Abstract
Studies reported that women in a low‐risk cohort with stage 1 hypertension defined as 130–139 mmHg/80–89 mmHg, according to the American College of Cardiology/American Heart Association, are more likely to develop into preeclampsia than women with normotensive in the early gestation. In this study, the authors investigated whether preeclampsia was more likely to occur in stage 1 hypertensive women compared to the normotensive pregnant women in a high‐risk cohort, which was based on the randomized controlled trial of "Low‐dose Aspirin in the Prevention of Preeclampsia in China." Meanwhile, the authors further evaluated the preventive effect of aspirin for preeclampsia in stage 1 hypertension subset. In women enrolled at or before 16 weeks of gestation, in the control group, the preeclampsia occurrence was significantly higher in stage 1 hypertensive woman than in the normotensive women (20.4% vs. 6.2%, aOR 3.960, 95% CI 1.299–12.074, p = .016), while no difference was observed in the aspirin group (4.5% vs. 4.2%, aOR 0.921, 95% CI 0.140–6.070, p = .932). In stage 1 hypertension, the incidences of preeclampsia and preterm birth were significantly lower in the aspirin group as compared to the control group (4.5% vs. 20.4%, aOR 0.139, 95% CI 0.027–0.716, p = .018; 4.5% vs. 18.4%, aOR 0.141, 95% CI 0.025–0.782, p = .025). Compared with the control group, the aspirin group displayed significantly prolonged gestational age at delivery (38.6 ± 1.2 vs. 37.4 ± 3.4, p = .042). This study indicates that the newly classified stage 1 hypertension might be an additional risk factor for preeclampsia in Chinese high‐risk pregnant women, and aspirin intervention might be useful.
Keywords: aspirin, preeclampsia, pregnancy, risk factors, stage 1 hypertension
1. INTRODUCTION
The American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Clinical Practice Guidelines have revised the recommendations for the blood pressure (BP) classification in adults, based on the evidence of the adverse effects of increased BP on the risk of long‐term cardiovascular diseases (CVDs). 1 According to the new guidelines, the "stage 1" hypertension category is now defined as systolic blood pressure (SBP) of 130–139 mmHg or diastolic blood pressure (DBP) of 80–89 mmHg, which belonged to the pre‐hypertension category in the Joint National Committee 7 report. 2 Women with chronic hypertension are at an increased risk of several pregnancy‐related complications, including superimposed preeclampsia (PE), fetal growth restriction, placental abruption, preterm birth, and cesarean section. 3 Chronic hypertension is not only a significant risk factor for PE (risk ratio 5.1, 95% confidence interval [CI] 4.0–6.5), but is also associated with increased risk of future CVDs. 4 , 5 A recent retrospective Chinese low‐risk cohort study estimated an increase in adverse pregnancy outcomes including preeclampsia associated with stage 1 hypertension in early gestation. Moreover, hypertensive disorders in pregnancy significantly increased with different body mass index (BMI)‐based groups. 6 Therefore, it is worthwhile to discuss whether stage 1 hypertension in prepregnant or early gestational women should be treated as a risk factor, and whether it requires additional management.
The role of aspirin in PE prevention has been studied since 1978, when Goodlin and colleagues 7 described a patient with recurrent PE and thrombocytopenia who seemed to benefit from aspirin. To date, the effect of aspirin on PE prevention has been extensively analyzed. However, there is no standardized protocol regarding aspirin use, because the dosage, initial time, and screening methods of the high‐risk population differ between studies. A study showed that effect of aspirin in the prevention of preterm PE may not apply in pregnancies with chronic hypertension. 8 And meta‐analysis showed that aspirin initiated at or before 16 weeks of gestation is associated with a greater reduction in the incidence of PE, perinatal death, and other adverse perinatal outcomes as compared to aspirin initiated after 16 weeks of gestation. 9 Hence, further studies are needed to determine the specific high‐risk population and active window.
In this study, we aimed to assess whether PE was more likely to occur in women with stage 1 hypertension or in those with normotension when entering pregnancy, screen based on the high‐ and moderate‐risk factors. And we aimed to determine the effect of 100 mg aspirin in the subgroup with stage 1 hypertension initially from 12 to 20 weeks of gestation (particularly in women enrolled at or before 16 weeks) in the Chinese population.
