Abstract
More stringent blood pressure (BP) goals have led to greater prevalence of apparent resistant hypertension (ARH), yet the long‐term prognostic impact of ARH diagnosed according to these goals in the general population remains unknown. We assessed the prognostic impact of ARH according to contemporary BP goals in 9612 participants of the Atherosclerosis Risk in Communities (ARIC) study without previous cardiovascular disease. ARH, defined as BP above goal (traditional goal <140/90 mmHg, more stringent goal <130/80 mmHg) despite the use of ≥3 antihypertensive drug classes or any BP with ≥4 antihypertensive drug classes (one of which was required to be a diuretic) was compared with controlled hypertension (BP at goal with 1‐3 antihypertensive drug classes). Cox regression models were adjusted for age, sex, race, study center, BMI, heart rate, smoking, eGFR, LDL, HDL, triglycerides, and diabetes. Using the traditional BP goal, 133 participants (3.8% of the treated) had ARH. If the more stringent BP goal was instead applied, 785 participants (22.6% of the treated) were reclassified from controlled hypertension to uncontrolled hypertension (n = 725) or to ARH (n = 60). Over a median follow‐up time of 19 years, ARH was associated with increased risk for a composite end point (all‐cause mortality, hospitalization for myocardial infarction, stroke, or heart failure) regardless of whether traditional (adjusted HR 1.50, 95% CI: 1.23‐1.82) or more stringent (adjusted HR 1.43, 95% CI: 1.20‐1.70) blood pressure goals were applied. We conclude that in patients free from cardiovascular disease, ARH predicted long‐term risk regardless of whether traditional or more stringent BP criteria were applied.
Keywords: antihypertensive therapy, epidemiology, resistant hypertension
1. INTRODUCTION
Elevated blood pressure (BP) is the most important risk factor for cardiovascular and renal death. 1 Although hypertension awareness and treatment have improved in the last decades, many patients still have elevated BP despite drug treatment. 2 , 3 , 4 Resistant hypertension is defined as BP above target despite use of antihypertensive medications from ≥3 drug classes, or the need to use antihypertensive medications from ≥4 drug classes to achieve the BP goal. 5 The etiology of resistant hypertension is believed to be multifactorial and to include obesity, increased renal sodium retention, and increased activity in the renin‐angiotensin‐aldosterone and sympathetic nervous systems. 6 The term “apparent resistant hypertension” (ARH) is used in the absence of confirmatory out‐of‐office BP measurements or in the absence of ascertained adherence to the antihypertensive drug regimen. 7 , 8 Apparent resistant hypertension is associated with cardiovascular risk factors and with prevalent cardiovascular disease. 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Prospective studies have shown that ARH is also a marker of increased risk for cardiovascular morbidity and mortality across a wide range of patient populations. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 In 2018, the American College of Cardiology/American Heart Association guidelines lowered both the diagnostic threshold and the BP treatment goal for hypertension from 140/90 mmHg to 130/80 mmHg. 24 Expectedly, the prevalence of ARH increases when the lower threshold is applied, 8 but the long‐term prognostic impact of ARH diagnosed with the lower blood pressure threshold has not been investigated in a primary preventive setting. The cardiovascular prognosis in patients who change hypertension categories if the more stringent BP criteria are applied is also not known. The aim of this analysis of a prospective observational study was to assess the prevalence and prognostic significance of ARH, diagnosed with either the traditional (BP≥140/90 mmHg) or the more stringent (BP≥130/80 mmHg) criteria, in a community‐based cohort of persons without known cardiovascular disease. We also explored the cardiovascular outcomes in participants who changed hypertension categories if the hypertension criteria were changed, and the prognostic significance of the number of antihypertensive drug classes used.
2. METHODS
2.1. Participants
The Atherosclerosis Risk in Communities (ARIC) study is an observational cohort study which recruited 15 792 mostly Caucasian and African American men and women aged 45‐64 years from four US communities (Forsyth County, NC; Jackson, MS; Minneapolis, MN and Washington County, MD). 25 The study protocol was approved by the institutional review boards of all participating centers. All participants provided written informed consent. Recruitment and enrollment (visit 1) took place between 1987 and 1989, and participants were thereafter followed up prospectively on triennial follow‐up visits through 1996‐1998. In this analysis, the third follow‐up visit (visit 4), which took place between 1996 and 1998 and was completed by 11 656 participants, was used as baseline. We first excluded participants with a prevalent or prior diagnosis of (or missing data for) coronary heart disease (n = 699) and/or stroke (n = 298) and/or heart failure (n = 846), participants who were enrolled in the randomized trial ALLHAT (Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial) (n = 56) or who had missing data (n = 15) for baseline BP (Figure 1). We thereafter excluded 401 participants (Figure 1) who had incomplete baseline data for one or more of the following variables: BMI (body mass index), heart rate, smoking status, eGFR (estimated glomerular filtration rate), LDL (low‐density lipoprotein), and HDL (high‐density lipoprotein) cholesterol, TG (triglycerides), or prevalent diabetes status (defined as fasting plasma glucose ≥126 mg/dl, non‐fasting glucose ≥200 mg/dl, self‐reported use of diabetes medications, or self‐reported physician diagnosis of diabetes), and those who were of a race other than Black or White or who were non‐White participants at the Minneapolis or Washington County Centers. This yielded a study cohort of 9612 participants.
FIGURE 1.
