the prevalence of low HDL cholesterol was higher in users of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, diuretics/β‐blockers, or insulin than their non‐user counterparts (P ≤.02). The corresponding adjusted odds ratios (95% CI) were 1.32 (1.04‐1.68, P =.02), 1.43 (1.09‐1.87, P =.01), and 1.55 (1.18‐2.02, P = 0. 002), respectively (Figure 2).
FIGURE 2.
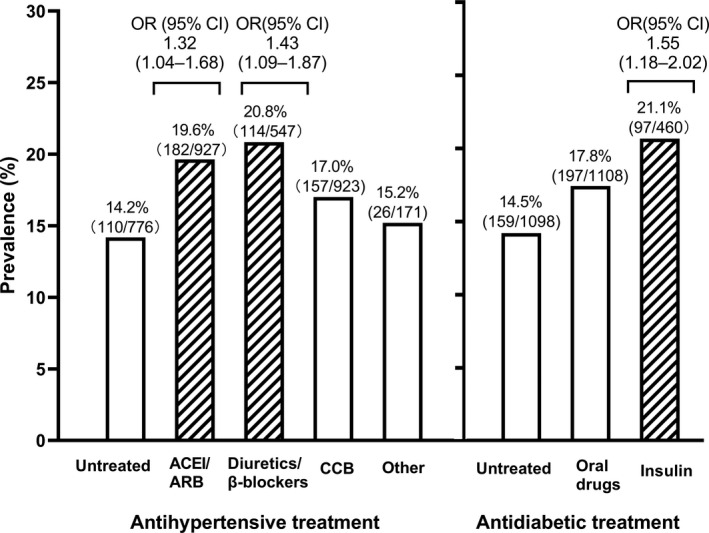
Prevalence of low high‐density lipoprotein (HDL) cholesterol by antihypertensive and antidiabetic treatments in all patients. The number and percentage of patients are given for all categories along the symbols. Odds ratios (OR, 95% confidence interval [CI]) were adjusted for age, sex, body mass index, current smoking and alcohol intake and are given above the bar for the significant ones. ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium‐channel blockers
4. DISCUSSION
A major finding of the present study was that the prevalence of dyslipidemia and the use of statins and other lipid‐lowering agents differed between treated and untreated hypertension and diabetes mellitus in favor of treatment, in spite of some compromise in serum HDL cholesterol with the use of several classes of antihypertensive or antidiabetic medications.
The observation on the positive and negative effects of treating hypertension and diabetes mellitus on serum lipids is noteworthy. There is some evidence on the positive effect. 10 , 11 A possible reason for the positive effect is the associated use of statins and other lipid‐lowering agents, as observed in the present study. Some antihypertensive or antidiabetic agents, such as α1‐blockers and thiazolidinediones, have beneficial metabolic effects. 12 , 13 , 14 However, these drugs are rarely used in the current routine management of hypertension or diabetes mellitus. There is also some evidence on the negative effect of antihypertensive and antidiabetic agents on serum lipids. 15 Indeed, both thiazide diuretics and β‐blockers, especially in combination and long‐term use, do have adverse metabolic effects. 16 , 17
The observed adverse metabolic profile associated with the use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers can probably be explained by indication bias. These two classes of drugs have been found to have beneficial metabolic effects. 18 , 19 Current hypertension guidelines recommend the use of these drugs in patients with diabetes mellitus or the metabolic syndrome. 20 , 21 , 22 The same indication bias mechanism may also apply for the adverse metabolic profile associated with the use of insulin, because some of the previous studies did show beneficial metabolic effects of insulin on lipid profile, characterized of lower total and LDL cholesterol, lower triglycerides, and higher HDL cholesterol. 23 , 24
The study results had to be interpreted within the context of several limitations. First, the study had a cross‐sectional design and hence does not allow any causal inference. Second, our study had a relatively small sample size with few measurements of biological markers. Third, the information on the dose and duration of antihypertensive or antidiabetic drugs was not collected. It was not possible to do any quantitative estimation on the studied associations with the use of these drugs.
5. CONCLUSIONS
In conclusion, the prevalence of dyslipidemia differed between treated and untreated hypertension and diabetes mellitus in favor of treatment, in spite of some compromise in HDL cholesterol with the use of some antihypertensive or antidiabetic medications. The study provides further evidence on the association of the currently recommended antihypertensive and antidiabetic medications with dyslipidemia. Future research should address management and prevention of dyslipidemia in treated and untreated patients with hypertension and diabetes mellitus.
CONFLICT OF INTEREST
Dr Wang reports receiving lecture and consulting fees from Merck, Novartis, Omron, Servier, and Takeda. The other authors declared no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
All authors participated in the development of the analysis plan, in interpretation of the results, and in reviewing and editing the manuscript. All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the participation of the patients and the contribution of the investigators. The participating hospitals are listed in an online supplemental Appendix 1 (http://links.lww.com/HJH/A634).