2. METHODS
2.1. Study design
This study was based on the "Low‐dose aspirin in the prevention of PE in China (APPEC)" study, which was a multicenter, open‐label, randomized controlled trial conducted in 13 tertiary hospitals in 11 provinces. 10 In the original study, the inclusion criteria for the participants were maternal age ≥18 years and <55 years, singleton pregnancy with a live fetus at gestational age 12–20 weeks, and a high risk of developing PE. The evaluation of high risk of PE was based on the following clinical risk factors: presence of at least one of the high‐risk factors of history of PE, preexisting diabetes, or chronic hypertension; or presence of ≥2 intermediate‐risk factors of obesity (BMI ≥ 28 kg/m2), advanced maternal age (≥35 years), family history of PE, or nulliparity. The exclusion criteria were allergy to aspirin, asthma, peptic ulcers, severe cardiac, hepatic, or renal diseases, rheumatic immune disease, mental disease, alcohol or drug abuse, in vitro fertilization, previous registration in another drug trial within the last 3 months, and difficulty in undergoing the procedure specified in the protocol. The study process was explained to all the participants in this study, and written informed consent was obtained. The enrolled participants were evaluated before 20 weeks of gestation at their first antenatal care, where they were randomly assigned to the aspirin and control groups if they met the inclusion criteria. The women in the aspirin group received 100 mg/day aspirin (enteric‐coated aspirin tablets, Bayer Corporation) starting from 12–20 weeks to 34 weeks of gestation. All participants underwent standard antenatal care prescribed for high‐risk women. The primary outcome of the original study was the occurrence of PE. This trial was approved by the Ethics Committee of the Peking University First Hospital (reference number 2016 [1109]) and was registered with ClinicalTrials.gov (NCT02797249) before the inclusion of the first patient. Participants' compliance was assessed using a diary to record the daily aspirin intake maintained by the participants themselves and evaluated by the study staff at every antenatal care visit.
2.2. Participants and pregnancy outcomes
In this analysis, women with chronic hypertension, diagnosed as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg before pregnancy or 20 weeks of gestation, were excluded. Additionally, those with missing BP data at the first antenatal visit were excluded. Women with SBP of 130–139 mmHg or DBP of 80–89 mmHg were categorized as the stage 1 hypertension group, as per the new ACC/AHA guidelines. The normotension group included women with SBP < 130 mmHg and DBP < 80 mmHg. 1 The classification of stage 1 hypertension or normotension was based on the BP data measured at the first antenatal care before 20 weeks of gestation. BP was measured by well‐trained nurses using a mercury sphygmomanometer with patients seated comfortably and quietly after 5 min rest, with their arms well supported at the level of their heart and an appropriate‐sized adult cuff. The first and last Korotkoff sounds were taken as SBP and DBP. Two BP measurements were performed at 1‐min intervals, and the average of readings was recorded. The primary outcome of this analysis was the occurrence of PE in each group, while the secondary outcomes included the incidence of PE before 37 weeks, and at 37 weeks or more of gestation, and the incidence of preterm birth, placental abruption, postpartum hemorrhage, and small for gestational age (SGA). The demographics and clinical characteristics recorded in this analysis included maternal age, BMI before pregnancy, SBP and DBP at enrollment, gestational age at delivery, birth weight, and incidence of advanced maternal age, obesity, preexisting diabetes, PE history, nulliparity, and family history of PE.
2.3. Diagnostic criteria for outcomes
The diagnosis of PE should be based on the recommendations of the ACOG, 2013. 11 For PE diagnoses, SBP should be ≥140 mmHg and DBP should be ≥90 mmHg on at least two occasions 4 h apart, developing after 20 weeks of gestation in a woman with previously normal blood pressure (SBP < 140 mmHg and DBP < 90 mmHg), and accompanied by proteinuria, or in the absence of proteinuria, new onset hypertension with the new onset on any of the following: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and cerebral or visual symptoms. Proteinuria is diagnosed by any of the following criteria: Protein in urine is ≥300 mg/24 h of urine collection (or can be extrapolated from a timed collection), protein/creatinine ratio is ≥0.3 mg/dl, and dipstick reading is 2+ (used only if other quantitative methods are not available). Preterm delivery was defined as delivery before 37 weeks of gestation. The diagnosis of placental abruption was based on the clinical signs of vaginal bleeding and uterine tenderness. Postpartum hemorrhage was defined as ≥500 ml blood loss in the vaginal delivery or ≥1000 ml in cesarean delivery, within 24 h after delivery. The SGA was identified as birth weight <10th percentile for singletons. 12
2.4. Statistical analyses
Statistical analysis was conducted using Statistical Package for the Social Sciences software, version 22 (SPSS Inc). Continuous statistics were expressed as mean ± SD. Student's t test was used for comparison between two groups, while one‐way analysis of variance was used for more than two groups. Categorical statistics were expressed as frequencies with proportions and analyzed using chi‐square test or Fisher's exact test where appropriate. Logistic regression was used to assess the risk of stage 1 hypertension and the effect of aspirin treatment on adverse pregnancy outcomes by estimating the odds ratio (OR) and 95% CIs while controlling for maternal age, prepregnancy BMI, and nulliparity. A p value <.5 was considered statistically significant.