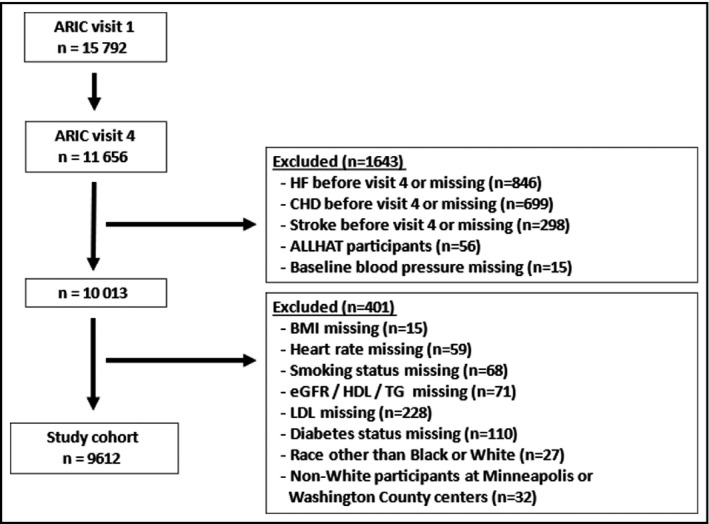
Study flowchart. ARIC, atherosclerosis risk in communities; ALLHAT, antihypertensive and lipid lowering treatment to prevent heart attack trial; BMI, body mass index; CHD, coronary heart disease; eGFR; estimated glomerular filtration rate; HDL, high density lipoprotein; HF, heart failure; MI, myocardial infarction; LDL, low density lipoprotein; TG, triglycerides
2.2. Antihypertensive drugs
The following drug classes were identified as antihypertensives: angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), calcium channel blockers, diuretics (thiazides/thiazide‐like diuretics/loop diuretics/potassium‐sparing diuretics), beta‐adrenergic blockers, mineralocorticoid receptor antagonists (spironolactone), alpha‐adrenergic blockers, centrally acting sympatholytics (clonidine/reserpine/methyl‐dopa/guanfacine), and vasodilators (hydralazine/minoxidil). Single‐pill combinations were categorized into their component classes. The number of antihypertensive drug classes were summed for each participant.
2.3. Hypertension categories
Baseline BP was measured in the sitting position. The BP values reported represent means of two measurements performed at the same visit. For each of the two BP goals (<140/90 mmHg and <130/80 mmHg, respectively), participants were classified as having either no hypertension (BP below goal and no prescribed antihypertensives), controlled hypertension (BP below goal with use of antihypertensives from one, two, or three drug classes), untreated hypertension (BP above goal and no antihypertensives), uncontrolled hypertension (BP above goal despite use of antihypertensives from one or two drug classes), or ARH (BP above goal despite use of antihypertensives from ≥3 drug classes or any BP with use of antihypertensives from ≥4 drug classes, one of which was required to be a diuretic).
2.4. End points
Participants were followed until the occurrence of a composite end point (first hospitalization for incident stroke or heart failure, or hospitalization for myocardial infarction, or death of any cause) or to December 31, 2019 (except in Jackson, where end point information was available only throughout 2017). The ARIC end point definitions and adjudication procedures have been described previously. 26 , 27 , 28
2.5. Statistics
Descriptive data are presented as mean ± SD (standard deviation) for continuous variables. Trends for differences across hypertension groups were tested for statistical significance with linear regression for continuous variables, and with a chi‐squared test for trend for categorical variables. Using participants with controlled hypertension as the reference group, Cox regression models were used to evaluate the impact of the different hypertension categories on time to first occurrence of the composite end point. Each of the components of the composite end point were also explored separately. For each hypertension category, the unadjusted HR (hazard ratio) with its 95% CI (confidence interval) was estimated, as well as the adjusted hazard ratio (aHR). Adjustments were made for the following baseline variables: age, sex, race, and study center, BMI, heart rate, current smoking, diabetes, and levels of eGFR, LDL, HDL, and TG. Cox regression models were also used to explore the prognostic impact of the number of prescribed antihypertensive drugs. P values <0.05 were considered as statistically significant. Statistical analyses were performed in STATA, version 14 (College Station, TX).
3. RESULTS
3.1. Baseline characteristics
Baseline hypertension categories and characteristics are shown in Table 1 and in Table 2. Regardless of whether the BP goal was <140/90 mmHg or <130/80 mmHg, participants with ARH were less likely to be current smokers, more likely to be Black and to have diabetes and were on average older, with lower levels of eGFR and HDL and higher levels of TG. Overall, 3473/9612 participants (36.1%) used ≥1 antihypertensive drug. The four most frequently used antihypertensive drug classes were diuretics, (n = 1507, 43.4% of the treated), followed by calcium channel blockers (n = 1025, 29.5% of the treated), ACE inhibitors or angiotensin receptor blockers (n = 1013, 29.2% of the treated), and beta‐adrenergic blockers (n = 1008, 29.0% of the treated). There were 2096 participants (60.4% of the treated) who used antihypertensive medications from only one drug class, 1075 participants (31.0% of the treated) who used antihypertensive medications from two drug classes, and 302 participants (8.7% of the treated) who used antihypertensive medications from ≥3 drug classes. The drug class most frequently used as monotherapy was diuretics (n = 505 participants, 24.1% of monotherapy participants).
TABLE 1.
