Abstract
Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are prescribed as conservative or adjunctive therapies for adult idiopathic nephrotic syndrome. However, studies on real‐world practice patterns are scarce. This study aimed to examine the prevalence and incidence of ACEI/ARB prescription and their associated factors. This nationwide cohort study included adult Japanese patients with idiopathic nephrotic syndrome including minimal change disease (MCD), membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), and others. The outcomes were the prevalence of ACEI/ARB prescription at baseline (date of renal biopsy or date of immunosuppressant initiation) and at 2 months after baseline. Of the 326 eligible patients, 122 (37.4%) had already been prescribed ACEIs/ARBs. Of the remaining 204 patients, 67 (32.7%) were newly prescribed within the 2‐month period. MN/FSGS (vs. MCD, adjusted odds ratio [AOR]: 4.96 [95% confidence interval {CI} 2.53–9.72] and 3.95 [95% CI 1.61–9.66], respectively), higher age (per 1‐yr increase, AOR: 1.02 [95% CI 1.00–1.04]), other hypertensive agents (AOR: 2.18 [95% CI 1.21–3.92]), antidiabetic drug (AOR: 6.57 [95% CI 1.77–24.4]) were associated with a higher prevalence of ACEI/ARB prescription. MN (vs. MCD, AOR: 6.00 [95% CI 2.57–14.0]) and higher baseline systolic blood pressure (SBP) (per 10‐mmHg increase, AOR: 1.36 [95% CI 1.09–1.70]) were associated with a higher incidence of ACEI/ARB prescription. On average, incidence of ACEI/ARB prescription increased from 19.2% to 40.8% as baseline SBP increased from 100 to 140 mmHg. Thus, Japanese nephrologists are likely to prescribe ACEIs/ARBs for nephrotic patients with MN or high baseline SBP, even below the hypertensive range.
Keywords: angiotensin receptor blocker, angiotensin‐converting enzyme inhibitor, blood pressure, focal segmental glomerulosclerosis, membranous nephropathy, minimal change disease, nephrotic syndrome, practice patterns, renin‐angiotensin‐aldosterone system inhibitor
1. INTRODUCTION
Nephrotic syndrome is a glomerular disorder which causes massive edema, impairing living function and sometimes irreversible renal insufficiency and end‐stage renal failure. The establishment of an effective treatment regimen aimed at inducing remission is thus required. For this purpose, while glucocorticoid and immunosuppressive agents are mainly implemented in idiopathic nephrotic syndrome, supportive therapies such as angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) are often also prescribed with some proven efficacy in a small number of randomized controlled and observational studies. 1 , 2 , 3 Indeed, the Japanese guidelines recommend these drugs as supportive therapy for patients with nephrotic syndrome complicated with hypertension, and the international guideline published by Kidney Disease Improving Global Outcomes also recommends them for pediatric cases. 4 , 5 , 6 However, the prescription patterns of ACEIs and ARBs are poorly studied.
Indications for prescription of ACEIs and ARBs may vary in terms of established efficacy, guideline recommendations, or refractoriness regarding underlying glomerular disease, and patient characteristics such as hemodynamics and renal function. While several practice pattern studies have shown that ACEIs and ARBs are prescribed for more than 80% of patients with membranous nephropathy (MN), prescription rates for other underlying glomerular disorders are unknown. 7 , 8 In addition, ACEIs and ARBs may be preferred as supportive therapy in older patients because glucocorticoids are usually started at a reduced dose during remission therapy. 9 Thus, clarifying the actual prescription patterns of ACEIs and ARBs, and the factors associated with their prescription, is clinically important as it may help resolve the guideline‐practice gap and serve as a basis for future studies on underlying glomerular diseases for which effectiveness of ACEIs and ARBs remains uncertain.
In the present study, using a nationwide cohort database called the Japan Nephrotic Syndrome Cohort Study (JNSCS), we aimed to examine the prevalence and incidence of ACEI/ARB prescription, and the clinical characteristics associated with their prescription in idiopathic nephrotic syndrome.
2. METHODS
2.1. Design, setting, and participants
The JNSCS is a cohort study which was originally planned to investigate incidence rates of the clinical outcomes and treatment effectiveness in primary nephrotic syndrome. The sampling method applied in the JNSCS was described in detail previously. 10 The participants of the JNSCS were recruited within the Japan Renal Biopsy Registry (J‐RBR), which was a multi‐center prospective registry of 1,986 patients with primary nephrotic syndrome who underwent renal biopsies at 129 facilities between 2008 and 2011. 11 The JNSCS enrolled patients with biopsy‐confirmed primary nephrotic syndrome, involving 56 facilities during the entry period between January 2009 and December 2010 and had a 5‐year observation period. According to the JNSCS protocol, the baseline date were set as the first day of treatment for patients who received immunosuppressive therapy or the date of kidney biopsy for those who did not undergo immunosuppressive therapy. 10 Data regarding patients’ drug prescriptions and characteristics, such as age, serum creatinine, and urinary protein per day, were obtained at baseline and at 1, 2, 6, 12, 24, 36, 48, and 60 months after baseline. The representativeness of the JNSCS relative to the J‐RBR was investigated in a previous study with similar clinical characteristics among patients aged > 18 years with minimal change disease (MCD) and MN and severer clinical activity among patients with focal segmental glomerular sclerosis (FSGS). 10 In the present study, eligible participants were patients with biopsy‐proven primary nephrotic syndrome who were aged ≥ 18 years and had a urinary protein creatinine ratio (UPCR) obtained via spot urine ≥ 3.5 g/gCr or pooled daily urinary protein ≥ 3.5 g/day at the time of renal biopsy or beginning of immunosuppressive agents. This study conformed with the Declaration of Helsinki and was approved by the Research Ethics Committee of Nagoya University Graduate School of Medicine (approved number 21(1–2)).
