Abstract
Aims:
Radiation-associated angiosarcomas (RT-AS) of the breast are rare tumours with poor prognosis. MYC amplification is considered the hallmark of RT-AS and is sometimes used as a diagnostic tool to distinguish from other radiation-associated vascular lesions. However, a small subset of RT-AS lacks MYC amplification, which may be associated with better outcome. Loss of H3K27me3 expression by immunohistochemistry (IHC) has been recently postulated as an additional diagnostic marker for RT-AS. This study aimed to evaluate the impact of MYC amplification as detected by fluorescence in situ hybridization and/or next-generation sequencing on clinicopathologic features and outcome in a large cohort of RT-AS, compare outcome with radiation-associated sarcomas of the breast (RT-S) other than angiosarcoma, and evaluate expression of H3K27me3 IHC in these groups.
Methods and Results:
Eighty-one RT-AS were identified, including 73 MYC amplified and 8 (10%) non-amplified. MYC amplified RT-AS were diagnosed in older patients (median age 69 vs 61 years). The 5-year disease specific survival and overall survival were 56% and 47%, respectively. Older age, larger tumour size, positive margin and MYC amplification were associated with worse prognosis. None of the RT-AS showed complete loss of H3K27me3 IHC expression. All 18 RT-S were MYC non-amplified, and complete loss of H3K27me3 expression was seen in 2. We found no difference in prognosis between RT-AS and RT-S.
Conclusions:
RT-AS is associated with a poor prognosis. Older age at diagnosis, larger tumour size, positive margin at excision and MYC amplification are associated with worse prognosis.
Keywords: Angiosarcoma, breast, MYC, radiation-associated sarcomas
Graphical Abstract
INTRODUCTION
Radiation-associated angiosarcomas of the breast (RT-AS) are rare tumours with an overall poor prognosis(1–3). MYC amplification is regarded as the genetic hallmark of these tumours and is used in the differential diagnosis with atypical vascular lesion (AVL), which also occur in the post-radiation setting but have a benign behaviour(4–7). However, a small subset of RT-AS lacks MYC amplification, posing a further diagnostic challenge, particularly on review of small biopsy material. Lack of MYC amplification could possibly be associated with a distinct pathogenesis and outcome. Very few studies have looked at the clinicopathologic features of MYC negative RT-AS. In 2010, Manner et al. first described MYC amplification in RT-AS(8). They reported 15 (45%) MYC non-amplified cases and did not find any differences in grade and proliferation index between amplified and non-amplified subsets. In 2015 Fraga-Guedes et al. reported a series of 37 RT-AS of the breast, 46% lacking MYC amplification(9). While they found no differences in clinicopathologic features at presentation between MYC amplified and non-amplified groups, MYC negative tumours were associated with longer overall survival (OS).
Distinguishing AVL from RT-AS in small biopsy material can be challenging. Loss of histone H3K27 trimethylation (H3K27me3) is an epigenetic event that has been found in 90–100% of radiation-associated malignant peripheral nerve sheath tumours (MPNST)(10, 11). Mentzel et al. investigated H3K27me3 expression by immunohistochemistry (IHC) in radiation-associated vascular lesions of the breast(12). Loss of H3K27me3 expression was documented in the majority of RT-AS, but in none of the benign vascular lesions and AVLs in the study, suggesting that this marker could be used as an additional diagnostic tool.
In this study we sought to examine the impact of MYC gene amplification as detected by fluorescence in situ hybridization (FISH) and/or next generation sequencing (NGS) on clinicopathologic features and outcome in a large cohort of RT-AS managed at our institution, compare outcome with radiation-associated sarcomas of the breast (RT-S) other than angiosarcoma, and evaluate expression of H3K27me3 IHC in these groups.
MATERIALS AND METHODS
Patients and Samples
Upon approval from our Institutional Review Board, we searched the pathology database for cases of RT-AS from the breast and/or chest wall diagnosed between January 1998 and August 2019. A separate search for cases of radiation induced sarcomas (RT-S) other than angiosarcoma diagnosed between January 2014 and August 2019 was performed. All patients had been previously treated with radiotherapy for breast cancer (BC). Clinicopathologic variables and follow-up data were obtained from the pathology reports and electronic medical records. These included age, clinical presentation, latency interval from BC treatment to RT-AS/RT-S diagnosis, tumour type, gross/microscopic margin status at initial surgery, details of treatment and outcomes.
Fluorescence In Situ Hybridization
FISH on interphase nuclei of tissue sections was performed by applying custom probes from bacterial artificial chromosomes (BACs) covering MYC (RP11–440N18; 8q24.21:128,596,756–128,777,986)(4). BAC DNA was isolated and labeled with different fluorochromes in a nick translation reaction. The slides were pretreated, denatured, and hybridized by probes. After overnight incubation, the slides were serially washed and mounted with DAPI in an antifade solution. Two hundred successive nuclei were manually scored using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). MYC amplification was defined as >10% tumour cells with a MYC to control centromeric probe ratio >10, or the presence of tight clustered signals characteristic of homogenous staining regions.