Baseline characteristics by HT (hypertension) status. No HT = BP (blood pressure) <140/90 mmHg and no antihypertensives; Controlled HT = BP < 140/90 mmHg with 1‐3 antihypertensives; Untreated HT = BP ≥ 140/90 mmHg and no antihypertensives; Uncontrolled HT = BP ≥ 140/90 mmHg with 1‐2 antihypertensives; Resistant HT = BP ≥ 140/90 mmHg with ≥3 antihypertensives or ≥4 antihypertensives regardless of BP, one antihypertensive required to be a diuretic
|
No HT n = 5029 |
Controlled HT n = 2306 |
Untreated HT n = 1110 |
Uncontrolled HT n = 1034 |
Resistant HT n = 133 |
P (trend) | |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | 118 ± 12 | 122 ± 11 | 152 ± 12 | 154 ± 14 | 151 ± 20 | NA |
| Diastolic BP (mmHg) | 68 ± 9 | 69 ± 9 | 79 ± 10 | 78 ± 11 | 75 ± 14 | NA |
| Pulse pressure (mmHg) | 49 ± 11 | 52 ± 11 | 73 ± 15 | 75 ± 15 | 76 ± 19 | NA |
| Heart rate (bpm) | 62 ± 9 | 63 ± 11 | 64 ± 10 | 63 ± 11 | 64 ± 13 | <0.001 |
| Age (years) | 61.7 ± 5.5 | 63.0 ± 5.6 | 63.6 ± 5.6 | 64.6 ± 5.7 | 65.2 ± 5.8 | <0.001 |
| Female sex, n (%) | 2845 (56.6%) | 1329 (57.6%) | 653 (58.8%) | 645 (62.4%) | 77 (57.9%) | 0.001 |
| Black race, n (%) | 743 (14.8%) | 595 (25.8%) | 309 (27.8%) | 321 (31.0%) | 52 (39.1%) | <0.001 |
| BMI (kg/m2) | 27.5 ± 4.9 | 30.0 ± 5.8 | 28.8 ± 5.8 | 29.9 ± 5.8 | 32.6 ± 5.8 | <0.001 |
| Current smoker, n (%) | 815 (16.2%) | 288 (12.5%) | 177 (15.9%) | 120 (11.6%) | 7 (5.3%) | <0.001 |
| Diabetes mellitus, n (%) | 461 (9.2%) | 485 (21.0%) | 138 (12.4%) | 248 (24.0%) | 52 (39.1%) | <0.001 |
| eGFR (ml/min/1.73 m2) | 83 ± 16 | 81 ± 19 | 85 ± 18 | 81 ± 20 | 75 ± 21 | <0.001 |
| LDL (mg/dl) | 124 ± 33 | 120 ± 33 | 126 ± 34 | 125 ± 34 | 117 ± 33 | 0.93 |
| HDL (mg/dl) | 52 ± 17 | 49 ± 16 | 53 ± 18 | 50 ± 16 | 49 ± 16 | 0.06 |
| TG (mg/dl) | 131 ± 65 | 143 ± 67 | 130 ± 62 | 148 ± 72 | 155 ± 77 | <0.001 |
| Diuretic, n (%) | 1035 (44.9%) | 339 (32.8%) | 133 (100.0%) | NA | ||
| CCB, n (%) | 630 (27.3%) | 317 (30.7%) | 78 (58.6%) | NA | ||
| BB, n (%) | 632 (27.4%) | 305 (29.5%) | 71 (53.4%) | NA | ||
| AB, n (%) | 252 (10.9%) | 104 (10.1%) | 30 (22.6%) | NA | ||
| ACEi, n (%) | 589 (25.5%) | 280 (27.1%) | 76 (57.1%) | NA | ||
| Central sympatholytics, n (%) | 96 (4.2%) | 58 (5.6%) | 28 (21.1%) | NA | ||
| ARB, n (%) | 45 (2.0%) | 14 (1.4%) | 12 (9.0%) | NA | ||
| Spironolactone, n (%) | 36 (1.6%) | 4 (0.4%) | 3 (2.3%) | NA | ||
| Vasodilators, n (%) | 17 (0.7%) | 4 (0.4%) | 15 (11.3%) | NA |
Abbreviations: AB, alpha adrenergic blockers; ACEi, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BB, beta‐adrenergic blockers; BMI, body mass index; BPM, beats per minute; CCB, calcium channel blockers, eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; TG, triglycerides.
TABLE 2.
Baseline characteristics by HT (hypertension) status. No HT = BP (blood pressure) <130/80 mmHg and no antihypertensive drugs; Controlled HT = BP < 130/80 mmHg with 1‐3 antihypertensives; Untreated HT = BP ≥ 130/80 mmHg and no antihypertensives; Uncontrolled HT = BP ≥ 130/80 mmHg with 1‐2 antihypertensives; Resistant HT = BP ≥ 130/80 mmHg with ≥3 antihypertensives or ≥4 antihypertensives regardless of BP, one antihypertensive required to be a diuretic
|
No HT n = 3857 |
Controlled HT n = 1521 |
Untreated HT n = 2282 |
Uncontrolled HT n = 1759 |
Resistant HT n = 193 |
P (trend) | |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | 114 ± 10 | 116 ± 9 | 141 ± 14 | 145 ± 15 | 145 ± 19 | NA |
| Diastolic BP (mmHg) | 66 ± 7 | 67 ± 8 | 77 ± 9 | 77 ± 10 | 75 ± 13 | NA |
| Pulse pressure (mmHg) | 47 ± 9 | 50 ± 10 | 64 ± 16 | 68 ± 16 | 70 ± 19 | NA |
| Heart rate (bpm) | 62 ± 9 | 62 ± 10 | 64 ± 10 | 63 ± 11 | 63 ± 13 | <0.001 |
| Age (years) | 61.6 ± 5.4 | 62.8 ± 5.6 | 62.9 ± 5.7 | 64.1 ± 5.6 | 64.5 ± 5.9 | <0.001 |
| Female sex, n (%) | 2223 (57.6%) | 879 (57.8%) | 1275 (55.9%) | 1058 (60.1%) | 114 (59.1%) | 0.36 |
| Black race, n (%) | 511 (13.2%) | 345 (22.7%) | 541 (23.7%) | 549 (31.2%) | 74 (38.3%) | <0.001 |
| BMI (kg/m2) | 27.2 ± 4.8 | 29.8 ± 5.7 | 28.7 ± 5.5 | 30.1 ± 5.8 | 32.5 ± 6.1 | <0.001 |
| Current smoker, n (%) | 666 (17.3%) | 214 (14.1%) | 326 (14.3%) | 190 (10.8%) | 11 (5.7%) | <0.001 |