2.2. Candidate factors
The candidate factors in this exploratory analysis are the characteristics of patients with nephrotic syndrome such as age, pathology patterns, baseline systolic blood pressure (SBP), serum creatinine level, UPCR, antihypertensive drugs other than ACEI/ARB, the prescription of antidiabetic drugs, and the prescription of immunosuppressive agents including glucocorticoid. These chosen predictors were assumed to determine indication of ACEI/ARB prescription based on clinical expertise or previous research. Data of the factors collected at baseline (ie, the time of initiation of immunosuppressive agents or at the time of the renal biopsy) were used for the primary and sensitivity analyses, respectively, as described below.
2.3. Outcomes
We investigated the associations of the candidate factors with both prevalence and incidence of ACEI/ARB prescriptions. The prevalence was defined as the percentage of ACEI/ARB prescriptions at baseline. The candidate factors were used to examine the association with the prevalence, collected at baseline. The incidence of ACEI/ARB prescription was defined as a new prescription during the observation period (Figure 1). The time frame to observe the incidence was defined as within two months of baseline. The period between the date of commencement of immunosuppressive agents and renal biopsy was relatively short compared to the two‐month at‐risk period. This is further explained in the “Results” section of this paper.
Figure 1.
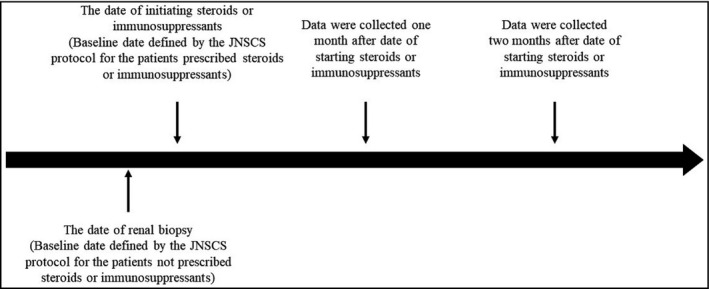
Timeline of the JNSCS protocol. The baseline date defined by the JNSCS protocol was the date of initiating steroid or immunosuppressant therapy for patients prescribed with these agents and the date of renal biopsy for patients not treated with these agents. Data on patients’ clinical characteristics were collected at baseline. Longitudinal data were collected with prespecified time intervals after the date. The median interval between the date of renal biopsy and the start of steroids or immunosuppressants is 9 days (25‐percent quartile, 1 day; 75‐percent quartile, 14 days)
2.4. Statistical analysis
For descriptive statistics, continuous variables were expressed as medians (interquartile range) and categorical variables were expressed as numbers and percentages, while the number of missingness of values for candidate factors was described as numbers. We evaluated the associations of the prevalence of ACEI/ARB prescription with the candidate factors described above using a logistic regression model, with serum creatinine and UPCR being log‐transformed. As a reference category for pathology patterns, MCD was selected because the prescription pattern of ACEIs/ARBs was expected to differ between patients with MCD and those with MN or FSGS, for whom the efficacy of ACEIs/ARBs for proteinuria has been suggested in previous studies. 1 , 2 , 3 We also evaluated the association with the incidence of ACEI/ARB prescription for the patients not being prescribed with ACEI/ARB at the baseline using a logistic regression model using the same candidate factors as for the prevalence of ACEI/ARB. To estimate the predicted probabilities for incidence of ACEI/ARB prescription across the baseline SBP values, we calculated the probabilities standardized to the total study population with all other variables set to their original values. 12 The baseline SBP was used as a proxy for SBP at the initiation of ACEIs/ARBs, as SBP measurement was not included in the JNSCS protocol. Missing values were multiply imputed. The results across 10 imputed datasets were combined by averaging, and standard errors were adjusted to reflect both within‐imputation and between‐imputation variability. These estimates and their standard errors were combined according to Rubin's rules. A p‐value of < .05 was considered statistically significant. All analyses were performed using STATA version 15 (Stata LP, College Station, TX, USA).
2.5. Sensitivity analysis
To examine the robustness of the association of the prevalence of ACEI/ARB prescription with patients’ characteristics, we conducted the sensitivity analyses where the baseline was set as the time of renal biopsy. Patients whose treatment with immunosuppressive agents was initiated prior to the renal biopsy were excluded from the sensitivity analysis for the assessment of prevalence with the candidate factors not affected by immunosuppressive agents. We evaluated the association using a logistic regression model using the same candidate factors as for the primary analysis except for the prescription of immunosuppressive agents.
