Abstract
Improved understanding of the current burden of hypertension, including awareness, treatment, and control, is needed to guide relevant preventative measures in Nigeria. A systematic search of studies on the epidemiology of hypertension in Nigeria, published on or after January 1990, was conducted. The authors employed random‐effects meta‐analysis on extracted crude hypertension prevalence, and awareness, treatment, and control rates. Using a meta‐regression model, overall hypertension cases in Nigeria in 1995 and 2020 were estimated. Fifty‐three studies (n = 78 949) met our selection criteria. Estimated crude prevalence of pre‐hypertension (120‐139/80‐89 mmHg) in Nigeria was 30.9% (95% confidence interval [CI]: 22.0%‐39.7%), and the crude prevalence of hypertension (≥140/90 mmHg) was 30.6% (95% CI: 27.3%‐34.0%). When adjusted for age, study period, and sample, absolute cases of hypertension increased by 540% among individuals aged ≥20 years from approximately 4.3 million individuals in 1995 (age‐adjusted prevalence 8.6%, 95% CI: 6.5‐10.7) to 27.5 million individuals with hypertension in 2020 (age‐adjusted prevalence 32.5%, 95% CI: 29.8‐35.3). The age‐adjusted prevalence was only significantly higher among men in 1995, with the gap between both sexes considerably narrowed in 2020. Only 29.0% of cases (95% CI: 19.7‐38.3) were aware of their hypertension, 12.0% (95% CI: 2.7‐21.2) were on treatment, and 2.8% (95% CI: 0.1‐5.7) had at‐goal blood pressure in 2020. Our study suggests that hypertension prevalence has substantially increased in Nigeria over the last two decades. Although more persons are aware of their hypertension status, clinical treatment and control rates, however, remain low. These estimates are relevant for clinical care, population, and policy response in Nigeria and across Africa.
Keywords: clinical management, high blood pressure, hypertension, Nigeria, prevalence
From 53 studies covering a population of 78 949 Nigerians, we estimated an age‐adjusted prevalence of hypertension of 8.6% in 1995 representing 4.3 million persons aged ≥20 years. Age‐adjusted prevalence increased to 32.5% (27.5 million individuals) in 2020. Of these, 29.0% of (95% CI: 19.7‐38.3) were aware of their hypertension, 12.0% (95% CI: 2.7‐21.2) were on treatment, and 2.8% (95% CI: 0.1‐5.7) had at‐goal blood pressure. Being the most populous country in Africa, our findings offer insights on the current status of hypertension in Africa, and are highly relevant for international comparisons. Improving hypertension awareness and clinical management is a public health priority.

1. INTRODUCTION
Hypertension (HTN) is a leading risk factor for cardiovascular disease (CVD) worldwide. 1 , 2 Low‐ and middle‐income countries (LMICs), including Nigeria, appear to be worst hit, with relatively higher number of cases and limited awareness, treatment, and control rates, against the trend observed in developed countries. 3 , 4
In Nigeria, HTN is the most frequently diagnosed CVD risk equivalent, with HTN‐related complications accounting for approximately a quarter of emergency admissions in urban hospitals. 5 , 6 The Nigerian population's mean blood pressure is higher than that of populations in Europe and the United States. 7 In prior work, 8 we reported that one in four adult Nigerians is hypertensive and that HTN unawareness is a likely contributor to deaths from CVD in the country. 8 , 9
Though numerous prior studies have provided estimates on the prevalence of HTN in Nigeria, 7 , 9 , 10 , 11 few studies have examined HTN trends over time. These data may be particularly informative in light of the substantial and more recent demographic shifts occurring in the Nigerian population. 3
The goal of this systematic review was to estimate both the prevalence of pre‐HTN and HTN in Nigeria, and the level of awareness, treatment, and control of HTN. Further, we sought to examine for evidence of geographic, urban/rural, and sex‐based differences in these estimates. These data are required to understand the likely trajectory of HTN in the country (useful for regional and global comparisons) and guide relevant country‐wide strategies to address the burden of HTN‐related disease.
2. METHODS
2.1. Search strategy
We conducted a systematic search of four databases—MEDLINE, EMBASE, Global Health, and Africa Journals Online (AJOL)—for studies on the prevalence of HTN in Nigeria. We also searched for studies on cardio‐metabolic risk as we identified from an initial scoping exercise that a high proportion of such studies report on the prevalence of HTN (search terms are shown in Table 1). Unpublished (gray) documents were mainly sourced from Google Scholar and Google searches. Titles and abstracts of studies were reviewed, and full texts of relevant studies were accessed for further screening. The reference lists of accessed full texts were hand‐searched for additional studies. Authors of selected papers were contacted for any missing information.
TABLE 1.
Search terms on hypertension in Nigeria
| No. | Searches |
|---|---|
| 1 | africa/ or africa, sub‐sahara/ or africa, western/ or nigeria/ |
| 2 | exp vital statistics/ |
| 3 | (incidence* or prevalence* or morbidity or mortality).tw. |
| 4 | (disease adj3 burden).tw. |
| 5 | exp “cost of illness”/ |
| 6 | case fatality rate.tw |
| 7 | hospital admissions.tw |
| 8 | Disability adjusted life years.mp. |
| 9 | (initial adj2 burden).tw. |
| 10 | exp risk factors/ |
| 11 | 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 |
| 12 | exp hypertension/ or high blood pressure/ or hypertensive heart disease/ or cardiovascular risks/ or cardio‐metabolic risks |
| 13 | 1 and 11 and 12 |
| 14 | Limit 13 to “1990‐current” |
2.2. Selection criteria
Studies were selected if they were (i) original population (or community)‐based studies reporting on the prevalence of HTN in Nigeria, (ii) published on or after January 1, 1990, (iii) conducted among individuals aged at least 15 years, and (iv) providing estimates on the prevalence, awareness, control, or treatment of HTN in Nigeria. We excluded hospital‐based reports, studies on Nigerians in diaspora, reviews, viewpoints, and commentaries.
2.3. Case definitions
The main outcome measures in this study were (i) prevalence of pre‐HTN, (ii) prevalence of HTN, (iii) awareness of HTN (expressed as percent of HTN cases aware of their status, (iv) treatment of HTN (expressed as percent of HTN cases on antihypertensive medication), and (v) control of HTN (expressed as percent of HTN cases with blood pressure controlled). The American College of Cardiology (ACC) and American Heart Association (AHA) recently published an updated report on the prevention, detection, evaluation, and management of HTN in adults. 12 In this report, stage 1 HTN was defined as a systolic blood pressure (SBP) of 130‐139 mmHg or a diastolic blood pressure (DBP) of 80‐89 mmHg, and stage 2 as SBP of 140 mmHg or more or a DBP of 90 mmHg or more. However, as this classification has not been employed by several epidemiologic studies in Nigeria, we maintained the definition of HTN by the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. 13 , 14 Therefore, we defined HTN as SBP of 140 mmHg or more, a DBP of 90 mmHg or more, taking antihypertensive medication, or having been diagnosed as hypertensive by a physician (corresponding to stage 2 HTN in the 2017 ACC/AHA classification). When available, we also extracted the prevalence of pre‐HTN (defined as SBP of 120‐139 mmHg or a DBP of 80‐89 mmHg). For the awareness of HTN, we defined this as self‐reported prior diagnosis of HTN by a doctor or a certified health worker, excluding women who were diagnosed with HTN during pregnancy. 15 We defined treatment of HTN as self‐reported medication use to lower blood pressure at the time of interview. 15 Control of HTN was defined as SBP below 140 mmHg and DBP below 90 mmHg while currently on antihypertensive medication. 15
2.4. Data extraction
Literature searches and assessment of eligible studies were conducted independently by three reviewers (DA, EOO, and AA), according to the selection criteria to ensure consistency in final selection of studies. Disagreements in study selection were resolved by consensus. Data on the study site, period, design, setting (urban or rural), sample size, and mean age of the population were extracted. These were matched with corresponding data on the number of HTN cases and prevalence of HTN in each study.