| Diabetes mellitus, n (%) | 314 (8.1%) | 320 (21.0%) | 285 (12.5%) | 402 (22.9%) | 63 (32.6%) | <0.001 |
| eGFR (ml/min/1.73m2) | 83 ± 16 | 81 ± 20 | 84 ± 17 | 82 ± 20 | 76 ± 21 | 0.001 |
| LDL (mg/dl) | 123 ± 33 | 119 ± 32 | 126 ± 34 | 124 ± 34 | 117 ± 33 | 0.21 |
| HDL (mg/dl) | 52 ± 17 | 49 ± 16 | 52 ± 17 | 50 ± 16 | 48 ± 15 | <0.001 |
| TG (mg/dl) | 129 ± 65 | 143 ± 67 | 133 ± 64 | 145 ± 70 | 153 ± 71 | <0.001 |
| Diuretic, n (%) | 695 (45.7%) | 619 (35.2%) | 193 (100.0%) | NA | ||
| CCB, n (%) | 366 (24.1%) | 541 (30.8%) | 118 (61.1%) | NA | ||
| BB, n (%) | 451 (29.7%) | 458 (26.0%) | 99 (51.3%) | NA | ||
| AB, n (%) | 177 (11.6%) | 171 (9.7%) | 38 (19.7%) | NA | ||
| ACEi, n (%) | 366 (24.1%) | 474 (26.9%) | 105 (54.4%) | NA | ||
| Central sympatholytics, n (%) | 60 (3.9%) | 85 (4.8%) | 37 (19.2%) | NA | ||
| ARB, n (%) | 30 (2.0%) | 28 (1.6%) | 13 (6.7%) | NA | ||
| Spironolactone, n (%) | 28 (1.8%) | 11 (0.6%) | 4 (2.1%) | NA | ||
| Vasodilators, n (%) | 7 (0.5%) | 10 (0.6%) | 19 (9.8%) | NA |
Abbreviations: AB, alpha adrenergic blockers; ACEi, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BB, beta‐adrenergic blockers; BMI, body mass index; BPM, beats per minute; CCB, calcium channel blockers, eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; TG, triglycerides.
3.2. Untreated participants
There were 6139 (63.9%) participants who did not use antihypertensive medications. Among them, the majority (n = 5029, 81.9% of the untreated) had BP <140/90 mmHg (no hypertension), and the remaining 1110 participants (18.1% of the untreated) had BP ≥140/90 mmHg (untreated hypertension). If the more stringent hypertension criteria ≥130/80 mmHg were instead applied, 1172 participants (19.1% of the untreated) were reclassified from no hypertension to untreated hypertension.
3.3. Treated participants
There were 3473 participants (36.1%) who used ≥1 antihypertensive medication at baseline. Applying the traditional blood pressure goal <140/90 mmHg, the number of participants with controlled hypertension was 2306 (66.4% of the treated), 1034 participants (29.8% of the treated) had uncontrolled hypertension and 133 participants (3.8% of the treated) fulfilled the criteria for ARH. If the more stringent blood pressure goal <130/80 mmHg was instead applied, 785 participants (22.6% of the treated) were reclassified from controlled hypertension to either uncontrolled hypertension (n = 725, 20.9% of the treated) or to ARH (n = 60, 1.7% of the treated) so that the number of participants with controlled hypertension decreased to 1521 (43.8% of the treated), the number of participants with uncontrolled hypertension increased to 1759 (50.6% of the treated) and the number of participants with ARH increased to 193 (5.6% of the treated).
3.4. Outcomes by hypertension categories
The cumulative incidence of the composite outcome is shown in Figure 2 by hypertension categories. Median follow‐up time was 19 [quartile one: 12; quartile three: 22] years. Compared with patients with controlled hypertension, patients with no hypertension had significantly lower risk of experiencing the composite outcome (Table 3), regardless of BP goal (aHR 0.77, 95% CI: 0.71‐0.82 when hypertension control was defined as BP<140/90 mmHg, and aHR 0.74, 95% CI: 0.68‐0.80 when hypertension control was defined as BP<130/80 mmHg). The incidence rates for the composite end point were progressively higher from untreated hypertension to uncontrolled and to ARH, regardless of BP goal (Table 3). Compared with controlled hypertension, ARH was associated with significantly higher risk for the composite end point, regardless of whether hypertension control was defined as BP <140/90 mmHg (aHR 1.50, 95% CI: 1.23‐1.82) or as BP <130/80 mmHg (aHR 1.43, 95% CI: 1.20‐1.70). Despite similar between‐group BP levels, ARH was associated with a significantly higher risk compared also with uncontrolled hypertension (aHR 1.31, 95% CI: 1.07‐1.60, P = .010 with BP goal<140/90 mmHg, aHR 1.31, 95% CI: 1.11‐1.56, P =.002 with BP goal<130/80 mmHg). Incidence rates and hazard ratios for the different hypertension categories are presented for the individual outcomes in Tables S1‐S4.
FIGURE 2.