Targeted-DNA Next Generation Sequencing (MSK-IMPACT)
Details of the MSK-IMPACT assay have been previously described(13, 14). MSK-IMPACT is a comprehensive molecular profiling assay that involves hybridization capture and deep sequencing of all exons and selected introns of up to 468 oncogenes and tumour suppressor genes, allowing the detection of point mutations, small and large insertions or deletions, and rearrangements. In addition, the assay captures more than 1000 intergenic and intronic single nucleotide polymorphism, allowing assessment of genome-wide copy number. DNA from formalin-fixed paraffin-embedded (FFPE) tissue of normal samples was used as a comparator for copy number alteration (CNA) analysis. MYC gene amplification was defined as a fold change (FC) ≥2.0. In 11 of 99 cases (81 RT-AS and 18 RT-S) the MYC status was available from the MSK-IMPACT results. A subset of cases which were reported as amplified by NGS with fold change between 2 and 10, were further validated and confirmed by FISH as being amplified.
Immunohistochemistry
Immunohistochemistry for H3K27me3 was performed using whole tissue sections on a Leica Bond III automated staining platform (Leica, Buffalo Grove, IL, USA) using a monoclonal antibody (clone: C36B11, dilution: 1:100, Cell Signaling, Danvers, Massachusetts, USA). Expression was scored according to Mentzel et al: 0 = no staining; 1+ = 1–25% of positive tumor nuclei; 2+ = 26–50% of positive tumor nuclei; 3+ = 51–75% of positive tumor nuclei; 4+ = 76–100% of positive tumor nuclei(12). 0 and 1+ were interpreted as negative, 2+ and 3+ as heterogeneous, and 4+ as positive.
Statistical analysis
Fisher’s exact test and two-tailed Student’s t-test was used for comparison of categorical and continuous variables, respectively. OS was calculated as time from diagnosis to death or date of last follow-up. Disease specific survival (DSS) was calculated as time from diagnosis to death due to disease. Local recurrence-free survival (LRFS) was calculated as time from diagnosis to first local recurrence for patients that presented with primary disease and had negative gross margins at surgery. Distant metastasis-free survival (DMFS) was calculated as time from diagnosis to date of first distant recurrence or date for patients that presented with primary disease. Univariate survival analysis was performed using log rank test for categorical variables and Cox Proportional hazard model for continuous variables. Variables significant on univariate analysis were subsequently included in a multivariate analysis using Cox Proportional Hazards Model. Cases with gross positive margins at initial surgery were not included in LRFS and DMFS analysis. P-values <0.05 were considered significant.
RESULTS
Clinical presentation of radiation-associated angiosarcomas
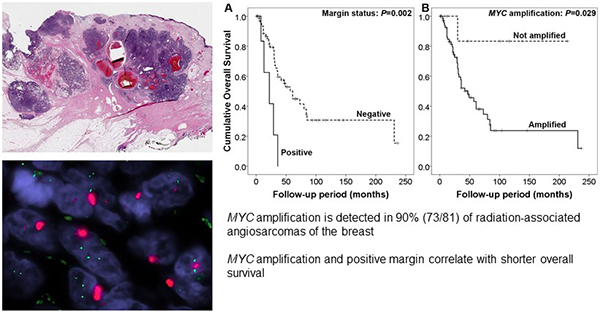
We identified 81 RT-AS cases with available MYC amplification status (74 tested by FISH, 3 by MSK-IMPACT, and 4 by both methods). All patients were women with a median age at diagnosis of 69 years (range 48–95). The median post-radiation latency interval was 7 years (range 3–25). Clinical presentation included skin erythema with or without skin thickening (50/79; 64%), palpable masses (27/79; 33%), or multiple papules (1/79; 1%)(Figure 1a). One tumour was detected on screening mammogram as a poorly defined retroareolar lesion in a 67-year-old woman with no associated skin changes; by ultrasound examination it appeared as a 1.5 cm echogenic irregular mass.
Figure 1.
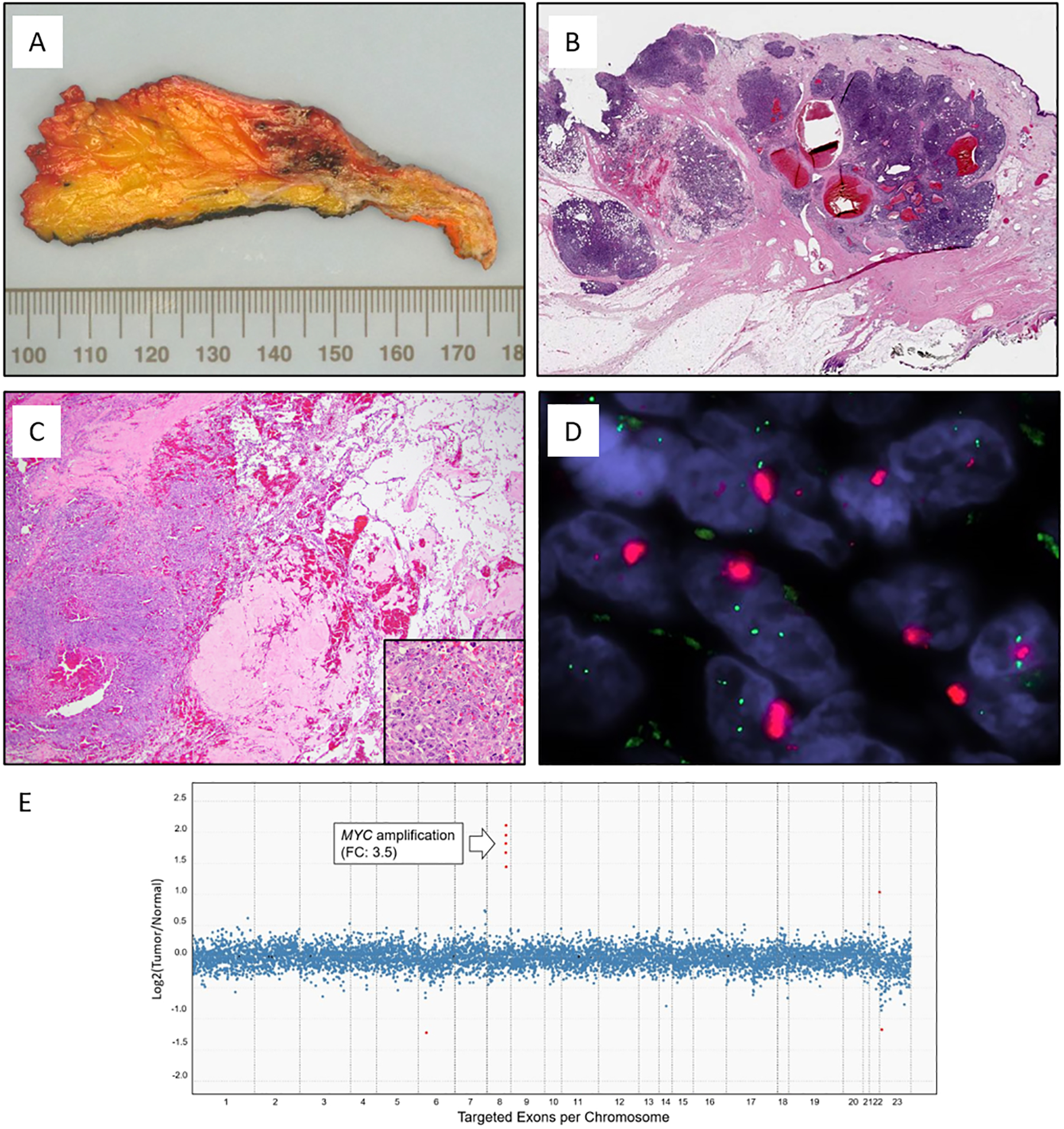
