Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly devastating disease with a dismal 5-year survival rate. PDAC has a complex tumour microenvironment; characterised by a robust desmoplastic stroma, extensive infiltration of immunesuppressive cells such as immature myeloid cells, tumour-associated macrophages, neutrophils and regulatory T cells, and the presence of exhausted and senescent T cells. The cross-talk between cells in this fibrotic tumour establishes an immune-privileged microenvironment that supports tumour cell escape from immune-surveillance, disease progression and spread to distant organs. PDAC tumours, considered to be non-immunogenic or cold, express low mutation burden, low infiltration of CD8+ cytotoxic lymphocytes that are localised along the invasive margin of the tumour border in the surrounding fibrotic tissue, and often display an exhausted phenotype. Here, we review the role of T cells in pancreatic cancer, examine the complex interactions of these crucial effector units within pancreatic cancer stroma and shed light on the increasingly attractive use of T cells as therapy.
Keywords: Immunosuppression, T cell exhaustion, Tumour microenvironment, Pancreatic ductal adenocarcinoma, Pancreatic cancer stroma
Core Tip: Pancreatic ductal adenocarcinoma (PDAC) is a highly devastating disease with a dismal 5-year survival of less than 5% in patients with metastatic disease, and is predicted to become the second cause of cancer-related death by 2030. Here, we discuss the complexity of the PDAC immunosuppressive tumour microenvironment, the mechanisms involved in T cell dysfunction, and potential immunotherapeutic strategies for treating PDAC.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a highly devastating disease with a dismal 5-year survival of less than 5% in patients with metastatic disease[1], and is predicted to become the second cause of cancer-related death by 2030[2]. Late detection and incredibly aggressive biology are significant challenges determining therapeutic failure[3,4]. PDAC has a complex tumour microenvironment (TME) characterised by a robust desmoplastic stroma[5], and an expanded pool of immunosuppressive immune cells shielding the malignant cells harbouring aberrant expression of oncogenic pathways. The interplay between various cell types in this fibrotic TME supports tumour cell escape from immunosurveillance, disease progression and spread to distant organs [6,7], highlighting this cancer’s ability to evade immune recognition and its extra-ordinary metastatic potential. In this review, we discuss the interactions between T cells and the other components of the PDAC TME and highlight the impact of these interactions on the phenotype and function of T cells. Emerging immune-therapeutic strategies employed in overcoming T cell dysfunction and improve patient survival are also discussed.
PDAC IMMUNE LANDSCAPE
PDAC carcinogenesis is characterised by an abundant fibro-inflammatory reaction and subsequent oncogene activation on epithelial cells, resulting in a pro-tumorigenic microenvironment[8]. At early stages of cancer development, oncogenic KRAS expression in pancreatic cells results in the formation of pancreatic intraepithelial neoplasia (PanIN), and drives an inflammatory reaction that modulates the recruitment and infiltration of immunosuppressive myeloid and lymphoid cell subsets. KRAS-mutated pancreatic cells regulate the maintenance of immunoregulatory microenvironment by inducing the release of interleukin (IL)-6, IL-10 and transforming growth factor (TGF-β) cytokines. In the setting of sustained chronic inflammation, PanIN progression to malignant lesion is accompanied by mutations in genes such as TP53, CDKN2A and SMAD4 frequently, which further contribute to shape the immune microenvironment. For example, the mutant tumour suppressor gene TP53 are implicated in sustaining the tissue damage and chronic inflammation by enhancing the expression of NF-kB, secretion of vascular endothelial growth factor (VEGF) and activation of fibroblasts. Decreased infiltration of T and B cells and elevated numbers of Tregs were significantly correlated with CDKN2A mutations while SMAD4 mutations are involved with enhanced invasion, metastasis and immunosuppressive effects of TGF-β on immune response[9].
Chemotactic factors associated with the recruitment of dysfunctional bone marrow-derived myeloid cells include granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte-colony-stimulating factor (G-CSF), IL-3, VEGF, and the interaction of the C-X-C chemokine ligand 12 (CXCL12)/C-X-C chemokine receptor 4 (CXCR4) or C-C chemokine ligand 2 (CCL2)/C-C chemokine receptor 2 (CCR2), amongst others[10]. Stromal-associated fibroblasts produce C-X-C chemokine ligand 13 (CXCL13), which recruits IL-35-producing regulatory B cells (Breg) into the TME, further contributing to PDAC progression through IL35-mediated stimulation of tumour cell proliferation[10]. Copious infiltration of immature myeloid cells, tumour-associated macrophages (TAMs), neutrophils and regulatory immune cells ultimately establishes an immune-privileged microenvironment that protects the malignant cells from T cell immunosurveillance and sustains tumour growth[11].
Therefore, pancreatic cancer evolves to establish a complex and heterogeneous immune microenvironment, characterised by high numbers of strongly suppressive immune cells, and a modest infiltration of lymphocytes with anti-tumour properties[12-14]. As such, PDAC tumours are considered to be non-immunogenic or cold, displaying low infiltration of CD8+ cytotoxic lymphocytes (CTLs) that are localised along the invasive margin of the tumour border or trapped in the surrounding fibrotic tissue but are not present within the tumour core. Moreover, infiltrated CD8+ T cells in PDAC tumours often display minimal signs of activation[11,15,16]. T cell exclusion from TME has been demonstrated both in genetically engineered KPC (KRasLSL_G12D/+, Trp53LSL_R172H/+, Pdx1-Cre) mouse models[16] and PDAC patients[17].
Macrophages compose the most abundant immune cells in PDAC[11]. They play a critical role in the exclusion of T cells from tumours, maintenance of fibrosis through the secretion of pro-fibrotic cytokines[18] and induction of angiogenesis by secreting VEGF[19]. Increases in TAMs correlate with poor prognosis[20,21]. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immunosuppressive cells, including immature monocytes, granulocytes and dendritic cells (DCs). These cells show potent ability to inhibit proliferation and induce apoptosis of both CD4+ and CD8+ T cells, secrete elevated amounts of immunosuppressive cytokines IL-10 and TGF-β, which collaborate to the recruitment of regulatory CD4+ T cells (Tregs), and decrease the infiltration of natural killer (NK) and NKT cells into the tumour[22]. MDSCs accumulation has been described in the spleen, tumours and metastatic lesions in KPC models of PDAC, and its accumulation negatively correlated with CD8+ T cells infiltration[19].