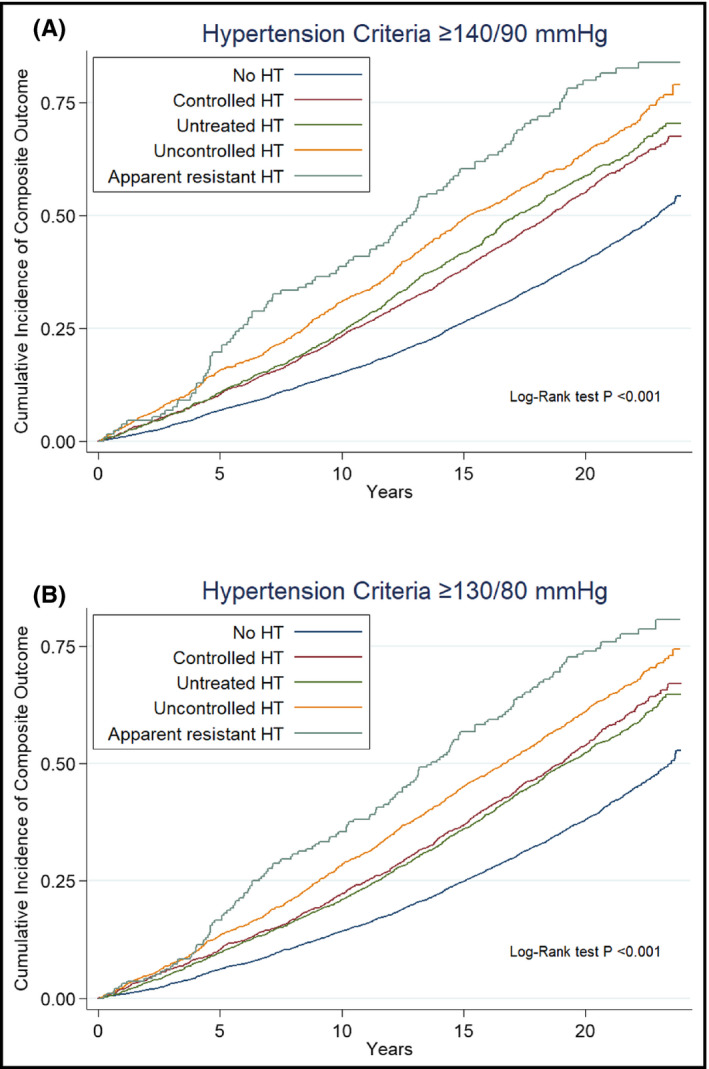
Cumulative incidence of the primary composite outcome by hypertension categories. Hypertension (HT) defined by (A) traditional criteria (BP ≥ 140/90 mmHg) or by (B) more stringent criteria (BP ≥ 130/80 mmHg). Composite outcome: first hospitalization for either incident myocardial infarction or stroke or heart failure, or death.
TABLE 3.
Outcomes (first hospitalization for incident myocardial infarction or stroke or heart failure, or death) by hypertension (HT) categories
| Traditional BP goal (<140/90 mmHg) |
Events Event rate |
Unadjusted HR (95% CI) P value |
Adjusted a HR (95% CI) P value |
|---|---|---|---|
|
No HT n = 5029 |
2328 events 26.3/1000 py |
0.64 (0.60‐0.69) P < .001 |
0.77 (0.71‐0.82) P < .001 |
|
Controlled HT n = 2306 |
1401 events 38.7/1000 py |
Ref. |
Ref. |
|
Untreated HT n = 1110 |
706 events 41.7/1000 py |
1.09 (1.00‐1.19) P = .06 |
1.06 (0.97‐1.16) P = .23 |
|
Uncontrolled HT n = 1034 |
715 events 48.8/1000 py |
1.31 (1.19‐1.43) P < .001 |
1.14 (1.05‐1.25) P = .004 |
|
Apparent resistant HT n = 133 |
108 events 65.3/1000 py |
1.84 (1.51‐2.23) P < .001 |
1.50 (1.23‐1.82) P < .001 |
|
Stricter BP goal (<130/80 mmHg) |
|||
|
No HT n = 3857 |
1715 events 24.9/1000 py |
0.62 (0.57‐0.68) P < .001 |
0.74 (0.68‐0.80) P < .001 |
|
Controlled HT n = 1521 |
914 events 38.0/1000 py |
Ref. | Ref. |
|
Untreated HT n = 2282 |
1319 events 36.0/1000 py |
0.94 (0.87‐1.02) P = .16 |
0.96 (0.88‐1.04) P = .33 |
|
Uncontrolled HT n = 1759 |
1163 events 45.0/1000 py |
1.22 (1.12‐1.33) P < .001 |
1.09 (1.00‐1.19) P = .06 |
|
Apparent resistant HT n = 193 |
147 events 58.2/1000 py |
1.65 (1.39‐1.97) P < .001 |
1.43 (1.20‐1.70) P < .001 |
Adjusted for baseline age, sex, race, and study center, BMI, heart rate, current smoking, eGFR, LDL, HDL, TG, and diabetes status.
3.5. Outcomes by reclassification of hypertension categories
The 1172 participants who were reclassified from no hypertension to untreated hypertension if the diagnostic threshold was changed from 140/90 mmHg to 130/80 mmHg were at significantly higher risk for the primary composite outcome than the 3857 participants who were not reclassified (HR 1.28, 95% CI: 1.17‐1.41, P < .001; aHR 1.16, 95% CI: 1.05‐1.27, P = .002). However, those 785 participants who were reclassified from controlled to uncontrolled hypertension or to ARH were not at significantly higher risk than the 1521 participants who were not reclassified (HR 1.08, 95% CI: 0.97‐1.20, P = .18; aHR 1.03, 95% CI: 0.92‐1.15, P = .61).
3.6. Outcomes by number of antihypertensive
The cumulative incidence of the composite outcome is shown in Figure 3 by numbers of antihypertensive drug classes. Among the 2259 participants with BP ≥140/90 mmHg and among the 4224 participants with BP ≥130/80 mmHg, the incidence rates of the composite end point increased with increasing numbers of antihypertensive drug classes (Table S5). Compared with treatment with no antihypertensive drugs, the aHR for treatment with ≥3 antihypertensive drug classes was 1.50, 95% CI: 1.21‐1.85 among participants with BP ≥140/90 mmHg, and 1.62, 95% CI: 1.36‐1.92 among participants with BP ≥130/80 mmHg. Among the 7353 participants with BP <140/90 mmHg and among the 5388 participants with BP<130/80 mmHg, the incidence rates of the composite end point also increased with increasing number of antihypertensive drug classes (Table S6). Compared with untreated normotensive participants, the aHR for treatment with ≥3 antihypertensive drug classes was 1.73, 95% CI: 1.44‐2.08 among participants with BP <140/90 mmHg and 1.71, 95% CI: 1.36‐2.17 among participants with BP<130/80 mmHg.