Representative images of radiation-associated angiosarcoma (RT-AS). A) Gross cut surface showing hemorrhagic nodules present in the dermis and subcutaneous tissue. B) RT-AS showing a typical multinodular growth pattern. C) Most tumors had a heterogeneous histology with solid growth admixed with vasoformative areas and high nuclear atypia with numerous mitoses (insert). D) MYC FISH (red signal, MYC; green signal, centromeric probe) with amplification detected as tight clustered signals characteristic of homogeneous staining regions. E) Copy number plot determined by MSK-IMPACT next-generation sequencing assay. Each dot represents a probe set, the values on the y-axis show the normalized log2 transformed fold change (FC) of tumor versus normal, and the x-axis is the targeted exons per chromosome.
Treatment
Initial surgery consisted of mastectomy in 69/79 (88%) patients and wide local excision in 8/79 (10%) patients, of which 6 had prior mastectomies and 2 prior lumpectomies for BC. Two patients (2%) had extensive breast involvement at diagnosis and did not undergo surgery. Eight of 75 patients were treated with neoadjuvant chemotherapy and 7 received adjuvant chemotherapy. Adjuvant radiation therapy was administered to 3 of 75 patients.
Clinicopathologic features of MYC amplified and MYC non-amplified radiation-associated angiosarcomas
Eight cases (10%) lacked MYC amplification. Non-amplified RT-AS were diagnosed in younger patients compared to MYC amplified tumours with a median age at diagnosis of 61 and 69 years, respectively (P=0.026, see Table 1). The overall median tumour size was 4 cm (range 0.6–13.6 cm). Six of 73 cases (8%) had microscopic positive margins at initial surgery. One patient (87-year-old) had gross residual disease post-mastectomy. There were no differences in tumour size, margin status, and post-radiation latency interval between MYC amplified and non-amplified RT-AS (Table 1). All cases with slides available for review (n=66) were histologically high grade (Figure 1).
Table 1.
Clinicopathologic features of MYC amplified and non- amplified radiation-associated angiosarcomas
| Clinicopathologic variable | All cases (n=81) |
MYC amplified (n=73) |
MYC not amplified (n=8) |
P value | |
|---|---|---|---|---|---|
| Age (years) | Median (range) | 69 (48–95) | 69 (49–95) | 61 (48–76) | 0.026 |
| Tumor size (cm) | Median (range) | 4 (0.6–13.6) | 3.8 (0.6–7.5) | 5.5 (0.8–13.6) | 0.235 |
| Margin a | Negative | 66 (82%) | 59 (81%) | 7 (88%) | 1.000 |
| Positive | 6 (7%) | 6 (8%) | 0 (0%) | ||
| Unknown | 9 (11%) | 8 (11%) | 1 (12%) | ||
| Latency (years) | Median (range) | 7.5 (3–25) | 7.5 (3–15) | 7.5 (4–25) | 0.249 |
| Chemotherapy | None | 51 | 7 | 0.487 | |
| Adjuvant | 9 | 0 | |||
| Neoadjuvant | 8 | 0 | |||
| Unknown | 5 | 1 | |||
| Adjuvant radiation | Yes | 3 | 0 | 1.000 | |
| No | 65 | 7 | |||
| Unknown | 5 | 1 | |||
Microscopic margin status
Clinicopathologic features associated with outcome in radiation-associated angiosarcomas
Follow-up was available for 80 patients (median follow-up of 30 months; range 0–237). The median follow-up for MYC amplified and non-amplified groups were 30 months (range 0–231) and 74 months (range 1–214), respectively. The 5-year DSS and 5-year OS of the entire cohort were 56% and 47%, respectively. Forty-two patients developed local recurrences (53%) and 28 distant metastases (35%). On univariate analysis, older age (P=0.047), larger tumour size (P=0.012), positive margin (P=0.002) and presence of MYC amplification (p=0.029) were associated with decreased OS (Figure 2). In addition, age, tumour size, and margin status were also predictive of DSS and LRFS (Table 2). On multivariate analysis (Table 3), tumour size and MYC amplification were associated with worse OS (tumour size: hazard ratio=1.2444, 95% confidence interval=1.096–1.412, P=0.001; MYC amplification: hazard ratio=20.076, 95% confidence interval=1.986–202.986, P=0.011), while positive margin was associated with worse DSS (hazard ratio=3.799, 95% confidence interval=1.096–13.168, P=0.035) and LRFS (hazard ratio=5.706, 95% confidence interval=1.887–17.252, P=0.002).