Likewise, Tregs upregulate the expression of CTL-associated antigen 4 (CTLA-4)[23], interact with DCs suppressing the expression of the co-stimulatory ligands, such as CD80 and CD86, necessary for T cell activation, secrete immunosuppressive cytokines, and directly suppress CD8+ T cells anti-tumour immunity[24]. Infiltration of Tregs occurs at early stages of PDAC formation, and increased numbers of both circulating and intra-tumoural. Tregs have been observed in pancreatic cancer patients[19]. Additionally, the presence of tumour-infiltrating IL-17-producing CD4+ T cells and γδT cells also contribute to tumour immune evasion and progression[11,25].
Similar to PDAC, in inflammatory conditions of the pancreas, such as pancreatitis, the inflammatory reaction leads to the infiltration of myeloid cells, such as monocytes and neutrophils. Although macrophages comprise a significant population within the inflamed pancreas, T cells are also present, and infiltration of CD4+ T cells has been implicated in the progression of acute pancreatitis in mice. As pancreatitis progresses, the ratio of CD4+ and CD8+ T cell increases, with increased numbers of immunosuppressive Tregs observed in patients with chronic pancreatitis.
T CELL INTERACTIONS AND IMMUNE DYSFUNCTION IN PANCREATIC CANCER
T cell infiltration is observed in patients with surgically-resected PDAC and correlates with improved outcomes suggesting the anti-tumour potential of tumour-infiltrating CD4+ and CD8+ T cells[26]. However, as PDAC progresses, tumour-infiltrating T cell composition shifts to a decrease in CD8+ T cells and elevated percentage of Tregs within the CD4+ T cell subset[27]. While CD4+ Tregs are a prominent feature of the immune infiltrate, CD8+ T cells are rare in the PDAC microenvironment[24]. Consequently, PDAC is considered to be a poorly immune responsive cancer, with T cells present within the tumour microenvironment often showing lack of activation, or an exhausted phenotype[28-30]. This observation demonstrates that infiltrated CD8+ T cell may recognise and mount a response against these tumours, but the unfavourable TME halts optimal cytotoxic function.
Spatial localisation of the immune cells in these tumours reflect the challenging biology of PDAC TME. Tumour-infiltrating CD8+ T cells are localised at the periphery, within the surrounding fibrotic stroma in PDAC tissues[6,21,31,32]. CD8+ T cells migrate away from the juxta-tumoural compartment by favouring their movement towards CXCL12-rich stroma laid by activated pancreatic stellate cells (PSCs)[6]. The proximity of intra-tumoral CD8+ T cells to tumour cells correlates with patient survival[32].
PSCs play a central role in shaping the architecture of PDAC by modulating the ECM components and producing a physical barrier that limits T cell infiltration, migration and direct interaction with neoplastic cells[33]. These cells can also act as non-professional antigen-presenting cells (APCs) and secrete cytokines and growth factors that boost the recruitment of immunosuppressive cells and inhibit T cell responses, resulting in increased disease aggressiveness and decreased overall survival[34]. Therefore, in conjunction with the immunosuppressive cells, PSCs are crucial players in the orchestration of an immuno-privileged PDAC microenvironment by combination of secreted cytokines, chemokines and extra-cellular matrix proteins as well as direct cell-cell contact.
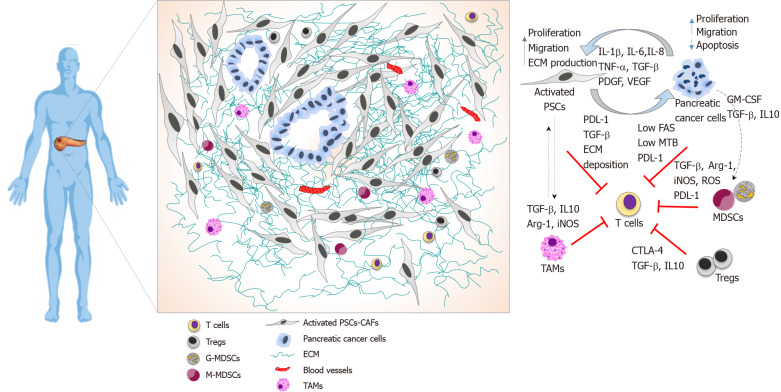
Cancer cell-intrinsic factors also impact T cell function. Overexpression of immune checkpoint mediators like programmed death-1 receptor (PD-1)-ligand (PD-L1) is one mechanism by which cancers suppress T cell immunity. PD-L1 is overexpressed in PDAC cells, and this overexpression correlates with worse prognosis[20]. Pancreatic cancer cells can also downregulate Fas, a cell surface receptor associated with the induction of Fas-mediated apoptosis in tumour cells. CD8+ T cells use the Fas-FasL and perforin–granzyme pathways as major effector mechanisms of cytotoxicity, and loss of Fas expression in PDAC tumours result in cancer immune evasion[7,35]. Spatial localisation and T cell interactions within the PDAC tumour microenvironment are shown in Figure 1.
Figure 1.