2.5. Quality assessment
We adapted a previously used quality assessment criteria for studies on chronic diseases 16 to provide insights on the quality of selected studies. We screened for explicit description of methods, protocols, case ascertainment, and sampling and representativeness of reported estimates within the larger geopolitical zone. We graded studies as high (4‐5), moderate (2‐3), or low quality (0‐1) (see Supplemental Material for details of quality grading).
2.6. Data analysis
We conducted a random‐effects meta‐analysis, using the DerSimonian and Laird Method, 17 on the individual study estimates to generate national and subnational pooled crude estimates of the respective study outcomes in Nigeria. Standard errors were determined from the reported crude estimates and population denominators, assuming a binominal (or Poisson) distribution. We assessed heterogeneity between studies using I‐squared (I 2) statistics. Subgroup analysis was conducted to detect causes of heterogeneity. We investigated publication bias using the Funnel plot and Egger's test. As described in previous studies, 8 , 16 we constructed a meta‐regression epidemiologic model accounting for study sample, year, and mean age to determine age‐adjusted prevalence distribution of HTN by age of the Nigerian population. Model expressed as:
where is the prevalence of hypertension in percentage, α is the constant, β 1 and β 2 are regression coefficients for mean age and study year, and ui represents study‐level variance.
From this model and the age‐adjusted prevalence rates, we estimated the absolute number of adult individuals with HTN in Nigeria at midpoints of the United Nation (UN) population 5‐year age groups for Nigeria for the years 1995 and 2020. 18 All statistical analyses were conducted on STATA (Stata Corp V.14, Texas, USA). The study was conducted in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines. 19 The complete dataset employed in this review is available in the Supplemental Material. Further details on searches, data extraction, and analysis are available on reasonable request from corresponding author.
3. RESULTS
3.1. Search results
Our searches returned 4154 studies—4132 from the four databases (MEDLINE, 1691; EMBASE, 2123; Global Health, 246; and AJOL, 72), and an additional 22 studies identified from Google Scholar, Google searches, and reference lists of relevant studies. After duplicates were removed, 1897 titles were screened for relevance (ie, for evidence of a population‐based study on HTN in Nigeria). On applying the selection criteria, 1709 studies were excluded. We assessed 188 full texts, which were screened explicitly using the selection and quality criteria. Fifty‐three studies were finally selected for both qualitative and quantitative syntheses (Figure 1).
FIGURE 1.

Flowchart of selection of studies
3.2. Study characteristics
The 53 studies were selected across the six geopolitical zones of Nigeria and covering a total population of 78 949 individuals (Table 2). The South‐west and South‐east were represented by 15 studies each, followed by the South‐south with 14 studies. North‐central had three studies, North‐west, three; and North‐east, two. One study was conducted across multiple sites in the country. Twenty‐one studies each were conducted in urban and rural settings, with 13 in mixed urban‐rural settings. We rated 32 studies as high quality, and the remaining 21 rated as moderate quality. Study periods ranged from 1995 to 2017, with most studies (90%) were conducted within a one‐year period. Sample mean age ranged from 23.0 to 71.1 years. Heterogeneity was high across studies, with I‐squared (I 2) estimated at 99.1% (P < .001). The Funnel plot suggests no publication bias, with the Egger's test (P = .309) further confirming no small study effects (Supplemental Material).
TABLE 2.
Characteristics of studies on prevalence of hypertension in Nigeria
| Author | Study period | Location | Geopolitical zone | Study design | Study setting | HTN prevalence % |
|---|---|---|---|---|---|---|
| Abegunde and Owoaje 47 | 2011 | Oyo State | South‐west | Descriptive cross‐sectional study | Mixed | 34.8 |
| Adedoyin et al 48 | 2008 | Ile‐Ife, Osun State | South‐west | Descriptive cross‐sectional study | Semi‐urban | 36.6 |
| Adedoyin et al 49 | 2012 | Maiduguri, Borno State | North‐east | Population‐based cross‐sectional study | Semi‐urban | 25.2 |
| Ahaneku et al 50 | 2011 | Enugu, Enugu State | South‐east | Population‐based cross‐sectional study | Rural | 44.5 |
| Alikor et al 51 | 2013 | Port‐Harcourt, River State | South‐south | Descriptive cross‐sectional study | Rural | 20.2 |
| Amira et al 52 | 2010 | Lagos State | South‐west | Descriptive cross‐sectional study | Urban | 33.0 |
| Amole et al 53 | 2008 | Ogbomoso, Oyo State | South‐west | Descriptive cross‐sectional study | Mixed | 50.5 |
| Asekun‐Olarinmoye et al 54 | 2011 | Osogbo, Osun State | South‐west | Population‐based cross‐sectional study | Rural | 13.2 |
| Cooper et al 55 | 1995 | Ibadan, Oyo State | South‐west | Descriptive cross‐sectional study | Mixed | 14.5 |
| Ejim et al 56 | 2006 | Enugu, Enugu State | South‐east | Population‐based cross‐sectional study | Rural | 46.4 |
| Ekanem et al 57 | 2012 | Abak, Akwa Ibom State | South‐south | Descriptive cross‐sectional study | Semi‐urban | 47.0 |
| Ekwunife et al 58 | 2009 | Nsukka, Enugu State | South‐east | Population‐based cross‐sectional study | Mixed | 21.1 |
| Erhun et al 59 | 2003 | Ile‐Ife, Osun State | South‐west | Descriptive cross‐sectional study | Semi‐urban | 21.0 |
| Hendriks e al 60 | 2011 | Ilorin, Kwara State | North‐central | Population‐based cross‐sectional study | Rural | 21.0 |
| Isezuo et al 61 | 2010 | Sokoto, Sokoto State | North‐west | Population‐based cross‐sectional study | Mixed | 24.8 |
| Kadiri et al 23 | 1998 | Ibadan, Oyo State | South‐west | Descriptive cross‐sectional study | Urban | 9.3 |
| Mbah et al 62 | 2012 | Nsukka, Enugu State | South‐east | Population‐based cross‐sectional study | Semi‐urban | 32.5 |