FIGURE 3.
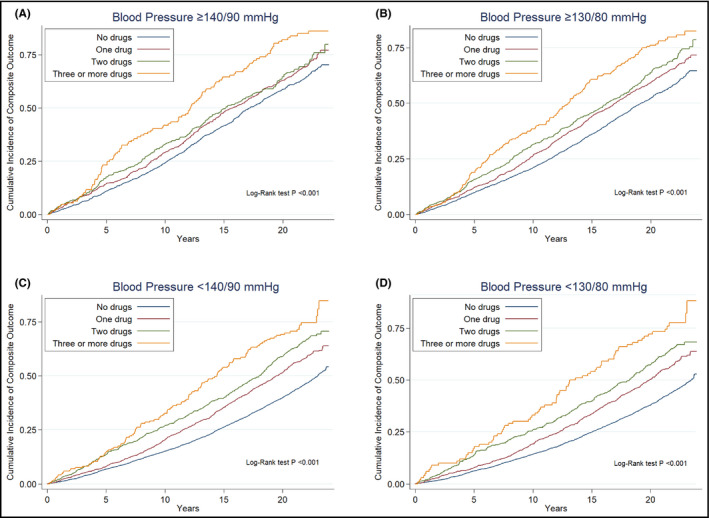
Cumulative incidence of the primary composite outcome by numbers of antihypertensive drug classes in patients with BP ≥ 140/90 mmHg (A), BP ≥ 130/80 mmHg (B), BP < 140/90 mmHg (C), and BP < 130/80 mmHg (D). Composite outcome: first hospitalization for incident myocardial infarction or stroke or heart failure, or death.
4. DISCUSSION
In ARIC participants without prior cardiovascular diseases, long‐term risks for cardiovascular morbidity and all‐cause mortality were significantly higher in participants with ARH at baseline than in participants with controlled hypertension at baseline, regardless of whether the traditional or the stringent BP criteria were applied. Long‐term risk was significantly higher in participants with ARH also when compared with participants with uncontrolled but non‐resistant hypertension, despite similar BP levels.
4.1. Prevalence of ARH
In the ARIC cohort, the prevalence of ARH was lower than in other population‐based cohorts. 7 , 8 , 10 The reason for this discrepancy remains unclear, but we believe that exclusion of participants with known cardiovascular diseases, and differences in demographic characteristics and prescription patterns, contributed. Less than ten percent of the treated participants in the present cohort used antihypertensive drugs from ≥3 drug classes at baseline. If the more stringent BP criteria were applied instead of the traditional BP criteria, almost one in four treated participants were reclassified from controlled to uncontrolled hypertension or to ARH. However, we did not observe a significantly increased risk in these participants. In contrast, untreated participants who were reclassified from no hypertension to untreated hypertension if the BP criteria were lowered were at higher risk than untreated participants whose hypertension categories did not change. This may imply that for the purpose of long‐term risk prediction, utilization of the more stringent blood pressure criteria is of larger importance in untreated than in treated people. Several markers of cardiovascular risk were elevated in participants with ARH, but smoking was less common, an apparently paradoxical association reported also from other cohorts. 29 , 30
4.2. Prognostic significance of resistance to antihypertensive treatment
Increased cardiovascular risk in patients with ARH has been described in several cohorts of patients with and without cardiovascular comorbiditites, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 with the exception of patients with heart failure. 31 Novel findings in the present study were that the prognostic significance of ARH, diagnosed on a single occasion, was retained during a median follow‐up time of almost 20 years. To the best of our knowledge, this is one of the longest follow‐up times reported for outcomes in patients with ARH. Furthermore, we found increasing incidence rates of the composite outcome with use of more antihypertensive drugs, both in patients with uncontrolled and controlled BP. This suggests that other factors besides BP goal achievement contribute to the increased risk associated with resistance to antihypertensive treatment. The number of antihypertensives required to achieve a certain BP level may be a marker for more advanced vascular and metabolic disease burden which makes BP control harder to achieve and increases cardiovascular risk. Indeed, ARIC participants who were strictly normotensive (SBP<130 mmHg) on repeated measurement occasions have been shown to have higher levels of circulating cardiac biomarkers, impaired cardiac structure and function evaluated with echocardiography, and higher prevalence of comorbidities if one or more antihypertensive drugs were used than if no antihypertensive drugs were used, and to have a higher risk of incident heart failure. 32 Resistant hypertension has been associated with endothelial dysfunction, increased arterial stiffness, 33 elevated plasma levels of aldosterone 34 and inflammatory biomarkers, 35 and with a high prevalence of obstructive sleep apnea. 36 Undiagnosed causes of secondary hypertension, such as primary hyperaldosteronism, Cushing's syndrome, or atherosclerotic renovascular disease, may also be more prevalent among participants with ARH, possibly contributing to their increased risk.
4.3. Prognostic significance of BP control
Compared with participants with untreated hypertension, we did not observe significantly lower risk in participants with controlled hypertension. This finding should not be interpreted as evidence against initiation of antihypertensive drug treatment in primary preventive patients. Elevated BP at a single occasion is not sufficient to establish a definitive diagnosis of hypertension in a previously untreated patient. 24 Furthermore, ARIC participants were recommended to consult their physician if the BP was found to be elevated. Therefore, participants with untreated hypertension at baseline may have received treatment during follow‐up. Likewise, some participants with controlled hypertension at baseline may have had uncontrolled hypertension during follow‐up. This might have attenuated any prognostic impact of their baseline hypertension status.