3. RESULTS
3.1. The patients’ characteristics
A total of 326 patients were eligible to investigate the prevalence of ACEI/ARB. 122 (37.4%) patients were already prescribed with ACEI/ARB. After excluding those patients from the baseline population, 204 patients were included to evaluate the incidence of ACEI/ARB. The process of selecting eligible patients is shown in Figure 2. Among 326 patients, 301 were prescribed immunosuppressive agents at the baseline. The median (25th and 75th percentiles) interval between the time of renal biopsy and the start of immunosuppressive medications was 6 (1–14) days. The patients’ characteristics for the analysis of prevalence are shown in Table 1. The median (25th and 75th percentiles) age was 60 (41–72) years and 191 (58.6%) patients were male. Among those who were not prescribed ACEI/ARB at baseline, a total of 52 (34.2%) and 67 (32.8%) patients were newly prescribed within 1 and 2 months after the commencement of immunosuppressive treatment, respectively. According to the pathological pattern, new prescriptions were observed in 12 of 113 (10.6%) patients with MCNS, 28 of 54 (51.9%) patients with MN, and 4 of 20 (20%) patients with FSGS after 1 month. After 2 months, new prescriptions were observed in 19 of 113 (16.8%) patients with MCNS, 32 of 54 (59.3%) patients with MN, and 7 of 20 (35%) patients with FSGS.
Figure 2.
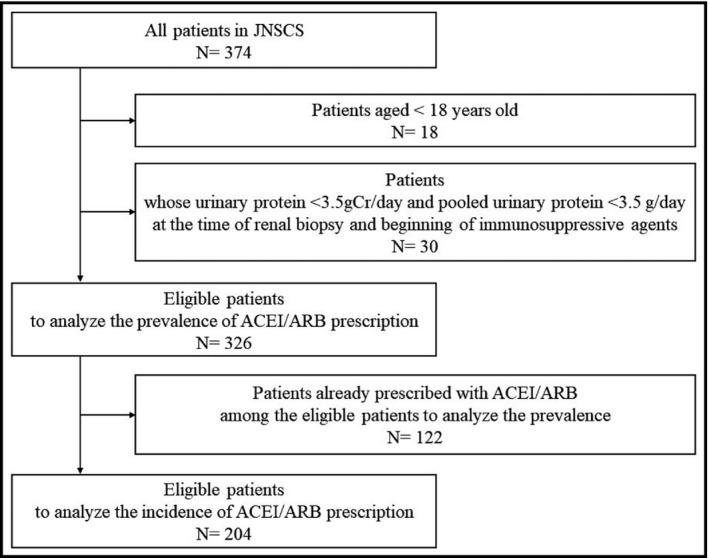
Flow of selecting eligible patients. ACEI/ARB; angiotensin‐converting enzyme inhibitor / angiotensin II receptor blocker, JNSCS; Japan Nephrotic Syndrome Cohort Study
Table 1.
Patients’ characteristics for the analysis of prevalence
| All (n = 326) | Patients with prevalence of ACEI/ARB prescription (n = 122) | Patients without prevalence of ACEI/ARB prescription (n = 204) | |
|---|---|---|---|
| Age | 60 (41–72) | 65 (58–74) | 51 (34–69) |
| Gender (male) | 191 (58.6) | 73 (59.8) | 118 (57.8) |
| Pathology patterns | |||
| MCD | 134 (41.1) | 21 (17.2) | 113 (55.4) |
| MN | 125 (38.3) | 71 (58.2) | 54 (26.5) |
| FSGS | 37 (11.4) | 17 (13.9) | 20 (9.8) |
| Others | 30 (9.2) | 13 (10.7) | 17 (8.3) |
|
Systolic blood pressure (mmHg) (Missing n = 3) |
126 (115–140) |
130 (120–142) Missing n = 2 |
124 (112–138) Missing n = 1 |
|
Diastolic blood pressure (mmHg) (Missing n = 3) |
76 (66–83) |
77 (68–84) Missing n = 2 |
74 (65–82) Missing n = 1 |
| Serum creatinine (mg/dl) | 0.95 (0.73–1.37) | 1.02 (0.80–1.44) | 0.93 (0.70–1.29) |
|
Pooled urinary protein (g/day) Missing n = 75 |
5.7 (3.9–8.7) |
5.1 (3.8–6.4) Missing n = 33 |
6.1 (4.1–9.1) Missing n = 42 |
|
Urinary protein (g/gCr) (Missing n = 40) |
6.8 (4.6–10.4) |
6.0 (4.2–9.4) Missing n = 12 |
7.5 (5.0–10.7) Missing n = 28 |
| Antihypertensive drug other than ACEI/ARB | 98 (30.1) | 59 (48.4) | 39 (19.1) |
| Antidiabetic drug | 16 (4.9) | 12 (9.8) | 4 (2.0) |
| Immunosuppressive medications | 301 (92.3) | 109 (89.3) | 192 (94.1) |
Data are presented as medians (interquartile ranges) for continuous data or numbers (%) for categorical data.
