Abstract
In the 1997-98 academic year, we conducted a longitudinal study of meningococcal carriage and acquisition among first-year students at Nottingham University, Nottingham, United Kingdom. We examined the dynamics of long-term meningococcal carriage with detailed characterization of the isolates. Pharyngeal swabs were obtained from 2,453 first-year students at the start of the academic year (October), later on during the autumn term, and again in March. Swabs were immediately cultured on selective media, and meningococci were identified and serologically characterized. Nongroupable strains were genetically grouped using a PCR-based assay. Pulsed-field gel electrophoresis was used to determine the link between sequential isolates. Of the carriers initially identified in October, 44.1% (98 of 222) were still positive later on in the autumn (November or December); 57.1% of these remained persistent carriers at 6 months. Of the index carriers who lost carriage during the autumn, 16% were recolonized at 6 months. Of 344 index noncarriers followed up, 22.1% acquired carriage during the autumn term and another 13.7% acquired carriage by March. Overall, 43.9% (397 of 904) of the isolates were noncapsulated (serologically nongroupable); by PCR-based genogrouping, a quarter of these belonged to the capsular groups B and C. The ratio of capsulated to noncapsulated forms for group B and C strains was 2.9 and 0.95, respectively. Sequential isolates of persistent carriers revealed that individuals may carry the same or entirely different organisms at different times. We identified three strains that clearly switched their capsular expression on and off at different times in vivo. One student developed invasive meningococcal disease after carrying the same organism for over 7 weeks. The study revealed a high rate of turnover of meningococcal carriage among students. Noncapsulated organisms are capable of switching their capsular expression on and off (both ways) in the nasopharynx, and group C strains are more likely to be noncapsulated than group B strains. Carriage of a particular meningococcal strain does not necessarily protect against colonization or invasion by a homologous or heterologous strain.
Neisseria meningitidis is the commonest cause of pyogenic meningitis, capable of causing outbreaks of invasive disease. A number of these have been reported at British universities. The natural habitat of N. meningitidis is the human nasopharynx, from where the organism may invade the bloodstream, causing bacteremia and a number of different clinical syndromes depending on the virulence of the meningococcal strain, host immunity, and other poorly understood factors. These syndromes vary in severity from transient harmless carriage to fatal meningitis and/or septicemia.
Currently, meningococcal disease is endemic in many parts of the world, with relatively large-scale outbreaks occurring in many countries. It is interesting that the highest attack rates of invasive meningococcal disease in the United Kingdom are in the first year of life (50/105) and among teenagers (5/105), whereas the highest carriage rate (5 to 15%) is found among teenagers and young adults (3). Estimating the rates of carriage across different groups within a community at any one time is difficult because several factors potentially affect the results. These include factors related to the organism, host, environment, and swabbing techniques and methodological ones relating to number of swabs per round and the sensitivity of different laboratory methods (3). Study results are therefore generally underestimates of true carriage.
Based on antigenic differences in their capsular polysaccharide, 13 serogroups of N. meningitidis have been identified. Virtually all disease-associated isolates are capsulated, with serogroups A, B, and C responsible for over 90% of invasive meningococcal infections worldwide. In the United Kingdom, group B is responsible for around two-thirds of the cases, followed by group C (around 30 to 40%). A small minority of United Kingdom cases are due to a mixture of serogroups A, W135, X, and Y. In contrast, up to half of the carrier strains are noncapsulated and, therefore, serologically nongroupable (NG). Until recently, NG isolates were considered nonpathogenic. However, it is now increasingly clear that capsular expression is phase variable (16), and the loss of capsule is believed to enhance the organism's ability to colonize the nasopharynx. The capsule, vital for evasion of human defense mechanisms, is thought to be expressed upon invasion of the bloodstream or cerebrospinal fluid. However, evidence for capsular switching on and off (in both directions) in vivo in the same carrier has not been previously reported.