3. RESULTS
Of the 898 participants at high risk for PE, 441 women were with chronic hypertension (200 in control and 231 in aspirin group), which is now classified as "stage 2" hypertension according to the new ACC/AHA guidelines. We excluded the chronic hypertension participants, as we want to clarify whether PE was more likely to occur in stage 1 hypertensive pregnant women than the normotensive. However, in our whole cohort, in the subset with chronic hypertension, the rate of PE was 25.7% (62/241) in the aspirin group and 27.0% (54/200) in the control group, without significant difference (p = .762) (not shown in the Tables). Finally, 397 participants (44.2%) were included in this analysis, of which 137 (93 initialed at or before 16 weeks) were in the stage 1 hypertension group, and 260 (190 initialed at or before 16 weeks) were in the normotension group, after excluding 60 women for missing BP data at the first antenatal visit (Figure 1). Both groups had similar maternal age and prepregnancy BMI values. The percentage of women with risk factors including advanced age, obesity, preexisting diabetes, PE history, and family history of PE did not differ significantly between both the groups. The percentage of nulliparity in the normotension group was significantly higher than that of the stage 1 hypertension group (39.6% vs. 25.5%, p = .005) (Table 1). Moreover, this difference in nulliparity between the normotension group and stage 1 hypertension group mainly existed in the aspirin‐taking participants (36.6% vs. 21.0%, p = .029). We included nulliparity in the adjustment covariates along with maternal age and prepregnancy BMI, based on previous studies. 13 The clinical characteristics of the participants taking and not taking aspirin (aspirin group and control group, respectively) were similar in the normotension and stage 1 hypertension groups (Table 2, Table S1).
FIGURE 1.
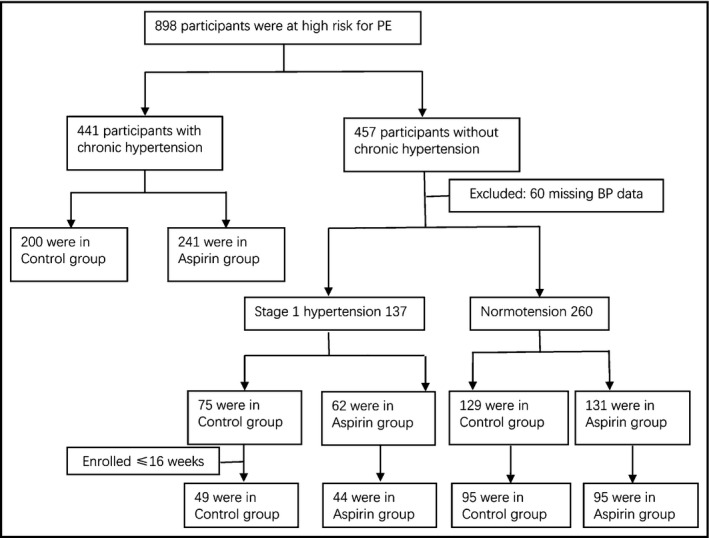
Flow chart of the study participants
TABLE 1.
Clinical characteristics of the women in normotension and stage 1 hypertension group
|
Normotension 260 |
Stage 1 hypertension 137 |
p value | |
|---|---|---|---|
| Age (years) | 32.4 ± 4.7 | 32.1 ± 4.2 | .641 |
| Prepregnancy BMI (kg/cm2) | 24.9 ± 4.3 | 25.5 ± 4.8 | .185 |
| Advanced age, n (%) | 95 (36.5) | 47 (34.3) | .659 |
| Obesity, n (%) | 68 (26.2) | 45 (32.8) | .160 |
| Preexisting diabetes, n (%) | 96 (36.9) | 55 (40.1) | .529 |
| PE history, n (%) | 104 (40.0) | 55 (40.1) | .977 |
| Nulliparity, n (%) | 103 (39.6) | 35 (25.5) | .005 |
| Family history of PE, n (%) | 11 (4.2) | 6 (4.4) | .944 |
| SBP at enrollment (mmHg) | 113.8 ± 8.7 | 124.9 ± 8.0 | <.001 |
| DBP at enrollment (mmHg) | 68.9 ± 6.3 | 82.8 ± 4.7 | <.001 |
| Gestational age at delivery (week) | 38.1 ± 2.9 | 38.1 ± 2.4 | .940 |
| Birth weight (g) | 3159.0 ± 696.5 | 3183.6 ± 657.3 | .746 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
TABLE 2.
Clinical characteristics of women in the aspirin group vs. control group with normotension or stage 1 hypertension when initialed at or before 16 weeks of gestation
| Normotension | Stage 1 hypertension | |||||
|---|---|---|---|---|---|---|
| Control | Aspirin | p value | Control | Aspirin | p value | |
| 95 | 95 | 49 | 44 | |||
| Age (years) | 32.5 ± 4.3 | 32.8 ± 4.8 | .705 | 32.5 ± 4.1 | 32.1 ± 4.6 | .627 |
| Prepregnancy BMI (kg/cm2) | 24.9 ± 4.1 | 24.8 ± 3.9 | .857 | 26.2 ± 4.1 | 24.5 ± 4.3 | .063 |
| Advanced age, n (%) | 36 (37.9) | 37 (38.9) | .881 | 20 (40.8) | 12 (27.3) | .170 |
| Obesity, n (%) | 27 (28.4) | 24 (25.3) | .623 | 16 (32.7) | 13 (29.5) | .747 |
| Preexisting diabetes, n (%) | 37 (38.9) | 34 (35.8) | .653 | 21 (42.9) | 18 (40.9) | .849 |
| PE history, n (%) | 34 (35.8) | 36 (37.9) | .764 | 20 (40.8) | 19 (43.2) | .817 |
| Nulliparity, n (%) | 43 (45.3) | 36 (37.9) | .303 | 14 (28.6) | 8 (18.2) | .239 |
| Family history of PE, n (%) | 4 (4.2) | 2 (2.1) | .407 | 2 (4.1) | 1 (2.3) | .622 |
| SBP at enrollment (mmHg) | 113.5 ± 8.3 | 114.9 ± 7.8 | .253 | 127.0 ± 7.9 | 123.8 ± 7.6 | .055 |
| DBP at enrollment (mmHg) | 68.6 ± 6.6 | 68.6 ± 6.0 | .945 | 83.1 ± 5.5 | 82.4 ± 3.7 | .486 |
| Gestational age at delivery (week) | 37.8 ± 3.2 | 38.1 ± 2.8 | .512 | 37.4 ± 3.4 | 38.6 ± 1.2 | .042 |
| Birth weight (g) | 3145.1 ± 745.4 | 3195.7 ± 646.1 | .638 | 3123.8 ± 678.5 | 3335.0 ± 631.0 | .154 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Among the control group participants (n = 204), 129 participants were in the normotension group and 75 were in the stage 1 hypertension group, and their clinical characteristics were consistent. The incidence of PE was significantly higher in the stage 1 hypertension group (12/75; 16.0%) than in the normotension group (8/129; 6.2%) (adjusted OR [aOR] 2.790, 95% CI 1.063–7.320; p = .037) (Table S2). The aspirin group participants (n = 193), of which 131 participants were in the normotension group and 62 were in the stage 1 hypertension group, showed similar clinical characteristics in both groups, except for the nulliparity percentage. The difference in PE incidence between the normotension and stage 1 hypertension groups seen in the control group was eliminated in the aspirin group (aOR 0.908, 95% CI, 0.288–2.867). The incidences of preterm birth, placental abruption, postpartum hemorrhage, and SGA between the stage 1 hypertension and normotension groups in both control and aspirin‐taking participants did not differ significantly (Table S2).
In the stage 1 hypertension group, the PE incidence in the control group was 16.0%, and it was lower than that of the aspirin group (8.1%), showing a very slight trend toward significance (aOR 0.428, 95% CI 0.140–1.312; p = .138) (Table S3). In the normotension group, there was no significant difference in PE incidence between the aspirin group and control group (8.4% vs. 6.2%, aOR 1.325, 95% CI 0.503–3.491; p = .569). Among the stage 1 hypertension group participants, the gestational age at delivery and birth weight in the aspirin group were slightly more than that of the control group (38.5 ± 1.5 vs. 37.8 ± 3.0; p = .089 and 3256.8 ± 653.1 vs. 3125.16 ± 659.6; p = .270, respectively), and tended to approach significance (Table S1).
