Key Points
Question
What are recent trends in oropharyngeal cancer (OPC) incidence, stage at diagnosis, and mortality in all 50 US states and the District of Columbia?
Findings
This cross-sectional study including 260 182 patients with OPC found that the incidence of OPC has increased 2.7% per year during 2001 through 2017 among men, with the most pronounced rise (3.1% per year) occurring for patients diagnosed with regional stage. Among men, increases of over 3.5% per year were largely clustered in the Southeastern and Midwestern states, and among women, an increase of over 2% per year was also mostly concentrated in the Southeast and Midwest; overall OPC incidence-based mortality increased 2.1% per year among men.
Meaning
Improved OPC prevention to combat rising disease burden and mortality is needed, and future research is also required to understand geographic disparities.
Abstract
Importance
Oropharyngeal cancer (OPC) incidence is rising among men in the US. Comprehensive assessments of nationwide trends in OPC incidence and mortality by demographics, tumor characteristics at diagnosis, and geography are lacking.
Objective
We examined secular trends in OPC incidence and mortality rates in all 50 US states and the District of Columbia (DC).
Design, Setting, and Participants
In this cross-sectional study, we used the US Cancer Statistics data set to examine OPC incidence trends from 2001 through 2017. Observed and incidence-based mortality trends were evaluated using data from the National Center for Health Statistics and Surveillance Epidemiology and End Results program, respectively. Data analysis was conducted from January to April 2021.
Results
Nationwide, 260 182 OPC cases were identified; 209 297 (80%) occurred in men, 168 674 (65%) with regional stage, and 142 068 (55%) in the Southeast and Midwest regions, during 2001 to 2017. Incidence of OPC increased nationally 2.7% per year among men, with a notable (over 3% per year) rise among non-Hispanic White men and in men aged 65 years and older. Overall, among women, the annual percentage change was 0.5% (95% CI, −0.28% to 1.22%). Among men, with a 3.1% per year rise (95% CI, 2.4% to 3.8%), regional-stage OPC incidence increased nearly 2-fold. Among women, regional-stage OPC incidence increased 1.0% per year (95% CI, 0.3% to 1.7%). Among men, OPC incidence increased in all states and regions except Alaska, DC, and Wyoming. Among men, the most pronounced increases (more than 3.5% per year) were clustered in the Southeast and Midwest regions. Among women, a rise of more than 2% per year was also concentrated in the Southeast and Midwest regions. Among men, OPC incidence-based mortality increased 2.1% per year (95% CI, 1.0% to 3.2%) overall in recent years (from 2006 to 2017). In contrast, among women, the annual percentage change in OPC incidence-based mortality was −1.2% (95% CI, −2.5% to 0.1%).
Conclusions and Relevance
The findings of this cross-sectional study suggest that the incidence of OPC has continued to increase nationally among men in the US, with rapid increases among the elderly population. The notable rise in regional-stage OPC and the concurrent recent rise in mortality among men is troubling and calls for urgent improvements in prevention. Distinct geographic patterns with notable rises in the Midwest and Southeast regions imply the need for improved and targeted prevention as well as future studies to understand etiological reasons for geographic disparities.
This cross-sectional study examines incidence and mortality of oropharyngeal cancer within various demographic groups in the US.
Introduction
Oropharyngeal cancer (OPC) incidence has continued to rise among men since the 1970s in the US.1 Notably, OPC incidence and the annual number of cases (burden) among men have surpassed those of cervical cancer, making OPC the most common cancer caused by human papillomavirus infection (HPV) in the US.2 Unlike cervical cancer, screening for OPC is not possible owing to the current inability to detect precancerous lesions and inadequate diagnostic technology to identify localized tumors.3 In the absence of screening, the rise in OPC incidence may be attributable to an increase in the advanced disease stage; however, such evidence is currently lacking. The rise in incidence might be expected to be accompanied by an increase in mortality. However, contemporary trends in OPC mortality rates have not been evaluated.
In addition to HPV, OPC risk factors include smoking and alcohol use.4,5,6,7 Heterogeneous patterns in these risk factors have been observed across the states in the US,8,9,10 implying that there may be state-level variation in OPC incidence trends. Previous studies documenting the rise in OPC incidence have used data from cancer registries participating in the Surveillance Epidemiology and End Results (SEER) program, representing only 28% of the US population limited to 10 states and selected population subgroups within 4 additional states. Currently, a comprehensive description of OPC incidence trends and burden for each state is unavailable. Such data could provide a complete view of the national-level and state-level trajectories in OPC disease and could have important implications for OPC prevention.
To provide a comprehensive view of OPC disease in the US, we examined secular trends in OPC incidence and mortality rates at national and state levels for all 50 US states and the District of Columbia (DC). Incidence and mortality trends were further characterized by demographic and tumor characteristics at the time of OPC diagnosis.
Methods
Data Sources
We analyzed the US Cancer Statistics data set that comes from the 2 federally funded population-based sources of data—the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and the National Cancer Institute’s SEER Program. This data set includes cancer incidence information collected from central cancer registries that use uniform data items and codes as documented by the North American Association of Central Cancer Registries. We identified OPC diagnoses from 2001 to 2017 from 50 states and DC, covering 100% of the US population.
Data regarding OPC mortality were derived from information recorded in death certificates ascertained by the National Center for Health Statistics (NCHS). The information regarding cancer diagnostic characteristics (ie, histology, stage, tumor size) are not collected on death certificates; therefore, to evaluate incidence-based mortality (ie, specific to tumor diagnostic characteristics), we used the SEER-13 registry incidence-based mortality file that links the SEER-13 cancer incidence file with death certificate information.11 To prevent underestimation of incidence-based mortality (IBM) rates, we considered diagnoses during 1992-2016 and deaths during 2001-2017. The institutional review board of the University of Texas Health Science Center deemed this study exempt from review owing to the use of publicly available deidentified data.
Demographic Characteristics
The demographic characteristic information submitted to each cancer registry was abstracted from patient medical records. We identified sex, age at cancer diagnosis, and race/ethnicity. Race/ethnicity was classified as non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic other races, including American Indian/Alaska Native, Asian/Pacific Islander, and unknown. Information on age at death was extracted from death certificates.
Case Definitions and Tumor Characteristics
Oropharyngeal cancers that are generally attributable to HPV include the base of the tongue, lingual tonsil, soft palate and uvula, tonsil, oropharynx, and Waldeyer ring (based on International Classification of Diseases for Oncology, Third Revision [ICD-O-3] site codes C01.9, 02.4, 02.8, 05.1-05.2, 09.0-09.1, 09.8-09.9, 10.0-10.4, 10.8-10.9, 14.0, 14.2, 14.8, and according to the classification suggested by the CDC).3,12,13,14,15 All cancers were malignant, and histology codes 8050-8084 and 8120-8131 were used to confirm squamous cell histologies. The SEER Summary Stage 2000 variable was used to classify stage at diagnosis as localized (confined to oropharynx), regional (spread outside the oropharynx area to nearby structures or lymph nodes), distant (spread to distant parts of the body), or unknown stage.
Statistical Analysis
Persons of unknown age and sex and those whose cancer was diagnosed at autopsy or was first documented on the death certificate were excluded. Only microscopically confirmed cases and the first matching records were included in the final analytical sample. We estimated OPC incidence rates overall and by subgroups of sex, age at diagnosis, race and ethnicity, stage at diagnosis, tumor size, and state of residence during tumor diagnosis. We used SEER*Stat version 8.3.9 to estimate incidence and mortality rates. Person-years were estimated by summing population sizes across calendar years. Incidence estimates were age-adjusted to the 2000 US standard population and were expressed per 100 000 person-years.
To examine trends in incidence rates and mortality over time and to calculate annual percentage changes (APCs) and average APCs (AAPCs), we used the National Cancer Institute’s Joinpoint Regression Analysis program (version 4.8.0).16 The APC characterizes trend, a single regression line on a log scale fitted over a fixed interval, whereas the AAPC is a weighted average of the APCs from the joinpoint model with the weights equal to the length of the APC interval. To determine whether the trends were different from 0, a t test was used for zero joinpoints, and a z test was used for one or more joinpoints. Statistical significance was assessed at a level of P < .05, and all hypotheses were 2-sided.
Results
Of 295 365 OPC cases diagnosed nationally, 260 182 (88%) met the case definition and were included in the incidence analysis (eFigure 1 in the Supplement). Men (209 297 [80%]), White patients (217 956 [84%]), and those with regional stage (168 674 [65%]) comprised the majority of cases (eTable 1 in the Supplement). More than 50% of all cases were diagnosed in the Southeastern (32%) and Midwestern (22%) states. The annual number of cases and incidence rates are provided in eFigure 2 and eTables 2, 3, 4, 5, 6, and 7 in the Supplement.
Incidence of OPC by Age, Sex, and Race and Ethnicity
Age-specific incidence rates for early (2001-2003) vs recent (2015-2017) years by race and ethnicity are presented in Figure 1. Corresponding rate ratios are presented in eFigure 3 and eTables 8 and 9 in the Supplement. Incidence of OPC increased among White men aged 40 years or older, with the peak incidence during early years (22.0 per 100 000 person-years) and recent years (42.0 per 100 000 person-years) occurring among those aged 60 to 64 years. The absolute incidence was lower among White men aged younger than 40 in recent years than in earlier years; however, the change was not significant. The incidence mostly decreased across age groups among Black men and remained stable among Hispanic men. Among White women, the peak incidence during early and recent years was observed among patients 70 to 74 years old. The incidence mostly declined across age groups among Black women and was stable among Hispanic women.
Figure 1. Age-Specific and Race-Specific Incidence Rates of Oropharyngeal Cancer During 2001-2003 and 2015-2017.
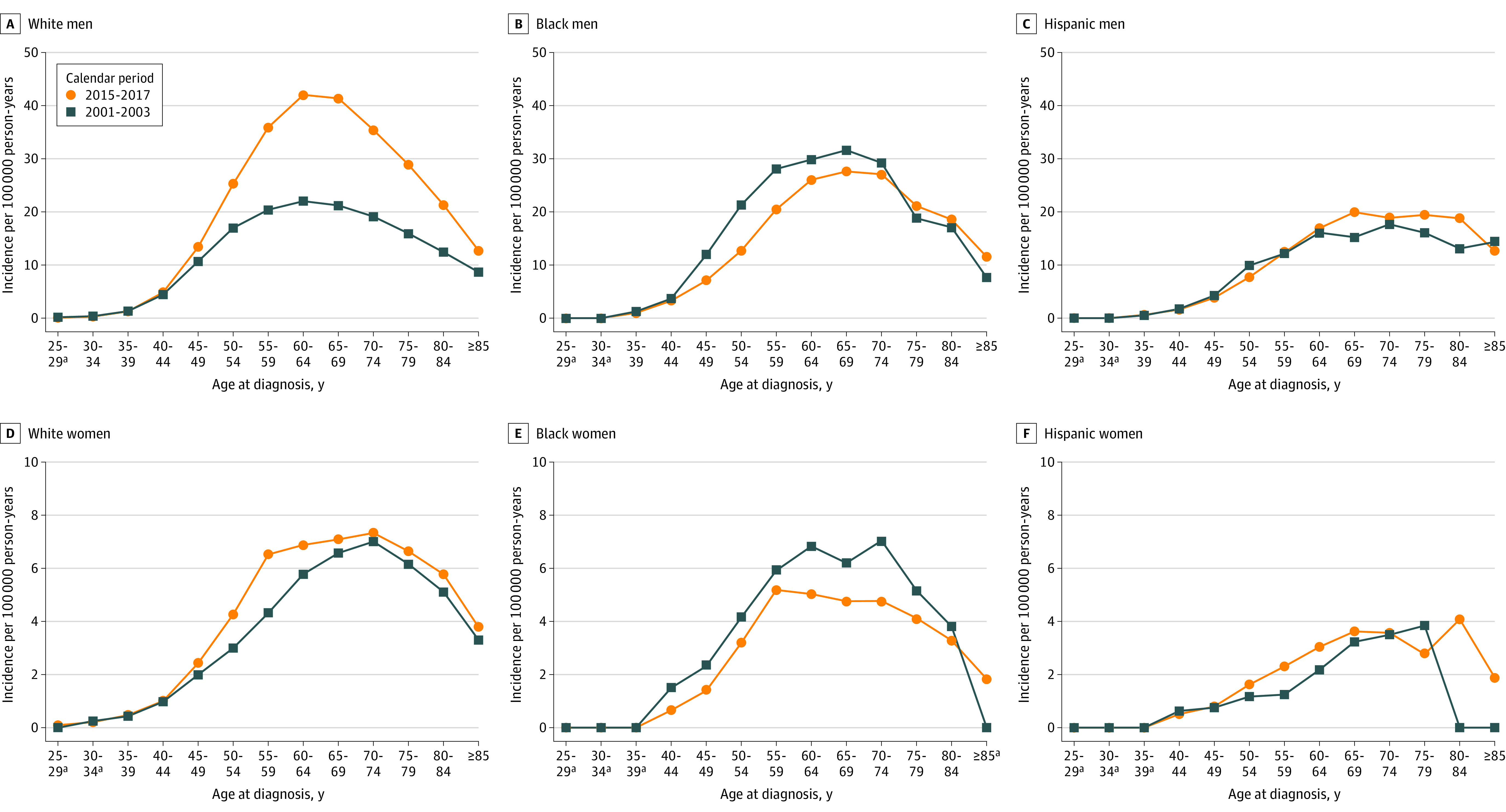
Panels A, B, C, D, E, and F show incidence rates according to 5-year age group among non-Hispanic White men, non-Hispanic Black men, Hispanic men, non-Hispanic White women, non-Hispanic Black women, and Hispanic women, respectively. Data were taken from the National Program of Cancer Registries and Surveillance Epidemiology and End Results program. Rates were calculated as number of cases per 100 000 person-years and age-adjusted to the 2000 US standard population.
aData suppressed owing to <16 cases in the time interval.
Trends in OPC Incidence Overall and According to Race, Age, and Stage at Diagnosis
Overall, from 2001 to 2017, OPC incidence increased 2.7% per year (95% CI, 2.5% to 2.9%) among men, rising from 5.9 per 100 000 to 8.9 per 100 000. No significant change occurred among women (1.6 per 100 000 to 1.7 per 100 000) (eTables 2, 10, and 11 in the Supplement).
The most pronounced increase occurred among White men (AAPC = 3.5%; 95% CI, 3.0% to 3.9%), followed by other races and ethnic groups (AAPC = 2.0%; 95% CI, 1.2% to 2.7%) (Figure 2A). Incidence declined among Black men (AAPC = −1.4%; 95% CI, −1.8% to −1.0%), while no significant change occurred among Hispanic men. The increase was relatively slower for White women (AAPC = 1.1%; 95% CI, 0.3% to 1.9%) (Figure 2B). The incidence rates declined among Black women (AAPC = −1.9%; 95% CI, −2.3% to −1.5%), but remained stable among Hispanic and other women (Figure 2B). Burden of OPC among men (81% in 2001 and 85% in 2017) and women (82% in 2001 and 83% in 2017) over the study duration was predominantly of White patients (eFigure 2 in the Supplement). The number of new cases among Black men and women remained stable from 2001 to 2017.
Figure 2. Trends in Annual Incidence Rates of Oropharyngeal Cancer According to Race and Ethnicity and Age at Diagnosis Among Men and Women.
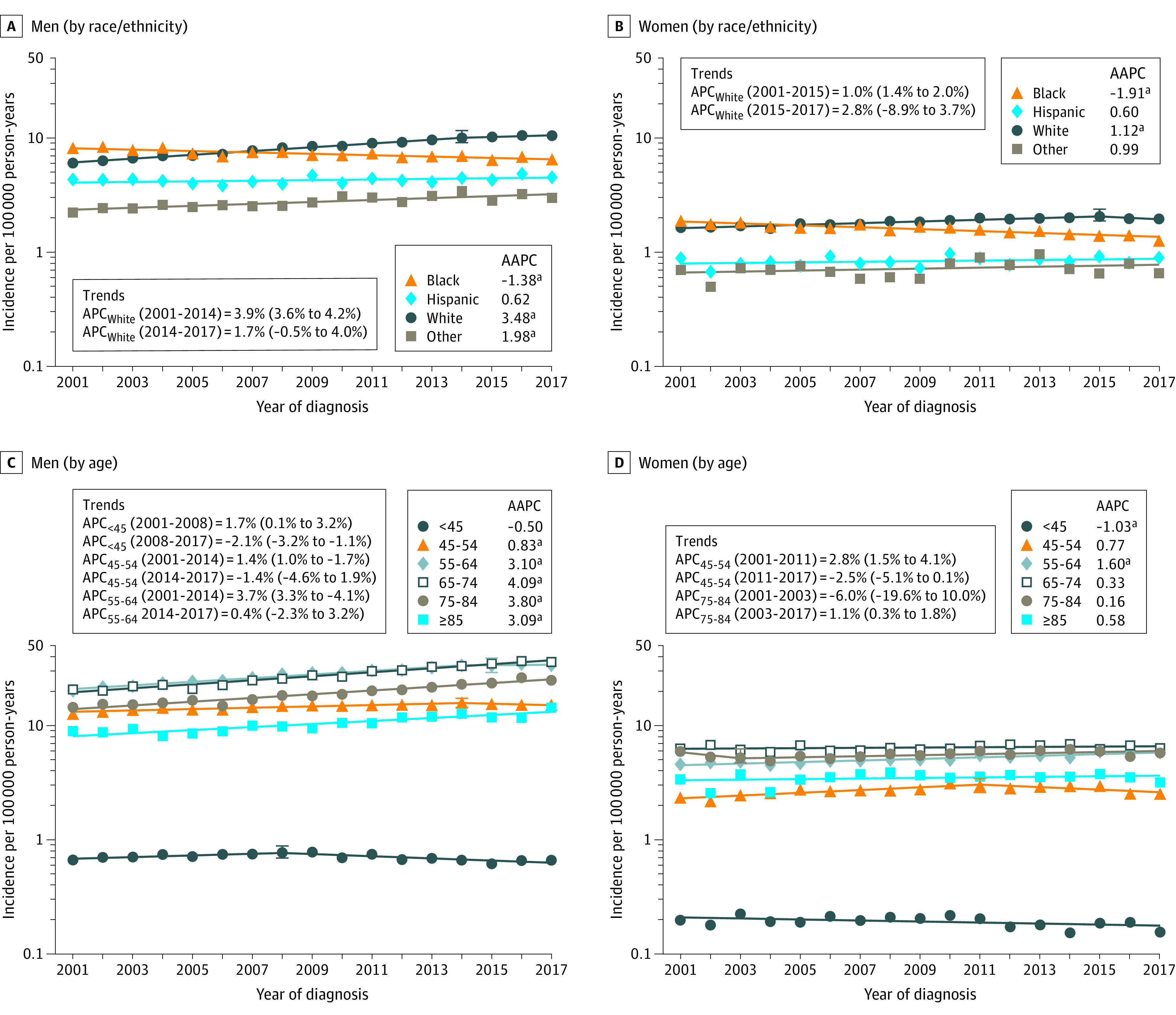
Data markers represent the observed incidence rates (cases per 100 000 person-years) of oropharyngeal cancer. The slope of the lines represents the annual percent change (APC). Panels A and B show incidence trends according to race/ethnicity in men and women, respectively. Panels C and D show incidence trends according to age at diagnosis in men and women, respectively. Data were taken from the National Program of Cancer Registries and Surveillance Epidemiology and End Results program. Rates were calculated as number of cases per 100 000 person-years and age-adjusted to the 2000 US standard population. Abbreviations: APC, annual percentage change; AAPC, average annual percentage change.
aIndicates statistically significant incidence trend (P < .05).
Among men aged younger than 45 years, after an initial increase of 1.7% per year (95% CI, 0.1% to 3.2%) from 2001 to 2008, OPC incidence declined from 2008 to 2017 (APC = −2.1%; 95% CI, −3.2% to −1.1%) (Figure 2C). From 2014 to 2017, incidence plateaued among men aged 45 to 54 and 55 to 64 years after initial increases. The incidence among men increased over 3% per year among all age groups 65 years and older. Among women, a decline occurred among the youngest (<45 years old) age group (AAPC = −1.0%; 95% CI, −2.0% to −0.1%), while incidence increased in 55 to 64-year-old women (AAPC = 1.6%; 95% CI, 1.3% to 1.9%) (Figure 2D). During 2001-2017, nationally, the proportion of patients aged 55 years and older increased from 61.9% in 2001 to 78.3% in 2017 among men and from 73.1% to 80.7% among women (eFigure 2 in the Supplement).
When characterized by stage, a marked increase was observed for the regional stage with an AAPC of 3.1% (95% CI, 2.4% to 3.8) among men and 1.0% (95% CI, 0.3% to 1.7%) among women (Figure 3A and 3B). Decrease in localized stage occurred for women (AAPC = −0.9%; 95% CI, −1.4% to −0.4%) but not for men (AAPC = −0.3%; 95% CI, −1.0% to 0.4%). Overall, no change in AAPC was observed for the distant or unknown stages. The proportion of patients diagnosed with regional stage increased from 65.3% in 2001 to 73.7% in 2017 among men and from 58.3% to 66.1% in women (eFigure 2 in the Supplement). Among both men and women, OPC incidence increased for every tumor size category, with prominent increases occurring for tumor sizes of 2 to 4 cm (AAPC among men = 4.1% [95% CI, 2.6% to 5.6%] and among women = 2.6% [95% CI, 1.6% to 3.7%]), and greater than 4 cm (AAPC among men = 4.6% [95% CI, 3.7% to 5.5%] and among women = 2.2% [95% CI, 1.1% to 3.4%]) (eTables 10 and 11 in the Supplement).
Figure 3. Trends in Annual Incidence Rates of Oropharyngeal Cancer According to Stage at Diagnosis Among Men and Women.
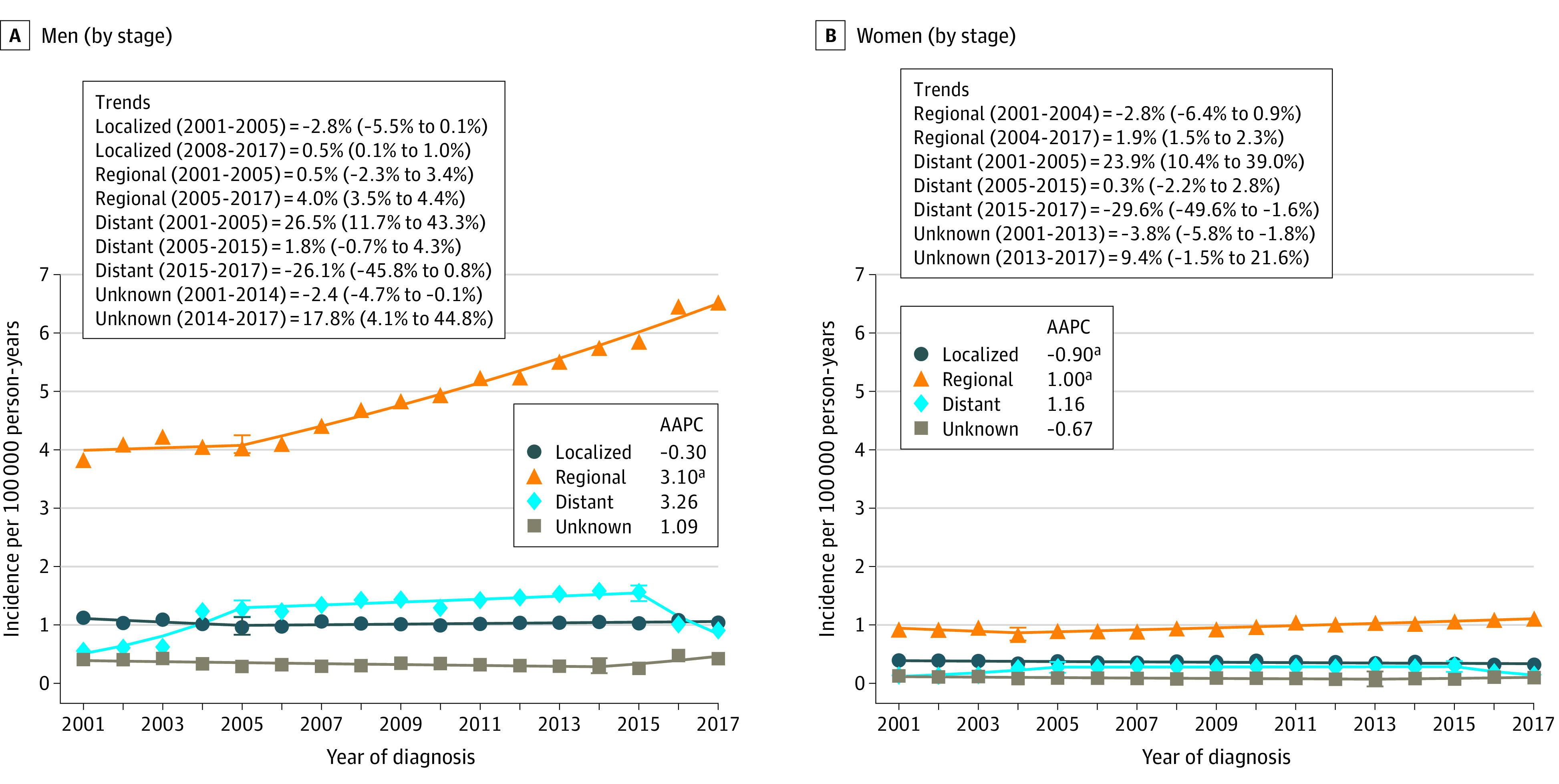
Data markers represent the observed incidence rates (cases per 100 000 person-years) of oropharyngeal cancer. The slope of the lines represents the annual percent change (APC). Panels A and B show incidence trends in men and women according to the SEER summary stage at diagnosis. Data taken from the National Program of Cancer Registries and Surveillance Epidemiology and End Results program. Rates were calculated as number of cases per 100 000 person-years and age-adjusted to the 2000 US standard population.
aIndicates statistically significant incidence trend (P < .05).
Trends in OPC Incidence and Burden by State
Incidence of OPC in 2001-2003 and 2015-2017 and calendar trends (AAPCs) among men and women by US state are presented in Figure 4; eTables 12 and 13 in the Supplement. Among men, OPC incidence increased in all states except Alaska, DC, and Wyoming. Of the top 15 states with the most rapid notable increase, 11 were in the Midwest (South Dakota [6.0%], Kansas [4.7%], Iowa [4.0%], Ohio [3.7%], Indiana [3.6%], Minnesota [3.5%], Missouri [3.4%], Nebraska [3.4%]) and the Southeast (Kentucky [3.9%], West Virginia 3.8%], Tennessee [3.8%]). Among women, the incidence decreased significantly in California (AAPC = −0.8%) and New Hampshire (AAPC = −2.2%). In contrast, a marked rise was largely concentrated in Southeastern states (Louisiana [AAPC = 3.1%], Kentucky [AAPC = 2.7%], Arkansas [AAPC = 2.6%]), Mississippi [AAPC = 2.4%], North Carolina [AAPC = 1.3%], Maryland [AAPC = 1.2%]), and Midwestern states (Indiana [AAPC = 2.4%], Ohio [AAPC = 2.2%], Iowa [AAPC = 2.0%], and Missouri [AAPC = 1.3%]). Throughout 2001-2017, more than 50% of all OPC cases were diagnosed in the Midwest and Southeast (eFigure 4 in the Supplement). Average annual percent changes in decreasing order are presented schematically in eFigure 5 in the Supplement. Incidence rates and burden for each state for 2001 and 2017 are presented in eFigures 6 and 7 in the Supplement.
Figure 4. Oropharyngeal Cancer Incidence Rates and Trends Among Men and Women by US State and District of Columbia.
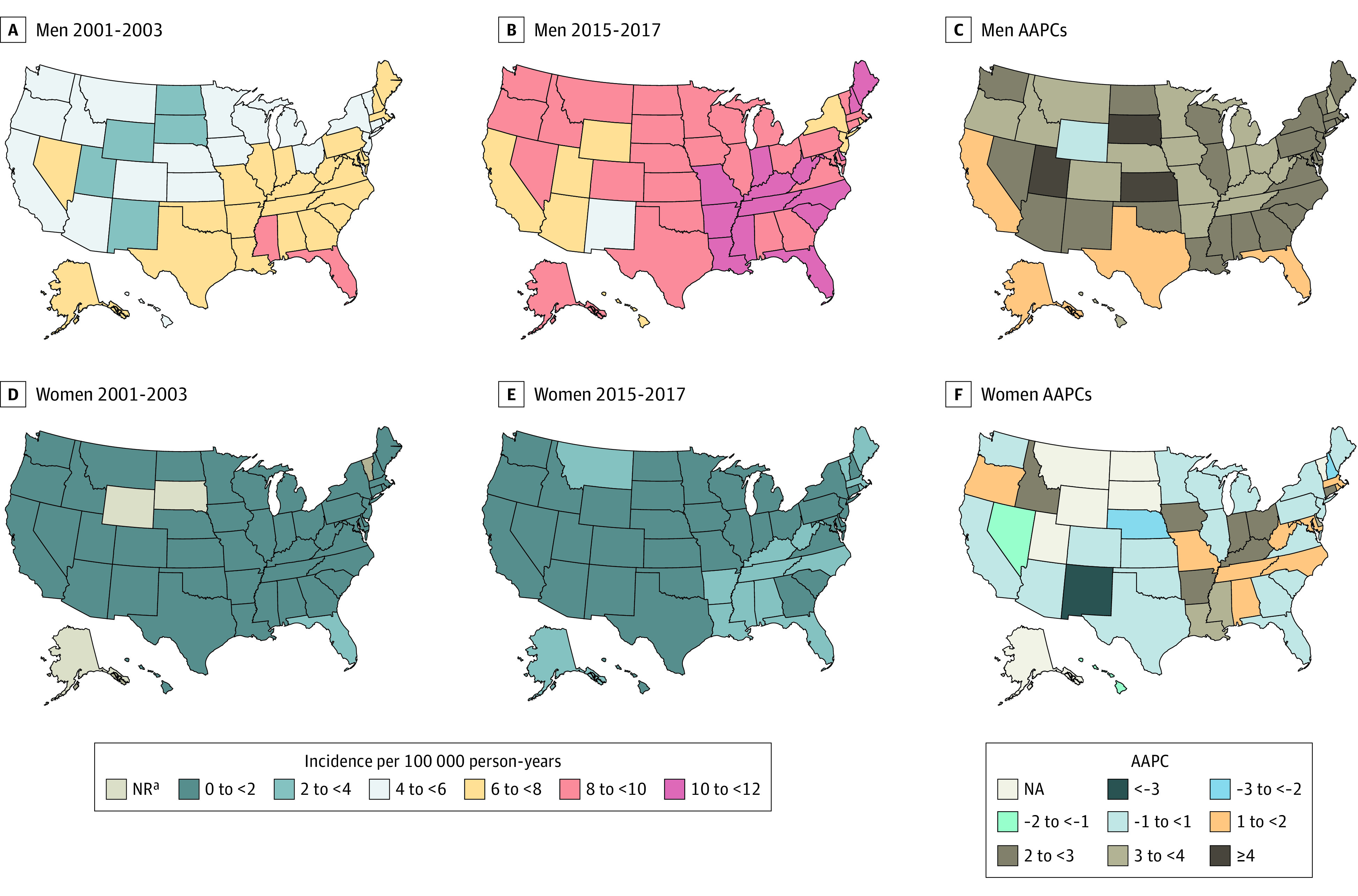
Panels A and B illustrate oropharyngeal cancer incidence rates among men in 2001-2003 and 2015-2017; panel C illustrates the average annual percentage change in incidence from 2001 to 2017. Panels D and E illustrate oropharyngeal cancer incidence rates among women in 2001-2003 and 2015-2017; panel F illustrates the average annual percentage change in incidence from 2001 to 2017. Data taken from the National Program of Cancer Registries and Surveillance Epidemiology and End Results program. Rates were calculated as number of cases per 100 000 person-years and age-adjusted to the 2000 US standard population. Abbreviations: AAPC, average annual percentage change; NA, not applicable; NR, not reported due to fewer than 16 cases diagnosed.
Trends in Observed and OPC Incidence-Based Mortality
A total of 111 291 OPC deaths were identified nationally during 2001-2017. For IBM analysis, 5522 deaths were identified among eligible OPC cases in SEER-13 regions. The annual number of deaths and observed and IBM rates are provided in eTable 14 in the Supplement.
Nationally, OPC observed mortality rates decreased in men (APC = −1.3%; 95% CI, −1.9% to −0.7%) and women (APC = −1.7%; 95% CI, −2.5% to −1.0%) during 2001 to 2009 and then increased in men at 1.1% per year (95% CI, 0.5% to 1.7%) after 2009 but no change occurred in women (Table). Overall, mortality rates increased in White men (AAPC = 0.6%; 95% CI = 0.3% to 1.0%), whereas the rates declined among Black men (AAPC = −3.3%; 95% CI, −3.9% to −2.7%); the observed mortality rate across 2001-2017 was greater among Black men (5.6 per 100 000 person-years in 2001, 3.4 per 100 000 person-years in 2017) than White men (3.0 per 100 000 person-years in 2001, 3.3 per 100 000 person-years in 2017).
Table. Trends in Observed and Incidence-Based Oropharyngeal Cancer Mortality Rates According to Race and Ethnicity, Age at Death, Age at Diagnosis, and Tumor Size During 2001-2017 Among Men and Women in the USa.
| Characteristics | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | APC (95% CI)b | P value | AAPC (95% CI)b | P value | Year | APC (95% CI)b | P value | AAPC (95% CI)b | P value | |
| Observed US oropharyngeal cancer mortality rates | ||||||||||
| Overall | 2001-2009 | −1.3 (−1.9 to −0.7) | <.001 | −0.1 (−0.5 to 0.3) | .60 | 2001-2009 | −1.7 (−2.5 to −1.0) | <.001 | −0.8 (−1.3 to −0.3) | <.001 |
| 2009-2017 | 1.1 (0.5 to 1.7) | <.001 | 2009-2017 | 0.1 (−0.6 to 0.8) | .73 | |||||
| Race/ethnicity | ||||||||||
| White | 2001-2009 | −0.5 (−1.1 to 0.2) | .13 | 0.6 (0.2 to 1.0) | <.001 | 2001-2013 | −0.9 (−1.3 to −0.5) | <.001 | −0.2 (−0.7 to 0.4) | .51 |
| 2009-2017 | 1.8 (1.2 to 2.4) | <.001 | 2013-2017 | 2.1 (−0.1 to 4.3) | .05 | |||||
| Black | 2001-2017 | −3.3 (−3.9 to −2.7) | <.001 | −3.3 (−3.9 to −2.7) | <.001 | 2001-2017 | −2.3 (−3.1 to −1.4) | <.001 | −2.3 (−3.1 to −1.4) | <.001 |
| Hispanic | 2001-2011 | −2.2 (−3.3 to −1.1) | <.001 | −0.7 (−1.7 to 0.2) | .11 | 2001-2017 | 0.2 (−0.9 to 1.4) | .65 | 0.2 (−0.9 to 1.4) | .65 |
| 2011-2017 | 1.8 (−0.2 to 3.8) | .07 | ||||||||
| Otherc | 2001-2009 | −4.2 (−7.2 to −1.1) | .01 | 0.1 (−1.7 to 1.9) | .95 | 2001-2017 | −1.1 (−2.6 to 0.4) | .13 | −1.1 (−2.6 to 0.4) | .13 |
| 2009-2017 | 4.5 (1.9 to 7.1) | <.002 | ||||||||
| Age at death, y | ||||||||||
| <45 | 2001-2017 | −2.2 (−2.3 to −1.5) | <.001 | −2.2 (−3.0 to −1.5) | <.001 | 2001-2017 | −2.4 (−3.6 to −1.2) | <.001 | −2.4 (−3.6 to −1.2) | <.001 |
| 45-54 | 2001-2017 | −1.8 (−2.3 to −1.3) | <.001 | −1.8 (−2.3 to −1.3) | <.001 | 2001-2017 | 0.6 (−0.2 to 1.4) | .14 | 0.6 (−0.2 to 1.4) | .14 |
| 55-64 | 2001-2006 | −3.2 (−5.8 to −0.5) | .02 | −0.2 (−1.0 to 0.7) | .68 | 2001-2011 | −1.9 (−3.1 to −0.6) | <.01 | 0.1 (−1.0 to 1.1) | .90 |
| 2006-2017 | 1.2 (0.5 to 1.9) | .003 | 2011-2017 | 3.4 (1.0 to 5.9) | .01 | |||||
| 65-74 | 2001-2010 | −0.8 (−1.4 to −0.2) | .01 | 0.4 (−0.1 to 0.8) | .06 | 2001-2012 | −2.4 (−3.2 to −1.7) | <.001 | −1.3 (−2.1 to −0.6) | <.001 |
| 2010-2017 | 2.0 (1.3 to 2.8) | <.001 | 2012-2017 | 1.1 (−1.2 to 3.5) | .32 | |||||
| 75-84 | 2001-2017 | 0.4 (0.0 to 0.8) | .05 | 0.4 (0.0 to 0.9) | .05 | 2001-2008 | −2.6 (−3.9 to −1.3) | <.001 | −1.1 (−1.8 to −0.4) | <.01 |
| 2008-2017 | 0.1 (−0.8 to 1.0) | .87 | ||||||||
| ≥85 | 2001-2017 | 0.4 (−0.4 to 1.3) | .34 | 0.4 (−0.5 to 1.3) | .34 | 2001-2017 | −0.4 (−0.9 to −0.1) | .02 | −0.4 (−0.8 to −0.1) | .02 |
| Incidence-based mortalityd | ||||||||||
| Overall | 2001-2006 | −2.8 (−6.7 to 1.3) | .16 | 0.5 (−0.8 to 1.9) | .45 | 2001-2017 | −1.2 (−2.5 to 0.1) | .06 | −1.2 (−2.5 to 0.1) | .06 |
| 2006-2017 | 2.1 (1.0 to 3.2) | .002 | ||||||||
| Race/ethnicity | ||||||||||
| White | 2001-2017 | 1.9 (0.9 to 2.8) | <.001 | 1.9 (0.9 to 2.8) | <.001 | 2001-2017 | −0.9 (−2.2 to 0.4) | .16 | −0.9 (−2.2 to 0.4) | .16 |
| Black | 2001-2017 | −2.5 (−4.3 to −0.6) | .01 | −2.5 (−4.3 to −0.6) | .01 | 2001-2017 | −1.9 (−4.5 to 0.7) | .14 | −1.9 (−4.5 to 0.7) | .14 |
| Hispanic | 2001-2017 | 1.3 (−2.0 to 4.8) | .41 | 1.3 (−2.0 to 4.8) | .41 | 2001-2017 | 0.8 (−4.6 to 6.5) | .77 | 0.8 (−4.6 to 6.5) | .77 |
| Other | 2001-2017 | 0.3 (−2.7 to 3.3) | .84 | 0.3 (−2.7 to 3.3) | .84 | 2001-2017 | 2.3 (−2.5 to 7.4) | .33 | 2.3 (−2.6 to 7.4) | .33 |
| Age at diagnosis, y | ||||||||||
| <45 | 2001-2017 | −2.5 (−5.5 to 0.6) | .10 | −2.5 (−5.5 to 0.5) | .10 | 2001-2016 | −3.9 (−10.1 to 2.7) | .21 | −3.9 (−10.1 to 2.7) | .21 |
| 45-54 | 2001-2017 | −0.4 (−2.0 to 1.2) | .56 | −0.4 (−2.0 to 1.2) | .56 | 2001-2017 | −0.5 (−4.1 to 3.3) | .78 | −0.5 (−4.1 to 3.3) | .78 |
| 55-64 | 2001-2017 | 2.0 (1.0 to 3.1) | <.001 | 2.0 (1.0 to 3.1) | <.001 | 2001-2017 | 0.2 (−1.7 to 2.2) | .81 | 0. 2 (−1.7 to 2.2) | .81 |
| 65-74 | 2001-2005 | −8.6 (−15.8 to −0.8) | .03 | −0.4 (−2.5 to 1.6) | .67 | 2001-2017 | −1.5 (−3.5 to 0.6) | .14 | −1.5 (−3.5 – 0.6) | .14 |
| 2005-2017 | 2.5 (1.0 to 3.9) | .003 | ||||||||
| 75-84 | 2001-2017 | 1.6 (0.4 to 2.9) | .01 | 1.6 (0.4 to 2.9) | .01 | 2001-2017 | −2.8 (−5.1 to −0.5) | .02 | −2.8 (−5.1 to −0.5) | .02 |
| ≥85 | 2001-2017 | 1.8 (−1.6 to 5.3) | .27 | 1.8 (−1.6 to 5.3) | .27 | 2001-2017 | −0.1 (−3.3 to 3.2) | .94 | −0.1 (−3.3 to 3.2) | .94 |
| Stage at diagnosis | ||||||||||
| Localized | 2001-2017 | −1.8 (−4.3 to 0.7) | .14 | −1.8 (−4.3 to 0.7) | .14 | 2001-2017 | −2.0 (−4.8 to 0.8) | .15 | −2.0 (−4.8 to 0.8) | .15 |
| Regional | 2001-2005 | −5.9 (−12.3 to 0.9) | .08 | −4.0 (−7.0 to −1.0) | .01 | 2001-2017 | −2.6 (−4.7 to −0.4) | .02 | −2.6 (−4.7 to −0.4) | .02 |
| 2005-2015 | 0.5 (−1.5 to 2.6) | .57 | ||||||||
| 2015-2017 | −20.8 (−37.4 to 0.2) | .05 | ||||||||
| Distant | 2001-2017 | 2.5 (−0.1 to 5.3) | .06 | 2.5 (−0.1 to 5.3) | .06 | 2001-2017 | −1.2 (−4.10 to 1.7) | .39 | −1.23 (−4.10 to 1.73) | .39 |
| Unknown | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Tumor size | ||||||||||