| Odugbemi et al 63 | 2010 | Tejuosho, Lagos | South‐west | Descriptive cross‐sectional study | Urban | 34.8 |
| Ogah et al 64 | 2012 | Umuahia, Abia State | South‐east | Population‐based cross‐sectional study | Mixed | 31.4 |
| Oghagbon et al 65 | 2007 | Ilorin, Kwara State | North‐central | Population‐based cross‐sectional study | Urban | 27.1 |
| Oladapo et al 66 | 2005 | Egbeda, Oyo State | South‐west | Descriptive cross‐sectional study | Rural | 20.8 |
| Omorogiuwa et al 67 | 2008 | Ekpoma, Edo State | South‐south | Descriptive cross‐sectional study | Urban | 33.0 |
| Omuemu et al 68 | 2004 | Edo State | South‐south | Population‐based cross‐sectional study | Rural | 20.2 |
| Suleiman et al 69 | 2011 | Amassoma, Bayelsa State | South‐south | Descriptive cross‐sectional study | Semi‐urban | 15.0 |
| Ulasi et al 70 | 2008 | Enugu State | South‐east | Population‐based cross‐sectional study | Mixed | 32.8 |
| Ulasi et al 71 | 2010 | Enugu State | South‐east | Population‐based cross‐sectional study | Mixed | 42.2 |
| Agaba et al 72 | 2014 | Jos, Plateau State | North‐central | Descriptive cross‐sectional study | Urban | 48.5 |
| Akinbodewa et al 73 | 2014 | Akure & Ondo, Ondo State | South‐west | Descriptive cross‐sectional study | Mixed | 43.4 |
| Emerole et al 74 | 2007 | Owerri, Imo State | South‐east | Descriptive cross‐sectional study | Urban | 29.1 |
| Ibekwe 75 | 2012 | Oghara, Delta State | South‐south | Descriptive cross‐sectional study | Rural | 21.0 |
| Ige et al 76 | 2013 | Ibadan, Oyo State | South‐west | Descriptive cross‐sectional study | Urban | 21.5 |
| Okaka and Eiya 77 | 2013 | Ovia, Edo state | South‐south | Population‐based cross‐sectional study | Rural | 19.3 |
| Oyeyemi and Adeyemi 78 | 2013 | Maiduguri, Borno State | North‐east | Population‐based cross‐sectional study | Semi‐urban | 23.1 |
| Oguoma et al 79 | 2015 | Kwale, Delta State | South‐south | Population‐based cross‐sectional study | Mixed | 35.5 |
| Ezejimofor et al 80 | 2014 | Niger Delta, Rivers State | South‐south | Community‐based cross‐sectional study | Rural | 51.1 |
| Adebayo et al 81 | 2013 | Ife North, Osun State | South‐west | Population‐based cross‐sectional study | Rural | 26.4 |
| Andy et al 20 | 2012 | Cross River & Akwa Ibom States | South‐south | Population‐based cross‐sectional study | Rural | 23.6 |
| Akpan et al 82 | 2015 | Akwa Ibom State | South‐south | Population‐based cross‐sectional study | Urban | 28.6 |
| Egbi et al 83 | 2013 | Yenagoa, Bayelsa State | South‐south | Population‐based cross‐sectional study | Rural | 21.3 |
| Bello‐Ovosi et al 22 | 2017 | Kawo, Kaduna State | North‐west | Population‐based cross‐sectional study | Urban | 55.9 |
| Chukwuonye et al 84 | 2013 | Abia State | South‐east | Population‐based house‐to‐house survey | Mixed | 40.2 |
| Ekpe et al 85 | 2015 | Adim, Cross River State | South‐south | Population‐based cross‐sectional study | Rural | 19.9 |
| Ezeala‐Adikaibe et al 86 | 2016 | Enugu State | South‐east | Population‐based cross‐sectional study | Urban | 52.5 |
| Ezekwesili et al 21 | 2016 | Anambra State | South‐east | Population‐based cross‐sectional study | Mixed | 22.8 |
| Iloh et al 87 | 2009 | Imo State | South‐east | Descriptive cross‐sectional study | Rural | 16.3 |
| Iloh et al 88 | 2008 | Imo State | South‐east | Descriptive cross‐sectional study | Rural | 18.4 |
| Murthy et al 32 | 2013 | National | National | Population‐based cross‐sectional study | Mixed | 44.9 |
| Ofuya 89 | 2007 | Niger Delta, Rivers State | South‐south | Population‐based cross‐sectional study | Rural | 13.8 |
| Okafor et al 90 | 2014 | Enugu, Enugu State | South‐east | Population‐based cross‐sectional study | Urban | 47.7 |
| Olamoyegun et al 91 | 2016 | Ekiti State | South‐west | Population‐based cross‐sectional study | Semi‐urban | 55.5 |
| Shittu et al 92 | 2016 | Oke‐Ogun, Oyo State | South‐west | Population‐based cross‐sectional study | Semi‐urban | 38.5 |
| Ugwuuja et al 93 | 2015 | Igbeagu, Ebonyi State | South‐east | Population‐based cross‐sectional study | Rural | 23.2 |
| Wahab et al 94 | 2006 | Katsina, Katsina State | North‐west | Population‐based cross‐sectional study | Urban | 16.0 |
3.3. Prevalence of pre‐HTN in Nigeria
The prevalence of pre‐HTN (SBP 120‐139 mmHg or DBP 80‐89 mmHg) reported in studies ranged from 17.2% estimated from two cities in the South‐south in 2012, 20 to 42.5% recorded in Anambra State, South‐east Nigeria, in 2016. 21 The estimated pooled crude prevalence of pre‐HTN in Nigeria was 30.9% (95% CI: 22.0‐39.7) (Figure 2). No sex‐specific estimates were reported.
FIGURE 2.
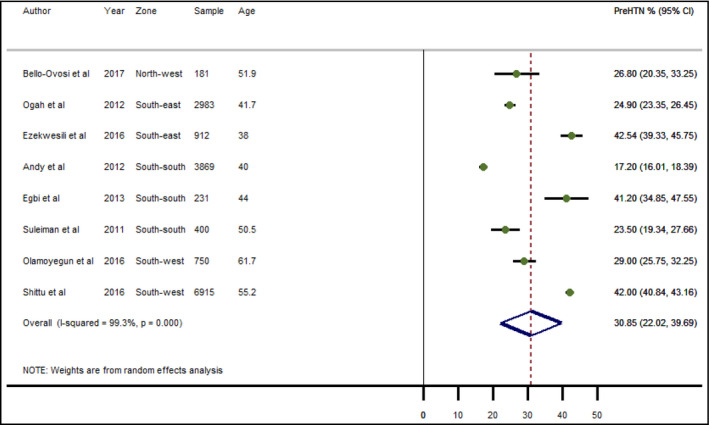
Crude prevalence of pre‐hypertension in Nigeria
3.4. Crude prevalence of HTN in Nigeria
From individual study estimates, the highest prevalence of HTN (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) was recorded in an urban community in Kaduna State, North‐west Nigeria, at 55.9% in 2018, 22 with the lowest prevalence estimated in Ibadan Oyo State, South‐west Nigeria, at 9.3% in 1999. 23 When pooled crude prevalence of HTN across the geopolitical zones was considered, pooled estimates in the four zones were relatively similar and above 30%. The highest prevalence was in the South‐east at 33.3% (95% CI: 27.3‐39.4), closely followed by the North‐central with a prevalence of 32.2% (95% CI: 13.5‐34.0); while the North‐west had 31.9% (95% CI: 14.9‐48.9), and the South‐west had 30.2% (95% CI: 23.6‐36.8) (Figure 3). The North‐east and the South‐south had a pooled HTN prevalence of 24.7% (95% CI: 22.4‐27.1) and 27.6% (95% CI: 21.4‐33.9), respectively. The overall pooled crude prevalence of HTN in Nigeria was 30.6% (95% CI: 27.3‐34.0) (Figure 3). Although no significant difference, the prevalence was slightly higher among women (30.4%, 95% CI: 25.2‐35.6) than among men (29.5%, 95% CI: 25.4‐33.6) (Table 3 and Supplemental Material). Across both sexes, the pooled prevalence was consistently higher among urban dwellers (33.5%, 95% CI: 25.1‐42.0) than among rural dwellers (25.5%, 95% CI: 21.1‐29.9) (Table 3).
FIGURE 3.
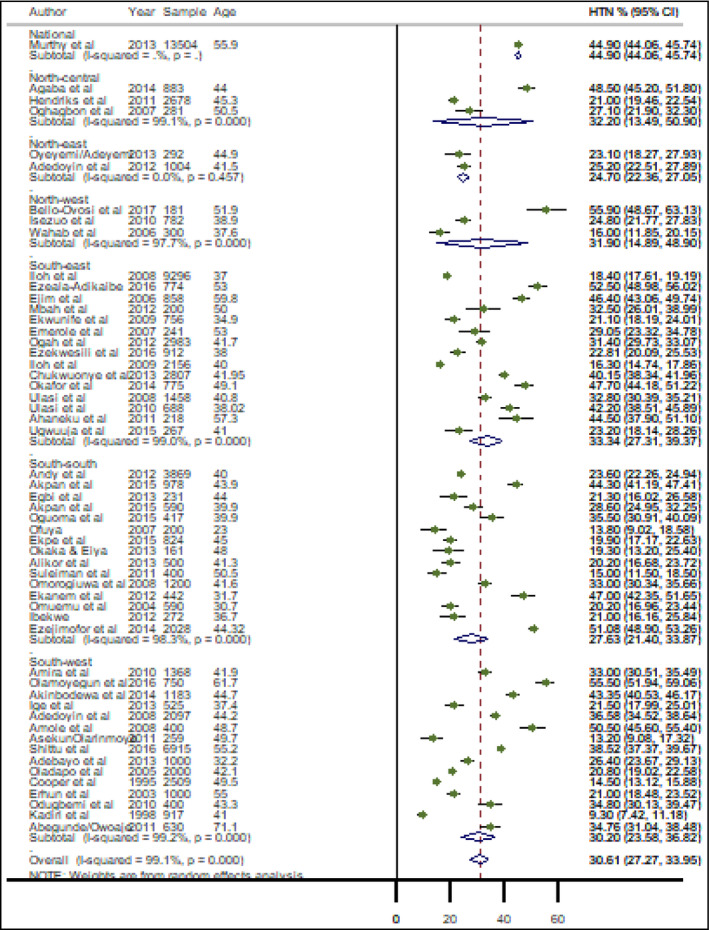
Crude prevalence of hypertension in Nigeria
TABLE 3.