3.2. Association of patients’ characteristics with prevalence of ACEI/ARB prescription
The adjusted odds ratio (AOR) of each candidate factor is shown in Table 2. The pathology patterns such as MN (AOR, 4.96; 95% CI, 2.53–9.72), FSGS (AOR, 3.95; 95% CI, 1.61–9.66), and other pathology patterns (AOR, 3.07; 95% CI, 1.14–8.26) were significantly associated with a higher likelihood of being prescribed compared with MCD. Furthermore, age (per 1‐year increase, AOR, 1.02; 95% CI, 1.00–1.04), prescription of antihypertensive drugs other than ACEI/ARB (AOR, 2.18; 95% CI, 1.21–3.92), and the prescription of antidiabetic drugs (AOR, 6.57; 95% CI, 1.77–24.4) were also significantly associated with ACEI/ARB prescription, respectively.
Table 2.
Association of patients’ characteristics with prevalence of ACEI/ARB prescription (N = 326)
| Adjusted OR | p‐value | ||
|---|---|---|---|
| Point estimates | 95% CI | ||
| Age, per 1‐year | 1.02 | (1.00 to 1.04) | .04 |
| Pathology patterns | |||
| MCD | Ref. | ||
| MN | 4.96 | (2.53 to 9.72) | <.001 |
| FSGS | 3.95 | (1.61 to 9.66) | .003 |
| Others | 3.07 | (1.14 to 8.26) | .03 |
| Log‐transformed serum creatinine, per 1‐unit | 0.98 | (0.57 to 1.69) | .94 |
| Log‐transformed urinary protein (gCr/day), per 1‐unit | 0.66 | (0.43 to 1.03) | .07 |
| Baseline SBP, per 10 mmHg | 1.03 | (0.88 to 1.21) | .70 |
| Antihypertensive drug other than ACEI/ARB | 2.18 | (1.21 to 3.92) | .01 |
| Antidiabetic drug | 6.57 | (1.77 to 24.4) | .01 |
| Immunosuppressive agents | 1.08 | (0.41 to 2.80) | .88 |
Adjusted odds were estimated using a logistic regression model. Bold font indicates significant associations with the outcomes.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AOR, adjusted odds ratio; ARB, angiotensin receptor blockerCI, confidence interval; Cr, creatinine; FSGS, focal segmental sclerosis; MCD, minimal change disease; MN, membranous nephropathy; SBP, systolic blood pressure.
3.3. Association of patients’ characteristics with incidence of ACEI/ARB prescription
The AOR of each candidate factor is shown in Table 3. The pathology patterns of MN (AOR, 6.00; 95% CI, 2.57–14.0) and other pathology patterns (AOR, 3.89; 95% CI, 1.17–12.9) were significantly associated with the incident prescription compared with MCD. The association of FSGS with incident prescription was not evident (AOR, 1.79; 95% CI, 0.58–5.52). Baseline SBP (AOR per 10 mmHg, 1.36; 95% CI, 1.09–1.70) was also significantly associated with incident prescription.
Table 3.
Association of patients’ characteristics with incidence of ACEI/ARB prescription (N = 204)
| Adjusted OR | p‐value | ||
|---|---|---|---|
| Point estimates | 95% CI | ||
| Age, per 1‐year | 1.01 | (0.98 to 1.03) | .632 |
| Pathology patterns | |||
| MCD | Ref. | ||
| MN | 6.00 | (2.57 to 14.0) | <.001 |
| FSGS | 1.79 | (0.58 to 5.52) | .31 |
| Others | 3.89 | (1.17 to 12.9) | .03 |
| Log‐transformed serum creatinine, per 1‐unit | 1.18 | (0.62 to 2.24) | .62 |
| Log‐transformed urinary protein (gCr/day), per 1‐unit | 1.00 | (0.61 to 1.65) | .99 |
| Baseline SBP, per 10 mmHg | 1.36 | (1.09 to 1.70) | .01 |
| Antihypertensive drug other than ACEI/ARB | 0.81 | (0.32 to 2.02) | .65 |
| Antidiabetic drug | 1.02 | (0.09 to 11.4) | .98 |
| Immunosuppressive agents | 0.90 | (0.22 to 3.78) | .89 |
Adjusted odds ratios were estimated using a logistic regression model. Bold font indicates significant association with the outcomes.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AOR, adjusted odds ratio; ARB, angiotensin receptor blocker; CI, confidence interval; Cr, creatinine; FSGS, focal segmental sclerosis; MCD, minimal change disease; MN, membranous nephropathy; SBP, systolic blood pressure.