Pancreatic ductal adenocarcinoma immune landscape and T cell immunosuppression. Illustrative image showing spatial localisation of T cells in the pancreatic ductal adenocarcinoma tumour microenvironment and cellular interactions that collectively prevent T cell infiltration and function. T cells are localised at the periphery of tumours preventing direct contact with cancer cells. Pancreatic stellate cells produce elevated amounts of extracellular matrix driving a fibrotic tissue that entraps infiltrated T cells, alongside with immunosuppressive cytokine to and expression of programmed death-ligand 1 (PDL-1). Pancreatic cancer cells avoid T cell killing by downregulating Fas, exhibiting low tumour mutational burden, expressing PDL-1 and secreting growth factors and cytokines that recruits immunosuppressive cells. Myeloid-derived-suppressor cells express PDL-1 and suppress T cells functions by several mechanisms, including depleting of arginase 1, the release of reactive oxygen species, and secretion of cytokines. Tregs directly suppress T cells, express cytotoxic T-lymphocyte-associated protein 4 and secrete cytokines. TAMs play a role in sequestering T cells at the periphery and secrete immunosuppressive cytokines. PSC: Pancreatic stellate cells; TAMs: Tumour-associated macrophages; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; GM-CSF: Granulocyte-macrophage colony-stimulating factor; Arg-1: Arginase 1; PDL-1: Programmed death-ligand 1; iNOS: Inducible nitric oxide; MDSC: Myeloid-derived-suppressor cells; ROS: Reactive oxygen species; ECM: Extracellular matrix; TBM: Tumour mutational burden.
PDAC has a low mutation burden, resulting in low neoantigen burden and the scarcity of tumour-infiltrating effector T cells. Only a few PDAC tumour antigens capable of inducing an anti-tumour immune response have been identified. Low mutation burden with minimal expression of neoantigens, and consequently marginal T cell infiltration is a classical feature in KPC models[36], and in PDAC patients[29,37]. In a recent study aimed to identify T cell neoantigens in long-term survival patients, it appears that the total neoantigen burden does not correlate with increased survival, but the presence of high-quality neoantigens played an essential role in the immunosurveillance of long-term survival patients. This study also highlighted the correlation of prolonged survival with granzyme B+ CD8+ T cells[26]. In keeping with this hypothesis, the total number of infiltrated CD8+ T cells after vaccine immunotherapy did not show correlation with survival, but the subset of granzyme B+ CD8+ T cells was associated with long-term survivors[38]. These findings suggested that T cell quality may be more important than the total number of T cells for adequate anti-tumour immunity[39].
Identification of multiple dense lymphocyte aggregates, known as tertiary lymphoid structures (TLS) has also been observed in PDAC[38]. Importantly, detection of TLS in tumour tissue of PDAC patients was an independent prognostic factor for prolonged survival[40-42]. Although TLS can occur intra-tumoral or at the tumour periphery, only the presence of intra-tumoral TLS correlates with survival[41]. TLS aggregates contain T- and B-cell areas co-localised with myeloid and follicular DCs, and high-endothelial venules, displaying similar organisation to secondary lymphoid organs. They comprise ectopic lymphoid sites where T-cell activation and proliferation takes place[41]. Nevertheless, PDAC immune microenvironment is enriched with both exhausted and senescent T cells, and a diverse pool of highly immunosuppressive cells[43].
T CELL PHENOTYPE AND FUNCTIONS
Mature T cells can be classified as CD8+ T cells (CTLs) and CD4+ helper T cells (Th), which further differentiate into Th1, Th2, Th17 and Tregs[17]. CD4+ Th1 cells secrete the pro-inflammatory cytokine interferon-γ (IFN-γ) which activates and supports CTLs cytotoxicity, while CD4+ Th2 cells exhibit tumour-promoting functions by producing a plethora of cytokines, sustaining fibrosis through ECM and collagen deposition, and contributing to the differentiation of macrophages into a M2-immunosuppressive phenotype[44]. Polarisation towards Th2 cell subset is a common trait in pancreatic cancer, and this shift from Th1 to Th2 cells is correlated with decreased patient survival[45]. In PDAC patients, CD4+ Th17 cells functions are mediated by the secretion of IL-17 cytokine. Although not very well understood, infiltration of this population has been associated with immune tolerance and reduced survival in murine models[46]. Tregs are an essential component of the T cell population. PDAC patients have increased numbers of Tregs that are inversely associated with CD8+ T cells, therefore, they are often used as a negative prognostic biomarker in PDAC[45]. These cells can be identified by the expression of CD4+CD25+FOXP3+ phenotype[24] (Table 1).
Table 1.
T cell phenotype and functions
| T cell phenotype | Surface markers | Immune response | Effector functions |
| Cytotoxic T cell | |||
| CTLs | CD8 | Tumour killing | IFN-γ, TNF-α cytokines, granzymes, FasL |
| Helper T cell | |||
| Th1 | CD4 STAT4 T-bet | Tumour killing | IFN-γ, IL-2 cytokines, increase CTL activity |
| Th2 | CD4 STAT6 GATA3 | Tumour tolerance | IL-4, IL-5, IL-13 cytokines, decrease CTL activity |
| Th17 | STAT3 RORγt | Tumour tolerance | IL-17 cytokine |
| γδ T cells | TCRγ/δ | Tumour tolerance | IL-4, IL-10, TGF-β cytokines and CTL activity |
| Regulatory T cell | |||
| Tregs | CD4 CD25 FOXP3 | Tumour tolerance | IL-10, TGF-β cytokines, CTLA-4 |
CTL: Cytotoxic lymphocyte; IFN-γ: Interferon-γ; TNF-α: Tumour necrosis factor α; IL: Interleukin; TGF-β: Transforming growth factor β.
CTLs are the preferred immune cells for targeting tumours. For durable and efficient immune responses, naïve T cells are primed in the lymph nodes with tumour antigens through interactions with APCs. Upon activation, they rapidly proliferate, differentiate into antigen-specific CTLs and migrate to tumour sites to perform their cytotoxic functions[47]. Elimination of tumour cells by CTLs occurs via the release of cytotoxic granzymes, IFN-γ and tumour necrosis factor α (TNF-α), or by induction of FasL-mediated apoptosis[48]. Following a cytotoxic immune response, the majority of CTLs will undergo apoptosis while a small fraction of them will further differentiate into diverse subsets of multipotent, long-lived memory CD8+ T cells endowed with self-renewal ability[47]. The integration of three coordinated signals regulates T cells activation, expansion, survival, and memory formation: T cell receptor (TCR) stimulation by antigens, engagement of co-stimulatory molecules (CD28, CD27, 4-1BB, and OX40) expressed by CD8+ T cells, and the release of inflammatory cytokines. In the absence of co-stimulatory signals, antigenic stimulation induces tolerance or clonal deletion in peripheral lymphoid organs[49]. The pro-inflammatory cytokines IL-12, IL-2 and IFN-γ, are crucial for satisfactory naïve CD8+ T cell activation, expansion and differentiation whereas IL-7 and IL-15 are predominantly required for formation maintenance of memory CD8+ T cells. In pancreatic cancer patients, both number and functions are altered within the CD8+ T cell population. These patients show a decrease in circulating CD8+ T cells and a decrease in perforin expression within these cells compared to healthy subjects. Moreover, intra-tumoural CD8+ T infiltrates often display abnormal exhausted phenotype[44].