| ≤1 | 2001-2017 | −0.7 (−4.4 to 3.2) | .72 | −0.7 (−4.4 to 3.2) | .72 | 2001-2017 | −1.5 (−8.4 to 6.0) | .66 | −1.5 (−8.45 to 6.0) | .66 |
| >1 to ≤2 | 2001-2017 | 3.2 (1.4 to 5.0) | <.001 | 3.2 (1.4 to 5.0) | <.001 | 2001-2017 | 1.6 (−2.6 to 6.0) | .43 | 1.6 (−2.58 to 6.0) | .43 |
| >2 to ≤4 | 2001-2017 | 1.9 (1.1 to 2.8) | <.001 | 1.9 (1.07 to 2.8) | <.001 | 2001-2017 | 2.0 (0.1 to 3.9) | .04 | 2.0 (0.1 to 3.9) | .04 |
| >4 | 2001-2017 | 4.5 (2.9 to 6.0) | <.001 | 4.5 (2.9 to 6.0) | <.001 | 2001-2017 | 2.7 (−0.3 to 5.8) | .07 | 2.7 (−0.3 to 5.8) | .07 |
| Unknown | 2001-2017 | −3.2 (−4.4 to −2.0) | <.001 | −3.2 (−4.4 to −2.0) | <.001 | 2001-2017 | −7.4 (−9.3 to −5.4) | <.001 | −7.4 (−9.3 to −5.4) | <.001 |
Abbreviations: APC, annual percentage change; AAPC, average annual percentage change.
Rates were calculated as number of deaths per 100 000 person-years and age adjusted to the 2000 US standard population.
The calendar period of each segment was defined based on the identification of calendar years when a statistically significant change in the APC occurred (ie, the joinpoint).
Other includes Asian/Pacific Islander, American Indian/Alaskan Native, and other unspecified.
Based on cases diagnosed during 1992-2017.
Among men, IBM increased 2.1% per year (95% CI, 1.0% to 3.2%) during 2006-2017, particularly for persons who were diagnosed with OPC at age 55 years or older. Throughout 2001-2017, OPC incidence-based mortality among men increased for tumors larger than 1 cm (AAPC for tumors 1.1-2 cm = 3.2%, AAPC for tumors 2.1-4 cm = 1.9%, and AAPC for tumors 4 cm or larger = 4.5%), while no significant change occurred for tumors smaller than 1 cm. The AAPC for IBM among women was −1.2% (95% CI, −2.5% to 0.1%).
Discussion
To our knowledge, the present study is the first to describe trends in OPC incidence in all 50 US states and DC and evaluate OPC incidence-based mortality trends by demographic and tumor characteristics at diagnosis. Overall, OPC incidence continued to increase rapidly among men, particularly among those age 65 years and older. An important finding from this study was the marked increase (nearly 2-fold) in regional-stage OPC among men. Incidence-based mortality for oropharyngeal cancer increased in men in recent years, which is likely owing to increased incidence among elderly age groups and an increasing number of patients diagnosed with advanced-stage OPC. In our state-level analysis, the most pronounced increase in OPC incidence was concentrated in the Midwest and Southeast regions. Notably, these regions also contribute to more than 50% of all new OPC cases diagnosed nationally.
After years of increases, OPC incidence began to decline among young men. Notably, for the first time in several decades, absolute incidence among young White men was lower in recent years (HPV vaccination era) compared with the prevaccination era. A recent study reported a very rapid reduction (22% per year from 2010 to 2017) in cervical cancer incidence among young women not eligible for cervical cancer screening.17 Recent studies evaluating cervical cancer incidence in Puerto Rico18 and anal cancer incidence nationally19 reported similar findings among young age groups.20 A prior analysis21 showed a nearly 100% reduction in vaccine-type oral HPV infection prevalence among vaccinated men. Notably, a 38% reduction in vaccine-type oral HPV infection prevalence among unvaccinated men also occurred in the US from 2009 through 2016.22 It has been nearly 15 years since the initial implementation of HPV vaccination. The present study findings, along with these data, indicate that we may have started to see early benefits of HPV vaccination, possibly driven by herd immunity through female vaccination and direct effects. Given the long latency between HPV acquisition and cancer development, the decline may not be attributable to HPV vaccination and may reflect a recent decline in the number of sex partners or a reduction in smoking among recent birth cohorts.23,24 Future research is needed to understand the reasons for the declining trend.
Overall, OPC incidence has continued to rise rapidly among White men and those aged 65 years and older. The rising rates among men aged 45-64 years have stabilized in recent years. A modeling study projected that OPC incidence would begin to stabilize among men age 65 years and older starting from the early 2030s, and a decline will not be observed at least until the late 2040s.25 It is further projected that the annual number of OPC diagnoses among elderly men will continue to rise, with an increasing proportion of cases attributable to HPV during the next 3 to 4 decades.25 The rising OPC burden among birth cohorts who will not benefit from HPV vaccination, increases in advanced-stage disease in those age groups, and concurrent rise in mortality increase the importance of the need for alternative prevention or early detection strategies in the form of screening. While continued progress has been made in identifying biomarkers of HPV-positive OPC,26,27,28 the value of such markers for population-wide OPC screening remains low, limiting their implementation.29 Continued research is needed to develop risk-stratification tools and methods to identify precancers and develop cost-effective screening algorithms.29,30
The state-specific findings revealed distinct incidence patterns, where the increase was most prominent in the Southeast and Midwest among both men and women. This particular geographic pattern may be attributable to smoking, as these states have some of the highest proportion of adults who are current smokers.9 In contrast, in California, where smoking prevalence is lowest in the nation, OPC incidence appeared to have stabilized among men while a decline occurred among women. Future research is needed to understand whether these state-specific trends can be explained by smoking patterns or other likely etiological risk factors or behaviors.
The state-specific findings of the present study also have important implications for cancer prevention. Unfortunately, Midwestern and Southeastern states have some of the lowest HPV vaccination rates in the nation and at least 55% of parents of unvaccinated adolescents in each state are hesitant to initiate HPV vaccination.31,32,33,34 Rising OPC rates in the context of suboptimal HPV vaccination coverage, high smoking prevalence, and high vaccine hesitancy in these states indicate that if HPV vaccination coverage is not urgently improved, current geographic disparities in OPC incidence will magnify.34,35
The present study finding of a marked rise in OPC incidence-based mortality among men in recent years is troubling. This rise is likely attributable to an increasing proportion of new patients with OPC who are elderly or immunosuppressed,4,36 who may have impaired HPV clearance or immunological surveillance leading to the growth of the advanced stage tumors. This is evident from findings that show an increasing number of patients with OPC who are elderly, a growing proportion of cases with advanced tumor stage, and a marked recent rise in incidence-based mortality among OPC cases diagnosed among older age groups. Future research is needed to understand further the reasons for rising mortality, particularly among older men. Parallel with OPC incidence trends, OPC mortality rates also increased recently among White men, while a decline occurred among Black men. Despite the decrease in mortality, the absolute OPC mortality rate was higher among Black patients than White patients. Lower HPV positivity in Black individuals diagnosed with OPC and relatively lower survival among HPV-negative OPC patients compared with those who are HPV-positive may explain this disparity.37,38,39
Strengths and Limitations
The principal strength of the present study is that it reports the most comprehensive and contemporary data to date on national trends in OPC incidence in each state and mortality rates using high-quality population-based cancer registries. The study has certain limitations. First, US cancer registry data do not include information regarding risk factors, limiting us to speculate on potential causes. Second, the patients with OPC who have unknown tumor stage may have influenced secular trends by stage at diagnosis; however, the fact that there was no significant change in unknown stage among men and women suggests that such an effect is likely to be minimal. Owing to reporting delays for OPC cases largely diagnosed in outpatient facilities, trends in incident OPC cases may erroneously appear to have decreased in the most recent years. Another limitation is that cancers were classified based on histologic criteria and not the actual assessment of individual tumors for the presence of HPV DNA; however, in the absence of robust data on the HPV status in cancer registries, this approach is widely accepted for reporting HPV-associated cancer incidence trends.2 Since 2010, SEER has collected data on the HPV status of patients with head and neck tumors. However, given that the HPV status is known for a limited population, the use of this data set for estimation of HPV-positive OPC incidence is strongly discouraged.40 Finally, given the lack of histology information in the National Center for Health Statistics data, the observed death rate may overestimate OPC mortality, which is evident from our reported differences in observed and incidence-based mortality rates. For this reason, we estimated incidence-based mortality to account for OPC cases that are largely attributable to HPV. Given this caveat, observed mortality findings should be interpreted with caution.
Conclusions
In conclusion, OPC incidence overall has continued to rise among US men with notable (more than 3% per year) increases among elderly age groups and for patients diagnosed with the regional stage. In recent years, OPC incidence-based mortality has also increased among men, particularly among patients diagnosed with advanced-stage tumors. These findings call for future research prioritizing screening and early detection advancements to combat the disease. For the first time in several decades, OPC incidence has decreased in recent years among young non-Hispanic white men, which may reflect the effect of HPV vaccination. The present study also documents geographic differences in OPC trends with marked increases largely concentrated in the Midwestern and Southeastern states, implying the need to understand etiological reasons for geographic variation and urgency to improve targeted prevention (eg, HPV vaccination) in states that are seeing marked increases in OPC incidence.
eFigure 1. Diagram depicting the sample flow of the study population
eFigure 2. Rate Ratios for oropharyngeal cancer among men and women in the incidence rates, comparing incidence rates in 2015-2017 versus 2001-2003, among age groups of major race/ethnic subgroups
eFigure 3. Burden (annual number of cases) of oropharyngeal cancer according to race/ethnicity, age, and stage at diagnosis among men and women: NPCR and SEER (2001-2017)
eFigure 4. Annual number of oropharyngeal cancers diagnosed among men and women according to geographic regions
eFigure 5. Average annual percentage change in oropharyngeal cancer incidence among men and women by US state and Washington, District of Columbia: NPCR and SEER (2001-2017)
eFigure 6. Oropharyngeal cancer incidence among men and women in 2001 and 2017 by US state and Washington, District of Columbia: NPCR and SEER (2001-2017)
eFigure 7. Oropharyngeal cancer burden among men and women in 2001 and 2017 by US state and Washington, District of Columbia: NPCR and SEER (2001-2017)
eTable 1. Overall oropharyngeal cancer incidence among men and women by race/ethnicity, age at diagnosis, SEER summary stage at diagnosis, US region, and tumor size during 2001-2017 in the United States
eTable 2. Overall and Sex, Race and SEER Summary Stage specific Oropharyngeal Squamous cell carcinoma Incidence Rates During 2001-2017 in the United States
eTable 3. Sex and race-stratified oropharyngeal cancer incidence rates over calendar year during 2001-2017 in the United States
eTable 4. Age-specific oropharyngeal cancer incidence rates over calendar year during 2001-2017 among men in the United States
eTable 5. Age-specific oropharyngeal cancer incidence rates over calendar year during 2001-2017 among Women in the United States
eTable 6. Oropharyngeal cancer incidence rates during 2001-2017 according to SEER summary stage at diagnosis among men in the United States
eTable 7. Oropharyngeal cancer incidence rates during 2001-2017 according to SEER Summary Stage at Diagnosis among women in the United States
eTable 8. Rate ratios for oropharyngeal cancer incidence rates during 2001-2017 among men in the United States
eTable 9. Rate ratios for oropharyngeal cancer incidence rates during 2001-2017 among Women
eTable 10. Trends in oropharyngeal cancer incidence rates during 2001-2017 among men in the United States
eTable 11. Trends in oropharyngeal cancer incidence rates during 2001-2017 among women in the United States
eTable 12. Trends in oropharyngeal cancer incidence rates according to state residency during 2001-2017 among men in the United States
eTable 13. Trends in oropharyngeal cancer incidence rates according to state residency during 2001-2017 among men in the United States
eTable 14. Observed and incidence-based Oropharyngeal Cancer Mortality Ratesa by Sex During 2001-2017
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612-619. doi: 10.1200/JCO.2007.14.1713 [DOI] [PubMed] [Google Scholar]
- 2.Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus-associated cancers—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918-924. doi: 10.15585/mmwr.mm6733a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreimer AR, Chaturvedi AK, Alemany L, et al. Summary from an international cancer seminar focused on human papillomavirus (HPV)-positive oropharynx cancer, convened by scientists at IARC and NCI. Oral Oncol. 2020;108:104736. doi: 10.1016/j.oraloncology.2020.104736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101(16):1120-1130. doi: 10.1093/jnci/djp205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anantharaman D, Muller DC, Lagiou P, et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int J Epidemiol. 2016;45(3):752-761. doi: 10.1093/ije/dyw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, D’Souza G, Gillison ML, Katki HA. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral Oncol. 2016;60:61-67. doi: 10.1016/j.oraloncology.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 7.Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167(10):714-724. doi: 10.7326/M17-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration . Results from the 2016. National Survey on Drug Use and Health: Detailed Tables. Accessed November 8, 2021. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