3.4. Baseline systolic blood pressure and incidence of ACEI/ARB prescription
The probabilities of incidence of ACEI/ARB prescription (ie, starting treatment with ACEI/ARB) standardized to the total study population across baseline SBP is shown in Figure 2. Among 67 patients with ACEI/ARB added, 40 patients had a baseline SBP less than 140 mmHg at baseline. As shown in Figure 3, the probabilities of starting ACEI/ARB increased as the baseline SBP increased, even with a baseline SBPs of less than 140 mmHg: for example, at baseline SBPs of 100, 120, and 140 mmHg, the probability of starting ACEI/ARB was 19.2% (95%CI 9.5%‐28.8%), 28.9% (95%CI 22.4%‐35.5%), and 40.8% (95%CI 31.9%‐49.8%), respectively.
Figure 3.
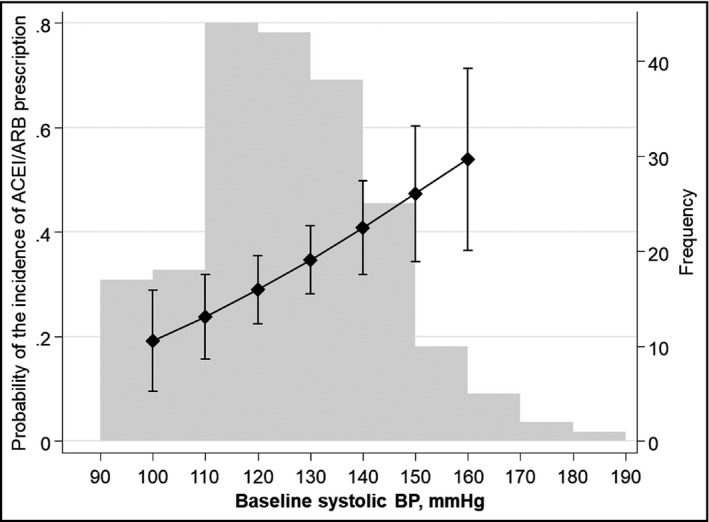
Probability of incident ACEI/ARB prescription by baseline Systolic Blood Pressure (SBP). Using the multivariable adjusted logistic model, adjusted probability by baseline SBP was predicted. The left vertical axis shows probability of incident ACEI/ARB prescription. The connected line indicates point estimates. The vertical lines indicate 95% confidence intervals. Gray bars indicate frequency of the baseline SBP values. The right vertical axis shows frequency of each gray bar
3.5. Sensitivity analysis for prevalence of ACEI/ARB prescription
After excluding patients whose immunosuppressive therapy started before their renal biopsy from the original cross‐sectional population, 269 patients were eligible to evaluate the prevalence of ACEI/ARB prescription at the time of renal biopsy. The process of selecting eligible patients is shown in Figure S1. The patients’ characteristics for the sensitivity analysis are shown in Table S1. Among those patients, 95 (35.3%) were prescribed with ACEI/ARB at the time of renal biopsy. Associations of the candidate factors chosen in the primary analysis with the prevalent prescription are shown in Table S2. As shown in the primary analysis, the pathology patterns such as MN (AOR, 2.57; 95% CI, 1.20–5.54) and FSGS (AOR, 3.03; 95% CI, 1.06–8.67) were associated with the prevalent prescription compared to MCD. Lower log‐transformed urinary protein (AOR, 0.56 per 1‐unit increase; 95%CI, 0.34–0.94), prescription of other antihypertensive agents (AOR, 3.77; 95% CI, 1.96–7.26), and prescription of antidiabetic drugs (ORs, 14.3; 95% CI, 1.40–145) were also associated with the prescription of ACEI/ARB at baseline.
4. DISCUSSION
This is the first study to clarify the variations in ACEI/ARB prescription among patients with idiopathic nephrotic syndrome and to determine the factors that predicts its prescription. We confirmed several important clinical factors associated with increased prevalence and incidence of ACEI/ARB prescription, such as MN and the baseline SBP.
We found that the prescription of antihypertensive drugs other than ACEI/ARBs, along with antidiabetic drug prescription and pathology patterns other than MCD, was associated with the prevalence with ACEI/ARB prescription. Several possibilities may explain these associated factors. Higher prevalence of ACEI/ARB prescription associated with antidiabetic drugs may be ascribed to evidence‐based practice for patients having diabetes as a comorbidity: ACEIs/ARBs are indicated for reducing proteinuria and for preventing the progression of diabetic kidney disease and cardiovascular disease. 13 , 14 , 15 , 16 , 17 For the patients with pathology patterns other than MCD, ACEIs/ARBs tend to be prescribed more often compared to those with MCD. For MCD, which is typically characterized by rapid onset of heavy proteinuria, immediate renal biopsy and subsequent glucocorticoid therapy usually precede consideration of ACEI/ARB prescription. The associations of older age with higher likelihood of prescription suggest that patients with older age could be started on conservative treatment using ACEI/ARB rather than immunosuppressive treatment for avoidance of adverse events from glucocorticoids and other immunosuppressive agents.