Figure 2.
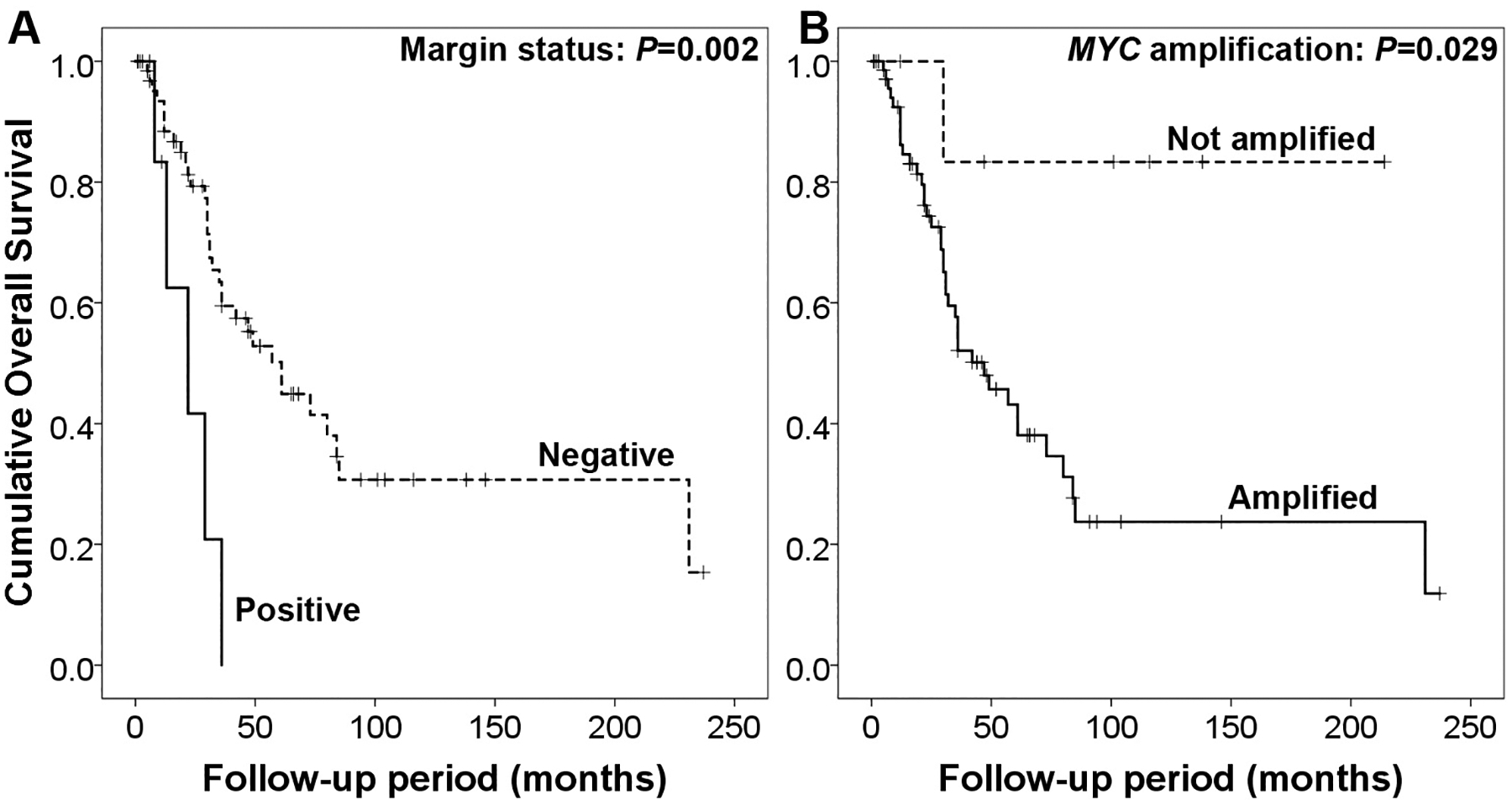
Overall survival for patients with radiation-associated angiosarcoma according to A) margin and B) MYC amplification status.
Table 2.