- 9.State Tobacco Activities Tracking and Evaluation (STATE) System. Map of Current Cigarette Use Among Adults. Accessed February 2, 2021.. https://www.cdc.gov/statesystem/cigaretteuseadult.html
- 10.Berenson AB, Hirth JM, Chang M. Geographical disparities in human papillomavirus herd protection. Cancer Med. 2020;9(14):5272-5280. doi: 10.1002/cam4.3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451-1461. doi: 10.1016/0895-4356(94)90089-2 [DOI] [PubMed] [Google Scholar]
- 12.Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of methods. Cancer. 2008;113(10)(suppl):2841-2854. doi: 10.1002/cncr.23758 [DOI] [PubMed] [Google Scholar]
- 13.Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus-Associated Cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666. doi: 10.15585/mmwr.mm6526a1 [DOI] [PubMed] [Google Scholar]
- 14.Saraiya M, Ahmed F, White M, Lawson H, Unger ER, Eheman C. Toward using National Cancer Surveillance data for preventing and controlling cervical and other human papillomavirus-associated cancers in the US. Cancer. 2008;113(10)(suppl):2837-2840. doi: 10.1002/cncr.23753 [DOI] [PubMed] [Google Scholar]
- 15.Ellington TD, Henley SJ, Senkomago V, et al. Trends in incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69(15):433-438. doi: 10.15585/mmwr.mm6915a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670-3682. doi: 10.1002/sim.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mix JM, Van Dyne EA, Saraiya M, Hallowell BD, Thomas CC. Assessing impact of HPV vaccination on cervical cancer incidence among women aged 15-29 years in the United States, 1999-2017: an ecologic study. Cancer Epidemiol Biomarkers Prev. 2021;30(1):30-37. doi: 10.1158/1055-9965.EPI-20-0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz AP, Ortiz-Ortiz KJ, Colón-López V, et al. Incidence of cervical cancer in Puerto Rico, 2001-2017. JAMA Oncol. 2021;7(3):456-458. doi: 10.1001/jamaoncol.2020.7488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshmukh AA, Suk R, Shiels MS, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst. 2020;112(8):829-838. doi: 10.1093/jnci/djz219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshmukh AA, Suk R, Shiels MS, et al. Incidence trends and burden of human papillomavirus-associated cancers among women in the United States, 2001-2017. J Natl Cancer Inst. 2021;113(6):792-796. doi: 10.1093/jnci/djaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262-267. doi: 10.1200/JCO.2017.75.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi AK, Graubard BI, Broutian T, et al. Prevalence of oral HPV infection in unvaccinated men and women in the United States, 2009-2016. JAMA. 2019;322(10):977-979. doi: 10.1001/jama.2019.10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda P, Mercer CH, Ghaznavi C, Herbenick D. Trends in frequency of sexual activity and number of sexual partners among adults aged 18 to 44 years in the US, 2000-2018. JAMA Netw Open. 2020;3(6):e203833. doi: 10.1001/jamanetworkopen.2020.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twenge JM, Sherman RA, Wells BE. Declines in sexual frequency among American adults, 1989-2014. Arch Sex Behav. 2017;46(8):2389-2401. doi: 10.1007/s10508-017-0953-1 [DOI] [PubMed] [Google Scholar]
- 25.Damgacioglu H, Sonawane K, Chhatwal J, Giuliano AR, Deshmukh AA. making oropharyngeal cancer a rare cancer among US men: the assessment of improving human papillomavirus vaccination coverage. 43rd Annual Meeting of the Society for Medical Decision Making 2020. [Google Scholar]
- 26.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708-2715. doi: 10.1200/JCO.2012.47.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreimer AR, Johansson M, Yanik EL, et al. Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst. 2017;109(8). doi: 10.1093/jnci/djx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreimer AR, Ferreiro-Iglesias A, Nygard M, et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium. Ann Oncol. 2019;30(8):1335-1343. doi: 10.1093/annonc/mdz138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tota JE, Gillison ML, Katki HA, et al. Development and validation of an individualized risk prediction model for oropharynx cancer in the US population. Cancer. 2019;125(24):4407-4416. doi: 10.1002/cncr.32412 [DOI] [PubMed] [Google Scholar]
- 30.Suk R, Mahale P, Sonawane K, et al. Trends in risks for second primary cancers associated with index human papillomavirus-associated cancers. JAMA Netw Open. 2018;1(5):e181999. doi: 10.1001/jamanetworkopen.2018.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109-1116. doi: 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718-723. doi: 10.15585/mmwr.mm6833a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonawane K, Zhu Y, Montealegre JR, et al. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: a nationwide, cross-sectional survey. Lancet Public Health. 2020;5(9):e484-e492. doi: 10.1016/S2468-2667(20)30139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonawane K, Lin YY, Damgacioglu H, et al. Trends in human papillomavirus vaccine safety concerns and adverse event reporting in the United States. JAMA Netw Open. 2021;4(9):e2124502. doi: 10.1001/jamanetworkopen.2021.24502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonawane K, Zhu Y, Lin YY, et al. HPV vaccine recommendations and parental intent. Pediatrics. 2021;147(3):e2020026286. doi: 10.1542/peds.2020-026286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dʼsouza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65(5):603-610. doi: 10.1097/QAI.0000000000000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiron J, Sethi S, Ali-Fehmi R, et al. Racial disparities in human papillomavirus (HPV) associated head and neck cancer. Am J Otolaryngol. 2014;35(2):147-153. doi: 10.1016/j.amjoto.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261-269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 39.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Cancer Institute Surveillance Epidemiology and End Results Program . Head and Neck with HPV Status Database (2010-2016). 2021. https://seer.cancer.gov/seerstat/databases/hpv/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Diagram depicting the sample flow of the study population
eFigure 2. Rate Ratios for oropharyngeal cancer among men and women in the incidence rates, comparing incidence rates in 2015-2017 versus 2001-2003, among age groups of major race/ethnic subgroups
eFigure 3. Burden (annual number of cases) of oropharyngeal cancer according to race/ethnicity, age, and stage at diagnosis among men and women: NPCR and SEER (2001-2017)
eFigure 4. Annual number of oropharyngeal cancers diagnosed among men and women according to geographic regions
eFigure 5. Average annual percentage change in oropharyngeal cancer incidence among men and women by US state and Washington, District of Columbia: NPCR and SEER (2001-2017)
eFigure 6. Oropharyngeal cancer incidence among men and women in 2001 and 2017 by US state and Washington, District of Columbia: NPCR and SEER (2001-2017)
eFigure 7. Oropharyngeal cancer burden among men and women in 2001 and 2017 by US state and Washington, District of Columbia: NPCR and SEER (2001-2017)
eTable 1. Overall oropharyngeal cancer incidence among men and women by race/ethnicity, age at diagnosis, SEER summary stage at diagnosis, US region, and tumor size during 2001-2017 in the United States
eTable 2. Overall and Sex, Race and SEER Summary Stage specific Oropharyngeal Squamous cell carcinoma Incidence Rates During 2001-2017 in the United States
eTable 3. Sex and race-stratified oropharyngeal cancer incidence rates over calendar year during 2001-2017 in the United States
eTable 4. Age-specific oropharyngeal cancer incidence rates over calendar year during 2001-2017 among men in the United States
eTable 5. Age-specific oropharyngeal cancer incidence rates over calendar year during 2001-2017 among Women in the United States
eTable 6. Oropharyngeal cancer incidence rates during 2001-2017 according to SEER summary stage at diagnosis among men in the United States
eTable 7. Oropharyngeal cancer incidence rates during 2001-2017 according to SEER Summary Stage at Diagnosis among women in the United States
eTable 8. Rate ratios for oropharyngeal cancer incidence rates during 2001-2017 among men in the United States
eTable 9. Rate ratios for oropharyngeal cancer incidence rates during 2001-2017 among Women
eTable 10. Trends in oropharyngeal cancer incidence rates during 2001-2017 among men in the United States
eTable 11. Trends in oropharyngeal cancer incidence rates during 2001-2017 among women in the United States
eTable 12. Trends in oropharyngeal cancer incidence rates according to state residency during 2001-2017 among men in the United States
eTable 13. Trends in oropharyngeal cancer incidence rates according to state residency during 2001-2017 among men in the United States
eTable 14. Observed and incidence-based Oropharyngeal Cancer Mortality Ratesa by Sex During 2001-2017