We also found that the incidence of ACEI/ARB prescription was associated with the presence of MN and baseline SBP. However, age, antidiabetic drugs, and the presence of FSGS were not associated with prescription incidence. There are several potential explanations for the discrepancies in the factors associated with the prescription prevalence and incidence. First, the discrepancies could be ascribed to differences in the clinical characteristics between patients examined for the prevalence and those examined for the incidence. For example, patients examined for the incidence were younger than those examined for the prevalence. Further, the baseline SBP was used in the incidence analysis only among those who were not exposed to ACEIs/ARBs, but may have been altered by ACEIs/ARBs among those who were already receiving ACEIs/ARBs in the prevalence analysis. Second, with regard to MN, previous research suggests remission could be induced by conservative therapy including ACEI/ARB. 3 Thus, the patients with MN could be treated using only ACEI/ARB in actual practice settings. In contrast, there was insufficient evidence that proteinuria in FSGS may be reduced with ACEI/ARB prescription. Third, evidence of associations of antidiabetic drugs with increased prevalence but not with increased incidence of ACEI/ARB prescription, suggests that ACEI/ARB prescription tends to precede renal biopsy or immunosuppressive treatment. As noted previously, ACEI/ARB had been prescribed for prevention of complications related to comorbid diabetes.
Notably, we could show that higher baseline SBP was associated with higher likelihood of new ACEI/ARB prescription. In addition, our findings revealed that ACEI/ARB was started for some patients with a baseline SBP of less than 140 mmHg. The probabilities of the incidence of prescription monotonically increased from 19.2% to 40.8% when the baseline SBP increased from 100 to 140 mmHg. Of note, about 20% of patients with a baseline SBP of 100 mmHg were started with ACEIs/ARBs suggesting nephrologists’ expectations for ACEI/ARB to reduce proteinuria independent of a blood pressure‐lowering effect. These findings suggest the presence of an actual prescription pattern for ACEI/ARB even for patients with well‐controlled blood pressure and warrant further investigation to clarify whether newly prescribed ACEI/ARB is associated with remission of proteinuria.
The present study has several strengths. Firstly, use of the nationwide JNCNS survey enabled us to detect several important clinical factors associated with the prevalence and incidence of ACEI/ARB prescription in a wide spectrum of idiopathic nephrotic syndrome with a large sample size. Secondly, as JNCNS was conducted at multiple centers across Japan; our findings of prescription patterns and their associated factors are applicable at a nationwide level.
However, there are also several limitations in the present study. Firstly, the JNCNS study collected prescription status data for only two months after the start of glucocorticoid or immunosuppressive agents. As noted in the methods section, since data collection starting from the date of renal biopsy was not performed for all patients, we could not evaluate the incidence of ACEI/ARB prescription just two months after renal biopsy. However, our results show that the interval between the time of renal biopsy and the start of immunosuppressive medications was so short that the influence of this interval was negligible. Secondly, the dose of the medications such as ACEI/ARB and the other antihypertensive drugs were not recorded and we could only examine newly prescribed ACEI/ARB, not the increase in dose of ACEI/ARB. Thirdly, the SBP levels at prescription of ACEI/ARB were not included in the dataset and we need to be careful to interpret the magnitude of associations of SBP with newly ACEI/ARB prescription as SBP measured before the prescription could have been higher than that measured at baseline. This is especially relevant among patients with an SBP under 140 mmHg at baseline and who could have been prescribed with ACEI/ARB when their SBP increased to 140 mmHg. If data on SBP immediately prior to prescription had been available, the slope of the curve would have been gentler and would have shifted to a higher SBP range than that observed in the present study. However, as the interval between the baseline and outcome measurements was two months, we believe that the change in the SBP during this short period was small. Moreover, SBP is prone to decreasing after starting treatment resulting in the association being unchanged even if SBP just before prescription is used for statistical modeling. Fourth, the small number (n = 4) of patients receiving antidiabetic drugs among those examined for the incidence might have contributed to the wide 95%CIs for the association of the incidence prescription with antidiabetic drugs.
In conclusion, we conducted the exploratory analyses to investigate the association of prevalence and incidence of ACEI/ARB prescription with patients’ characteristics in idiopathic nephrotic syndrome. We found that several factors were associated with the prevalence and incidence of ACEI/ARB prescription. More especially, we indicated the actual practice pattern of ACEI/ARB prescription for patients with well‐controlled blood pressure. These findings could be helpful for further studies to investigate the effectiveness of ACEI/ARB on renal outcomes in this population.
CONFLICTS OF INTEREST
All authors declare that they have no relevant financial or other conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Study concept and design: HN, KN, SS, YS, NK; data collection: YS, RY, KN, TT, SU, AT, and the other members of the JNSCS Study Group; data analysis: HN, KN, NK; data interpretation: HN, KN, SS, YS, RY, KN, TT, SU, AT, HO, IN, YI, NK; manuscript preparation: HN, KN, NK.
Supporting information
Figure S1 Flow of selecting eligible patients for sensitivity analysis of prevalence of ACEI/ARB prescription.
Table S1 Patients’ characteristics for sensitivity analysis.
Table S2 Association of patients’ characteristics with prevalence of ACEI/ARB prescription at renal biopsy.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Appendix 1.