In participants enrolled in the trial at or before 16 weeks of gestation, the aspirin group of the stage 1 hypertension group showed significantly decreased incidence of PE (4.5% vs. 20.4%, aOR 0.139, 95% CI 0.027–0.716; p = .018) and preterm birth (4.5% vs. 18.4%, aOR 0.141, 95% CI 0.025–0.782; p = .025) as compared to the control group. Moreover, the gestational age at delivery was significantly prolonged (38.6 ± 1.2 vs. 37.4 ± 3.4; p = .042) and the birth weight was slightly increased (3335.0 ± 631.0 vs. 3123.8 ± 678.5; p = .154) in the aspirin group as compared to the control group, without a statistically significant difference (Table 3). Further, in the control group, the PE rate was significantly higher in the stage 1 hypertension group compared to the normotension group (aOR 3.960, 95% CI 1.299–12.074; p = .016), whereas in the aspirin group, the PE rates had no significance between two groups (aOR 0.921, 95% CI 0.140–6.070; p = .932) (Table 4).
TABLE 3.
The aspirin effect on pregnancy outcomes initiated at or before 16 weeks of gestation in women with stage 1 hypertension or normotension
| Normotension | Stage 1 hypertension | |||||||
|---|---|---|---|---|---|---|---|---|
|
Control 95 |
Aspirin 95 |
aOR (95% CI) | p value |
Control 49 |
Aspirin 44 |
aOR (95% CI) | p value | |
| Preeclampsia, n (%) | 6 (6.3) | 4 (4.2) | 0.511 (0.121–2.159) | .361 | 10 (20.4) | 2 (4.5) | 0.139 (0.027–0.716) | .018 |
| Delivery at <37 gestational weeks | 4 (4.2) | 1 (1.1) | 0.247 (0.026–2.344) | .223 | 5 (10.2) | 1 (2.3) | 0.105 (0.010–1.137) | .064 |
| Delivery at ≥37 gestational weeks | 2 (2.1) | 3 (3.2) | 1.095 (0.147–8.176) | .929 | 5 (10.2) | 1 (2.3) | 0.184 (0.020–1.703) | .136 |
| Preterm birth, n (%) | 17 (17.9) | 10 (10.5) | 0.506 (0.212–1.210) | .126 | 9 (18.4) | 2 (4.5) | 0.141 (0.025–0.782) | .025 |
| Placental abruption, n (%) | 9 (9.5) | 9 (9.5) | 1.030 (0.384–2.760) | .953 | 3 (6.1) | 4 (9.1) | 2.076 (0.346–12.457) | .424 |
| Postpartum hemorrhage, n (%) | 9 (9.5) | 9 (9.5) | 0.871 (0.312–2.437) | .793 | 1 (2.0) | 4 (9.1) | 6.264 (0.602–65.171) | .125 |
| SGA (<10th percentile), n (%) | 5 (5.3) | 9 (9.5) | 2.101 (0.668–6.608) | .204 | 4 (8.2) | 2 (4.5) | 0.489 (0.082–2.904) | .431 |
Abbreviations: aOR, adjusted odds ratio, adjusted for maternal age, prepregnancy body mass index, nulliparity; CI, confidence interval; NA, not applicable; SGA, small for gestational age.
TABLE 4.
Risk of adverse pregnancy outcomes in women with stage 1 hypertension to the normotension, respectively, in the control or aspirin group when initialed at or before 16 weeks of gestation
| Control | Aspirin | |||||
|---|---|---|---|---|---|---|
|
Normotension 95 |
Stage 1 hypertension 49 |
p value |
Normotension 95 |
Stage 1 hypertension 44 |
p value | |
| Preeclampsia | 1.00 (ref) | 3.960 (1.299–12.074) | .016 | 1.00 (ref) | 0.921 (0.140–6.070) | .932 |
| Delivery at <37 gestational weeks | 1.00 (ref) | 3.281 (0.791–13.617) | .102 | 1.00 (ref) | 1.828 (0.101–32.955) | .683 |
| Delivery at ≥37 gestational weeks | 1.00 (ref) | 4.359 (0.788–24.109) | .092 | 1.00 (ref) | 0.584 (0.050–6.887) | .669 |
| Preterm birth | 1.00 (ref) | 1.157 (0.458–2.926) | .758 | 1.00 (ref) | 0.432 (0.087–2.148) | .305 |
| Placental abruption | 1.00 (ref) | 0.731 (0.179–2.990) | .663 | 1.00 (ref) | 1.305 (0.353–4.823) | .690 |
| Postpartum hemorrhage | 1.00 (ref) | 0.134 (0.016–1.122) | .064 | 1.00 (ref) | 0.999 (0.274–3.648) | .999 |
| SGA (<10th percentile) | 1.00 (ref) | 1.732 (0.424–7.076) | .444 | 1.00 (ref) | 0.392 (0.076–2.013) | .262 |
The data were shown as aOR (95% CI). The normotension group, respectively, in the control group or the aspirin group was referent.