Univariate survival analysis of radiation-associated angiosarcomas
| Variables | OS | DSS | LRFS | DMFS |
|---|---|---|---|---|
| Univariate Cox proportional model P value | ||||
| Age (continuous variable) | 0.047 | 0.021 | 0.027 | 0.003 |
| Tumor size (continuous variable) | 0.012 | 0.028 | 0.037 | 0.049 |
| Log-rank P value | ||||
| Margin status a : positive vs. negative | 0.002 | 0.003 | <0.001 | 0.911 |
| MYC amplification: amplified vs. non-amplified | 0.029 | 0.069 | 0.372 | 0.117 |
| Chemotherapy: no chemotherapy vs. adjuvant chemotherapy vs. neoadjuvant chemotherapy | 0.819 | 0.805 | 0.422 | 0.266 |
| Adjuvant radiation: yes vs. no | 0.352 | 0.871 | 0.234 | 0.269 |
OS=overall survival; DSS= disease specific survival; LRFS=local recurrence free survival; DMFS=distant metastasis free survival;
only microscopic margin status included
Table 3.
Multivariate survival analysis of radiation-associated angiosarcomas
| Overall survival | Disease specific survival | Local recurrence free survival | Distant metastasis free survival | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | |
| Age (continuous) | 0.986 (0.950–1.023) |
0.444 | 0.995 (0.959–1.031) |
0.768 | 1.024 (0.992–1.057) |
0.142 | 0.974 (0.934–1.015) |
0.205 |
| Tumor size (continuous) | 1.244 (1.096–1.412) |
0.001 | 1.095 (0.990–1.211) |
0.078 | 0.993 (0.892–1.105) |
0.892 | 1.036 (0.916–1.172) |
0.574 |
| Margin status: positive vs. negative | 2.417 (0.820–7.130) |
0.110 | 3.799 (1.096–13.168) |
0.035 | 5.706 (1.887–17.252) |
0.002 | ||
| MYC amplification: amplified vs. non-amplified | 20.076 (1.986–202.986) |
0.011 | ||||||
H3K27me3 immunohistochemistry in radiation-associated angiosarcomas
Immunohistochemistry for H3K37me3 was performed in 20 RT-AS, including 17 MYC amplified and 3 non-amplified. None of the tumors showed complete loss of expression (score 0). Among MYC amplified RT-AS, 8 (47%) tumours were positive and 7 (41%) yielded heterogeneous results. Only 2 cases showed expression in 1–25% tumor nuclei (negative, score 1+) (Table 4, Figure 3). All MYC non-amplified RT-AS were positive for H3K27me3 expression. Intensity ranged from moderate to strong in heterogeneous and positive cases, and weak to moderate in negative tumours. There was no correlation between H3K27me3 expression and MYC amplification status.
Table 4.
H3K27me3 immunohistochemistry in radiation-associated angiosarcomas
| H3K27me3 IHC results | All cases | MYC amplified | MYC not amplified | ||
|---|---|---|---|---|---|
| Negative | 0 (0%) | 0 | 0 | 0 | P=0.459 |
| 1+ (1–25%) | 2 | 2 (100%) | 0 | ||
| Heterogeneous | 2+ (26–50%) | 4 | 4 (100%) | 0 | |
| 3+ (51–75%) | 3 | 3 (100%) | 0 | ||
| Positive | 4+ (76–100%) | 11 | 8 (73%) | 3 (27%) | |
| Total | 20 | 17 | 3 | ||
Figure 3.