JAPAN NEPHROTIC SYNDROME COHORT STUDY GROUP
Enyu Imai (Nakayamadera Imai Clinic), Shoichi Maruyama (Department of Nephrology, Nagoya University Graduate School of Medicine), Toshinobu Sato (Department of Nephrology, JCHO Sendai Hospital), Hiroshi Sato (Department of Nephrology, Endocrinology, and Vascular Medicine, Tohoku Univeristy Gradaute School of Medicine), Takashi Wada (Department of Nephrology and Laboratory Medicine, Kanazawa University), Hiroki Hayashi (Department of Nephrology, Fujita Health University School of Medicine), Yasuhiro Akai (First Department of Internal Medicine, Nara Medical University), Megumu Fukunaga (Division of Nephrology, Department of Internal Medicine, Toyonaka Municipal Hospital), Kazuhiko Tsuruya (Department of Integrated Therapy for Chronic Kidney Disease, Graduate School of Medical Sciences, Kyushu University), Kosuke Masutani (Division of Nephrology and Rheumatology, Department of Internal Medicine, Faculty of Medicine, Fukuoka University), Tsuneo Konta (Department of Cardiology, Pulmonology, and Nephrology, Yamagata University School of Medicine), Hitoshi Yokoyama (Department of Nephrology, Kanazawa Medical Univeristy School of Medicine), Tatsuya Shoji (Department of Kidney Disease and Hypertension, Osaka General Medical Center), Takeyuki Hiramatsu (Department of Nephrology, Konan Kosei Hospital), Shunsuke Goto (Division of Nephrology and Kidney Center, Kobe University Graduate School of Medicine), Hitoshi Sugiyama (Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences), Hirofumi Tamai (Department of Nephrology, Anjo Kosei hospital), Saori Nishio (Division of Rheumatology, Endocrinology and Nephrology, Hokkaido University Graduate School of Medicine), Arimasa Shirasaki (Department of Nephrology, Ichinomiya Municipal Hospital), Kojiro Nagai (Department of Nephrology, Institute of Biomedical Sciences, Tokushima University Graduate School), Kunihiro Yamagata (Department of Nephrology, Faculty of Medicine, University of Tsukuba), Hajime Hasegawa (Department of Nephrology and Hypertension, Saitama Medical Center, Saitama Medical University), Hidemo Yasuda (Internal Medicine 1, Hamamatsu University School of Medicine), Shizunori Ichida (Department of Nephrology, Japanese Red Cross Nagoya Daiichi Hospitail), Tomohiko Naruse (Department of Nephrology, Kasugai Municipal Hospital), Kei Fukami (Division of Nephrology, Department of Medicine, Kurume University School of Medicine), Tomoya Nishino (Department of Nephrology, Nagasaki University Hospital), Hiroshi Sobajima (Department of Diabetology and Nephrology, Ogaki Municipal Hospital), Satoshi Tanaka (Department of Nephrology, Shizuoka General Hospital), Toshiyuki Akahori (Department of Nephrology, Chutoen General Medical Center), Takafumi Ito (Division of Nephrology, Shimane University Hospital), Yoshio Terada (Department of Endocrinology, Metabolism and Nephrology, Kochi Medical School, Kochi University), Ritsuko Katafuchi (Kideny Unit, National Fukuoka Higashi Medical Center), Shouichi Fujimoto (Department of Hemovascular Medicine and Artificial Organs, Faculty of Medicine, University of Miyazaki), Eiji Ishimura (Department of Nephrology, Osaka City University Graduate School of Medicine), Junichiro James Kazama (Department of Nephrology and Hypertension, Fukushima Medical University School of Medicine), Keiju Hiromura (Department of Nephrology and Rheumatology, Gunma University Graduate School of Medicine), Tetsushi Mimura (Department of Nephrology, Gifu Prefectural Tajimi Hospital), Satashi Suzuki (Department of Nephrology, Kainan Hospital), Yosuke Saka (Department of Nephrology, Yokkaichi Municipal Hospital), Tadashi Sofue (Department of Cardiorenal and Cerebrovascular Medicine, Kagawa University), Yusuke Suzuki (Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine), Kiyoki Kitagawa (Division of Internal Medicine, National Hospital Organization Kanazawa Medical Center,), Kunio Morozumi (Department of Nephrology, Masuko Memorial Hospital), Yoshiro Fujita (Department of Nephrology, Chubu Rosai Hospital), Makoto Mizutani (Department of Nephrology, Handa City Hospital), Takashi Shigematsu (Department of Nephrology, Wakayama Medical University), Naoki Kashihara (Department of Nephrology and Hypertension, Kawasaki Medical School), Seiichi Matsuo (Department of Nephrology, Nagoya University Graduate School of Medicine).
Nishiwaki H, Niihata K, Shimizu S, et al; Japan Nephrotic Syndrome Cohort Study group . Incidence and factors associated with prescribing renin‐angiotensin‐system inhibitors in adult idiopathic nephrotic syndrome: A nationwide cohort study. J Clin Hypertens. 2021;23:999–1007. 10.1111/jch.14224
Hiroki Nishiwaki and Kakuya Niihata contributed equally to this work.