Pooled crude estimates of prevalence of hypertension in Nigeria
| Region | Both sexes | Men | Women | |||
|---|---|---|---|---|---|---|
| Prevalence % (95% CI) | I2, P‐value | Prevalence % (95% CI) | I2, P‐value | Prevalence % (95% CI) | I2, P‐value | |
| Nation‐wide | ||||||
| Hypertension | 30.6 (27.3‐34.0) | 99.1, <.001 | 29.5 (25.4‐33.6) | 98.2, <.001 | 30.4 (25.2‐35.6) | 99.2, <.001 |
| Pre‐hypertension | 30.9 (22.0‐39.6) | 99.3, <.001 | ‐ | ‐ | ‐ | ‐ |
| Awareness a | 29.0 (19.7‐38.3) | 98.9, <.001 | ‐ | ‐ | ‐ | ‐ |
| Treatment a | 12.0 (2.7‐21.2) | 97.9, <.001 | ‐ | ‐ | ‐ | ‐ |
| Control a | 2.8 (0.0‐5.7) | 83.1, .015 | ‐ | ‐ | ‐ | ‐ |
| SBP (mmHg) | 130.9 (128.4‐133.4) | 90.7, <.001 | ‐ | ‐ | ‐ | ‐ |
| DBP (mmHg) | 81.1 (79.5‐82.8) | 95.0, <.001 | ‐ | ‐ | ‐ | ‐ |
| Geopolitical zone | ||||||
| North‐central | 32.2 (13.5‐34.0) | 99.1, <.001 | 35.9 (21.6‐50.2) | 93.3, <.001 | 24.8 (21.3‐28.4) | 97.1, <.001 |
| North‐east | 24.7(22.4‐27.1) | 0.0, .457 | 23.3 (19.5‐27.1) | 28.7. .236 | 24.8 (21.3‐28.4) | 0.0, .867 |
| North‐west | 31.9 (14.9‐48.9) | 97.7, <.001 | 20.1 (7.2‐33.1) | 90.9, <.001 | 34.0 (13.9‐54.1) | 97.2, <.001 |
| South‐east | 33.3 (27.3‐39.4) | 99.0, <.001 | 40.8 (33.7‐48.0) | 95.1, <.001 | 35.8 (29.0‐42.6) | 96.1, <.001 |
| South‐south | 27.6 (21.4‐33.9) | 98.3, <.001 | 24.4 (18.9‐29.8) | 92.2, <.001 | 20.9 (15.6‐26.3) | 94.6, <.001 |
| South‐west | 30.2 (23.6‐36.8) | 99.2, <.001 | 27.3 (17.3‐37.2) | 98.0, <.001 | 30.9 (20.0‐41.8) | 99.4, <.001 |
| Settings | ||||||
| Urban | 33.6 (25.1‐42.0) | 98.8, <.001 | 27.2 (17.3‐37.2) | 97.9, <.001 | 34.5 (23.2‐45.8) | 98.3, <.001 |
| Rural | 25.5 (21.1‐29.9) | 98.6, <.001 | 26.4 (20.6‐32.2) | 94.5, <.001 | 22.9 (17.8‐28.1) | 95.5, <.001 |
| Mixed | 33.7 (26.7‐40.7) | 99.3, <.001 | 35.8 (27.8‐43.7) | 98.6, <.001 | 34.1 (25.0‐43.1) | 99.1, <.001 |
Awareness, treatment, and control rates expressed as percent of HTN cases.
3.5. Pooled mean SBP and DBP in Nigeria
The pooled mean population SBP in Nigeria was 130.9 mmHg (128.4‐133.4) and the pooled mean DBP was 81 mmHg (79.5‐82.8) (Table 3 and Supplemental Material).
3.6. Awareness, treatment, and control of HTN in Nigeria
The pooled HTN awareness rate (expressed as a percentage of all HTN cases) was 29.0% (95% CI: 19.7‐38.3), while 12.0% (95% CI: 2.7‐21.2) were on antihypertensive medications, and 2.8% (95% CI: 0.1‐5.7) of these had at‐goal blood pressure (Figure 4).
FIGURE 4.
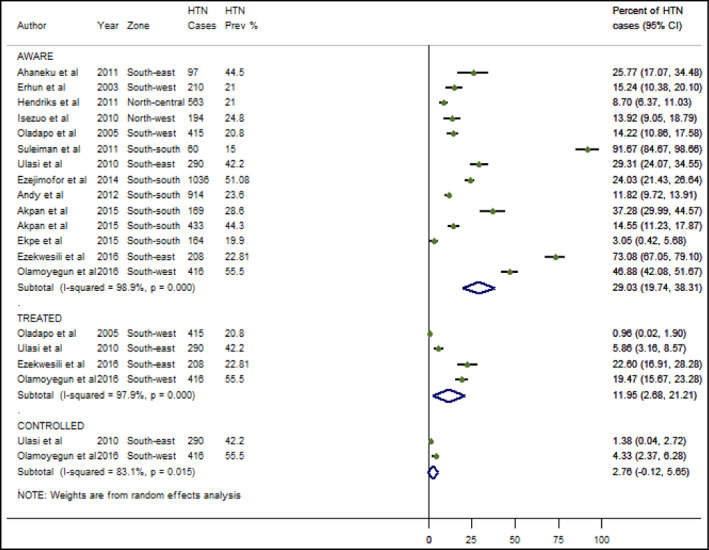
Awareness, treatment, and control of hypertension in Nigeria
3.7. Estimated number of individuals with HTN in Nigeria
The meta‐regression epidemiologic modeling showed that age and study period were statistically significant determinants of HTN prevalence in Nigeria, P < .001 (Supplemental Material). The prevalence of HTN increased significantly with advancing age and year of study. For example, in 1995, the prevalence of HTN at ages 20‐24 and 50‐59 years was 1.0% and 15.6%, respectively, and by 2020, the prevalence rates at these age brackets had increased to 23.5% and 40.0%, respectively (Table 4). Using the United Nations demographic projections for Nigeria, we estimated that approximately 4.3 million individuals over the age of 19 had HTN in Nigeria in 1995 (age‐adjusted prevalence of 8.6%, 95% CI: 6.5‐10.7). This figure increased by 540% to 27.5 million individuals over the age of 19 with HTN in 2020 (age‐adjusted prevalence of 32.5%, 95% CI: 29.8‐35.3) (Table 4). When the sexes were considered, cases increased significantly from 3 million (12.0%, 95% CI: 9.3‐14.8) to 14.2 million (33.5%, 95% CI: 29.3‐37.7) among men, and 1.3 million (5.2%, 95% CI: 2.5‐7.9) to 13.2 million (31.5%, 95% CI: 27.0‐35.9) among women between 1995 and 2020, respectively. The age‐adjusted prevalence was only significantly higher in men in 1995, with the gap in prevalence between both sexes considerably narrowed in 2020.
TABLE 4.
Absolute number of hypertensive individuals in Nigeria (age ≥ 20 years), during 1995 and 2020
| Age (years) | Both sexes | Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | 2020 | 1995 | 2020 | 1995 | 2020 | |||||||
| Prevalence % (SE) | Cases (000) | Prevalence % (SE) | Cases (000) | Prevalence % (SE) | Cases (000) | Prevalence % (SE) | Cases (000) | Prevalence % (SE) | Cases (000) | Prevalence % (SE) | Cases (000) | |
| 20‐24 | 1.0 (0.09) | 95.6 | 23.5 (0.34) | 3752.2 | 1.3 (0.16) | 66.1 | 29.6 (0.51) | 2409.6 | 0.6 (0.11) | 29.4 | 17.1 (0.43) | 1342.6 |
| 25‐29 | 1.8 (0.15) | 139.0 | 26.2 (0.37) | 3686.8 | 2.6 (0.25) | 102.1 | 30.9 (0.55) | 2207.9 | 1.0 (0.16) | 36.9 | 21.4 (0.49) | 1479.0 |
| 30‐34 | 4.5 (0.26) | 299.0 | 29.0 (0.41) | 3509.6 | 7.8 (0.47) | 258.8 | 32.2 (0.60) | 1981.5 | 1.2 (0.19) | 40.2 | 25.7 (0.57) | 1528.1 |
| 35‐39 | 7.3 (0.35) | 404.0 | 31.8 (0.47) | 3170.5 | 12.9 (0.64) | 355.7 | 33.5 (0.66) | 1704.6 | 1.7 (0.25) | 48.2 | 30.0 (0.66) | 1465.8 |
| 40‐44 | 10.1 (0.44) | 463.9 | 34.5 (0.54) | 2681.4 | 15.8 (0.76) | 359.1 | 34.7 (0.75) | 1383.7 | 4.5 (0.43) | 104.9 | 34.3 (0.77) | 1297.7 |
| 45‐49 | 12.8 (0.54) | 499.3 | 37.3 (0.62) | 2240.1 | 18.6 (0.88) | 361.6 | 36.0 (0.89) | 1094.6 | 7.1 (0.58) | 137.7 | 38.6 (0.89) | 1145.5 |
| 50‐54 | 15.6 (0.63) | 519.0 | 40.0 (0.69) | 1999.6 | 21.5 (1.01) | 354.7 | 37.1 (1.01) | 914.3 | 9.8 (0.73) | 164.2 | 42.9 (0.98) | 1085.2 |
| 55‐59 | 18.3 (0.75) | 493.6 | 42.8 (0.77) | 1774.6 | 24.3 (1.18) | 319.5 | 38.1 (1.18) | 760.2 | 12.6 (0.89) | 174.0 | 47.2 (1.07) | 1014.4 |
| 60‐64 | 21.1 (0.89) | 441.2 | 45.6 (0.86) | 1515.3 | 27.2 (1.40) | 273.4 | 38.9 (1.40) | 611.8 | 15.5 (1.09) | 167.8 | 51.5 (1.19) | 903.5 |
| 65‐69 | 23.9 (1.08) | 368.5 | 48.3 (0.99) | 1234.3 | 30.0 (1.69) | 219.8 | 40.1 (1.69) | 486.2 | 18.3 (1.36) | 148.8 | 55.8 (1.36) | 748.1 |
| 70‐74 | 26.6 (1.37) | 274.7 | 51.1 (1.17) | 930.5 | 32.9 (2.15) | 157.4 | 41.0 (2.14) | 351.2 | 21.2 (1.74) | 117.3 | 60.1 (1.58) | 579.3 |
| 75‐79 | 29.4 (1.89) | 170.9 | 53.8 (1.52) | 580.2 | 35.7 (2.97) | 93.2 | 41.4 (2.97) | 203.9 | 24.2 (2.39) | 77.7 | 64.4 (1.98) | 376.4 |
| 80+ | 32.4 (2.49) | 114.5 | 56.9 (1.84) | 410.5 | 38.9 (4.04) | 56.6 | 40.7 (4.04) | 126.4 | 27.9 (3.11) | 58.0 | 69.1 (2.3) | 284.1 |
| All (20+) | 8.6 | 4283.2 | 32.5 | 27 485.6 | 12.0 | 2978.1 | 33.5 | 14 235.8 | 5.2 | 1305.1 | 31.5 | 13 249.8 |
| Lower CI | 6.5 | 3224.6 | 29.8 | 25 154.2 | 9.3 | 2295.9 | 29.3 | 12 449.5 | 2.5 | 638.8 | 27.0 | 11 369.8 |
| Upper CI | 10.7 | 5341.8 | 35.3 | 29 816.9 | 14.8 | 3660.4 | 37.7 | 16 022.0 | 7.9 | 1971.3 | 35.9 | 15 129.8 |
Abbreviations: CI, 95% confidence interval; SE, standard error.
4. DISCUSSION
In this systematic review of 53 studies on the prevalence of HTN in Nigeria, we found strong evidence that HTN has become far more common among Nigerian adults in recent years and that awareness of the condition remains alarmingly low. We also provide the first country‐specific estimates of pre‐HTN for Nigeria, and present strong evidence of geographic heterogeneity in this condition. These results have strong implications for future preventative and educational campaigns.
Our results suggest that between 1995 and 2020, HTN cases in Nigeria increased by over 540% from four million individuals to 28 million individuals. We estimated that the age‐adjusted HTN prevalence in 2020 was 32.5%, a substantially higher number than the 28.0% prevalence we estimated in 2010 in a prior study. 8 Likely contributors to this high and steady increase in HTN include population aging, increased urbanization, unhealthy lifestyles, and the absence of effective nation‐wide preventative measures. Our results lend credence to concerns that HTN and related complications may soon represent the most significant public health and economic threat in many African countries, overshadowing epidemics such as malaria and other infectious diseases. 7 , 24 , 25
Given our pooled mean SBP and DBP estimates of 131 mmHg and 81 mmHg, respectively, our study findings may indicate that many Nigerians are also in the pre‐hypertensive stage. Though pre‐HTN does not connote inevitable progression to HTN, studies had long shown that the presence of pre‐HTN increases the risk for HTN, cardiovascular complications, and target organ damage by 30% in the absence of lifestyle modifications or treatment. 26 , 27 , 28 We estimated that nearly one in three Nigerians is pre‐hypertensive. Our estimate is in the range of pre‐HTN prevalence estimates from Ghana (30.7%) and South Africa (29.4%), but higher than the pooled estimate in four sub‐Saharan African countries that included Nigeria (21.0%). 29 , 30 In contrast, Chow et al 31 estimated that pre‐HTN prevalence was 36.8% from a multicountry study, although this does not include Nigeria. Comparison between prior studies and ours may be challenging due to the differences in the methodology between studies. However, several studies 29 , 30 , 31 including the current have pointed to a high prevalence of pre‐HTN and HTN in Nigeria and neighboring countries, calling for more awareness and education.
We found evidence of substantial regional variation in the prevalence of HTN in Nigeria, which ranged from 25% to 33% across the geopolitical zones. The highest prevalence was in the South‐east and North‐central at 33.3% and 32.2%, respectively. Although the regional pattern of distribution may be subject to further studies, Murthy et al 32 reported high prevalence of HTN in 2013 among the Nupe and Igbo communities in the North‐central and South‐east at 50.5% and 40.4%, respectively. Dietary differences in these regions, particularly in the amount of oil and salt used in food, may be the contributing factor. The significant variations in socio‐economic conditions also have important implications on dietary choices, particularly in urban settings characterized by high consumption of processed foods, without population‐wide strategies promoting healthy diets. 33 , 34 Moreover, varying weather and climatic conditions in Nigeria considerably affect farming and the type of food crops produced, possibly another important factor for the dietary differences. Our findings of a higher prevalence of HTN among urban dwellers and those of advanced age are consistent with findings from numerous previous studies. 25 , 35 , 36 , 37
Our estimates clearly indicate a narrowing prevalence gap between men and women, with a difference of 7% in 1995 and 2% in 2020. In prior work, 8 we reported a 5% difference between the two sexes (30% men vs 25% women) in 2010. Although the current crude prevalence difference in both sexes was not statistically significant, this was slightly higher among women (30.4%) compared with men (29.5%). These findings are consistent with those from a 2013 study of 13 504 Nigerians, in which the prevalence of HTN was higher among women (46.8%) than among men (42.6%). The rising prevalence of HTN among women could be linked to increasing obesity, decreasing physical inactivity and unhealthy diets. 38 , 39 , 40 , 41 Moreover, women appear to suffer worse mental, psychological, and emotional consequences from increasing security challenges in Nigeria, with repeated anxiety and panic attacks likely having adverse effects on the overall cardiovascular health of many. In addition, it is worth noting that women are more likely to participate in community medical outreach efforts, possibly leading to selection bias and relatively higher prevalence reported for females. 8 , 9
Compared with our previous pooled awareness rate (17.4%), 8 HTN status awareness (expressed as a percentage of HTN cases in the country) increased to 29%. This rate is higher than recorded in some African countries (Gabon, Uganda, and Kenya), which ranged from 9% to 12%. 7 , 42 Despite the improvement in awareness of HTN in the country, the treatment and control rates of HTN (also expressed as percentage of HTN cases) were relatively low at 12% and 3%, respectively. These rates are very low compared with treatment and control rates in other African countries such as Zimbabwe and South Africa, which are above 30%. 40 , 41 , 43 Addressing the relatively low awareness, treatment, and control rates of HTN in Nigeria is key to reducing cardiovascular health burden. However, an important consideration are the challenges relating to acquiring and adhering to antihypertensive medications. 9 , 44 Although cardiology training program for medical doctors has relatively improved over the years, and there are ongoing studies and trials on medications, several doctors particularly in primary and secondary health facilities lack the requisite knowledge of the standard management for hypertension. Prescriptions are often too complex, and there is a lack of follow‐up of cases, resulting in both poor adherence and suboptimal control of HTN. 33 , 45
Targeted community‐wide programs, commonly organized by non‐governmental organizations and research groups, are an important avenue for blood pressure screening in Nigeria. 7 , 9 , 10 , 11 The government, ministry of health and other stakeholders can collaborate with these organizations and groups in observing the annual May Measurement Month (MMM) across primary care levels in the country to raise awareness and facilitate blood pressure screening in catchment communities. According to Beaney et al, 46 the MMM is an inexpensive intervention that can be employed to address a shortfall in screening of blood pressures across world regions, with over 35 000 (28%) newly diagnosed cases reported in sub‐Saharan Africa alone in 2017. In addition to initiatives and campaigns such as the MMM, primary health workers who are in regular contact with patients can play active roles in promoting screening and regular assessment of blood pressure for early identification of individuals at risk and timely provision of treatments where necessary.
Our study should be considered with respect to its limitations. Heterogeneity across studies was high, reflecting variations in population structures, blood pressure measurement protocols, and overall study designs. Of the 53 studies retained, only eight were conducted in the Northern regions, and data on age‐ and sex‐specific prevalence, and specific geographic settings, were not always provided. It is not so clear why studies are predominantly lower in the North, but this has been observed in previous studies, 8 , 16 possibly a reflection of the overall research capacity in the region. These factors resulted in some discrepancies in the crude sex estimates, although this was accounted for in the final model. Moreover, our estimates were based on JNC classifications, as there were no available studies based on the 2017 ACC/AHA guidelines. Moreover, we reported relatively high age‐adjusted estimates in the younger age groups in 2020; this should be cautiously interpreted due to sparse overall data for persons under 30 years. Largely, these limitations are balanced by the strengths of our study, including its large sample size, rigorous methodology, and its provision of the first nation‐wide estimates of the treatment and control of HTN in Nigeria.
5. CONCLUSIONS
In summary, the burden of HTN is increasing in Nigeria and our data suggest that many Nigerians are pre‐hypertensive. HTN prevalence appears to be increasing at a faster rate among Nigerian women than among men. Further, though the awareness of HTN has improved over time, more than half of hypertensive individuals in Nigeria are untreated and/or have poorly controlled blood pressure. Our results strongly support a need for improved and comprehensive nation‐wide population preventive strategies to mitigate the effects of HTN in Nigeria.
DISCLOSURE
DBO and MOH declare funding from the National Institutes of Health (NIH). All other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
DA conceived and designed the study. DA, EOO, and AA conducted the literature searches and data extraction. DA, EOO, and MOH wrote the first draft. DA and MOH conducted the analysis. DA, AA, DBO, MTD, TOO, OSO, CO, NE, RGM, EA, MG, WA, AOA, MOH, and other authors contributed to the final draft and checked for important intellectual content. All authors approved the manuscript as submitted.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the support of the Nigeria Federal Ministry of Health, the World Health Organization Country Office for Nigeria, and Resolve to Save Lives (Vital Strategies), Abuja, Nigeria, in the conduct of this study. Special thanks to Funke Davies‐Adeloye for proof‐reading the manuscript.
Adeloye D, Owolabi EO, Ojji DB, et al. Prevalence, awareness, treatment, and control of hypertension in Nigeria in 1995 and 2020: A systematic analysis of current evidence. J Clin Hypertens. 2021;23:963–977. 10.1111/jch.14220
Funding information
None.
REFERENCES
- 1. Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country‐years and 54 million participants. Lancet. 2011;377(9765):568‐577. [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990–2015. JAMA. 2017;317(2):165‐182. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Global brief on hypertension. Geneva: World Health Organization; 2013. [Google Scholar]
- 4. Akinyi H, Oti S, Olajide A, Agyemang C, Aboderin I, Kyobutungi C. Status report on hypertension in Africa–consultative review for the 6th Session of the African Union Conference of Ministers of Health on NCD's. Pan Afr Med J. 2013;16:38‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekere AU, Yellowe BE, Umune S. Mortality patterns in the accident and emergency department of an urban hospital in Nigeria. Niger J Clin Pract. 2005;8(1):14‐18. [PubMed] [Google Scholar]
- 6. Ogunniyi A, Baiyewu O, Gureje O, et al. Morbidity pattern in a sample of elderly Nigerians resident in Idikan community, Ibadan. West Afr J Med. 2001;20(4):227‐231. [PubMed] [Google Scholar]
- 7. van de Vijver S, Akinyi H, Oti S, et al. Status report on hypertension in Africa‐Consultative review for the 6th Session of the African Union Conference of Ministers of Health on NCD’s. Pan Afr Med J. 2014;16(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adeloye D, Basquill C, Aderemi AV, Thompson JY, Obi FA. An estimate of the prevalence of hypertension in Nigeria: a systematic review and meta‐analysis. J Hypertens. 2015;33(2):230‐242. [DOI] [PubMed] [Google Scholar]
- 9. Ogah OS, Okpechi I, Chukwuonye II, et al. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: a review. World J Cardiol. 2012;4(12):327‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akinlua JT, Meakin R, Umar AM, Freemantle N. Current prevalence pattern of hypertension in Nigeria: a systematic review. PLoS One. 2015;10(10):e0140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ekwunife OI, Aguwa CN. A meta analysis of prevalence rate of hypertension in Nigerian populations. J Public Health Epidemiol. 2011;3(13):604‐607. [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 13. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 6). Washington, DC: U.S. Department of Health and Human Services; 1997. [Google Scholar]
- 14. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). JAMA. 2003;289(19):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 15. Whitworth JA. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983‐1992. [DOI] [PubMed] [Google Scholar]
- 16. Adeloye D, Ige JO, Aderemi AV, et al. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: a systematic review and meta‐analysis. BMJ Open. 2017;7(5):e015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 18. United Nations . 2017 Revision of World Population Prospects. New York: United Nations; 2017. Available from https://esa.un.org/unpd/wpp/ [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andy JJ, Peters EJ, Ekrikpo UE, Akpan NA, Unadike BC, Ekott JU. Prevalence and correlates of hypertension among the Ibibio/Annangs, Efiks and Obolos: a cross sectional community survey in rural South‐South Nigeria. Ethn Dis. 2012;22(3):335‐339. [PubMed] [Google Scholar]
- 21. Ezekwesili CN, Ononamadu CJ, Onyeukwu OF, Mefoh NC. Epidemiological survey of hypertension in Anambra state, Nigeria. Niger J Clin Pract. 2016;19(5):659‐667. [DOI] [PubMed] [Google Scholar]
- 22. Bello‐Ovosi BO, Asuke S, Abdulrahman SO, et al. Prevalence and correlates of hypertension and diabetes mellitus in an urban community in North‐Western Nigeria. Pan Afr Med J. 2018;29:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kadiri S, Walker O, Salako BL, Akinkugbe O. Blood pressure, hypertension and correlates in urbanised workers in Ibadan, Nigeria: a revisit. J Hum Hypertens. 1999;13(1):23‐27. [DOI] [PubMed] [Google Scholar]
- 24. Bygbjerg I. Double burden of noncommunicable and infectious diseases in developing countries. Science. 2012;337(6101):1499‐1501. [DOI] [PubMed] [Google Scholar]
- 25. Adeloye D, Basquill C. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PLoS One. 2014;9(8):e104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collier SR, Landram MJ. Treatment of prehypertension: lifestyle and/or medication. Vasc Health Risk Manag. 2012;8:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ladapo TA, Fajolu IB, Adeniyi OF, et al. Blood pressure to height ratio as a screening tool for prehypertension and hypertension in adolescents. Niger J Clin Pract. 2016;19(3):401‐406. [DOI] [PubMed] [Google Scholar]
- 28. Okpokowuruk FS, Akpan MU, Ikpeme EE. Prevalence of hypertension and prehypertension among children and adolescents in a semi‐urban area of Uyo Metropolis, Nigeria. Pan Afr Med J. 2017;28:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gebreselassie KZ, Padyab M. Epidemiology of hypertension stages in two countries in sub‐Sahara Africa: factors associated with hypertension stages. Int J Hypertens. 2015;2015:959256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guwatudde D, Nankya‐Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub‐Saharan Africa: a four‐country cross sectional study. BMC Public Health. 2015;15:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310(9):959‐968. [DOI] [PubMed] [Google Scholar]
- 32. Murthy GV, Fox S, Sivasubramaniam S, et al. Prevalence and risk factors for hypertension and association with ethnicity in Nigeria: results from a national survey. Cardiovasc J Afr. 2013;24(9–10):344‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bello M. Nigerians wake up to high blood pressure. Bull World Health Organ. 2013;91(4):242‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elias SO, Azinge EC, Umoren GA, Jaja SI, Sofola OA. Salt‐sensitivity in normotensive and hypertensive Nigerians. Niger Q J Hosp Med. 2011;21:85‐91. [PubMed] [Google Scholar]
- 35. Addo J, Agyemang C, Smeeth L, de‐Graft Aikins A, Edusei AK, Ogedegbe O. A review of population‐based studies on hypertension in Ghana. Ghana Med J. 2012;46(2 Suppl):4‐11. [PMC free article] [PubMed] [Google Scholar]
- 36. Twagirumukiza M, De Bacquer D, Kips JG, de Backer G, Stichele RV, Van Bortel LM. Current and projected prevalence of arterial hypertension in sub‐Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens. 2011;29(7):1243‐1252. [DOI] [PubMed] [Google Scholar]
- 37. Anchala R, Kannuri NK, Pant H, et al. Hypertension in India: a systematic review and meta‐analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32(6):1170‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Commodore‐Mensah Y, Samuel LJ, Dennison‐Himmelfarb CR, Agyemang C. Hypertension and overweight/obesity in Ghanaians and Nigerians living in West Africa and industrialized countries: a systematic review. J Hypertens. 2014;32(3):464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dalal S, Holmes MD, Laurence C, et al. Feasibility of a large cohort study in sub‐Saharan Africa assessed through a four‐country study. Global Health Action. 2015;8:27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adeniyi OV, Yogeswaran P, Longo‐Mbenza B, Ter Goon D. Uncontrolled hypertension and its determinants in patients with concomitant type 2 diabetes mellitus (T2DM) in rural South Africa. PLoS One. 2016;11(3):e0150033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Owolabi EO, Ter Goon D, Adeniyi OV, Seekoe E. Social epidemiology of hypertension in Buffalo City Metropolitan Municipality (BCMM): cross‐sectional study of determinants of prevalence, awareness, treatment and control among South African adults. BMJ Open. 2017;7(6):e014349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Assah FK, Ekelund U, Brage S, Mbanya JC, Wareham NJ. Urbanization, physical activity, and metabolic health in sub‐Saharan Africa. Diabetes Care. 2011;34(2):491‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goverwa T, Masuka N, Tshimanga M, et al. Uncontrolled hypertension among hypertensive patients on treatment in Lupane District, Zimbabwe, 2012. BMC Res Notes. 2014;7(1):703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dzudie A, Kengne AP, Muna WFT, et al. Prevalence, awareness, treatment and control of hypertension in a self‐selected sub‐Saharan African urban population: a cross‐sectional study. BMJ Open. 2012;2(4):e001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dzudie A, Rayner B, Ojji D, et al. Roadmap to achieve 25% hypertension control in Africa by 2025. Global Heart. 2018;13(1):45‐59. [DOI] [PubMed] [Google Scholar]
- 46. Beaney T, Schutte AE, Tomaszewski M, et al. May Measurement Month 2017: an analysis of blood pressure screening results worldwide. Lancet Global Health. 2018;6(7):e736‐e743. [DOI] [PubMed] [Google Scholar]
- 47. Abegunde KA, Owoaje ET. Health problems and associated risk factors in selected urban and rural elderly population groups of South‐West Nigeria. Ann Afr Med. 2013;12(2):90‐97. [DOI] [PubMed] [Google Scholar]
- 48. Adedoyin RA, Mbada CE, Balogun MO, et al. Prevalence and pattern of hypertension in a semiurban community in Nigeria. Eur J Cardiovasc Prev Rehabil. 2008;15(6):683‐687. [DOI] [PubMed] [Google Scholar]
- 49. Adedoyin RA, Mbada CE, Ismaila SA, Awotidebe OT, Oyeyemi AL, Ativie RN. Prevalence of cardiovascular risk factors in a low income semi‐Urban community in the north‐east Nigeria. TAF Prev Med Bull. 2012;11(4):463‐470. [Google Scholar]
- 50. Ahaneku GI, Osuji CU, Anisiuba BC, Ikeh VO, Oguejiofor OC, Ahaneku JE. Evaluation of blood pressure and indices of obesity in a typical rural community in eastern Nigeria. Ann Afr Med. 2011;10(2):120‐126. [DOI] [PubMed] [Google Scholar]
- 51. Alikor CA, Emem‐Chioma PC, Odia OJ. Hypertension in a rural community in Rivers State, Niger Delta region of Nigeria: prevalence and risk factors. Niger Health J. 2013;13:18‐25. [Google Scholar]
- 52. Amira CO, Sokunbi DOB, Sokunbi A. The prevalence of obesity and its relationship with hypertension in an urban community: data from world kidney day screening programme. Int J Med Biomed Res. 2012;1(2):104‐110. [Google Scholar]
- 53. Amole IO, OlaOlorun AD, Odeigah LO, Adesina SA. The prevalence of abdominal obesity and hypertension amongst adults in Ogbomoso, Nigeria. Afr J Prim Health Care Fam Med. 2011;3(1):188. [Google Scholar]
- 54. Asekun‐Olarinmoye EO, Akinwusi PO, Adebimpe WO, et al. Prevalence of hypertension in the rural adult population of Osun State, southwestern Nigeria. Int J General Med. 2013;6:317‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cooper R, Rotimi C, Ataman S, et al. The prevalence of hypertension in seven populations of west African origin. Am J Public Health. 1997;87(2):160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ejim EC, Okafor CI, Emehel A, et al. Prevalence of cardiovascular risk factors in the middle‐aged and elderly population of a Nigerian rural community. J Trop Med. 2011;2011:308687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ekanem US, Opara DC, Akwaowo CD. High blood pressure in a semi‐urban community in south‐south Nigeria: a community‐based study. Afr Health Sci. 2013;13(1):56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ekwunife OI, Udeogaranya PO, Nwatu IL. Prevalence, awareness, treatment and control of hypertension in a Nigerian population. Health. 2010;2(7):731‐735. [Google Scholar]
- 59. Erhun WO, Olayiwola G, Agbani EO, Omotoso NS. Prevalence of hypertension in a university community in South West Nigeria. Afr J Biomed Res. 2005;8(1):15‐19. [Google Scholar]
- 60. Hendriks ME, Wit FWNM, Roos MTL, et al. Hypertension in sub‐Saharan Africa: cross‐sectional surveys in four rural and urban communities. PLoS One. 2012;7(3):e32638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Isezuo SA, Sabir AA, Ohwovorilole AE, Fasanmade OA. Prevalence, associated factors and relationship between prehypertension and hypertension: a study of two ethnic African populations in Northern Nigeria. J Hum Hypertens. 2011;25(4):224‐230. [DOI] [PubMed] [Google Scholar]
- 62. Mbah BO, Eme PE, Ezeji J. Prevalence and risk factors of hypertension among middle‐aged adults in Ahiazu Mbaise local government area, Imo State, Nigeria. Int J Basic Appl Sci. 2013;13(1):26‐30. [Google Scholar]
- 63. Odugbemi TO, Onajole AT, Osibogun AO. Prevalence of cardiovascular risk factors amongst traders in an urban market in Lagos, Nigeria. Niger Postgrad Med J. 2012;19(1):1‐6. [PubMed] [Google Scholar]
- 64. Ogah OS, Madukwe OO, Chukwuonye II, et al. Prevalence and determinants of hypertension in Abia State Nigeria: results from the Abia State non‐communicable diseases and cardiovascular risk factors survey. Ethn Dis. 2013;23(2):161‐167. [PubMed] [Google Scholar]
- 65. Oghagbon EK, Okesina AB, Biliaminu SA. Prevalence of hypertension and associated variables in paid workers in Ilorin, Nigeria. Niger J Clin Pract. 2008;11(4):342‐346. [PubMed] [Google Scholar]
- 66. Oladapo OO, Salako L, Sodiq O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south‐western Nigerian population: a population‐based survey. Cardiovasc J Afr. 2010;21(1):26‐31. [PMC free article] [PubMed] [Google Scholar]
- 67. Omorogiuwa A, Ezenwanne EB, Osifo C, Ozor MO, Ekhator CN. Comparative study on risk factors for hypertension in a University setting in Southern Nigeria. Int J Biomed Health Sci. 2009;5(2):103‐107. [Google Scholar]
- 68. Omuemu VO, Okojie OH, Omuemu CE. Awareness of high blood pressure status, treatment and control in a rural community in Edo State. Niger J Clin Pract. 2007;10(3):208‐212. [PubMed] [Google Scholar]
- 69. Suleiman IA, Amogu EO. Prevalence of hypertension in Amassoma, Southern Ijaw, Bayelsa state, Nigeria. Value in Health. 2012; Conference: 17th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research, ISPOR 2012 Washington, DC United States. Conference Start: 20120602 Conference End: 20120606. Conference Publication: (var.pagings). 15 (4):A116.
- 70. Ulasi II, Ijoma CK, Onodugo OD. A community‐based study of hypertension and cardio‐metabolic syndrome in semi‐urban and rural communities in Nigeria. BMC Health Serv Res. 2010;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ulasi II, Ijoma CK, Onwubere BJC, Arodiwe E, Onodugo O, Okafor C. High prevalence and low awareness of hypertension in a market population in Enugu, Nigeria. Int J Hypertens. 2011;2011:869675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Agaba EI, Akanbi MO, Agaba PA, et al. A survey of non‐communicable diseases and their risk factors among university employees: a single institutional study. Cardiovasc J Afr. 2017;28(6):377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Akinbodewa AA, Adejumo AO, Koledoye OV, et al. Community screening for pre‐hypertension, traditional risk factors and markers of chronic kidney disease in Ondo State, South‐Western Nigeria. Niger Postgrad Med J. 2017;24(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 74. Emerole CO, Aguwa EN, Onwasigwe CN, Nwakoby BA. Cardiac risk indices of staff of Federal University Of Technology Owerri, Imo State, Nigeria. Tanzan Health Res Bull. 2007;9(2):132‐135. [DOI] [PubMed] [Google Scholar]
- 75. Ibekwe R. Modifiable risk factors of hypertension and socio‐demographic profile in Oghara, Delta State; prevalence and correlates. Ann Med Health Sci Res. 2015;5(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ige OK, Owoaje ET, Adebiyi OA. Non communicable disease and risky behaviour in an urban university community Nigeria. Afr Health Sci. 2013;13(1):62‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Okaka E, Eiya B. Prevalence and pattern of dyslipidemia in a rural community in Southern Nigeria. Afr J Med Health Sci. 2013;12(2):82‐86. [Google Scholar]
- 78. Oyeyemi AL, Adeyemi O. Relationship of physical activity to cardiovascular risk factors in an urban population of Nigerian adults. Arch Public Health. 2013;71(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oguoma VM, Nwose EU, Skinner TC, Digban KA, Onyia IC, Richards RS. Prevalence of cardiovascular disease risk factors among a Nigerian adult population: relationship with income level and accessibility to CVD risks screening. BMC Public Health. 2015;15:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ezejimofor MC, Uthman OA, Maduka O, et al. The burden of hypertension in an oil‐ and gas‐polluted environment: a comparative cross‐sectional study. Am J Hypertens. 2016;29(8):925‐933. [DOI] [PubMed] [Google Scholar]
- 81. Adebayo RA, Balogun MO, Adedoyin RA, Obashoro‐John OA, Bisiriyu LA, Abiodun OO. Prevalence of hypertension in three rural communities of Ife North Local Government Area of Osun State, South West Nigeria. Int J Gen Med. 2013;6:863‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Akpan EE, Ekrikpo UE, Udo AI, Bassey BE. Prevalence of hypertension in Akwa Ibom State, South‐South Nigeria: rural versus urban communities study. Int J Hypertens. 2015;2015:975819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Egbi OG, Ogoina D, Oyeyemi A. Prevalence of hypertension and associated factors in a rural community in Bayelsa State. Int J Res Med Sci. 2018;6(4):1106‐1113. [Google Scholar]
- 84. Chukwuonye II, Chuku A, Onyeonoro U, et al. Prevalence of abdominal obesity in Abia State, Nigeria: results of a population‐based house‐to‐house survey. Diabetes Metab Syndr Obes. 2013;6:285‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ekpe EL, Elemi IA. Hypertension in a rural community in south‐South Nigeria. J Coll Physicians Surg Pak. 2016;26(10):868‐869. [PubMed] [Google Scholar]
- 86. Ezeala‐Adikaibe BA, Orjioke C, Ekenze OS, et al. Population‐based prevalence of high blood pressure among adults in an urban slum in Enugu, South East Nigeria. J Hum Hypertens. 2016;30(4):285‐291. [DOI] [PubMed] [Google Scholar]
- 87. Iloh G, Amadi AN, Nwankwo BO, Ugwu VC. Obesity in adult Nigerians: a study of its pattern and common primary co‐morbidities in a rural Mission General Hospital in Imo state, South‐Eastern Nigeria. Niger J Clin Pract. 2011;14(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 88. Iloh GU, Amadi AN, Nwankwo BO. Obesity in adult Nigerians: a study of its prevalence and common primary co‐morbidities in a semi‐urban Mission General Hospital in South‐Eastern Nigeria. Niger J Med. 2010;19(4):459‐466. [DOI] [PubMed] [Google Scholar]
- 89. Ofuya ZM. The incidence of hypertension among a select population of adults in the Niger Delta region of Nigeria. Southeast Asian J Trop Med Public Health. 2007;38(5):947‐949. [PubMed] [Google Scholar]
- 90. Okafor C, Anyaehie U, Ofoegbu E. The magnitude of obesity and its relationship to blood pressure among the residents of Enugu metropolis in South East Nigeria. Ann Med Health Sci Res. 2014;4(4):624‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Olamoyegun MA, Oluyombo R, Iwuala SO, Asaolu SO. Epidemiology and patterns of hypertension in semi‐urban communities, south‐western Nigeria. Cardiovasc J Afr. 2016;27(6):356‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shittu RO, Odeigah LO, Fakorede KO, et al. Prevalence and correlates of hypertension‐outcome of a free medical screening in Oke‐Ogun area of Oyo state, Nigeria, West Africa. J Am Soc Hypertens. 2018;12(4):268‐274. [DOI] [PubMed] [Google Scholar]
- 93. Ugwuja E, Ezenkwa U, Nwibo A, Ogbanshi M, Idoko O, Nnabu R. Prevalence and determinants of hypertension in an agrarian rural community in southeast Nigeria. Ann Med Health Sci Res. 2015;5(1):45‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wahab KW, Sani MU, Yusuf BO, Gbadamosi M, Gbadamosi A, Yandutse MI. Prevalence and determinants of obesity – a cross‐sectional study of an adult Northern Nigerian population. Int Arch Med. 2011;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


