Abstract
This study of fully vaccinated health care workers examines antibody levels and variant cross-neutralization after COVID-19 breakthrough infection.
Breakthrough infections after vaccination against SARS-CoV-2 are increasingly reported, possibly due to waning of vaccine-induced antibody levels.1 Moreover, emerging variants of concern with diminished susceptibility to vaccine-induced antibodies are responsible for most new cases.2,3 Studies have focused on determining the rate of vaccine breakthrough based on antibody levels after standard vaccination practices.4,5 We assessed antibody levels and variant cross-neutralization after breakthrough infection.
Methods
Fully vaccinated health care workers subsequently diagnosed with SARS-CoV-2 breakthrough infection based on a positive polymerase chain reaction (PCR) test result were sequentially recruited at the Oregon Health & Science University between January 31, 2021, and August 18, 2021. Only those with no history of previous infection whose test results were negative for nucleocapsid antibodies were included. Controls were fully vaccinated individuals without a breakthrough infection matched on sex, age, time between vaccine doses, and time between sample collection and most recent antigen exposure (PCR confirmation for those with breakthrough infection and final vaccine dose for controls). Full-length viral genomic sequencing was used to determine SARS-CoV-2 variant identity. Enzyme-linked immunosorbent assays were used to determine serum dilution titers with a 50% effective concentration (EC50) of IgG, IgA, and IgM antibodies specific to the SARS-CoV-2 spike receptor–binding domain. Live SARS-CoV-2 neutralizing serum dilution titers were determined by 50% focus reduction neutralization tests (FRNT50) against isolates of the original SARS-CoV-2 strain (WA1) and variants of concern (Alpha, Beta, Gamma, and Delta). Median breakthrough and control serum values were calculated in GraphPad Prism and compared with the Wilcoxon matched-pairs signed rank test with the Holm-Šídák correction. Delta-neutralizing potency was determined by comparing Delta- and WA1-neutralizing titers for sequence-confirmed Delta variant breakthrough cases, non-Delta breakthrough cases, and controls using the Kruskal-Wallis test with Dunn correction. Statistical significance was defined as a 2-tailed P < .05. Additional laboratory methods are provided in the Supplement. The Oregon Health & Science University institutional review board approved this study. Written informed consent was obtained.
Results
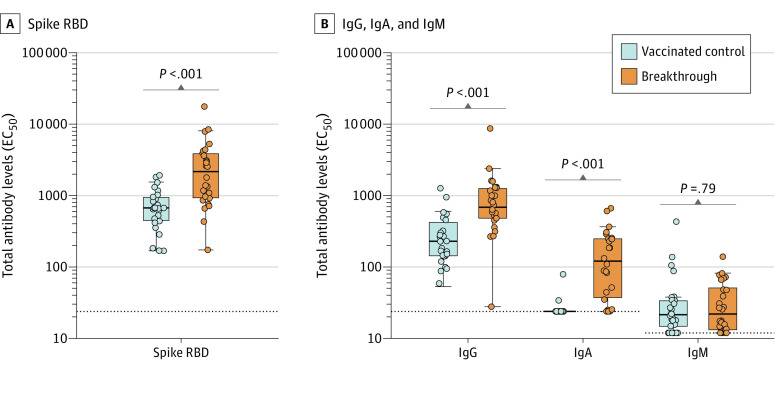
Twenty-six participants with breakthrough infections (mean age, 38 years; 20 [77%] women; 24 [92%] were vaccinated with BNT162b2, sampled a median 28 days after PCR date and 213.5 days after final vaccination; 21 [81%] with mild symptoms) were matched to 26 controls (mean age, 39 years; 21 [81%] women; 26 [100%] were vaccinated with BNT162b2, sampled a median 28 days after final vaccination). Total receptor-binding domain–specific immunoglobulin increased in participants with breakthrough infection with a median EC50 of 2152 (95% CI, 961-3596) compared with 668 (95% CI, 473-892) in controls (322% increase; P < .001) (Figure 1A). Median serum dilutions increased for both IgG and IgA. For example, the median IgA EC50 after breakthrough infection was 120 (95% CI, 44-246), compared with 24 (95% CI, 24-24) for controls (502% increase; P < .001). IgM levels were not significantly different between groups (Figure 1B).
Figure 1. SARS-CoV-2 Spike Receptor-Binding Domain (RBD)–Specific Antibody Levels After Vaccination and Breakthrough Infection.
Enzyme-linked immunosorbent assay measurement of serum dilution titers with a 50% effective concentration (EC50) of SARS-CoV-2 spike RBD-binding antibodies. The dotted lines indicate the assay limits of detection. Two-tailed P values were determined using the Wilcoxon matched-pairs signed rank test with the Holm-Šídák multiple comparison correction. Box plots were generated using the Tukey method. The large box displays the median and IQR. The error bars indicate 1.5 times the IQR or the furthest outlier, whichever is closer to the median. All individual data points are displayed as filled circles.
Among sequence-confirmed breakthrough cases, 10 were Delta and 9 were non-Delta infections. Among breakthrough cases, the median FRNT50 against WA1 was 4646 (95% CI, 2283-7053) vs 489 (95% CI, 272-822) for controls (950% increase; P < .001). FRNT50 results for Alpha, Beta, and Gamma variants are shown in Figure 2A. In breakthrough cases, median FRNT50 against the Delta variant was 2482 (95% CI, 1072-4923), compared with 243 (95% CI, 118-336) for controls (1021% increase; P < .001) (Figure 2A). Sera from Delta breakthrough cases showed improved potency against the Delta variant at 99% (95% CI, 73-151) of WA1 neutralization for each participant, compared with 36% (95% CI, 33-52) for non-Delta cases and 41% (95% CI, 24-56) for controls (Figure 2B).