Funding information
This study was supported in part by a Grant‐in‐Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan.
Contributor Information
Hiroki Nishiwaki, Email: nwacky1978@med.showa-u-ac.jp.
Japan Nephrotic Syndrome Cohort Study group:
Enyu Imai, Shoichi Maruyama, Toshinobu Sato, Hiroshi Sato, Takashi Wada, Hiroki Hayashi, Yasuhiro Akai, Megumu Fukunaga, Kazuhiko Tsuruya, Kosuke Masutani, Tsuneo Konta, Hitoshi Yokoyama, Tatsuya Shoji, Takeyuki Hiramatsu, Shunsuke Goto, Hitoshi Sugiyama, Hirofumi Tamai, Saori Nishio, Arimasa Shirasaki, Kojiro Nagai, Kunihiro Yamagata, Hajime Hasegawa, Hidemo Yasuda, Shizunori Ichida, Tomohiko Naruse, Kei Fukami, Tomoya Nishino, Hiroshi Sobajima, Satoshi Tanaka, Toshiyuki Akahori, Takafumi Ito, Yoshio Terada, Ritsuko Katafuchi, Shouichi Fujimoto, Eiji Ishimura, Junichiro James Kazama, Keiju Hiromura, Tetsushi Mimura, Satashi Suzuki, Yosuke Saka, Tadashi Sofue, Yusuke Suzuki, Kiyoki Kitagawa, Kunio Morozumi, Yoshiro Fujita, Makoto Mizutani, Takashi Shigematsu, Naoki Kashihara, and Seiichi Matsuo
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, HN, upon reasonable request.
REFERENCES
- 1. Randomised placebo‐controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non‐diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997;349(9069):1857‐1863. [PubMed] [Google Scholar]
- 2. Usta M, Ersoy A, Dilek K, et al. Efficacy of losartan in patients with primary focal segmental glomerulosclerosis resistant to immunosuppressive treatment. J Intern Med. 2003;253(3):329‐334. [DOI] [PubMed] [Google Scholar]
- 3. Polanco N, Gutierrez E, Covarsi A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21(4):697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishi S, Ubara Y, Utsunomiya Y, et al. Evidence‐based clinical practice guidelines for nephrotic syndrome 2014. Clin Exp Nephrol. 2016;20(3):342‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group . KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter 2012;2:139‐274. [Google Scholar]
- 6. The Special Study Group of the Progressive Renal Dysfunction Research Group of the Ministry of Health Labour and Welfare . Evidence‐based clinical practice guidelines for nephrotic syndrome 2017. Tokyo‐igakusha, Tokyo 2017. [Japanese].
- 7. Lonnbro‐Widgren J, Molne J, Haraldsson B, Nystrom J. Treatment pattern in patients with idiopathic membranous nephropathy‐practices in Sweden at the start of the millennium. Clin Kidney J. 2016;9(2):227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Shaughnessy MM, Troost JP, Bomback AS, et al. Treatment patterns among adults and children with membranous nephropathy in the cure glomerulonephropathy network (CureGN). Kidney Int Rep. 2019;4(12):1725‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niihata K, Nishiwaki H, Kurita N, et al. Variations in actual practice patterns and their deviations from the clinical practice guidelines for nephrotic syndrome in Japan: certified nephrologists' questionnaire survey. Clin Exp Nephrol. 2019;23(11):1288‐1297. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto R, Imai E, Maruyama S, et al. Regional variations in immunosuppressive therapy in patients with primary nephrotic syndrome: the Japan nephrotic syndrome cohort study. Clin Exp Nephrol. 2018;22(6):1266‐1280. [DOI] [PubMed] [Google Scholar]
- 11. Sugiyama H, Yokoyama H, Sato H, et al. Japan Renal Biopsy Registry: the first nationwide, web‐based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011;15(4):493‐503. [DOI] [PubMed] [Google Scholar]
- 12. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deferrari G, Ravera M, Deferrari L, Vettoretti S, Ratto E, Parodi D. Renal and cardiovascular protection in type 2 diabetes mellitus: angiotensin II receptor blockers. J Am Soc Nephrol. 2002;13(Suppl 3):S224‐229. [DOI] [PubMed] [Google Scholar]
- 14. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861‐869. [DOI] [PubMed] [Google Scholar]
- 15. Makino H, Haneda M, Babazono T, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1577‐1578. [DOI] [PubMed] [Google Scholar]
- 16. Andersen S, Brochner‐Mortensen J, Parving HH, et al. Kidney function during and after withdrawal of long‐term irbesartan treatment in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2003;26(12):3296‐3302. [DOI] [PubMed] [Google Scholar]
- 17. Imai E, Chan JC, Ito S, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo‐controlled study. Diabetologia. 2011;54(12):2978‐2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flow of selecting eligible patients for sensitivity analysis of prevalence of ACEI/ARB prescription.
Table S1 Patients’ characteristics for sensitivity analysis.
Table S2 Association of patients’ characteristics with prevalence of ACEI/ARB prescription at renal biopsy.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, HN, upon reasonable request.