So far, little is known about the genetic (and clonal) makeup of strains carried within large communities. The population of meningococcal carrier strains is known to be more diverse than the population associated with clinical disease (4). Nevertheless, only a small number of hypervirulent strains, with a particular genetic makeup, are thought to be capable of causing invasive disease and epidemic outbreaks. University students are considered to be a population at increased risk of invasive meningococcal disease (12). They originate from various parts of the country and abroad, and the carriage strains isolated on the first day(s) of the academic year constitute a representative sample of the prevalent strains in the country. However, the fate of individual strains (clones) of meningococci after this mass pooling is unknown. It is not clear whether a natural genetic equilibrium is maintained or whether certain clones will eventually dominate.
In the 1997-98 academic year, we carried out a study of meningococcal carriage among first-year university students in Nottingham, United Kingdom. During “freshers week,” all first-year students were targeted, and representative population samples in residential halls were approached again at intervals for reswabbing during the autumn (November or December) and spring (March) terms.
During freshers week, 2,453 students were screened over 4 consecutive days. The carriage rate rose rapidly in the first week of the term, from 6.9% on day 1, through 11.2% on day 2 and 19% on day 3, to 23.1% on day 4 (11). The average carriage rate in residents of residential halls where shared catering facilities are available during the first week was 13.9%. By November, the carriage rate was 31.0%, and in December it had reached 34.2%. In March, the rate was 28.0%.
In this paper, we report the dynamics of long-term host-pathogen interaction and provide strong evidence for the continued susceptibility of individuals to multiple meningococcal carriage and invasive disease. We also provide the evidence for in vivo switching of capsular polysaccharide expression, which may have important implications for future mass immunization with capsule-based conjugate vaccines.
MATERIALS AND METHODS
Recruitment of students and swabbing.
During the first week of October 1997 (freshers week), 2,453 of the first-year intake of students in Nottingham University were recruited and swabbed for meningococcal carriage (11); those residing in halls on campus where shared catering facilities are available were reswabbed in the first week of either November or December. A fourth round of swabbing in five randomly selected halls was undertaken in March 1998.
Culture and characterization of isolates.
Pharyngeal swabs were taken using cotton swabs and plated immediately onto a selective medium, GC agar (Oxoid) containing vancomycin, colistin, nystatin, and trimethoprim (VCNT selective supplement SR91; Oxoid). The plates were then incubated within 3 h of collection at 37°C in air with 5% carbon dioxide and examined at 24 and 48 h.
Typical colonies suggestive of Neisseria spp. were examined for positive oxidase reaction, and a single colony was then subcultured onto a new GC plate (with no antibiotics) to obtain pure cultures for further characterization. Overnight cultures were then tested by the Gonocheck system (EY Inc.) according to the manufacturer's instructions. All isolates identified as meningococci were frozen as duplicate samples, one of which was subsequently sent to the Meningococcal Reference Unit (Manchester Public Health Laboratory, Manchester, United Kingdom) for confirmation of the identification and serological characterization (6).
PCR-based genogrouping of the serologically NG isolates.
A total of 112 strains of N. meningitidis, previously characterized in the Meningococcal Reference Unit, were used in the developmental stage of the PCR assay. These included 3 reference strains (group A, NCTC 10025; group B, NCTC 10026; and group C, NCTC 8554) and 109 laboratory isolates, including 4 of group A, 49 of group B, 23 of group C, 9 of group 29E, 2 of group H, 7 of group W135, 6 of group X, 5 of group Y, and 4 of group Z. In addition, clinical isolates of Neisseria gonorrhoeae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, Streptococcus pneumoniae, and Streptococcus agalactiae were also used as negative controls.
Oligonucleotide primers were designed using the siaD sequence specific to capsular groups B and C, to amplify DNA fragments of a capsule-specific size (786 and 634 bp for groups B and C, respectively). The primers were designed from the group B sequence (EMBL accession no. M64289) and the group C sequence (GenBank U75650) (16) and were as follows: group B siaD forward, 677GTTAGTCAACGCTACC692, and reverse, 1463GGAGATCAGAAGTCAT1448; group C siaD forward, 529GTGGGTAACAACTTACA545, and reverse, 1163CCATCCTCTATACTTG1148.