Memory CD8+ T cells immediately proliferate upon antigen stimulation, execute cytotoxic functions, secrete effector cytokines, persist in greater numbers and exist in different metabolic, transcriptional, and epigenetic states[50]. Importantly, while the correlation between the numbers of memory CD8+ T cells and the efficacy of T cell immunity is firmly established, the quality (or functional ability) of memory CD8+ T cells also determines the degree of protection[47,48,50]. While memory T cell population are heterogeneous and consist of multiple subsets, the central memory T cells (TCM) and effector memory T cell (TEM) subsets have been best characterised. TCM cells express high levels of CD62L and CCR7 and efficiently home to lymph nodes, whereas TEM cells lack these molecules and reside mainly in non-lymphoid peripheral tissues but are able to migrate rapidy in response to cytokine gradient. TCM and TEM subsets can also be identified along with a terminally differentiated CD8+ T subset that expresses CD45RA (TEMRA). This way, the TCM subset is classified as CD45RA- CD27high CCR7+ cells and TEM subset as CD45RA- CD27low CCR7- cells. In contrast, TEMRA subset can be identified as CD45RA+ CD27lo CCR7- cells, and naïve T cells as CD45RA+ CD27high CCR7+ cells, but there are other methods of differentiating these sub-types[47,50].
A handful of other markers have been described to differentiate T cell populations during the effector-to-memory transition states. Increased expression of IL-7Rα (CD127) is functionally required for long-term survival and can be used to identify memory precursor CD8+ T cells. Other proteins co-expressing with CD127+ CD8+ T cells include Bcl-2, CD27, CXCR3, and CD28. Cells expressing these set of markers have the most remarkable capacity to develop into central memory CD8+ T cells (TCM), showing elevated ability to proliferate upon antigen stimulation, increased IL-2 secretion, and self-renewal. Conversely, CD8+ T cells with increased expression of KLRG1, CD57 and decreased expression CD127, CD27, CXCR3, and CD28 are associated with effector or memory CD8+ T cells that display cytotoxicity, elevated IFN-γ production and short-life span. Therefore, KLRG1+ CD127- CD8+ T cells can be considered effector memory CD8+ T cells (TEM), at least in murine models, though human equivalent data is awaited[47,50].
Transcriptional factors promote the development and function of TEM and TCM cells. Expression of T-bet, Blimp1, ID2, and STAT4 is associated with TEM cells, while high expression of TCF1, BCL-6, ID3, and STAT3 is linked to the formation of TCM cells[49,50]. Interesting, in B cells, Blimp-1 and BCL-6 are essential for the development of germinal centre B cells and long-lived plasma cell through reciprocally antagonising each other[51], suggesting that this set of transcription factors acts in a similar fashion, in the regulation of effector- memory T-cell transition. Moreover, Tcf7 and Lef1 transcription factors are found in self-renewing multipotent CD8+ T cells known as memory stem cells[52].
T CELL EXHAUSTION
Exhausted T cells differ from other dysfunctional T cells, including anergic T cells and senescent T cells. Anergic T cells are induced by suboptimal stimulation showing cells with low proliferative capacity and minimal effector function. Senescent T cells initiate from repeated stimulation, resulting in cells with low proliferative capacity, low expression of inhibitory receptors but show high effector functions despite shortened telomeres. Differently, exhausted T cells result from persistent antigenic stimulation causing Tcells with low proliferative capacity, low to moderate effector functions and elevated expression of multiple inhibitory receptors[53].
In cancers such as PDAC, T cells that go through the activation process will later differentiate into memory-like cells and will ultimately become terminally differentiated exhausted T cells. Exhausted T cells result from persistent antigen exposure featuring cells with low proliferative capacity, increased apoptosis, loss of their cytotoxic function, and elevated expression of multiple inhibitory receptors also known as immune checkpoints such as PD-1, CTLA-4, T cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene 3 (LAG-3), or T cell immunoreceptor with Ig and ITIM domains (TIGIT)[43,53]. Each inhibitory receptor binds to its ligand, typically expressed by APCs and tumour cells in the TME.
The surface receptor PD-1 (CD279) is the primary receptor involved in T cell inhibitory signalling. PD-1 has two ligands: PD-L1 (CD274) and PD-L2 (CD273) can be found on the surface of antigen-presenting, MDSCs, TAMs and cancer cells. IFN-γ is the main trigger for PD-L1 and PD-L2 upregulation, while induction of PD-1 expression on T cells results from cell receptor (TCR) stimulation or secretion of the cytokines IL-2, IL-7, IL-15, IL-21, and TGF-β. Engagement of PD-L1 or PD-L2 with PD-1 receptor on T cells, inhibits dephosphorylation of TCR signalling components, specifically CD28, resulting in decreased IL-2, IFN-γ, and TNF-α cytokine production, survival, proliferation and effector functions[54].
CTLA-4 (CD152) is a B7/CD28 family member that is constitutively expressed by Tregs. CTLA-4-mediated immunosuppression occurs by limiting signalling through the co-stimulatory receptor CD28 during antigen-presentation, by either binding or deleting CD80 and CD86 from APCs[55]. Thus, indirectly reducing T cell activation and immune responses to tumour antigens. Other T cell subsets such as CD4+ T cells can also upregulate this receptor upon activation[56]. LAG-3 exerts inhibitory effects on T cells through MHCII binding, which results in decreased T cell activation and cytotoxicity, and increased suppressive function in Tregs. In PDAC, upregulation of this receptor is observed in association with upregulation of both PD-1 and CTL-4[57].
The inhibitory receptor TIGIT compete with CD226 to bind the ligands CD112 and CD155 while Tim-3 binds to Galectin-9 and CEACAM1 proteins to inhibit T cell function[58]. Of note, upregulation of Tim-3 in patients with PDAC is correlated with decreased patient survival[57]. Transcription factors involved in the formation of dysfunctional T cells include T-bet, Eomes, Foxo1, Blimp-1, NFAT and IRF-4[53].
A recent study using multiplex immunohistochemistry imaging combined with single-RNA sequencing to evaluate T cell landscape and function in patients with pancreatic cancer, demonstrated that infiltrated CD8+ T cells displayed a senescent phenotype, identified by the expression of CD57+CD27-CD28- or CD45RA+ CD27-/LowCD28-/Low or an exhausted phenotype with elevated expression of TIGIT+ and CD39+ markers alongside PD-1low/intermediate expression[30]. Senescent and exhausted T cells as well as Tregs were also identified within the CD4+ population. Additionally, intra-tumoural Tregs exhibit highly suppressive phenotypes, highlighted by the expression of multiple (TIGIT, ICOS, CD39) inhibitory markers[30].
IMMUNOTHERAPY AND PANCREATIC CANCER
Strategies aiming to leverage the activity of CTLs or the reversal of T cell dysfunction are widespread and have shown clinical success across a variety of cancer[27,29,59]. However, efforts to translate immunotherapy to PDAC, have been met with substantial challenges. The presence of tumour-infiltrating lymphocytes (TILs) with effector and memory functions within the tumour microenvironment and the positive correlation between CD8+ T effector memory cells and patient survival highlight the significance of the T cell immune infiltrate in limiting cancer progression[48]. Hence, the lack of efficacy in existing immunotherapies reflects the challenging non-immunogenic PDAC TME[11,38,48,60].
Chimeric antigen receptor (CAR) T cells or tumour vaccines alone have not demonstrated a survival benefit in PDAC tumours[11,35,48,61]. However, work is ongoing on demonstrating novel targetable antigens or switchable CAR T cells which get activated on reaching the tumour[62,63]. Although most infiltrated CD8+ T cells in the PDAC stroma display features of an exhausted phenotype, demonstrated by cell surface expression of multiple inhibitory receptors, immunotherapy with single-agent immune checkpoint blockade (ICB) has been disappointing. KPC mouse models did not show anti-tumour responses to either CTLA4, PD-1, PDL-1 monotherapy or CTL-4 combined with PD-1/PDL-1 blockade[19,64]. Similarly, human clinical trials using ICB demonstrated insufficient clinical activity and minimal improvement on prognosis, with clinical benefit observed in only highly selected patients[27,39]. Equally, monotherapy with CTLA-4 antibodies and in combination with chemotherapy has not shown ideal clinical activity[59]. Furthermore, exciting avenues for targeting novel antigens such as CEACAM7 offers hope for CAR-T cell therapy[62,65,66].
The vast majority of trials targeted towards T cells in pancreatic cancer are centred around the use of immune inhibitory receptors against PD-1 and CTLA-4[67]. Most of these trials have enrolled patients with metastatic or borderline resectable pancreatic cancer and assessed the response to either single or double agent immunotherapy or combination therapy with chemotherapy/radiotherapy. The results regarding progression free survival or overall survival have been so far underwhelming[68]. In a meta-analysis on checkpoint inhibitors overall survival and progression-free survival showed no improvement in single agent therapy but a small number of studies on combination therapy have been more promising[69]. It is feasible that the limited tumor mutational burden of pancreatic cancer compared to immunotherapy responsive tumours, such as melanoma or non-small cell lung cancer, may be the key differentiating factor. The phase II KEYNOTE-185 study trying to assess the efficacy of pembrolizumab on patients with non-colorectal microsatellite unstable/mismatch repair deficient cancers enrolled 22 patients with pancreatic cancer, of which four patients showed response to treatment with increase in progression-free survival and median survival[70]. These results, although encouraging, demonstrate that there key barriers around identifying correct groups of patients that would benefit from T cell targeted therapies.
There are various explanations for ICB failure in PDAC tumours including low mutational burden and expression of neoantigens, minimal intra-tumoural infiltration of CD8+ T cells, expression of multiple inhibitory receptors in CD8+ T cells that infiltrate tumours, as well as decreased tumour and myeloid expression cell expression of PDL-1[31,57]. To improve PADC response to ICB, combined approaches have been investigated. Multi-agent immunotherapeutic protocols targeting multiple inhibitory receptors is a promising approach, and has proved more effective than single inhibitory receptor blockade in reversing dysfunctional CD8+ T cells PDAC[27,71]. In the same way, strategies with the goal to prime effector CD8+ T cells to increase their immunogenicity and responsiveness before the use of checkpoint inhibitor treatment represents an exciting opportunity in cancer immunotherapy[12,27,59,72,73]. Combinatory approaches utilising GM-CSF-secreting tumour cells vaccine (GVAX), to induce upregulation of PD-L1 expression into the PDAC TME, prior CTLA-4 and anti-PD-1/PDL-1 blockade has shown promising results in PDAC patients[57,72], and a dual blockade targeting CXCR4 and PD-1 demonstrated improvement in T cell infiltration with a decline in MDSCs[57].
Strategies with the co-stimulatory molecule agonist CD40 used to enhance APC capabilities of macrophages[74] combined with gemcitabine, PD-1 and CTL-4 ICB resulted in increased T-cell priming and infiltration in PDAC tumours[64,72]. Extraction and in vitro expansion of TILs from PDAC tumours also have been explored[40] and the results demonstrated autologous T cell killing activity[75,76].