4.4. Strengths and limitations
Strengths of this study were the community‐based cohort, the focus on participants without known cardiovascular disease likely to be representative of patients seen in general clinical practice aimed at primary prevention, and the long follow‐up period. The large cohort also made it possible to compare outcomes across the entire range of BP treatment phenotypes, from untreated participants with or without elevated BP to treated participants with controlled or uncontrolled hypertension, or ARH. However, several factors related to so‐called pseudo‐resistant hypertension, such as information about medication doses and adherence, were not assessed in the ARIC study and these represent important study limitations. Sub‐optimal dosing of antihypertensive drugs is common in patients with ARH. 37 Poor adherence is also common and is even more prevalent when assessed objectively with biochemical methods than when assessed with questionnaires or through pharmacy dispensing databases. 38 , 39 , 40 Another important limitation is that BP measurements were performed only at baseline and only on one visit. Fluctuations in BP values during follow‐up are likely to have occurred, but were not accounted for in this analysis. Furthermore, BP control was assessed by office BP measurements and was not confirmed by home or 24‐hour ambulatory BP measurements, which represent the gold standard, and no adjustments were made for changes in BP levels or treatment during follow‐up. Baseline investigations took place prior to the questioning of beta‐adrenergic blockers as first‐line antihypertensives, 41 and before the beneficial effects of spironolactone had been demonstrated in patients with resistant hypertension. 42 , 43 Recent guidelines also recommend initiation of antihypertensive drug therapy with two drug classes for many patients. 24 Therefore, the clinical care and the prescription pattern of antihypertensive drugs at baseline may not entirely mirror that seen in clinical practice today, which is a limitation. It is also possible that some drugs classified as antihypertensives were prescribed for other indications than for hypertension (for instance, beta‐adrenergic blockers may have been used for arrhythmias). Finally, since this is an observational study, the results should not be used to guide decisions about optimal blood pressure treatment targets or diagnostic criteria.
5. CONCLUSIONS
In this observational community‐based cohort of people who were free from cardiovascular disease at baseline, long‐term risk was elevated in participants with ARH, regardless of whether the traditional or the more stringent blood pressure criteria were applied. The increased risk was independent of traditional markers of risk. The risk increased also with increasing numbers of antihypertensive medications in patients with blood pressure below either the traditional or the more stringent blood pressure goal. This implies that resistance to antihypertensive medications can be used to identify patients at risk for developing cardiovascular disease and suggests that risk factor modification may be considered in these patients.
CONFLICTS OF INTEREST
MOW has served on advisory boards or lectured for MSD, Lilly, Novo Nordisk, and Sanofi and has organized a professional regional meeting sponsored by Lilly, Rubin Medical, Sanofi, Novartis, and Novo Nordisk. MVBM serves on the advisory board and/or receives speaker fees from Abbott, Bayer, Biolab Sanus, Libbs, and Novo Nordisk. BLC has received consulting fees for Amgen, Biogen, Corvia, Myokardia, and Novartis. AMS reports research support through Brigham and Women's Hospital from Novartis and Philips Ultrasound and consulting fees from Philips Ultrasound and Edwards Lifesciences. PSJ´s employer the University of Glasgow has been paid by AstraZeneca for his time working on the DAPA‐HF and DELIVER trials and by Novartis for his time working on the PARADIGM‐HF and PARAGON‐HF trials, and he reports advisory board or speaker´s fees from AstraZeneca, Novartis, Boehringer Ingelheim and grants from Boehringer Ingelheim. JC reports grants from NIH and NKF and is an advisor to Healthy.io. SDS has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, and Theracos and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer‐Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, and American Regent. SC, KM, and OV report no conflicts of interest.
AUTHOR CONTRIBUTIONS
MOW, MVBM, BLC, SDS, and OV designed the present study and drafted the manuscript. MOW and BLC conducted the statistical analyses. SC, KM, AMS, PSJ, and JC provided data interpretation and meaningful contributions to the revision of the manuscript. All authors read and approved the final version of the manuscript.
Supporting information
Table S1‐S6
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
Wijkman MO, Malachias MVB, Claggett BL, et al. Resistance to antihypertensive treatment and long‐term risk: The Atherosclerosis Risk in Communities study. J Clin Hypertens. 2021;23:1887–1896. 10.1111/jch.14269
Funding information
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). MOW is supported by grants from The Swedish Heart Association, The Swedish Society of Medicine and Region Östergötland, Sweden. AMS is supported by NIH/NHLBI grants R01HL135008, R01HL143224, R01HL150342, R01HL148218, and K24HL152008.