A fresh single colony per isolate was suspended in 300 μl of Chelex extraction buffer (Bio-Rad), heated to 100°C for 20 min, and centrifuged in a microcentrifuge (MSE Micro Centaur) at 13,000 rpm for 30 s. A 102 or 103 dilution of the supernatant was then used as template in the PCR, which consisted of 30 cycles of denaturation (95°C, 30 s), annealing (45°C, 1 min), and extension (72°C, 1 min).
PFGE.
Comparative genetic analysis was carried out on selected strains, using pulsed-field gel electrophoresis (PFGE) as described by Bevanger and colleagues (2).
RESULTS
Characterization of index carrier meningococcal isolates: capsular expression and distribution of serogroups, types, and subtypes.
Overall, 904 meningococcal isolates were obtained during the study period, and they were serologically grouped, typed, and subtyped. Table 1 shows that 30.5% of the isolates belonged to the more virulent serogroups B and C; 25.6% belonged to the apparently less virulent serogroups 29E, H, W135, X, Y, and Z; and up to 43.9% of isolates were NG. According to serological markers, virtually all of the phenotypically distinct strains, which were found in October, continued to be represented, although in varying ratios, throughout the academic year (data available on request). Serogroup H, first isolated in China (15), was found in only two individuals at the beginning of the academic year but not in subsequent rounds of swabbing.
TABLE 1.
Serogroup distribution among all the meningococcal isolates in this study
| Group | Value for sampling time
|
Total (n = 904)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Freshers week (October) (index round) (n = 300)
|
Autumn term (November or December) (n = 448)
|
Spring term (March) (n = 156)
|
||||||
| No. of isolates | % | No. of isolates | % | No. of isolates | % | No. of isolates | % overall | |
| B | 85 | 28.3 | 92 | 20.5 | 45 | 28.8 | 222 | 24.5 |
| C | 10 | 3.3 | 38 | 8.5 | 6 | 3.8 | 54 | 6 |
| 29E | 18 | 6 | 24 | 5.4 | 9 | 5.8 | 51 | 5.6 |
| H | 2 | 0.7 | 0 | 0 | 0 | 0 | 2 | 0.2 |
| W135 | 19 | 6.3 | 45 | 10 | 18 | 11.5 | 82 | 9.1 |
| X | 5 | 1.7 | 12 | 2.7 | 2 | 1.3 | 19 | 2.1 |
| Y | 24 | 8 | 37 | 8.3 | 11 | 7 | 72 | 8 |
| Z | 1 | 0.3 | 4 | 0.9 | 0 | 0 | 5 | 0.6 |
| NG | 136 | 45.3 | 196 | 43.7 | 65 | 41.7 | 397 | 43.9 |
With regard to serotype distributions, there were more serologically nontypeable (NT) strains than typeable ones (data available on request). For example, 38% (84 of 222) of all the serogroup B strains were NT, compared with 29.3 and 25% for serotypes 1 and 4, respectively. Similarly, serologically nonsubtypeable (NST) strains were also more prevalent than any individual subtypeable ones (data not shown). For example, among the 85 serogroup B strains in October, there were 19 NST strains, compared to 17, 10, 9, and 8 strains expressing serosubtype 15, 14, 9, and 4 antigens, respectively.
Serogroups B and C comprised 54.5% of all capsulated, serogroupable strains. In order to determine proportions of group B and C strains among the NG isolates, a PCR-based genogrouping assay was developed. This technique correctly amplified 49 of 49 known group B and 23 of 23 known group C strains, with no false negatives. No false-positive results were obtained from 60 capsulated non-group B or -C N. meningitidis isolates or the other nonmeningococcal capsulated bacteria examined (see Materials and Methods).
A random sample of 53 NG isolates from the October round of swabbing was then selected for PCR-based genogrouping. Eight (15.1%) of these genogrouped as B, and six (11.3%) genogrouped as C. This is in contrast to the ratio of their serogroupable counterparts among the capsulated isolates (43.8 and 10.7%, respectively). The ratio of capsulated to noncapsulated isolates was greater among group B strains than among group C strains. Also, the ratio of group B to group C strains among capsulated isolates was significantly greater than among the noncapsulated (NG) isolates (Fisher's exact test [two-tailed]: P < 0.05) (Table 2).
TABLE 2.
Proportions (percents) and different ratios of capsular group B and C strains among the capsulated (excluding NG) and noncapsulated (NG-only) meningococci
| Group | % (no. of isolates/total no.)
|
Capsulated/noncapsulated ratio | |
|---|---|---|---|
| Capsulated | Noncapsulated | ||
| B | 43.8 (222/507) | 15.1 (8/53) | 2.9 |
| C | 10.7 (54/507) | 11.3 (6/53) | 0.95 |
| Non-B non-C | 45.5 (231/507) | 73.6 (39/53) | 0.62 |
| B/C ratio | 4.1 | 1.3 | |
Dynamics of long-term carriage. (i) Turnover of carriage.
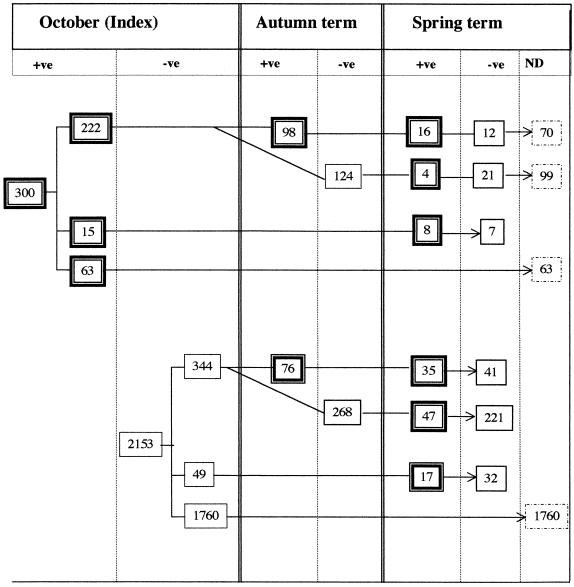
The follow-up results for the 630 index carriers and noncarriers who were reswabbed at least once more during the course of the academic year are shown in Fig. 1. Of the 222 index carriers reswabbed in autumn, 98 (44.1%) were still positive for N. meningitidis. Of the latter, 28 were screened again in March, of whom 16 (57.1%) continued to harbor meningococci. One hundred twenty-four (55.9%) index carriers apparently cleared the organism by the end of autumn. Twenty-five of these were reexamined in March, and four (19%) were found to be recolonized, indicating that the individual remained susceptible to subsequent meningococcal carriage despite eradication following an episode of colonization. Figure 1 also shows another 15 index carriers who were reswabbed in March (not in autumn), and more than half of these were clearly persistent carriers. In all, 68 index carriers were rescreened at 6 months and 28 (41.2%) were still carriers.
FIG. 1.
Long-term follow-up of index carriers and noncarriers. +ve, positive; −ve, negative; ND, not determined.
Of students who entered the university as noncarriers, 344 were reexamined in autumn and spring; 268 (77.9%) persisted as noncarriers in autumn; 76 (22.1%) became colonized in autumn, and another 47 (13.7%) became colonized by March. Two hundred twenty-one (64.2%) were apparently persistent noncarriers.
(ii) Identity of sequential isolates in persistent carriers.
To see whether persistent carriers predominantly harbored the same or different strains during their carriage period, the capsular serogroups of the sequential (paired) isolates from the 98 individual carriers in October who remained positive in the autumn were compared (Table 3). Almost a third of the autumn-round isolates expressed the same capsular serogroup as their paired index isolates, but only six of these expressed matching serotypes and subtype antigens (data not shown). The rest either expressed different type and subtype epitopes or were NT and NST (data available on request). Thus, based on serological markers alone, only six pairs of the sequential isolates can be considered highly likely to be the same.
TABLE 3.
Comparison of the capsular serogroups of sequential isolates obtained from individual persistent carriers in October and autumn rounds
| October status | Autumn status | No. of isolates (n = 98) | % |
|---|---|---|---|
| Grouped | Same group | 30 | 30.6 |
| Grouped | NG | 11 | 12.2 |
| Grouped | Other groups | 8 | 8.2 |
| NG | NG | 32 | 32.6 |
| NG | Grouped | 17 | 17.3 |
Table 4 shows details of the serological markers and the results of PFGE carried out on all sequential clinical isolates of the 16 persistent carriers, who were confirmed positive on all three rounds of swabbing. The serological characterization of the isolates suggested that only students S1 to S3 carried single strains over the 6-month period. The PFGE experiments, however, confirmed that these three students, and an additional four (S4 to S7) carried single strains from October until March, highlighting the unreliable nature of serological markers for linking strains. The other nine students (S8 to S16) were colonized with genetically and serologically heterologous strains at various time points over the 6-month period.
TABLE 4.
Serological characterization and PFGE results for all the sequential isolates from 16 persistent carriers
| Student | Group:type:subtype
|
PFGE profile of all three strains | Inference | ||
|---|---|---|---|---|---|
| October isolate | Autumn isolate | Spring isolate | |||
| S1 | B:NT:P1.9 | B:NT:P1.9 | B:NT:P1.9 | Indistinguishable | Continued carriage |
| S2 | B:1:P1.4 | B:1:P1.4 | B:1:P1.4 | Indistinguishable | Continued carriage |
| S3 | NG:1:P1.6 | NG:1:P1.16 | NG:1:P1.16 | Indistinguishable | Continued carriage |
| S4 | B:1:P1.13 | B:1:P1.13 | B:NT:NST | Indistinguishable | Continued carriage |
| S5 | 29E:NT:P1.5 | 29E:NT:NST | NG:NT:P1.2 | Indistinguishable | Continued carriage |
| S6 | NG:NT:P1.15 | 29E:NT:P1.15 | 29E:NT:P1.9 | Indistinguishable | Continued carriage |
| S7 | NG:NT:P1.5 | W135:NT:NST | NG:NT:NST | Indistinguishable | Continued carriage |
| S8 | NG:4:NST | NG:NT:P1.15 | NG:4:NST | Oct = springa | Recolonization or simultaneous carriage |
| S9 | W135:NT:P1.3,6 | Y:NT:P1.2 | W135:NT:P1.3 | Oct = springa | Recolonization or simultaneous carriage |
| S10 | NG:NT:P1.15 | NG:NT:P1.15 | NG:NT:P1.1 | Different | Heterologous carriage |
| S11 | NG:2b:P1.16 | B:2b:P1.10 | B:2b:P1.10 | Different | Heterologous carriage |
| S12 | Y:NT:NST | NG:1:P1.2,5 | NG:1:P1.2,5 | Different | Heterologous carriage |
| S13 | B:NT:P1.9 | B:NT:P1.9 | W135:NT:P1.3 | Different | Heterologous carriage |
| S14 | NG:15:NST | W135:NT:P1.3 | W135:15:P1.9 | Different | Heterologous carriage |
| S15 | NG:4:NST | B:1:P1.13 | B:4:P1.15 | Different | Heterologous carriage |
| S16 | B:NT:P1.16 | B:4:P1.16 | W135:NT:P1.2 | Different | Heterologous carriage |
October and spring isolates were indistinguishable.
(iii) Evidence for in vivo capsular switching.
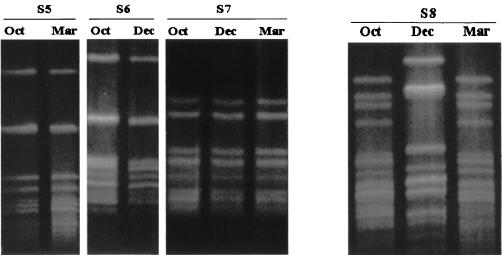
The three individual strains carried by students S5 to S7 had all clearly changed their serological markers at different points in time, including their capsular polysaccharide expression (Table 4). The strain carried by student S5 lost its capsule in March, whereas that of student S6 regained its capsule in autumn after being NG in October. The strain carried by student S7 was noncapsulated in October, switched on its capsular expression in autumn, and reverted to NG status in March. The PFGE profiles of S5 to S7 are shown in Fig. 2.
FIG. 2.
PFGE profile of sequential isolates of students S5 to S8. Oct, October; Dec, December; Mar, March.
(iv) Evidence for recolonization or simultaneous carriage.
The serological markers and PFGE profiles of the first and third isolates of students S8 (Fig. 2) and S9 (data not shown) were indistinguishable.
DISCUSSION
We studied the dynamics of interaction between meningococci and university student populations during the course of an academic year. In total, 904 meningococcal isolates were obtained; almost a third belonged to the more virulent serogroups B and C, and up to 43.9% were NG. The NG isolates consisted of a heterogeneous mixture of noncapsulated strains belonging to all capsular groups. Whereas serogroups B and C comprised more than half of all capsulated serogroupable strains, the proportion of the these two groups among the NG isolates was 26.4%. The ratio of capsulated to noncapsulated isolates seems to be lower among the non-B non-C strains (0.62) than among B and C ones. More interestingly, this ratio was greater among group B strains than among group C strains (2.9 versus 0.95, respectively). Also, the ratio of group B to group C strains among capsulated isolates was significantly greater than that among NG isolates. These data suggest that group C strains are more likely to switch off their capsular expression than group B ones. This may be due to greater pressure on serogroup C strains, generated by their more immunogenic capsule, compared with serogroup B. In this context, it will be interesting to see what impact mass vaccination with conjugated serogroup C vaccines will have on the carriage rate and/or capsular expression of serogroup C strains. Conventional capsular polysaccharide vaccines against serogroup C appear to have little impact on nasopharyngeal carriage of the organism. Recently, a number of large-scale phase II clinical trials were carried out on new-generation conjugated group C capsular polysaccharide vaccines (9, 10). These have been followed more recently by mass immunization in the United Kingdom covering all age groups up to university undergraduate level. In view of the recent findings that serogroup B and C strains can exchange capsular genes (16, 17), there may be a risk of forcing such escape mechanisms on hypervirulent strains as a result of the increased level of herd immunity generated by mass vaccination. It is therefore important to be aware of this risk during surveillance before and after mass vaccination. Conventional methods of meningococcal surveillance, based on morbidity and mortality combined with phenotypic characterization of isolates, will not detect capsular switching by hypervirulent strains or determine the success of vaccination. In collaboration with five other United Kingdom centers, we have recently embarked on a major “molecular surveillance” project to monitor pre- and postvaccination changes in the circulating meningococcal population. Large population samples (around 15,000 students), consisting of 15- to 16-year-old adolescents, have been swabbed before the recent vaccination campaign and will be reswabbed annually over the next 2 years. All meningococcal isolates will be subjected to multilocus sequence typing, in order to study their genetic structures and detect any possible changes in capsular expression of the currently prevalent group C strains, which belong to the ET-37 complex.
In this study, follow-up of index carriers and noncarriers during the course of the academic year indicated that individuals may carry meningococci for short or long periods (over 6 months) and those who eradicate one episode of meningococcal carriage remain susceptible to subsequent colonizations. Of students who entered the university as noncarriers, almost two-thirds were negative when screened in autumn and spring. These have been previously labeled persistent noncarriers. However, it is important to remember that the putative persistent noncarriers may have also carried an organism at some point in the past (or between swabs) but cleared it prior to swabbing. An alternative explanation is that their carriage (at the time of swabbing) was below detection threshold. The high turnover of carriage and continued susceptibility to further colonization suggest that the organism colonizes different individuals at different points in time and that most students are potential targets. This would secure continued circulation of the various meningococcal clones in the community, and conversely, the student population might gradually acquire herd immunity against the circulating strains.