Miao C‐Y, Ye X‐F, Zhang W, Ji L‐N, Wang J‐G; for the ATTEND investigators . Association between dyslipidemia and antihypertensive and antidiabetic treatments in a China multicenter study. J Clin Hypertens. 2021;23:1399–1404. 10.1111/jch.14264
Funding information
The registry was sponsored by Sanofi China (Shanghai, DIREG_L_05728). The study investigators were financially supported by grants from the National Natural Science Foundation of China (grants 91639203 and 82070435), Beijing, China and from the Shanghai Commissions of Science and Technology (grant 19DZ2340200), and Health (a special grant for ‘leading academics’), and Clinical Research Program, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (grant 2018CR010), Shanghai, China.
REFERENCES
- 1. Song PK, Man QQ, Li H, et al. Trends in lipids level and dyslipidemia among Chinese adults, 2002–2015. Biomed Environ Sci. 2019;32:559‐570. [DOI] [PubMed] [Google Scholar]
- 2. Wang ZH, Wang LH, Li YC, et al. Current status of diabetes, hypertension and dyslipidemia among older Chinese adults in 2010. Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:922‐926. [PubMed] [Google Scholar]
- 3. Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic‐based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886‐1892. [PubMed] [Google Scholar]
- 4. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981‐2997. [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, Grassi G, Zanchetti A. New‐onset diabetes and antihypertensive drugs. J Hypertens. 2006;24:3‐10. [DOI] [PubMed] [Google Scholar]
- 6. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305:2556‐2564. [DOI] [PubMed] [Google Scholar]
- 7. Song J, Sheng CS, Huang QF, et al. Management of hypertension and diabetes mellitus by cardiovascular and endocrine physicians: a China registry. J Hypertens. 2016;34:1648‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The ATTEND, investigators , Zhang W, Liu C‐Y, et al. Blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy in patients with hypertension and diabetes. J Clin Hypertens (Greenwich). 2020;22:212‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joint Committee for Guideline Revision . 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kintscher U, Marx N, Martus P, et al. Effect of high‐dose valsartan on inflammatory and lipid parameters in patients with Type 2 diabetes and hypertension. Diabetes Res Clin Pract. 2010;89:209‐215. [DOI] [PubMed] [Google Scholar]
- 11. REMOVAL Study Group , Petrie JR, Chaturvedi N, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double‐blind, randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2017;5:597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toth K, Nemes J, Czopf L, et al. Effects of doxazosin on hemorheological, hemodynamic and lipid parameters in patients with essential hypertension. Clin Hemorheol Microcirc. 1999;20:57‐61. [PubMed] [Google Scholar]
- 13. Rabkin SW, Huff MW, Newman C, et al. Lipids and lipoproteins during antihypertensive drug therapy. Comparison of doxazosin and atenolol in a randomized, double‐blind trial: the Alpha Beta Canada Study. Hypertension. 1994;24:241‐248. [DOI] [PubMed] [Google Scholar]
- 14. Davidson JA, Perez A, Zhang J, et al. Addition of pioglitazone to stable insulin therapy in patients with poorly controlled type 2 diabetes: results of a double‐blind, multicentre, randomized study. Diabetes Obes Metab. 2006;8:164‐174. [DOI] [PubMed] [Google Scholar]
- 15. Deano R, Sorrentino M. Lipid effects of antihypertensive medications. Curr Atheroscler Rep. 2012;14:70‐77. [DOI] [PubMed] [Google Scholar]
- 16. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995‐1003. [DOI] [PubMed] [Google Scholar]
- 17. Investigators ASCOT, Dahlöf B, Sever PS, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): A multicentre randomised controlled trial. Lancet. 2005;366:895‐906. [DOI] [PubMed] [Google Scholar]
- 18. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145‐153. [DOI] [PubMed] [Google Scholar]
- 19. Vitale C, Mercuro G, Castiglioni C, et al. Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol. 2005;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334‐1357. [DOI] [PubMed] [Google Scholar]
- 21. ESC Scientific Document Group , Williams B, Mancia G, et al. ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;2018(36):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 22. Joint Committee for Guideline Revision . 2018 Chinese guidelines for prevention and treatment of hypertension‐A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16:182‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geltner C, Lechleitner M, Föger B, et al. Insulin improves fasting and postprandial lipemia in type 2 diabetes. Eur J Intern Med. 2002;13:256‐263. [DOI] [PubMed] [Google Scholar]
- 24. Cieluch A, Uruska A, Grzelka A, et al. An increase in high‐density lipoprotein cholesterol concentration after initiation of insulin treatment is dose‐dependent in newly diagnosed type 1 diabetes. The results of the InLipoDiab1 study. Pol Arch Intern Med. 2018;128:69‐71. [DOI] [PubMed] [Google Scholar]