REFERENCES
- 1. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: comparative risk assessment . Lancet Diabetes Endocrinol. 1980;2014(2):634‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD‐RisC) . Long‐term and recent trends in hypertension awareness, treatment, and control in 12 high‐income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394:639‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross‐sectional study. BMJ Open. 2013;3:e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antikainen RL, Moltchanov VA, Chukwuma C Sr, et al. Trends in the prevalence, awareness, treatment and control of hypertension: The WHO MONICA project. Eur J Cardiovasc Prev Rehabil. 2006;13:13‐29. [DOI] [PubMed] [Google Scholar]
- 5. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension:detection, evaluation, and management: a scientific statement from the american heart association. Hypertension. 2018;72:e53‐e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hwang AY, Dietrich E, Pepine CJ, Smith SM. Resistant hypertension: mechanisms and treatment. Curr Hypertens Rep. 2017;19:56. [DOI] [PubMed] [Google Scholar]
- 7. Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment resistant hypertension in the United States. Hypertension. 2019;73:424‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmqvist L, Bostrom KB, Kahan T, et al. Prevalence of treatment‐resistant hypertension and important associated factors‐results from the Swedish primary care cardiovascular database. J Am Soc Hypertens. 2016;10:838‐846. [DOI] [PubMed] [Google Scholar]
- 10. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076‐1080. [DOI] [PubMed] [Google Scholar]
- 11. Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34:1204‐1214. [DOI] [PubMed] [Google Scholar]
- 12. Smith SM, Gong Y, Handberg E, et al. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. J Hypertens. 2014;32:635‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irvin MR, Booth JN, Shimbo D, et al. Apparent treatment‐resistant hypertension and risk for stroke, coronary heart disease, and all‐cause mortality. J Am Soc Hypertens. 2014;8:405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muntner P, Davis BR, Cushman WC, et al. Treatment‐resistant hypertension and the incidence of cardiovascular disease and end‐stage renal disease: Results from the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). Hypertension. 2014;64:1012‐1021. [DOI] [PubMed] [Google Scholar]
- 15. Smith SM, Huo T, Delia Johnson B, et al. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI‐sponsored WISE study. J Am Heart Assoc. 2014;3:e000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hung CY, Wang KY, Wu TJ, et al. Resistant hypertension, patient characteristics, and risk of stroke. PLoS One. 2014;9:e104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsioufis C, Kasiakogias A, Kordalis A, et al. Dynamic resistant hypertension patterns as predictors of cardiovascular morbidity: a 4‐year prospective study. J Hypertens. 2014;32:415‐422. [DOI] [PubMed] [Google Scholar]
- 20. Bangalore S, Fayyad R, Laskey R, et al. Prevalence, predictors, and outcomes in treatment‐resistant hypertension in patients with coronary disease. Am J Med. 2014;127:71–81.e1. [DOI] [PubMed] [Google Scholar]
- 21. Cardoso CRL, Leite NC, Bacan G, Ataide DS, Gorgonio LKC, Salles GF. Prognostic importance of resistant hypertension in patients with type 2 diabetes: the rio de janeiro type 2 diabetes cohort study. Diabetes Care. 2020;43:219‐227. [DOI] [PubMed] [Google Scholar]
- 22. Thomas G, Xie D, Chen HY, et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: Report from the chronic renal insufficiency cohort study. Hypertension. 2016;67:387‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holmqvist L, Bostrom KB, Kahan T, et al. Cardiovascular outcome in treatment‐resistant hypertension: results from the Swedish primary care cardiovascular database (SPCCD). J Hypertens. 2018;36:402‐409. [DOI] [PubMed] [Google Scholar]
- 24. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13‐e115. [DOI] [PubMed] [Google Scholar]
- 25. The ARIC Investigators . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687‐702. [PubMed] [Google Scholar]
- 26. Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861‐867. [DOI] [PubMed] [Google Scholar]
- 27. Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the atherosclerosis risk in communities (ARIC) cohort. Stroke. 1999;30:736‐743. [DOI] [PubMed] [Google Scholar]
- 28. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol. 2008;101:1016‐1022. [DOI] [PubMed] [Google Scholar]
- 29. Liu X, Byrd JB. Cigarette smoking and subtypes of uncontrolled blood pressure among diagnosed hypertensive patients: paradoxical associations and implications. Am J Hypertens. 2017;30:602‐609. [DOI] [PubMed] [Google Scholar]
- 30. Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in americans with diagnosed hypertension. Hypertension. 2008;51:1142‐1148. [DOI] [PubMed] [Google Scholar]
- 31. Jin CN, Liu M, Sun JP, et al. The prevalence and prognosis of resistant hypertension in patients with heart failure. PLoS One. 2014;9:e114958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teramoto K, Nadruz Junior W, Matsushita K, et al. Mid‐ to late‐life time‐averaged cumulative blood pressure and late‐life cardiac structure, function, and heart failure. Hypertension. 2020;76:808‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Figueiredo VN, Yugar‐Toledo JC, Martins LC, et al. Vascular stiffness and endothelial dysfunction: correlations at different levels of blood pressure. Blood Press. 2012;21:31‐38. [DOI] [PubMed] [Google Scholar]
- 34. Gaddam KK, Nishizaka MK, Pratt‐Ubunama MN, et al. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barbaro NR, Fontana V, Modolo R, et al. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press. 2015;24:7‐13. [DOI] [PubMed] [Google Scholar]
- 36. Pratt‐Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453‐459. [DOI] [PubMed] [Google Scholar]
- 37. Egan BM, Zhao Y, Li J, et al. Prevalence of optimal treatment regimens in patients with apparent treatment‐resistant hypertension based on office blood pressure in a community‐based practice network. Hypertension. 2013;62:691‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daugherty SL, Powers JD, Magid DJ, et al. The association between medication adherence and treatment intensification with blood pressure control in resistant hypertension. Hypertension. 2012;60:303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Jager RL, van Maarseveen EM, Bots ML, Blankestijn PJ. Medication adherence in patients with apparent resistant hypertension: findings from the sympathy trial. Br J Clin Pharmacol. 2018;84:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Irvin MR, Shimbo D, Mann DM, et al. Prevalence and correlates of low medication adherence in apparent treatment resistant hypertension. J Clin Hypertens (Greenwich). 2012;14:694‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis. Lancet. 2005;366:1545‐1553. [DOI] [PubMed] [Google Scholar]
- 42. Vaclavik J, Sedlak R, Plachy M, et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double‐blind, placebo‐controlled trial. Hypertension. 2011;57:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 43. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug‐resistant hypertension (PATHWAY‐2): a randomised, double‐blind, crossover trial. Lancet. 2015;386:2059‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S6