Figure 2. Live SARS-CoV-2 Variants Neutralization After Vaccination and Breakthrough Infection.
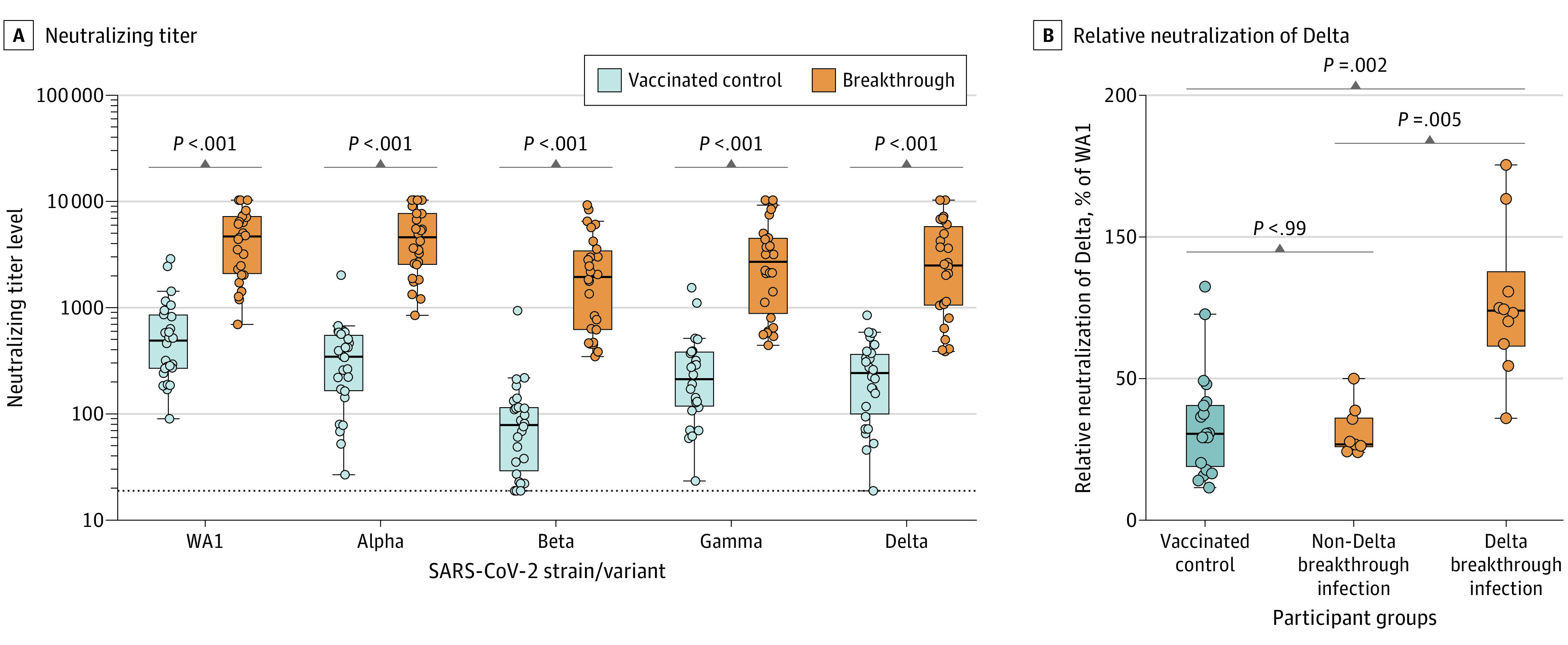
Live SARS-CoV-2 neutralization by focus-forming assay. A, The dotted line indicates the assay limit of detection. Two-tailed P values were determined using the Wilcoxon matched-pairs signed rank test with the Holm-Šídák multiple comparison correction. B, Participants with inconclusive sequencing information were excluded from this analysis. Two-tailed P values were determined using the Kruskal-Wallis test with the Dunn multiple comparison correction. Box plots were generated using the Tukey method. The large box displays the median and IQR. The error bars indicate 1.5 times the IQR or the furthest outlier, whichever is closer to the median. All individual data points are displayed as filled circles.
Discussion
Results of this study showed substantial boosting of humoral immunity after breakthrough infection, despite predominantly mild disease. Boosting was most notable for IgA, possibly due to the differences in route of exposure between vaccination and natural infection. In addition, breakthrough sera demonstrated improved variant cross-neutralization, and Delta breakthrough infections in particular exhibited improved potency against Delta vs WA1, suggesting that the protective immune response may be broadened through development of variant boosters with antigenic inserts matching the emerging SARS-CoV-2 variants. Limitations of this study include the small number of samples and the difference in time from initial vaccination to serum collection between the breakthrough and control groups, which emerging evidence suggests may contribute to the development of variant cross-neutralizing antibody responses.6
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
eMethods
References
- 1.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021. doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27(4):717-726. doi: 10.1038/s41591-021-01294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585-594. doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474-1484. doi: 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau NVV, Ngoc NM, Nguyet LA, et al. An observational study of breakthrough SARS-CoV-2 Delta variant infections among vaccinated healthcare workers in Vietnam. EClinicalMedicine. 2021;41:101143. doi: 10.1016/j.eclinm.2021.101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR, Frenck RW Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627-1629. doi: 10.1056/NEJMc2113468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods