Abstract
Purpose: Immunotherapy combined with chemotherapy have synergistic effects in multiple malignancies. We aimed to compare the efficacy and safety of toripalimab plus hepatic arterial infusion chemotherapy (HAIC) of oxaliplatin, fluorouracil, and leucovorin versus lenvatinib in advanced hepatocellular carcinoma (HCC). Materials and Methods: We conducted this retrospective study at 3 hospitals in China and eligible patients were 18 years or older and had a primary diagnosis of unresectable HCC with macroscopic vascular invasion and/or extrahepatic spread. These patients were treated with toripalimab plus HAIC or lenvatinib monotherapy. The primary endpoint was progression-free survival (PFS) and the secondary endpoints were overall survival (OS), disease control rate per response evaluation criteria in solid tumors (RECIST) 1.1, and objective response rate (ORR) per RECIST 1.1. The results were compared by Student's test or the chi-square test, and the survival curves were calculated by the Kaplan–Meier method, and propensity-score matching (PSM) was used to reduce bias. Results: A total of 118 patients were recruited for this study: 53 in the TorHAIC group and 65 in the lenvatinib group. We found that the TorHAIC group showed a longer PFS (9.3 [95% CI, 7.81-10.8] vs 4.8 months [95% CI, 3.31−6.29]; hazard ratio [HR] = 0.57, 95% CI, 0.38-0.85; p = .006), a longer OS (17.13 [95% CI, 13.99−20.27] vs 10.1 months [95% CI, 8.14−12.06]; HR = 0.5, 95% CI, 0.31 − 0.81; p = .005), a higher disease control rate (86.8% vs 69.2%, p = .002) and a higher ORR (47.2% vs 9.2%, p < .001) by RECIST criteria than the lenvatinib group. Both toripalimab plus HAIC and lenvatinib had acceptable safety profiles. No treatment-related deaths occurred in this study. In the propensity score-matched cohorts (47 pairs), the outcomes in the TorHAIC group were also better than those in the lenvatinib group (p < .05). Conclusion: Toripalimab plus HAIC was tolerable and effective in advanced HCC and the result needs to be confirmed in the phase III trial.
Keywords: Toripalimab, hepatic arterial infusion chemotherapy, oxaliplatin, 5-fluorouracil and leucovorin, lenvatinib, advanced hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) ranked sixth for cancer incidence and third for cancer deaths in 2020. 1 Until now, half of the patients are diagnosed at advanced disease stages (Barcelona Clinic Liver Cancer C stage). 2 These patients have an extremely poor prognosis, with a median survival time of only 4.2 to 7.9 months with supportive care.3,4 Lenvatinib and sorafenib are the standard systemic treatment for advanced HCC.3,5 However, the prognosis of these patients remains unsatisfactory, with a median survival time of 10.7 to 11.8 months.3,5
With recent technological advances, immunotherapies that inhibit the immune checkpoint interaction between programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1) have shown survival benefits in patients with advanced HCC.6,7 As a recombinant, humanized programmed cell death receptor-1 monoclonal antibody, toripalimab received conditional approval in China for the treatment of unresectable or metastatic melanoma. 8 However, HCC is known as an immune-tolerant malignancy, and phase 3 trials concerning PD-1 antibody for advanced HCC ended with failure.9,10 Thus, PD-1 antibody monotherapy may not be sufficiently effective for advanced HCC.
Recently, preclinical research has demonstrated that chemotherapy may enhance the endogenous immune response.11,12 Furthermore, in other malignancies such as lung cancer and nasopharyngeal carcinoma, PD-1 antibody combined with chemotherapy has displayed promising antitumor activities and manageable toxicity and had been recommended as the first-line treatment.13,14 However, systemic chemotherapy is not used routinely since HCC is considered a chemotherapy-resistant tumor. 15 On the other hand, growing evidence indicates that hepatic arterial infusion chemotherapy (HAIC) of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) provided significant survival benefits for patients with advanced HCC. 16 HAIC was also recommended as an alternative treatment for advanced HCC in Asia.17,18 Thus, PD-1 antibody combined with HAIC may have promising response rates and lead to improved survival for patients with advanced HCC.
Hence, we conducted this retrospective study to compare toripalimab plus HAIC with lenvatinib monotherapy for advanced HCC.
Materials and Methods
This retrospective study was performed at 3 medical sites in China. Consecutive HCC patients who were treated with toripalimab plus HAIC or lenvatinib monotherapy were identified. Ethical approval to report this case series was obtained from the Institutional Review Board of Sun Yat-sen University, First People's Hospital of Foshan, and Guangzhou No. 12 People’s Hospital (B2021-047-01), and was conducted in accordance with the Declaration of Helsinki. The patient with advanced HCC was informed that lenvatinib or sorafenib was the recommended treatment. However, patients may refuse lenvatinib or sorafenib due to the high cost or serious adverse events. For these patients, HAIC and PD-1 inhibitors were recommended based on previous studies.6,7,16 We informed patients that PD-1 inhibitor plus HAIC may gain promising antitumor activity, although this combined therapy was not the standard treatment for advanced HCC. The final decision was principally made by the patient, and the written informed consents were signed at the first hospitalization and kept in the medical record.
Eligible patients were 18 years or older and had a primary diagnosis of unresectable HCC with macroscopic vascular invasion and/or extrahepatic spread. Other eligibility criteria were as follows: no prior systemic treatment, including PD-1 antibody, PD-L1 antibody, molecularly targeted drugs, and systemic chemotherapy; an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2; Child-Pugh A class liver function; at least 1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. 19 Exclusion criteria consisted of the following: central nervous system metastases; a known medical history of human immunodeficiency virus infection; pregnancy or breastfeeding; other invasive malignant diseases; combined with other treatment; incomplete medical information; and loss to follow-up.
Lenvatinib and toripalimab were administered according to the instructions, respectively.5,8 The chemotherapy regimen used in the HAIC was FOLFOX as the previous study described.
Clinical data were retrospectively collected from the medical record. The first imaging assessment (upper abdomen-enhanced computed tomography [CT]/magnetic resonance imaging and chest-enhanced CT) was performed within 2 weeks before the first treatment, and the subsequent imaging assessments were performed every 6 weeks. All imaging data were independently assessed by 2 radiologists. If there was a discrepancy between the 2 radiologists, the final classification was made by another more experienced radiologist.
The primary endpoint was progression-free survival (PFS), calculated as the time from the commencement of treatment to progression by RECIST 1.1 criteria or death from any cause, whichever occurred first. The secondary endpoints were overall survival (OS; calculated as the time from the commencement of treatment to death from any cause), disease control rate (DCR; the proportion of patients with complete response, partial response, or stable disease according to RECIST version 1.1) and objective response rate (ORR; the proportion of patients with complete response or partial response according to RECIST version 1.1). Adverse events were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
The results were compared by Student's t-test or the chi-square test. OS and PFS were calculated with the Kaplan–Meier method and compared using the log-rank test. Any factors that were statistically significant with p<.10 in the univariate analysis were candidates for entry into a multivariable Cox proportional-hazards model. All p values were 2-sided, with p values <.05 considered significant. All statistical processing was performed by the Statistical Package for Social Science (SPSS) version 24.
To reduce the selection bias and other confounding factors, the propensity score matching (PSM) analysis was applied to form the propensity score-matched cohort.20,21 All parameters were included in PSM (age, gender, absence or presence of hepatitis B surface antigen, alpha-fetoprotein (AFP), tumor size, tumor number, absence or presence of portal vein tumor thrombus, absence or presence of hepatic vein tumor thrombus, ECOG PS, and absence or presence of extrahepatic metastasis). Matched pairs were then formed using a 1-to-1 nearest-neighbor caliper width of 0.2.
The study had followed the STROBE Statement and the complete relevant checklist had been submitted as a Supplemental File.
Results
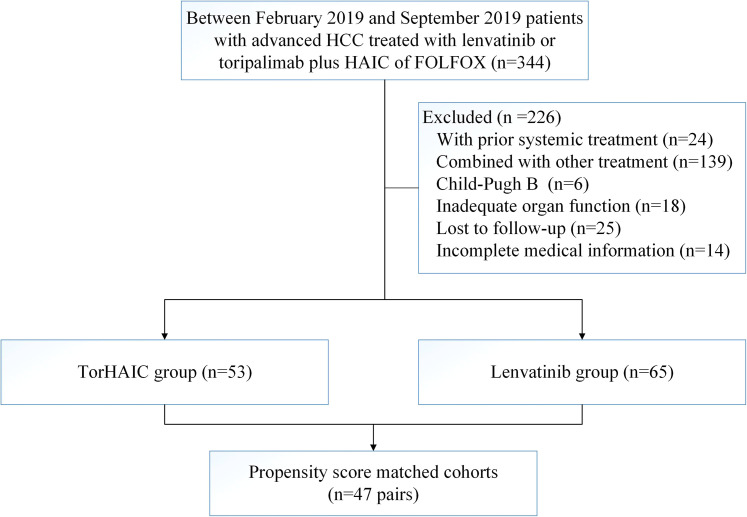
Between February 27, 2019, and September 9, 2019, 53 patients who received toripalimab plus HAIC (TorHAIC group) and 65 patients who received lenvatinib met the criteria for inclusion in this study (Figure 1). Data were last updated on October 26, 2020. There was no difference in the baseline characteristics between the groups (Table 1). Of the 118 patients, 105 (89%) were males and 104 (88.1%) were infected with hepatitis B virus infection (HBV). The mean age of the patients was 51.4 years (standard deviation [SD] 12.3), and the mean size of the maximum tumor measured by the RECIST criteria was 10 cm (SD 3.9). All the patients infected with hepatitis B virus infection received antiviral therapy within 2 weeks before the treatment. The median level of HBV-DNA was 2078 IU/ml before the treatment, and 54.4 IU/ml during the treatment. After PSM, we obtained 1-to-1 paired cohorts (47 patients in each group), and no significant difference was observed in the baseline characteristics between the 2 groups (Table 1).
Figure 1.
Patients’ selection flow.
Abbreviations: TorHAIC, Toripalimab plus hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; FOLFOX, oxaliplatin, fluorouracil, and leucovorin; PD-1, programmed cell death protein-1.
Table 1.
Patient baseline demographic and clinical characteristics.
| Initial cohort | Propensity-score-matched cohort | |||||
|---|---|---|---|---|---|---|
| Toripalimab plus HAIC (n = 53) | Lenvatinib (n = 65) | P | Toripalimab plus HAIC (n = 47) | Lenvatinib (n = 47) | P | |
| Gender | 1.00 | 1.00 | ||||
| Male | 47 (88.7%) | 58 (89.2%) | 42 (89.4%) | 41 (77.2%) | ||
| Female | 6 (11.3%) | 7 (10.8%) | 5 (10.6%) | 6 (12.8%) | ||
| Age, years | .68 | .84 | ||||
| ≤50 | 24 (45.3%) | 33 (50.8%) | 21 (44.7%) | 19 (40.4%) | ||
| >50 | 29 (54.7%) | 32 (49.2%) | 26 (55.3%) | 28 (59.6%) | ||
| ECOG | .66 | .57 | ||||
| 0 | 5 (9.4%) | 5 (7.7%) | 5 (10.6%) | 4 (8.5%) | ||
| 1 | 40 (75.5%) | 46 (70.8%) | 35 (74.5%) | 32 (68.1%) | ||
| 2 | 8 (15.1%) | 14 (21.5%) | 7 (14.9%) | 11 (23.4%) | ||
| HBsAg | .49 | 1.00 | ||||
| Positive | 45 (84.9%) | 59 (90.8%) | 43 (91.5%) | 42 (89.4%) | ||
| Negative | 8 (15.1%) | 6 (9.2%) | 4 (8.5%) | 5 (10.6%) | ||
| AFP, ng/ml | .86 | .83 | ||||
| ≤400 | 17 (32.1%) | 23 (35.4%) | 14 (29.8%) | 16 (34.0%) | ||
| >400 | 36 (67.9%) | 42 (64.6%) | 33 (70.2%) | 31 (66.0%) | ||
| Tumor size, cm | .59 | 1.00 | ||||
| ≤10 | 28 (52.8%) | 30 (46.2%) | 24 (51.1%) | 25 (53.2%) | ||
| >10 | 25 (47.2%) | 35 (53.8%) | 23 (48.9%) | 22 (46.8%) | ||
| Tumor number | .47 | 1.00 | ||||
| ≤3 | 10 (18.9%) | 8 (12.3%) | 9 (19.1%) | 8 (17%) | ||
| >3 | 43 (81.1%) | 57 (87.7%) | 38 (80.9%) | 39 (83%) | ||
| PVTT | .55 | 1.00 | ||||
| Absent | 12 (22.6%) | 19 (29.2%) | 10 (21.3%) | 10 (21.3%) | ||
| Present | 41 (77.4%) | 46 (70.8%) | 37 (78.7%) | 37 (78.7%) | ||
| HVTT | .44 | 1.00 | ||||
| Absent | 41 (77.4%) | 45 (69.2%) | 36 (75.6%) | 36 (75.6%) | ||
| Present | 12 (22.6%) | 20 (30.8%) | 11 (23.4%) | 11 (23.4%) | ||
| Extrahepatic metastasis | .72 | .67 | ||||
| Absent | 36 (68.9%) | 41 (63.1%) | 32 (68.1%) | 29 (61.7%) | ||
| Present | 17 (32.1%) | 24 (36.9%) | 15 (31.9%) | 18 (38.3%) | ||
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; ECOG, Eastern Cooperative Oncology Group; HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein; PVTT, portal vein tumor thrombus; HVTT, hepatic vein tumor thrombus; TACE, transarterial chemoembolization.
P-value was calculated by chi-square tests.
Patients in the TorHAIC group were treated with a total of 208 cycles of HAIC (median 4) and 389 cycles of toripalimab (median 8). Eleven patients discontinued the combined therapy due to disease progression, 8 patients due to radical resection, 15 patients due to poor blood supply of tumors, 13 patients due to adverse events, 3 patients due to withdrew consent, and 3 patients due to other reasons such as the economy. The median duration of study treatment for patients in the lenvatinib group was 4.6 months. Forty-one patients discontinued lenvatinib due to disease progression, 16 patients due to adverse events, 2 patients due to withdrew consent, 1 patient due to loss to follow-up, and 5 patients due to other reasons such as the economy. The subsequent treatments after the discontinuation of the study treatment are shown in Table 2. Curative surgical resection was performed for 8 patients (15.1%) in the TorHAIC group owing to downstaging, compared with no resection performed in the lenvatinib group (p = .001). Additionally, more patients in the lenvatinib group received subsequent PD-1 antibodies than those in the TorHAIC group (p = .04).
Table 2.
Treatment Administration.
| Toripalimab plus HAIC (n = 53) | Lenvatinib (n = 65) | P | |
|---|---|---|---|
| Study treatment, median (range) | |||
| HAIC cycle | 4 (1-7) | − | |
| Toripalimab cycle | 8 (1-19) | − | |
| Duration of lenvatinib, months | − | 4.6 (0.9-16.3) | |
| Subsequent treatment | |||
| HAIC | − | 12 | |
| Lenvatinib | 13 | − | |
| Resection | 8 | 0 | .001 |
| Sorafenib | 26 | 30 | .75 |
| PD-1 antibody | 16 a | 32 | .04 |
| Regorafenib | 22 | 33 | .32 |
| TACE | 11 | 10 | .45 |
| Radiotherapy | 4 | 2 | .27 |
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; PD-1, programmed cell death protein-1; TACE, transarterial chemoembolization.
Patient in the toripalimab plus HAIC group receive other PD-1 antibodies, such as nivolumab, pembrolizumab, sintilimab, and camrelizumab.
Efficacy
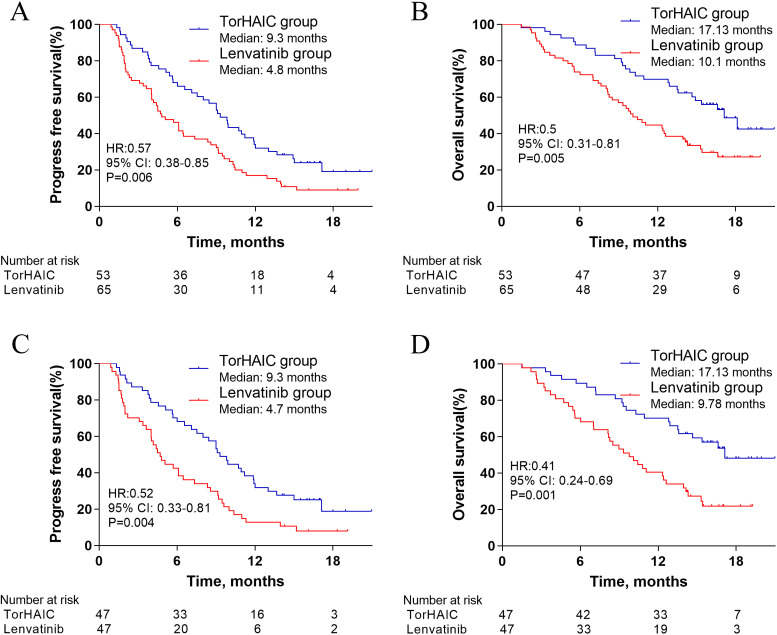
In the initial cohort, the median PFS in the TorHAIC group was higher than that in the lenvatinib group (9.3 months [95% CI, 7.81-10.8] vs 4.8 months [95% CI, 3.31-6.29]; hazard ratio [HR] = 0.57, 95% CI, 0.38-0.85; p = .006; Figure 2A). The median OS in the TorHAIC group was also higher than that in the lenvatinib group (17.13 months [95% CI, 13.99-20.27] vs 10.1 months [95% CI, 8.14-12.06]; HR = 0.5, 95% CI, 0.31-0.81; p = .005; Figure 2B). The results of univariable and multivariable analyses of PFS are shown in Table 3. The factors with a p-value <.1 in the univariable analysis were selected for multivariate analysis, including the type of treatment, ECOG PS, tumor size, absence or presence of extrahepatic metastasis, and AFP level. Multivariable analysis showed that independent risk factors for PFS were type of treatment (toripalimab plus HAIC vs lenvatinib, HR = 0.54; 95% CI, 0.36-0.82; p = .003), and absence or presence of extrahepatic metastasis (HR = 0.62; 95% CI, 0.41-0.94; p = .02). On the other hand, the results of univariable and multivariable analyses of OS are shown in Table 4. Multivariable analysis showed that independent risk factors for OS were type of treatment (toripalimab plus HAIC vs lenvatinib, HR = 0.51; 95% CI, 0.31-0.82; p = .006), and absence or presence of extrahepatic metastasis (HR = 0.43; 95% CI, 0.27-0.68; p < .001). In addition, patients in the TorHAIC group achieve higher DCR (86.8% vs 69.2%, p = .002) and ORR (47.2% vs 9.2%, p < .001) per RECIST 1.1 than patients in the lenvatinib group (Table 5). Computed tomography or magnetic resonance imaging scans of 4 representative patients who received toripalimab plus HAIC are shown in Supplemental figures.
Figure 2.
Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in the initial cohort, and progression-free survival (C) and overall survival (D) in the propensity-score-matched cohort.
Abbreviations: TorHAIC, Toripalimab plus hepatic arterial infusion chemotherapy; HR, hazard ratio; CI, confidence interval.
Table 3.
Univariate and Multivariate Analysis of Progression-Free Survival.
| Initial cohort | Propensity-score-matched cohort (1:1) | |||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||
| P* | HR (95% CI) | P** | P* | HR | P** | |
| Group (toripalimab plus HAIC vs lenvatinib) | .005 | 0.54 (0.36−0.82) | .003 | 0.003 | 0.51 (0.33−0.8) | .003 |
| Gender (male/female) | .39 | 0.64 | ||||
| Age, year (≤50 vs >50) | .19 | 0.42 | ||||
| ECOG (0-1 vs 2) | .09 | 0.73 (0.43−1.22) | .23 | 0.02 | 0.51 (0.29−0.87) | .01 |
| HBsAg (positive vs negative) | .37 | 0.21 | ||||
| AFP, ng/ml (≤400 vs >400) | .06 | 0.65 (0.42−1.01) | .06 | 0.16 | ||
| Tumor size, cm (≤10 vs >10) | .03 | 0.75 (0.49−1.14) | .18 | 0.15 | ||
| Tumor number (≤3 vs >3) | .36 | 0.51 | ||||
| PVTT (absent vs present) | .25 | 0.15 | ||||
| HVTT (absent vs present) | .69 | 0.88 | ||||
| Extrahepatic metastasis (absent vs present) | .04 | 0.62 (0.41−0.94) | .02 | 0.11 | ||
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PVTT, portal vein tumor thrombus; HVTT, hepatic vein tumor thrombus; HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein.
P* value was calculated with a 2-sided log-rank test. Any factors that were statistically significant at P<10% in the univariate analysis were candidates for entry into a multivariable Cox analysis.
P** value was calculated by multivariable Cox proportional-hazards analysis.
Table 4.
Univariate and Multivariate Analysis of Overall Survival.
| Initial cohort | Propensity-score-matched cohort (1:1) | |||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||
| P* | HR (95% CI) | P** | P* | HR | P** | |
| Group (toripalimab plus HAIC vs lenvatinib) | 0.004 | 0.51 (0.31−0.82) | 0.006 | 0.001 | 0.41 (0.24−0.7) | 0.001 |
| Gender (male/female) | 0.37 | 0.81 | ||||
| Age, year (≤50 vs >50) | 0.26 | 0.27 | ||||
| ECOG (0-1 vs 2) | 0.62 | 0.22 | ||||
| HBsAg (positive vs negative) | 0.36 | 0.5 | ||||
| AFP, ng/ml (≤400 vs >400) | 0.12 | 0.11 | ||||
| Tumor size, cm (≤10 vs >10) | 0.85 | 0.87 | ||||
| Tumor number (≤3 vs >3) | 0.51 | 0.41 | ||||
| PVTT (absent vs present) | 0.52 | 0.9 | ||||
| HVTT (absent vs present) | 0.21 | 0.14 | ||||
| Extrahepatic metastasis (absent vs present) | <0.001 | 0.43 (0.27−0.68) | <0.001 | 0.003 | 0.48 (0.28−0.8) | 0.005 |
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PVTT, portal vein tumor thrombus; HVTT, hepatic vein tumor thrombus; HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein.
P* value was calculated with a 2-sided log-rank test. Any factors that were statistically significant at P<10% in the univariate analysis were candidates for entry into a multivariable Cox analysis.
P** value was calculated by multivariable Cox proportional-hazards analysis.
Table 5.
Summary of best response based on the RECIST criteria.
| Overall response (before PSM) | Overall response (after PSM) | |||||
|---|---|---|---|---|---|---|
| Toripalimab plus HAIC (%) | Lenvatinib (%) | P | Toripalimab plus HAIC (%) | Lenvatinib (%) | P | |
| CR | 0(0) | 0(0) | — | 0(0) | 0(0) | — |
| PR | 25 (47.2%) | 6 (9.2%) | <0.001 | 22 (46.8%) | 4 (8.5%) | <0.001 |
| SD | 21 (39.6%) | 39 (60%) | 0.03 | 19 (40.4%) | 29 (61.7%) | 0.04 |
| PD | 7 (13.2%) | 20 (30.8%) | 0.002 | 6 (12.8%) | 14 (29.8%) | 0.04 |
| DCR | 46 (86.8%) | 45 (69.2%) | 0.002 | 41 (87.2%) | 33 (70.2%) | 0.04 |
| ORR | 25 (47.2%) | 6 (9.2%) | <0.001 | 22 (46.8%) | 4 (8.5%) | <0.001 |
Abbreviations: PSM, propensity-score matching; HAIC, hepatic arterial infusion chemotherapy; RECIST, response evaluation criteria in solid tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; ORR, objective response rate.
Statistical significance was assessed with the chi-square test.
In the propensity score-matched cohort, the median PFS in the TorHAIC group was 9.3 months (95% CI, 7.66-10.94) compared with 4.7 months (95% CI, 3.49-5.91) in the lenvatinib group (HR = 0.52; 95% CI, 0.33-0.81; p = .004; Figure 2C). The median OS in the TorHAIC group was 17.13 compared with 9.77 months in the lenvatinib group (HR = 0.41; 95% CI, 0.24-0.69; p = .001; Figure 2D). Multivariable analysis showed that type of treatment was an independent risk factor for PFS (HR = 0.51; 95% CI, 0.33-0.8; p = .003) (Table 3) and OS (HR = 0.41; 95% CI, 0.24-0.7; p = .001) (Table 4). In addition, compared with the lenvatinib group, higher DCR (87.2% vs 68.1%, p = .04) and ORR (46.8% vs 8.5%, p < .001) per RECIST 1.1 were also observed in the TorHAIC group (Table 5).
Safety
The treatment-related adverse events (AEs) with high incidence rates (≥10%) are shown in Table 6. The frequencies of all-grade neutropenia, fatigue, sensory neuropathy, elevated alanine aminotransferase, elevated aspartate aminotransferase, and hypoalbuminemia were significantly higher in the TorHAIC group, while the frequencies of all-grade hypertension, hand–foot skin reaction, and hypothyroidism were significantly higher in the lenvatinib group (p < .05). Compared with the TorHAIC group, grade 3 to 4 hypertension was more frequent in the lenvatinib group (p = .02). Moreover, specific abdominal pain associated with oxaliplatin infusion occurred in 12 (22.6%) patients in the TorHAIC group. This pain could be acute and severe but was quickly relieved by symptomatic treatment. In addition, 1 patient developed immune-related dermatitis in the TorHAIC group. There is no treatment-related death in our study.
Table 6.
Treatment-Related Adverse Events a .
| Adverse event | Toripalimab plus HAIC (n = 53) | Lenvatinib (n = 65) | P-value | |||
|---|---|---|---|---|---|---|
| Any grade (%) | Grade 3 to 4 (%) | Any grade (%) | Grade 3 to 4 (%) | Any grade | Grade 3 to 4 | |
| Neutropenia | 24 (45.3%) | 3 (5.7%) | 11 (16.9%) | 1 (1.5%) | 0.001 | 0.47 |
| Thrombocytopenia | 16 (30.2%) | 2 (3.8%) | 10 (15.4%) | 1 (1.5%) | 0.054 | 0.86 |
| Fatigue | 36 (67.9%) | 3 (5.7%) | 26 (40%) | 2 (3.1%) | <0.001 | 0.71 |
| Hypertension | 2 (3.8%) | 0 | 23 (35.4%) | 7 (10.8%) | <0.001 | 0.02 |
| Weight loss | 18 (34.0%) | 2 (3.8%) | 19 (29.2%) | 1 (1.5%) | 0.58 | 0.44 |
| Hypothyroidism | 2 (3.8%) | 0 | 10 (15.4%) | 0 | 0.04 | − |
| Hand–foot skin reaction | 0 | 0 | 15 (23.1%) | 2 (3.1%) | <0.001 | 0.50 |
| Rash | 4 (7.5%) | 0 | 8 (12.3%) | 0 | 0.45 | − |
| Nausea | 16 (30.2%) | 1 (1.9%) | 12 (18.5%) | 0 | 0.14 | 0.92 |
| Vomiting | 14 (26.4%) | 2 (3.8%) | 11 (16.9%) | 1 (1.5%) | 0.21 | 0.59 |
| Diarrhea | 14 (26.4%) | 1 (1.9%) | 23 (35.4%) | 2 (3.1%) | 0.3 | 1.00 |
| Abdominal pain | 17 (32.1%) | 2 (3.8%) | 12 (18.5%) | 0 | 0.09 | 0.2 |
| Sensory neuropathy | 17 (32.1%) | 0 | 0 | 0 | <0.001 | − |
| Proteinuria | 7 (13.2%) | 1 (1.9%) | 15 (23.1%) | 2 (3.1%) | 0.17 | 1.00 |
| Elevated ALT | 36 (67.9%) | 6 (7.5%) | 19 (29.2%) | 2 (3.1%) | <0.001 | 0.14 |
| Elevated AST | 38 (71.7%) | 7 (13.2) | 23 (35.4%) | 3 (4.6%) | <0.001 | 0.18 |
| Hyperbilirubinemia | 20 (37.8%) | 2 (3.8%) | 17 (26.2%) | 1 (1.5%) | 0.18 | 0.59 |
| Hypoalbuminemia | 37 (69.8%) | 1 (1.9%) | 3 (3.4%) | 0 | <0.001 | 0.92 |
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
P-value was calculated by a 2-sided chi-square test.
Listed are adverse events, as defined by the National Cancer Institute Common Terminology Criteria (version 4.03), that occurred in at least 10% of patients in either study group.
Discussion
The results of this study suggested that toripalimab plus HAIC significantly improved PFS compared with lenvatinib (9.3 vs 4.8 months). At the same time, toripalimab plus HAIC was also associated with a longer OS (17.13 vs 10.1 months), better DCR (86.8% vs 69.2%), and ORR (47.2% vs 9.2%) compared with lenvatinib alone. In multivariate analysis, type of treatment was 1 of the independent factors for PFS and OS, respectively. After using PSM analysis, patients receiving toripalimab plus HAIC also indicated a significant improvement in PFS, OS, DCR, and ORR compared with patients receiving lenvatinib. And the type of treatment was also 1 of the independent factors for PFS and OS in multivariate analysis. In addition, both toripalimab plus HAIC and lenvatinib had acceptable safety profiles.
The survival benefit reported in this study may be due to the synergistic antitumor effect of toripalimab and HAIC. At first, the adaptive immune system may be activated because chemotherapy increased HLA<AQ> expression and enhanced T-cell stimulation. 12 Second, chemotherapy may restore immunosurveillance by disrupting signal transducer and activator of transcription-6-mediated immunosuppression. 11 Last but not least, chemotherapy can induce immunogenic cell death of tumor cells and reduce “off-target” immunosuppression in the tumor microenvironment to increase antigenicity. 22
The design of this study may also help to capture the survival benefits. To eliminate the confounding effect of deaths unrelated to tumor progression, we selected patients with Child-Pugh class A, and adequate organ function so that patients could receive long-term treatments. In addition, as a primary outcome, PFS was important because it is not affected by subsequent treatment and can reflect the efficacy more accurately compared with OS.
In this study, the PFS and ORR in the lenvatinib group were lower than those observed in the REFLECT trial (4.8 vs 7.3 months; 9.2% vs 18.8%). 5 It can be explained that patients in our study had poorer ECOG PS, higher concentrations of AFP, and larger HCC compared with patients in the REFLECT trial, because these characteristics have been identified to be prognostic factors for patients with HCC. 23 Additionally, although patients receiving toripalimab plus HAIC in this study seemed to show better survival outcomes than those receiving HAIC monotherapy or sorafenib plus HAIC in previous studies (DCR: 86.8% vs 75.2% vs 78.9%%; PFS: 9.3 vs 7.1 vs 7.03 months), 16 whether toripalimab plus HAIC is better than HAIC alone or sorafenib plus HAIC needs support from further randomized trials.
Recently, atezolizumab plus bevacizumab has been shown to have a survival advantage over sorafenib in the first line (IMbrave150). 24 The PFS and ORR in the IMbrave150 trial were lower than those observed in this study (6.8 vs 9.4 months; 27.3% vs 47.2%). However, whether PD-1 antibody plus chemotherapy is superior to atezolizumab plus bevacizumab needed further study.
Moreover, the adverse events in patients receiving lenvatinib were consistent with those in the REFLECT trial, 5 and all HAIC-related or PD-1 antibody-related adverse events were consistent with those in previous studies.6,7,9,16 It is worth noting that the adverse events of liver enzyme elevation and hypoalbuminemia were more frequent in the TorHAIC group than in the lenvatinib group. However, most of these adverse events were grade 1 to 2, and no treatment-related death was observed in these patients. All adverse events were not unexpected and were manageable, the rates of grades 3 to 4 AEs were relatively low, and no treatment-related deaths were observed in the study.
In this study, toripalimab plus HAIC is not the first-line treatment for advanced HCC according to most clinical practice guidelines.25–27 However, many patients with advanced HCC in China cannot afford sorafenib, lenvatinib, or atezolizumab plus bevacizumab. Recently, HAIC monotherapy had been widely performed and may improve the OS of patients with advanced HCC,16,28 and 2020 Chinese clinical guidelines for the management of HCC have recommended HAIC for patients with advanced HCC. Our previous studies showed that lenvatinib plus toripalimab and HAIC of FOLFOX had promising antitumor activity and manageable toxicity, and might improve survival compared with lenvatinib monotherapy in advanced HCC.29,30 However, due to the high cost of triple combination therapy, some patients could not afford the cost of lenvatinib ($5225 per month for patients <60 kg and $7837 per month for patients over 60 kg), and received toripalimab plus HAIC ($3110 per month, including $1120 of toripalimab). A previous study had suggested that the prognosis of patients was significantly better with HAIC combined with PD-1 antibodies than with HAIC alone. 31 Additionally, chemotherapy combined with PD-1 antibodies has been recommended as the first-line treatment in other malignancies such as lung cancer and nasopharyngeal carcinoma. Toripalimab is cheaper than other PD-1 antibodies, including nivolumab and pembrolizumab. Therefore, we think HAIC plus toripalimab may be the promising treatments for these patients.
In addition, there were other limitations in this study. First, it was retrospective in nature, which might limit the interpretation of the results. Nevertheless, the baseline characteristics were well balanced, and PSM analysis was used to improve the intergroup comparability. Second, patients with HBV accounted for 88.1% of all cases in this study. As such, whether these findings may be applicable to Western countries, where HCC is more commonly caused by hepatitis C virus and alcohol use, 32 needs further study. Finally, >30% of patients had extrahepatic lesions in both groups. Generally speaking, the target of HAIC is intrahepatic lesions. However, a large sample retrospective study comparing HAIC with sorafenib reported that HAIC improve survival compared to sorafenib in patients with advanced HCC even though >50% of patients had extrahepatic lesions. 16 Thus, HAIC may be a promising treatment for patients with extrahepatic lesions.
In summary, compared with lenvatinib monotherapy, toripalimab plus HAIC might be tolerable, with a longer PFS, OS, and higher ORR, which needed to be confirmed in the future phase 3 trial.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338211063848 for Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma by Yu-Jie Xu, Zhi-Cheng Lai, Min-Ke He, Xiao-Yun Bu, Huan-Wei Chen, Yuan-Min Zhou, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Ming Shi and Qi-Jiong Li in Technology in Cancer Research & Treatment
Supplemental material, sj-doc-2-tct-10.1177_15330338211063848 for Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma by Yu-Jie Xu, Zhi-Cheng Lai, Min-Ke He, Xiao-Yun Bu, Huan-Wei Chen, Yuan-Min Zhou, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Ming Shi and Qi-Jiong Li in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338211063848 for Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma by Yu-Jie Xu, Zhi-Cheng Lai, Min-Ke He, Xiao-Yun Bu, Huan-Wei Chen, Yuan-Min Zhou, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Ming Shi and Qi-Jiong Li in Technology in Cancer Research & Treatment
Glossary
Abbreviations
- AFP
alpha-fetoprotein
- CI
confidence interval
- DCR
disease control rate
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- FOLFOX
oxaliplatin, fluorouracil, and leucovorin
- HAIC
hepatic arterial infusion chemotherapy
- HBV
hepatitis B virus infection
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ORR
objective response rate
- OS
overall survival
- PD-1
programmed cell death protein-1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- RECIST
response evaluation criteria in solid tumors
- SD
standard deviation.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Statements: Ethical approval to report this case series was obtained from the Institutional Review Board of Sun Yat-sen University Cancer Center (B2021-047-01), First People’s Hospital of Foshan (no. 201910), and Guangzhou No. 12 People’s Hospital (no. 2019092). The study was conducted in accordance with the Declaration of Helsinki. Each patient signed the informed consents before treatments at the first hospitalization and kept them in the medical record.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key R&D Program of China (2017YFA0505803), National Natural Science Foundation of China (Nos. 81625017 and 82072610], National Science and Technology Major Project of China (2018ZX10302205), and Research and Development Planned Project in Key Areas of Guangdong Province (2019B110233002).
ORCID iD: Qi-Jiong Li https://orcid.org/0000-0002-3149-1645
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249 [DOI] [PubMed] [Google Scholar]
- 2.Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy versus sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4(5):661-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. [DOI] [PubMed] [Google Scholar]
- 6.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. [DOI] [PubMed] [Google Scholar]
- 8.Keam SJ. Toripalimab: first global approval. Drugs. 2019;79(5):573-578. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As second-line therapy in patients With advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193-202. [DOI] [PubMed] [Google Scholar]
- 10.Yau T, Park JW, Finn RS, et al. Checkmate 459: a randomized, multi-center phase III study of nivolumab (NIVO) versus sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874-v875. [Google Scholar]
- 11.Lesterhuis WJ, Punt CJ, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121(8):3100-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WM, Fowler DW, Smith P, et al. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102(1):115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305-314. [DOI] [PubMed] [Google Scholar]
- 14.Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1535-1537. [DOI] [PubMed] [Google Scholar]
- 15.Johnson PJ. Systemic chemotherapy of liver tumors. Semin Surg Oncol. 2000;19(2):116-124. [DOI] [PubMed] [Google Scholar]
- 16.Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin versus sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60-69. [DOI] [PubMed] [Google Scholar]
- 17.Kudo M, Matsui O, Izumi N, et al. JSH Consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3-4):458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Martinelli E, Cheng A, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31(3):334-351. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 20.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J. Discussion of research using propensity-score matching: comments on ‘A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003’ by peter Austin, statistics in medicine. Stat Med. 2008;27(12):2055-2061; discussion 2066-2059. [DOI] [PubMed] [Google Scholar]
- 22.Mathew M, Enzler T, Shu CA, et al. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther. 2018;186:130-137. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999-1008. [DOI] [PubMed] [Google Scholar]
- 24.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. [DOI] [PubMed] [Google Scholar]
- 25.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301-1314. [DOI] [PubMed] [Google Scholar]
- 26.Heimbach JK, Kulik LM, Finn RS, et al. AASLD Guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358-380. [DOI] [PubMed] [Google Scholar]
- 27.European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. [DOI] [PubMed] [Google Scholar]
- 28.Lyu N, Zhao M. Hepatic arterial infusion chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase 3 trial (The FOHAIC-1 study). J Clin Oncol. 2021;39(15_suppl):4007. [DOI] [PubMed] [Google Scholar]
- 29.MinKe He SMZLQL. A phase II trial of lenvatinib plus toripalimab and hepatic arterial infusion chemotherapy as a first-line treatment for advanced hepatocellular carcinoma (LTHAIC study). In MinKe He SMZLQL, Department of Hepatobiliary Oncology SY-sUCCSKLoOiSCCICfCMGC, Department of Hepatobiliary Oncology SY-sUCCSKLoOiSCCICfCMGVAC, (Eds). ASCO Annual Meeting: American Society of Clinical Oncology 2021. [Google Scholar]
- 30.He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei J, Li SH, Li QJ, et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu MC, Yuan JM, Govindarajan S, et al. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol. 2000;14(8):703-709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338211063848 for Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma by Yu-Jie Xu, Zhi-Cheng Lai, Min-Ke He, Xiao-Yun Bu, Huan-Wei Chen, Yuan-Min Zhou, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Ming Shi and Qi-Jiong Li in Technology in Cancer Research & Treatment
Supplemental material, sj-doc-2-tct-10.1177_15330338211063848 for Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma by Yu-Jie Xu, Zhi-Cheng Lai, Min-Ke He, Xiao-Yun Bu, Huan-Wei Chen, Yuan-Min Zhou, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Ming Shi and Qi-Jiong Li in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338211063848 for Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma by Yu-Jie Xu, Zhi-Cheng Lai, Min-Ke He, Xiao-Yun Bu, Huan-Wei Chen, Yuan-Min Zhou, Li Xu, Wei Wei, Yao-Jun Zhang, Min-Shan Chen, Rong-Ping Guo, Ming Shi and Qi-Jiong Li in Technology in Cancer Research & Treatment