From the serological markers alone, it appears that virtually all of the phenotypically distinct strains, which were found in October, continued to be represented, although to varying ratios, throughout the academic year (data available on request). The proportion of the individual capsular groups, types, and subtypes fluctuated only marginally during the course of the academic year, suggesting that there may be a relatively stable natural equilibrium among the carried meningococcal population in this setting. A similar observation was made in the study reported by Andersen et al. which was carried out over a 3-month period on military recruits (1). In contrast, Jones and colleagues studied nine military recruits who acquired meningococci during the course of 30 weeks of basic training (8). They found that a dominant strain carried by one individual at the start of the course later colonized six more individuals by the end of the course. Although the study population was too small to provide solid conclusions, this observation may suggest that some strains (clones) are capable of displacing others in the carried meningococcal population. The fact that military recruits train and associate in small platoon-strength groups with considerable intimacy may explain why one clone can predominate. In contrast, in a larger university campus, the social interaction among students is more diverse and individuals are exposed less-continuously to a greater number of other persons. This phenomenon deserves further detailed examination. Similarly, the effect of the summer vacation on the meningococcal population and the fate of individual strains is also unknown.
Little is known about the host-pathogen interaction during long-term carriage, and it is not entirely clear whether the persistent carriers predominantly harbor the same or different strains during their carriage period. In this study, it is evident that carriage of particular strains did not prevent colonization with a heterologous strain; this is in agreement with the findings of Jones et al. (8). Given that a third of the autumn-round isolates expressed the same capsular serogroup as their paired index isolates (Table 3) but that only six could be considered highly likely to be the same, it is clear that capsular phase variation, coupled with the hypervariability of serotype and subtype antigens, makes it extremely difficult to rely on serological markers to determine the fate of individual meningococcal isolates or the clonal relation of disseminated strains. This latter issue is of epidemiological and public health importance during epidemic outbreaks of meningococcal disease. Only molecular techniques, such as PFGE and multilocus sequence typing, are sufficiently reliable ways to trace and link virulent strains (18).
The fact that the three individual strains carried by students S5 to S7 (Table 4) had all clearly changed their serological markers, including their capsular polysaccharide expression, at different points in time and yet were genetically indistinguishable demonstrates meningococcal in vivo capsular switching (on and off in both directions) among carriers. Very often, outbreak strains are not identified among carriers during outbreak investigations (e.g., in Cardiff University, Cardiff, and Southampton University, Southampton, United Kingdom) because only serologically groupable isolates are examined. Therefore, when searching for particular hypervirulent strains among carriers (e.g., during outbreaks), all NG isolates ought to be genogrouped for the capsular group in question.
The serological markers and PFGE profile of the first and third isolates of students S8 and S9 were indistinguishable, suggesting that the students were recolonized in March by their October isolates after harboring a heterologous strain in autumn (Fig. 2). If this were the case, it would imply that meningococcal carriage may not protect against even the homologous strain, let alone a heterologous one. Alternatively, these students might have carried both strains simultaneously at all times, but we failed to detect them together during the laboratory identification process, because we examined only pure growths originating from single colonies on the original culture plates. Andersen et al. (1) showed that a small minority (15 of 1,777) of swabs from military recruits yielded two simultaneous phenotypically distinct isolates of meningococci. Moreover, multiple isolates from patients with invasive meningococcal disease, where two distinct isolates were isolated (blood and cerebrospinal fluid samples) from the same patient have also been reported previously (5).
It has been suggested elsewhere that individuals acquiring new strains are at greater risk of invasive meningococcal disease and that carriage of Neisseria spp. protects against disease (7, 14). During the course of the autumn term, two students who were noncarriers in the first week of October developed invasive meningococcal disease. However, one student, who carried Neisseria lactamica in October and a serogroup B:NT:P1.4 strain in December, developed meningococcal meningitis in late January with an indistinguishable strain (13). This clearly indicates that nasopharyngeal carriage of N. meningitidis or N. lactamica will not necessarily protect against invasion even by the same meningococcal strain. There has been some interest in using commensal Neisseria bacteria or attenuated N. meningitidis as a potential live vaccine against meningococcal infection. Our findings suggest that this approach requires careful consideration.
ACKNOWLEDGMENTS
This project was partially supported by a grant from the Meningitis Research Foundation and Sir Halley Stewart's Trust.
We thank Keith Ashford and Carol Webster for their assistance during the initial isolation and molecular characterization of strains and the Meningococcal Reference Unit in Manchester for the serological characterization of all the meningococcal isolates.