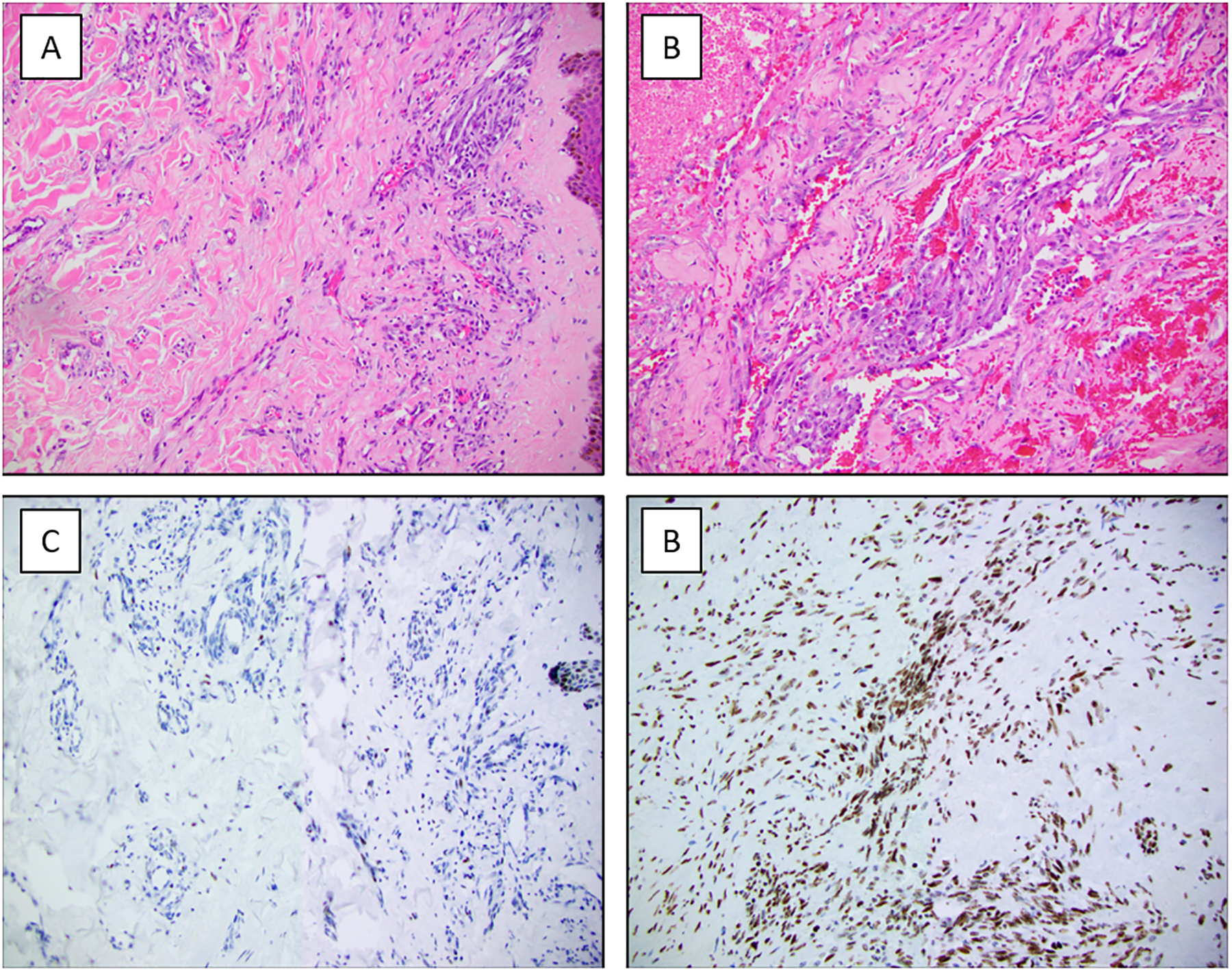
H3K27me3 immunohistochemistry in radiation-associated angiosarcoma (RT-AS) A and C) Loss of H3K27me3 expression in the tumor cells with retained expression in lymphocytes and squamous epithelium. B and D) RT-AS with retained H3K27me3 expression.
Clinicopathologic features of radiation-associated sarcomas of the breast other than angiosarcomas
We identified 18 cases of RT-S of the breast and chest wall secondary to radiotherapy for BC. All patients were female with a median age at diagnosis of 60 years (range 41–83). Median post-radiation latency was 9 years (range 2–19). Thirteen patients (72%) presented with a mass, 1 (6%) with skin erythema and swelling, and 4 (22%) had tumours detected on imaging (either breast screening or incidentally found on work-up for other medical reasons). There were 14 undifferentiated pleomorphic sarcomas (UPS) and spindle cell sarcomas NOS, 1 pleomorphic rhabdomyosarcoma, 1 MPNST, 1 osteosarcoma, and 1 myxofibrosarcoma. Median tumour size was 4.8 cm (range 1.1–11.5). All tumours had negative gross margins. Microscopic margin status was available for 16 cases, and was negative in 13 cases and positive in 3. None of the RT-S showed MYC amplification (14 cases were tested by FISH and 4 by MSK-IMPACT). One UPS showed MYC amplification by FISH as a very focal event in 5% of tumour cells. H3K27me3 IHC was performed in 12 cases, 5 of which were negative (1 UPS and 1 MPNST with score 0; 3 UPS with score 1+), 4 had heterogeneous expression (3 UPS and 1 osteosarcoma) and 3 were positive (3 UPS). Information on treatment and follow-up was available for 17 patients. Ten patients underwent surgical excision (including 3 previously treated with mastectomy and 7 with lumpectomy for BC), and six underwent mastectomy. One patient received neoadjuvant therapy and 2 adjuvant chemotherapy. None of the patients received adjuvant radiation therapy. One patient with extensive chest wall involvement was treated with palliative chemotherapy only. Median follow-up time was 24 months (range 3–153). Six patients (33%) died during the follow-up period and 11 were alive. Five-year OS, DSS, DMFS and LRFS were 53%, 65%, 69% and 81%, respectively.
Comparison of clinicopathologic features and outcome between radiation-associated angiosarcomas and radiation-associated sarcomas
Patients diagnosed with RT-AS were older compared to those with RT-S (median 69 vs 60 years; P=0.001). RT-S had a non-significant trend towards longer latency (9 vs. 7 years, P=0.055) with larger tumour size at presentation compared to RT-AS (median size 4.8 cm vs 4 cm, P=0.222). Although patients with RT-AS had worse OS, DSS, LRFS and DMFS compared to patients with RT-S the findings were not significantly different (Figure 4).
Figure 4.