Abbreviations: aOR, adjusted odds ratio, adjusted for maternal age, prepregnancy body mass index, nulliparity; CI, confidence interval; NA, not applicable; SGA, small for gestational age.
4. DISCUSSION
This study demonstrated that in the high‐risk pregnant women in the APPEC study, those with stage 1 hypertension according to the new ACC/AHA guidelines showed higher rates of PE than those with normotension (normal and elevated categories of BP as per the new guidelines). The incidence of PE was higher in the stage 1 hypertension group as compared to the normotension group. Furthermore, the control group received only the standard antenatal care for high‐risk women, and the significant difference in PE incidence was eliminated in the group additionally treated with aspirin. For women with stage 1 hypertension in this study, the rate of PE was 16.0% in the control group and 8.1% in the aspirin group (aOR 0.428, 95% CI 0.140–1.312), close to the limit of significant decrease (p = .138). In the normotension participants during early gestation, the PE incidence was 6.2% in the control group and 8.4% in the aspirin group (aOR 1.325, 95% CI 0.503–3.491; p = .569). When the aspirin group was compared to the control group, the PE incidence decreased in the stage 1 hypertension group without a significant difference. However, in the stage 1 hypertensive women, the straightforward comparison of the PE occurrence in the control and aspirin group had minimal and not statistically significant effect. But in the sensitivity analysis including participants enrolled at or before 16 weeks of gestation, the rate of PE decreased with aspirin treatment to 13.9% in the stage 1 hypertension group with a statistically significant difference (p = .018). In the normotension group in the sensitivity analysis, there was no significant difference between the aspirin and control groups (p = .361). These results indicated that aspirin had a certain preventive effect on the increased risk of developing PE in cases where BP elevated to stage 1 hypertension from normotension in early pregnancy, thus suggesting that stage 1 hypertension may be an additional risk factor for aspirin treatment in PE prevention.
With the redefinition of the BP classification according to the new ACC/AHA guidelines for adults, attention to pregnancy management in reproductive‐aged women with stage 1 hypertension has increased. 14 , 15 Previous literature reported that in a low‐risk cohort, participants with a lower range of stage 1 hypertension receiving placebo had a higher incidence of PE as compared to the normotension pregnant women, whereas women receiving aspirin showed a similar PE incidence. 16 For high‐risk participants with preexisting insulin‐dependent diabetes mellitus or previous PE, treatment with aspirin in those entering pregnancy as normotensive reduced the risk of PE incidence by 3%, as compared to a 39% reduction in those entering pregnancy with stage 1 hypertension. 13 These were secondary analyses of the data collected in the randomized controlled trials conducted in the 1990s with an initial dose of 60 mg aspirin administered from 12 to 26 weeks of gestation. 17 , 18 With the progress of research regarding aspirin in PE prevention, the recommended dosage of aspirin was 81 mg/day, and the risk factors were updated in the ACOG guidelines. 19 In our study, we included participants who were more likely to develop PE based on the high and moderate risk factors, and those at an initial dosage of 100 mg/day of aspirin from 12 to 20 weeks of gestation. The clinical characteristics of participants in our study were similar, except for nulliparity, and the effect of aspirin on PE prevention in women with stage 1 hypertension in early pregnancy was demonstrated.
In the women with stage 1 hypertension in this study, additional to other risk factors, the protective effect of aspirin was significant with respect to preterm birth when aspirin treatment was initiated at or before 16 weeks of gestation (aOR 0.141, 95% CI 0.025–0.782; p = .025). In contrast, there was a slight protective effect of aspirin against preterm birth in the normotensive women, without a significant difference (aOR 0.506, 95% CI 0.212–1.210; p = .126). A recently published study showed that the aspirin reduced the incidence of preterm births in nulliparous women with singleton pregnancies from low‐income and middle‐income countries. 20 In stage 1 hypertension women, the aspirin group participants had a significantly longer gestational age than that of the control group (38.6 ± 1.2 vs. 37.4 ± 3.4; p = .042). Thus, aspirin may have a better protective effect in women with stage 1 hypertension as compared to those with normotension when entering pregnancy.