REFERENCES
- 1.Andersen J, Berthelsen L, Bech-Jensen B, Lind I. Dynamics of meningococcal carrier state and characterisation of the carrier strains: a longitudinal study within three cohorts of military recruits. Epidemiol Infect. 1998;121:85–94. doi: 10.1017/s0950268898008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevanger L, Bergh G, Caugant D A, Froholm L O. Identification of nasopharyngeal carriage of an outbreak strain of Neisseria meningitidis by pulsed-field gel electrophoresis versus phenotypic methods. J Med Microbiol. 1998;47:993–998. doi: 10.1099/00222615-47-11-993. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright K. Meningococcal carriage and disease. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley and Sons; 1995. pp. 114–146. [Google Scholar]
- 4.Caugant D A, Kristiansen B E, Froholm L O, Bovre K, Selander R K. Clonal diversity of Neisseria meningitidis from a population of asymptomatic carriers. Infect Immun. 1988;56:2060–2068. doi: 10.1128/iai.56.8.2060-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Communicable Disease Report. Diagnosis of meningococcal disease by throat swab. Commun Dis Rep. 1998;8:49. [Google Scholar]
- 6.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 7.Gold R, Goldschneider I, Lepow M L, Deaper T F, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 8.Jones G R, Christodoulides M, Brooks J L, Miller A R O, Cartwright K A V, Heckels J E. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonisation. J Infect Dis. 1998;178:451–459. doi: 10.1086/515622. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald M E, Halperin S A, Law B J, Forrest B, Danzig L E, Granoff D M. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers. A randomized controlled trial. JAMA. 1998;280:1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 10.Miller E, Richmond P, Borrow R, Kaczmarski E, Cartwright K, Morris R, Thornton C. UK strategy for introduction of meningococcal C conjugate vaccines. In: Nassif X, Quentin-Millet M-J, Taha M-K, editors. Eleventh international pathogenic Neisseria conference. Paris, France: EDK; 1998. p. 57. [Google Scholar]
- 11.Neal K R, Nguyen-Van-Tam J S, Jeffrey N, Slack R C B, Madeley R J, Ait-Tahar K, Job K, Wale M C J, Ala'Aldeen D A A. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross-sectional study. Br Med J. 2000;320:846–849. doi: 10.1136/bmj.320.7238.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neal K R, Nguyen-Van-Tam J S, Monk P, O'Brien S J, Stuart J, Ramsay M. Invasive meningococcal disease among universities providing relatively large amounts of catered hall accommodations. Epidemiol Infect. 1999;122:351–357. doi: 10.1017/s0950268899002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neal K R, Nguyen-Van-Tam J S, Slack R C B, Kaczmarski E B, White A, Ala'Aldeen D A A. Seven week interval between acquisition of a meningococcus and the onset of invasive disease. Epidemiol Infect. 1999;123:507–509. doi: 10.1017/s0950268899003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reller L B, MacGregor R R, Beaty H N. Bactericidal antibody after colonization with Neisseria meningitidis. J Infect Dis. 1973;127:56–62. doi: 10.1093/infdis/127.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Shao-Qing D, Ren-Bang Y, Huan-Chun Z. Three new serogroups of Neisseria meningitidis. J Biol Stand. 1981;9:307–315. doi: 10.1016/s0092-1157(81)80056-x. [DOI] [PubMed] [Google Scholar]
- 16.Swartley J S, Marfin A A, Edupuganti S, Liu L-J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel U, Claus H, Frosch M. Rapid serogroup switching in Neisseria meningitidis. N Engl J Med. 2000;342:219–220. doi: 10.1056/NEJM200001203420319. [DOI] [PubMed] [Google Scholar]
- 18.Vogel U, Morelli G, Zurth K, Claus H, Kriener E, Achtman M, Frosch M. Necessity of molecular techniques to distinguish between Neisseria meningitidis strains isolated from patients with meningococcal disease and from healthy contacts. J Clin Microbiol. 1998;36:2465–2470. doi: 10.1128/jcm.36.9.2465-2470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]