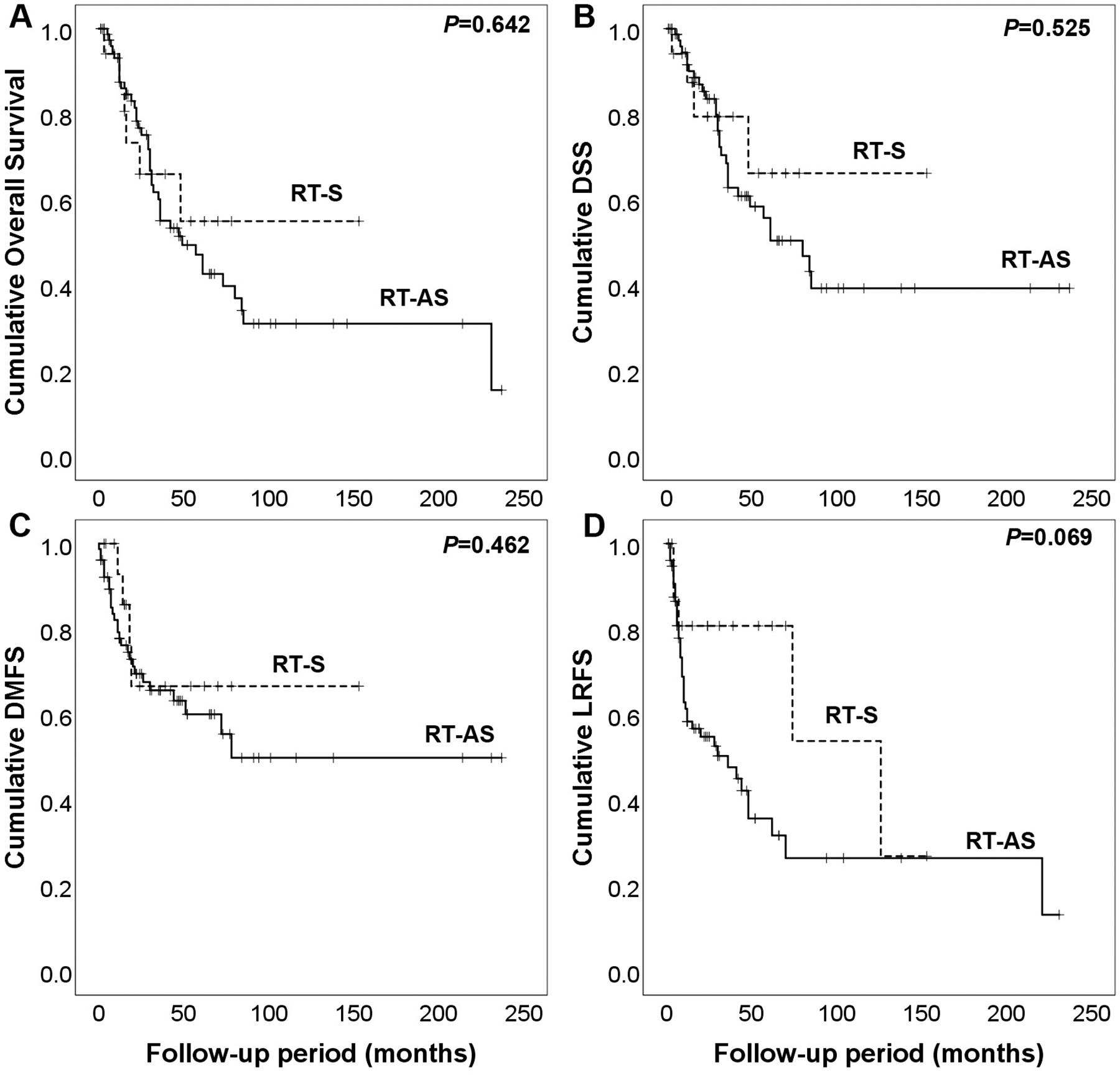
Radiation-associated angiosarcoma compared with other radiation-associated sarcomas. A) Overall survival, B) disease-specific survival (DSS), C) distant metastasis free survival (DMFS), and D) local recurrence free survival (LRFS).
DISCUSSION
MYC is a proto-oncogene located on long arm of chromosome 8 that promotes tumorigenesis through cell growth, proliferation, loss of differentiation and apoptosis(15). MYC amplification was first reported by Manner et al in 2010 in a study of 33 secondary angiosarcomas. Subsequent studies confirmed these findings with MYC amplification detected by FISH in 54 to 100% of RT-AS, with excellent concordance with protein expression by IHC(4–7, 9, 16). Consequently, MYC amplification has been considered the hallmark of RT-AS and is often used as a diagnostic tool to distinguish RT-AS from other vascular lesions of the breast such as AVL, which are associated with a good prognosis. MYC amplification has not been found in AVL, even in AVL adjacent to RT-AS(4). Nonetheless, RT-AS and AVL may have overlapping morphologic features and the differential diagnosis may be difficult, especially on small tissue biopsies(17, 18).
A subset of RT-AS, however, lacks MYC amplification. Information on the clinicopathologic features of this subset of RT-AS is limited. Manner et al did not find any difference in tumour grade, proliferation index and apoptotic rates (assessed as ki67 and terminal deoxynucleotidyl transferase dUTP nick-end labeling positive cells, respectively) between MYC amplified (18/33) and non-amplified (15/33) tumours from different sites(8). In a study of 37 RT-AS, Fraga-Guedes et al reported no difference in age at diagnosis, tumour grade, size, or latency (from radiation therapy to diagnosis) between these 2 groups, however, they found that patients with MYC amplified RT-AS (20/37, 54%) had significantly worse OS compared to those without MYC amplification (17/37, 46%).
We evaluated the clinicopathologic features and prognostic factors in the largest cohort to date of RT-AS with known MYC amplification status. We found that 10% of RT-AS lack MYC amplification, and these tumors are often diagnosed in younger patients and are associated with longer OS.