In the normotension group, the PE incidences were 6.2% (8/129) and 8.4% (11/131) in the control group and aspirin group, respectively. Although the difference between the two groups did not differ significantly (p = .569), the incidence of PE was slightly higher in the aspirin group than in the control group, which is inconsistent with the opinion that aspirin has a preventive effect in the high‐risk population. In the normotensive participants enrolled at or before 16 weeks of gestation, the PE incidence in the aspirin group was lower than that of the control group, with no significant difference (4.2% vs. 6.3%; p = .361), whereas the preterm PE incidence was 25% (1/4) in the aspirin group and 33.3% (2/6) in the control group. This could be because aspirin may have a better preventive effect on preterm PE when initiated at or before 16 weeks of gestation in the high‐risk population, which was suggested in subsequent studies. Meta‐analyses have shown that aspirin reduces the risk of preterm PE when it is initiated at or before 16 weeks of gestation, at a daily dose of 100 mg. 21 , 22 A study showed that the preventive effect of aspirin on preterm PE was substantial, and a comparison of the incidence of PE between the aspirin and placebo groups showed no significant difference. 23 A recent systematic review and meta‐analysis reported that the administration of low‐dose aspirin at <11 weeks of gestation in high‐risk women does not decrease the risk of PE, but it might reduce the risk of preterm delivery. 24 Combined with the results of previous study indicating that the minimal effect of aspirin in the prevention of preterm PE in pregnancies with chronic hypertension, the specific high‐risk population and active window for aspirin use need to be clarified. Our research indicated that although aspirin treatment does not seem to be efficient to prevent PE in chronic hypertension, it seems that considering stage 1 hypertension the stage 1 hypertension may be an additional risk factor for PE prevention of aspirin. However, the sample size of participants included in this study was small, and the effect of aspirin on the stage 1 hypertension subset should be studied in further clinical trials with a larger sample size. Further, the BP data we used in this study were measured on the first antenatal care before 20 weeks, it will be better for evaluating the maternal BP condition if there were more than one values.
To date, studies on pregnant women with elevated BP, classified as stage 1 hypertension according to the updated ACC/AHA guidelines, in the early gestation period are limited. Results of our research and other studies show that in low‐risk and high‐risk pregnant women, those with stage 1 hypertension in early pregnancy are at a higher risk of PE, gestational diabetes mellitus, and other adverse pregnancy outcomes. 6 , 13 , 16 As for the use of aspirin, the screening methods, initial time, and dosage for high‐risk populations are still unclear. According to the ACOG guidelines, chronic hypertension is one of the high‐risk factors for aspirin use. Stage 1 hypertension has been classified as hypertension by the updated ACC/AHA guidelines; hence, it is important to evaluate the effect of aspirin in this subset along with other risk factors. 19 Our results indicated that initial aspirin dose of 100 mg/day at or before 16–34 weeks of gestation may have a better preventive effect against PE in the stage 1 hypertension subset of the high‐risk Chinese population. However, the screening methods need to be improved. Our approach for PE screening was to identify risk factors from the demographic characteristics and medical history of the women, which led to heterogeneity in the effects of aspirin on PE prevention. Studies have shown that screening by maternal factors and biomarkers at 11–13 weeks of gestation can identify a high proportion of pregnancies that could develop early and preterm PE. 25 Overall, the pathophysiologies differed among the subgroups presenting different high‐risk factors, and the usage of aspirin needs to be more specific and personalized. 26 This study provides evidence for making clinical and public health decisions while managing reproductive‐aged women with stage 1 hypertension.
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Huixia Yang and Jing Huai contributed to the conception and design of the work; Jing Huai and Li Lin contributed to the acquisition, analysis, or interpretation of data for the work; Juan Juan contributed to the data analysis consulting; Boya Li and Yuchun Zhu drafted the original grant proposal and trial protocol; Jiahui Chen and Mengting Yu contributed to the data collection; Huixia Yang contributed to critical revision of the manuscript for important intellectual content. All the authors approved this study finally.
Funding information
This study was supported by the Major Program of the National Natural Science Foundation of China (grant No. 81490745) and the National Basic Research Program of China (grant No. 2015CB943304).
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
We thank all the other APPEC study group members, which are listed as follows: Mei‐hua Zhang (Maternal and Child Health Hospital of Taiyuan), Shi‐hong Cui (The Third Affiliated Hospital of Zhengzhou University), Xian‐lan Zhao (The First Affiliated Hospital of Zhengzhou University), Yu‐yan Ma (Qilu Hospital of Shandong University), Hong‐juan Ding (Nanjing Maternity and Child Health Care Hospital), Yang Mi (The Northwest Women and Children's Hospital), Yang‐yu Zhao (Peking University Third Hospital), Dun‐jin Chen (The Third Affiliated Hospital of Guangzhou Medical University), Wei‐she Zhang (Xiangya Hospital), Hong‐bo Qi (The First Affiliated Hospital of Chongqing Medical University), Xiao‐tian Li (Obstetrics and Gynecology Hospital of Fudan University), and Xiao‐tong Sun (Gansu Provincial People's Hospital). Also, we want to thank the patients, the doctors, and nurses who participated in this trial.