CONCLUSION
The PDAC tumour microenvironment is characterised by complex fibrotic stroma with substantial infiltration of tumour-promoting immunosuppressive cells and pronounced T cell exhaustion, favouring immune evasion that results in immunotherapeutic failures and poor clinical outcome. Therefore, understanding the complexity of PDAC immune landscape and the mechanisms involved in T cell dysfunction may contribute to identifying new immunotherapeutic strategies for treating PDAC and monitoring such response with novel technologies such as ctDNA to assess tumour lysis[77]. As such, unsuccessful immunotherapies could be reversed using combined approaches targeting multiple pathways that obstruct T cell anti-tumour immunity along with other strategies to target stroma[78,79].
A variety of preclinical studies highlighting the influence of PDAC stromal components on T cell anti-tumour responses provided rationale for the development of clinical trials incorporating combined approaches to enhance T cell responses[80]. CXCL12 from cancer-associated fibroblasts synergizes with anti-PD-L1 blockade resulting in activation of T cells and tumour regression in mice[6,81]. Similarly, dual blockade of TGF-β and anti-PD1 resulted in increased T cell responses and tumour regression[82]. Moreover, targeting of myeloid cells with CSF1R in combination with PD-1 or CTLA-4 blockade[83] or focal adhesion kinases inhibitors has been shown to decrease infiltration of suppressive myeloid populations with concomitant activation of T cells, and improved survival in mice models[84].
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 9, 2021
First decision: May 17, 2021
Article in press: November 28, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Falasca M S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
Contributor Information
Michelle R Goulart, Centre for Tumour Biology Barts Cancer Institute-A CRUK Centre of Excellence, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Konstantinos Stasinos, Centre for Tumour Biology Barts Cancer Institute-A CRUK Centre of Excellence, Queen Mary University of London, London EC1M 6BQ, United Kingdom; Barts and the London HPB Centre, The Royal London Hospital, Barts Health NHS Trust, London E1 1BB, United Kingdom.
Rachel Elizabeth Ann Fincham, Centre for Tumour Biology Barts Cancer Institute-A CRUK Centre of Excellence, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Francesca R Delvecchio, Centre for Tumour Biology Barts Cancer Institute-A CRUK Centre of Excellence, Queen Mary University of London, London EC1M 6BQ, United Kingdom; Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Hemant M Kocher, Centre for Tumour Biology Barts Cancer Institute-A CRUK Centre of Excellence, Queen Mary University of London, London EC1M 6BQ, United Kingdom; Barts and the London HPB Centre, The Royal London Hospital, Barts Health NHS Trust, London E1 1BB, United Kingdom. h.kocher@qmul.ac.uk.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 4.Neuzillet C, Rousseau B, Kocher H, Bourget P, Tournigand C. Unravelling the pharmacologic opportunities and future directions for targeted therapies in gastro-intestinal cancers Part 1: GI carcinomas. Pharmacol Ther. 2017;174:145–172. doi: 10.1016/j.pharmthera.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Froeling FE, Kocher HM. Homeostatic restoration of desmoplastic stroma rather than its ablation slows pancreatic cancer progression. Gastroenterology. 2015;148:849–850. doi: 10.1053/j.gastro.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 6.Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG, Ramsay AG, Kocher HM. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. doi: 10.1186/s12943-018-0858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dibra D, Mishra L, Li S. Molecular mechanisms of oncogene-induced inflammation and inflammation-sustained oncogene activation in gastrointestinal tumors: an under-appreciated symbiotic relationship. Biochim Biophys Acta. 2014;1846:152–160. doi: 10.1016/j.bbcan.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun H, Zhang B, Li H. The Roles of Frequently Mutated Genes of Pancreatic Cancer in Regulation of Tumor Microenvironment. Technol Cancer Res Treat. 2020;19:1533033820920969. doi: 10.1177/1533033820920969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6:247–255. doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone ML, Beatty GL. Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol Ther. 2019;201:202–213. doi: 10.1016/j.pharmthera.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orth M, Metzger P, Gerum S, Mayerle J, Schneider G, Belka C, Schnurr M, Lauber K. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol. 2019;14:141. doi: 10.1186/s13014-019-1345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee B, Gibbs P. Inflammation, Biomarkers and Immuno-Oncology Pathways in Pancreatic Cancer. J Pers Med. 2019;9 doi: 10.3390/jpm9020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Santiago I, Yau C, Heij L, Middleton MR, Markowetz F, Grabsch HI, Dustin ML, Sivakumar S. Immunophenotypes of pancreatic ductal adenocarcinoma: Meta-analysis of transcriptional subtypes. Int J Cancer. 2019;145:1125–1137. doi: 10.1002/ijc.32186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzunparmak B, Sahin IH. Pancreatic cancer microenvironment: a current dilemma. Clin Transl Med. 2019;8:2. doi: 10.1186/s40169-019-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 17.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, Schmid MC. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Knudsen ES, Vail P, Balaji U, Ngo H, Botros IW, Makarov V, Riaz N, Balachandran V, Leach S, Thompson DM, Chan TA, Witkiewicz AK. Stratification of Pancreatic Ductal Adenocarcinoma: Combinatorial Genetic, Stromal, and Immunologic Markers. Clin Cancer Res. 2017;23:4429–4440. doi: 10.1158/1078-0432.CCR-17-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan UM, Langhoff E, Goni E, Costello E, Greenhalf W, Halloran C, Ormanns S, Kruger S, Boeck S, Ribback S, Beyer G, Dombroswki F, Weiss FU, Neoptolemos JP, Werner J, D'Haese JG, Bazhin A, Peterhansl J, Pichlmeier S, Büchler MW, Kleeff J, Ganeh P, Sendler M, Palmer DH, Kohlmann T, Rad R, Regel I, Lerch MM, Mayerle J. Immune Cell and Stromal Signature Associated With Progression-Free Survival of Patients With Resected Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2018;155:1625–1639.e2. doi: 10.1053/j.gastro.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 24.Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep. 2017;20:558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK, Avanzi A, Tippens D, Narayanan R, Jang JE, Newman E, Pillarisetty VG, Dustin ML, Bar-Sagi D, Hajdu C, Miller G. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell. 2016;166:1485–1499.e15. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O Australian Pancreatic Cancer Genome Initiative; Garvan Institute of Medical Research; Prince of Wales Hospital; Royal North Shore Hospital; University of Glasgow; St Vincent’s Hospital; QIMR Berghofer Medical Research Institute; University of Melbourne, Centre for Cancer Research; University of Queensland, Institute for Molecular Bioscience; Bankstown Hospital; Liverpool Hospital; Royal Prince Alfred Hospital, Chris O’Brien Lifehouse; Westmead Hospital; Fremantle Hospital; St John of God Healthcare; Royal Adelaide Hospital; Flinders Medical Centre; Envoi Pathology; Princess Alexandria Hospital; Austin Hospital; Johns Hopkins Medical Institutes; ARC-Net Centre for Applied Research on Cancer, Gönen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson BA 3rd, Yarchoan M, Lee V, Laheru DA, Jaffee EM. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin Cancer Res. 2017;23:1656–1669. doi: 10.1158/1078-0432.CCR-16-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey P, Chang DK, Forget MA, Lucas FA, Alvarez HA, Haymaker C, Chattopadhyay C, Kim SH, Ekmekcioglu S, Grimm EA, Biankin AV, Hwu P, Maitra A, Roszik J. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci Rep. 2016;6:35848. doi: 10.1038/srep35848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivakumar S, Abu-Shah E, Ahern DJ, Arbe-Barnes EH, Mangal N, Reddy S, Rendek A, Easton A, Kurz E, Silva M, Soonawalla Z, Heij LR, Bashford-Rogers R, Middleton MR, Dustin ML. Activated regulatory T-cells, dysfunctional and senescent T-cells dominate the microenvironment of pancreatic cancer. 2020 Preprint. Available from: bioRxiv:2020.06.20.163071. [DOI] [PMC free article] [PubMed]
- 31.Stromnes IM, Hulbert A, Pierce RH, Greenberg PD, Hingorani SR. T-cell Localization, Activation, and Clonal Expansion in Human Pancreatic Ductal Adenocarcinoma. Cancer Immunol Res. 2017;5:978–991. doi: 10.1158/2326-6066.CIR-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, Kalluri R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. doi: 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Cui L, Yang L, Wang B, Zhuo Y, Zhang L, Wang X, Zhang Q, Zhang S. Pancreatic Stellate Cells Promote Tumor Progression by Promoting an Immunosuppressive Microenvironment in Murine Models of Pancreatic Cancer. Pancreas. 2020;49:120–127. doi: 10.1097/MPA.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 34.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tesfaye AA, Kamgar M, Azmi A, Philip PA. The evolution into personalized therapies in pancreatic ductal adenocarcinoma: challenges and opportunities. Expert Rev Anticancer Ther. 2018;18:131–148. doi: 10.1080/14737140.2018.1417844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans RA, Diamond MS, Rech AJ, Chao T, Richardson MW, Lin JH, Bajor DL, Byrne KT, Stanger BZ, Riley JL, Markosyan N, Winograd R, Vonderheide RH. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:569. doi: 10.1038/nrc.2017.74. [DOI] [PubMed] [Google Scholar]
- 38.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upadhrasta S, Zheng L. Strategies in Developing Immunotherapy for Pancreatic Cancer: Recognizing and Correcting Multiple Immune "Defects" in the Tumor Microenvironment. J Clin Med. 2019;8 doi: 10.3390/jcm8091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poschke I, Faryna M, Bergmann F, Flossdorf M, Lauenstein C, Hermes J, Hinz U, Hank T, Ehrenberg R, Volkmar M, Loewer M, Glimm H, Hackert T, Sprick MR, Höfer T, Trumpp A, Halama N, Hassel JC, Strobel O, Büchler M, Sahin U, Offringa R. Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:e1240859. doi: 10.1080/2162402X.2016.1240859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castino GF, Cortese N, Capretti G, Serio S, Di Caro G, Mineri R, Magrini E, Grizzi F, Cappello P, Novelli F, Spaggiari P, Roncalli M, Ridolfi C, Gavazzi F, Zerbi A, Allavena P, Marchesi F. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology. 2016;5:e1085147. doi: 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papalampros A, Vailas M, Ntostoglou K, Chiloeches ML, Sakellariou S, Chouliari NV, Samaras MG, Veltsista PD, Theodorou SDP, Margetis AT, Bergonzini A, Karydakis L, Hasemaki N, Havaki S, Moustakas II, Chatzigeorgiou A, Karamitros T, Patsea E, Kittas C, Lazaris AC, Felekouras E, Gorgoulis VG, Frisan T, Pateras IS. Unique Spatial Immune Profiling in Pancreatic Ductal Adenocarcinoma with Enrichment of Exhausted and Senescent T Cells and Diffused CD47-SIRPα Expression. Cancers (Basel) 2020;12 doi: 10.3390/cancers12071825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, Olive D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front Immunol. 2018;9:1044. doi: 10.3389/fimmu.2018.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4:210. doi: 10.3389/fphys.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barilla RM, Diskin B, Caso RC, Lee KB, Mohan N, Buttar C, Adam S, Sekendiz Z, Wang J, Salas RD, Cassini MF, Karlen J, Sundberg B, Akbar H, Levchenko D, Gakhal I, Gutierrez J, Wang W, Hundeyin M, Torres-Hernandez A, Leinwand J, Kurz E, Rossi JAK, Mishra A, Liria M, Sanchez G, Panta J, Loke P, Aykut B, Miller G. Specialized dendritic cells induce tumor-promoting IL-10+IL-17+ FoxP3neg regulatory CD4+ T cells in pancreatic carcinoma. Nat Commun. 2019;10:1424. doi: 10.1038/s41467-019-09416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 49.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 50.Martin MD, Badovinac VP. Defining Memory CD8 T Cell. Front Immunol. 2018;9:2692. doi: 10.3389/fimmu.2018.02692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, Zhang C, Lunceford JK, Joe A, Cheng J, Webber AL, Ibrahim N, Plimack ER, Ott PA, Seiwert TY, Ribas A, McClanahan TK, Tomassini JE, Loboda A, Kaufman D. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362 doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saka D, Gökalp M, Piyade B, Cevik NC, Arik Sever E, Unutmaz D, Ceyhan GO, Demir IE, Asimgil H. Mechanisms of T-Cell Exhaustion in Pancreatic Cancer. Cancers (Basel) 2020;12 doi: 10.3390/cancers12082274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia A, Zhang Y, Xu J, Yin T, Lu XJ. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Front Immunol. 2019;10:1719. doi: 10.3389/fimmu.2019.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young K, Hughes DJ, Cunningham D, Starling N. Immunotherapy and pancreatic cancer: unique challenges and potential opportunities. Ther Adv Med Oncol. 2018;10:1758835918816281. doi: 10.1177/1758835918816281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strauss J, Alewine C, Figg WD, Duffy A. Targeting the microenvironment of pancreatic cancer: overcoming treatment barriers and improving local immune responses. Clin Transl Oncol. 2016;18:653–659. doi: 10.1007/s12094-015-1459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ott PA, Wu CJ. Cancer Vaccines: Steering T Cells Down the Right Path to Eradicate Tumors. Cancer Discov. 2019;9:476–481. doi: 10.1158/2159-8290.CD-18-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raj D, Nikolaidi M, Garces I, Lorizio D, Castro NM, Caiafa SG, Moore K, Brown NF, Kocher HM, Duan X, Nelson BH, Lemoine NR, Marshall JF. CEACAM7 Is an Effective Target for CAR T-cell Therapy of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2021;27:1538–1552. doi: 10.1158/1078-0432.CCR-19-2163. [DOI] [PubMed] [Google Scholar]
- 63.Raj D, Yang MH, Rodgers D, Hampton EN, Begum J, Mustafa A, Lorizio D, Garces I, Propper D, Kench JG, Kocher HM, Young TS, Aicher A, Heeschen C. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut. 2019;68:1052–1064. doi: 10.1136/gutjnl-2018-316595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reader CS, Vallath S, Steele CW, Haider S, Brentnall A, Desai A, Moore KM, Jamieson NB, Chang D, Bailey P, Scarpa A, Lawlor R, Chelala C, Keyse SM, Biankin A, Morton JP, Evans TJ, Barry ST, Sansom OJ, Kocher HM, Marshall JF. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol. 2019;249:332–342. doi: 10.1002/path.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haider S, Wang J, Nagano A, Desai A, Arumugam P, Dumartin L, Fitzgibbon J, Hagemann T, Marshall JF, Kocher HM, Crnogorac-Jurcevic T, Scarpa A, Lemoine NR, Chelala C. A multi-gene signature predicts outcome in patients with pancreatic ductal adenocarcinoma. Genome Med. 2014;6:105. doi: 10.1186/s13073-014-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katayama ES, Hue JJ, Bajor DL, Ocuin LM, Ammori JB, Hardacre JM, Winter JM. A comprehensive analysis of clinical trials in pancreatic cancer: what is coming down the pike? Oncotarget. 2020;11:3489–3501. doi: 10.18632/oncotarget.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth MT, Cardin DB, Berlin JD. Recent advances in the treatment of pancreatic cancer. F1000Res. 2020;9 doi: 10.12688/f1000research.21981.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30. doi: 10.1016/j.ctrv.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torphy RJ, Schulick RD, Zhu Y. Understanding the immune landscape and tumor microenvironment of pancreatic cancer to improve immunotherapy. Mol Carcinog. 2020;59:775–782. doi: 10.1002/mc.23179. [DOI] [PubMed] [Google Scholar]
- 72.Vonderheide RH. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell. 2018;33:563–569. doi: 10.1016/j.ccell.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karamitopoulou E. Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br J Cancer. 2019;121:5–14. doi: 10.1038/s41416-019-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng Q, Liu Z, Rangelova E, Poiret T, Ambati A, Rane L, Xie S, Verbeke C, Dodoo E, Del Chiaro M, Löhr M, Segersvärd R, Maeurer MJ. Expansion of Tumor-reactive T Cells From Patients With Pancreatic Cancer. J Immunother. 2016;39:81–89. doi: 10.1097/CJI.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 76.Hall M, Liu H, Malafa M, Centeno B, Hodul PJ, Pimiento J, Pilon-Thomas S, Sarnaik AA. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. doi: 10.1186/s40425-016-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sivapalan L, Kocher HM, Ross-Adams H, Chelala C. Molecular profiling of ctDNA in pancreatic cancer: Opportunities and challenges for clinical application. Pancreatology. 2021;21:363–378. doi: 10.1016/j.pan.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D, deSouza NM, De Paepe KN, Goulart MR, Hughes C, Imrali A, Roberts R, Pawula M, Houghton R, Lawrence C, Yogeswaran Y, Mousa K, Coetzee C, Sasieni P, Prendergast A, Propper DJ. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11:4841. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, Erkan M, Kleeff J, Wilson J, Apte M, Tosolini M, Wilson AS, Delvecchio FR, Bousquet C, Paradis V, Hammel P, Sadanandam A, Kocher HM. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248:51–65. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ware MB, El-Rayes BF, Lesinski GB. Mirage or long-awaited oasis: reinvigorating T-cell responses in pancreatic cancer. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Principe DR, Park A, Dorman MJ, Kumar S, Viswakarma N, Rubin J, Torres C, McKinney R, Munshi HG, Grippo PJ, Rana A. TGFβ Blockade Augments PD-1 Inhibition to Promote T-Cell-Mediated Regression of Pancreatic Cancer. Mol Cancer Ther. 2019;18:613–620. doi: 10.1158/1535-7163.MCT-18-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H, Fields RC, Pachter JA, Lim KH, DeNardo DG. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. 2020;69:122–132. doi: 10.1136/gutjnl-2018-317424. [DOI] [PMC free article] [PubMed] [Google Scholar]