Huai J, Lin L, Juan J, et al. Preventive effect of aspirin on preeclampsia in high‐risk pregnant women with stage 1 hypertension. J Clin Hypertens. 2021;23:1060–1067. 10.1111/jch.14149
Jing Huai and Li Lin contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to the information that could compromise the privacy of research participants.
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206‐1252. [DOI] [PubMed] [Google Scholar]
- 3. Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254‐1261. [DOI] [PubMed] [Google Scholar]
- 4. Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre‐eclampsia determined in early pregnancy: systematic review and meta‐analysis of large cohort studies. BMJ (Clinical research ed). 2016;353:i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094‐1112. [DOI] [PubMed] [Google Scholar]
- 6. Wu DD, Gao L, Huang O, et al. Increased adverse pregnancy outcomes associated with stage 1 hypertension in a low‐risk cohort: evidence from 47 874 cases. Hypertension. 2020;75(3):772‐780. [DOI] [PubMed] [Google Scholar]
- 7. Goodlin RC, Haesslein HO, Fleming J. Aspirin for the treatment of recurrent toxaemia. Lancet (London, England). 1978;2(8079):51. [DOI] [PubMed] [Google Scholar]
- 8. Poon LC, Wright D, Rolnik DL, et al. Aspirin for evidence‐based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. 2017;217(5):585.e581‐585.e585. [DOI] [PubMed] [Google Scholar]
- 9. Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low‐dose aspirin: a meta‐analysis. Ultrasound Obstet Gynecol. 2013;41(5):491‐499. [DOI] [PubMed] [Google Scholar]
- 10. Lin L, Zhu Y, Li B, Yang H. Low‐dose aspirin in the prevention of pre‐eclampsia in China (APPEC study): protocol for a multicentre randomized controlled trial. Trials. 2018;19(1):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hypertension in pregnancy . Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122‐1131. [DOI] [PubMed] [Google Scholar]
- 12. Hadlock FP, Harrist RB, Martinez‐Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181(1):129‐133. [DOI] [PubMed] [Google Scholar]
- 13. Hauspurg A, Sutton EF, Catov JM, Caritis SN. Aspirin effect on adverse pregnancy outcomes associated with stage 1 hypertension in a high‐risk cohort. Hypertension. 2018;72(1):202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019;221(3):277.e271‐277.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLaren RA, Atallah F, Persad VVD, et al. Pregnancy outcomes among women with American College of Cardiology‐ American Heart Association defined hypertension. J Matern Fetal Neonatal Med. 2019;1‐6. 10.1080/14767058.2019.1704250; PMID: 31875736 [DOI] [PubMed] [Google Scholar]
- 16. Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal outcomes associated with lower range stage 1 hypertension. Obstet Gynecol. 2018;132(4):843‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caritis S, Sibai B, Hauth J, et al. Low‐dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal‐Fetal Medicine Units. N Engl J Med. 1998;338(11):701‐705. [DOI] [PubMed] [Google Scholar]
- 18. Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low‐dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal‐Fetal Medicine Units. N Engl J Med. 1993;329(17):1213‐1218. [DOI] [PubMed] [Google Scholar]
- 19. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):e1‐e25. [DOI] [PubMed] [Google Scholar]
- 20. Hoffman MK, Goudar SS, Kodkany BS, et al. Low‐dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double‐blind, placebo‐controlled trial. Lancet (London, England). 2020;395(10220):285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberge S, Bujold E, Nicolaides KH. Meta‐analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage. Am J Obstet Gynecol. 2018;218(5):483‐489. [DOI] [PubMed] [Google Scholar]
- 22. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287‐293.e281. [DOI] [PubMed] [Google Scholar]
- 23. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613‐622. [DOI] [PubMed] [Google Scholar]
- 24. Chaemsaithong P, Cuenca‐Gomez D, Plana MN, Gil MM, Poon LC. Does low‐dose aspirin initiated before 11 weeks' gestation reduce the rate of preeclampsia? Am J Obstet Gynecol. 2020;222(5):437‐450. [DOI] [PubMed] [Google Scholar]
- 25. Tan MY, Syngelaki A, Poon LC, et al. Screening for pre‐eclampsia by maternal factors and biomarkers at 11–13 weeks' gestation. Ultrasound Obstet Gynecol. 2018;52(2):186‐195. [DOI] [PubMed] [Google Scholar]
- 26. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre‐eclampsia: pathophysiology and clinical implications. BMJ (Clinical research ed). 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to the information that could compromise the privacy of research participants.