The frequency of MYC amplification in our study (90%) is in keeping with prior reports, in which MYC status was evaluated by FISH or IHC(5, 7, 19). However, a few European studies found MYC amplification to be considerably lower (<60%). Manner et al reported MYC amplification in 18 of 33 tumours (55%), however, these included RT-AS from different sites, including breast. While MYC amplification and overexpression have been reported in non-mammary RT-AS, these are seen at a significantly lower incidence, ranging from 9.5 to 50% of tumours (6, 20). Similarly, a German study found MYC amplification in 58% of RT-AS, however, when only RT-AS of the breast were counted, the frequency was 86%(21). Interestingly, in the study by Fraga-Guedes of 37 RT-AS of the breast, only 54% of tumors were MYC amplified. In contrast to our cohort, which consisted only of high-grade RT-AS, their study included 17 histologic grade 1 or 2 tumours. However, they found no difference in grade between amplified and non-amplified groups. Thus, their lower detection rate of MYC amplification cannot be attributed to tumor morphology and the difference with our results is not completely understood.
Our findings support the role of MYC amplification as an adverse prognostic factor in RT-AS and suggest that tumours lacking this genetic aberration may have different pathogenesis. The genetic alterations of this subset of RT-AS are unknown. Corradini et al demonstrated genetic heterogeneity among 17 RT-AS (with and without MYC expression by IHC), with higher mutational burden seen in high grade (grade 3) tumours.
In our series, all RT-AS with available slides for review were histologically high grade, regardless of MYC amplification status. Grading of RT-AS is controversial and not consistently reported given discrepant results regarding its prognostic value(9, 22–24). Recently, two studies have shown that histologic grade 3 RT-AS of the breast are associated with worse OS and DFS compared with grade 1 or 2 tumours (9, 23). In a study of 176 radiation sarcomas (including 67 RT-AS of the breast) Mito et al found that high grade tumours were associated with worse OS(25). Based on these findings, reporting the histologic grade in RT-AS is recommended, as it may have prognostic significance.
Upon our case review, we encountered a single case of possible low grade RT-AS which was excluded from the study as it did not reach a consensus diagnosis. This was a 48-year-old female with a history of ipsilateral BC status post-lumpectomy and radiation therapy 5 years prior. She presented with skin discoloration and palpable nodules. Her biopsy showed an atypical vascular proliferation concerning for RT-AS and underwent excision. A 5.5 cm hemorrhagic mass was identified on gross examination, which microscopically corresponded to an infiltrative vascular lesion mainly located in the breast parenchyma, and focally involving the dermis and adipose tissue. It was composed of open, inter-anastomosing vascular spaces with minimal nuclear enlargement and hyperchromasia. Mitoses, solid growth, bloody lakes, and necrosis were not seen. This vascular proliferation was MYC non-amplified. Upon re-review for this study, histologic features felt short for an unequivocal RT-AS, and the possibility of an atypical vascular lesion with unusual clinical presentation or a primary angiosarcoma could not be completely ruled out. This lesion did not recur, and the patient was alive at 138 months follow-up.
The overall prognosis for RT-AS in our cohort was poor with a 5-year OS and DSS of 47% and 56%, respectively. Local recurrences were seen in 53% of patients and 35% developed distant metastases. In addition to MYC amplification, we found that older age at diagnosis, larger tumour size, and positive margins at initial excision were adverse prognostic factors, similar to prior series(1, 2, 17, 25, 26).
We found that patients with RT-S were diagnosed at a younger age compared to patients with RT-AS, with a tendency to a longer latency. We observed a trend towards shorter OS, DSS, LRFS, and DMFS in patients with RT-AS compared to patients with RT-S, however, the differences were not statistically significant. Mito et al reported a significantly higher 3-year DMFS for RT-AS (n=67) compared to radiation-associated sarcomas from different sites (n=109) (82% vs. 67%) with a trend towards higher 3-year OS in RT-AS (84% vs. 68%), but similar 3-year LRFS. The difference prognosis may be due to a smaller number of RT-S and possibly the absence of any low-grade RT-AS in our cohort.
H3K27me3 has been recently postulated as an additional diagnostic tool in RT-AS, with complete loss of nuclear expression reported in the majority of RT-AS (12/20, 60%) and in none of the AVL (0/9), benign vascular lesions (0/8) and normal dermal vessels (0/4) studied(12). Given that absence of MYC amplification does not rule out a diagnosis of RT-AS, we evaluated expression of H3K27me3 IHC in a subset of cases, using the previously published scoring method for these tumours(12), to explore its possible diagnostic utility. We found that none of our cases showed complete loss of expression (score 0, 0% positive nuclei). A recent series by Panse et al reported a 20% rate of H3K27me3 loss in RT-AS(27). Differences between monoclonal and polyclonal antibodies for detection of complete H3K27me3 loss have been demonstrated in MPNST(28). However, the type of antibody cannot solely explain the discrepant results in H3K27me3 expression in RT-AS as one would expect higher rates of complete loss with monoclonal antibodies (as used in our study and by Panse et al) compared to polyclonal antibodies (used by Mentzel et al)(12, 27, 28) and additional studies are necessary to further investigate this. Based on these findings, we conclude that although RT-AS may show loss of H3K27me3, retained expression of this marker does not rule out the diagnosis. In addition, we found complete loss of expression in 2/12 (16%) RT-S (1/1 MPNST, 1/10 UPS). Three cases (all UPS) retained 1–25% nuclear expression. Panse et al studied H3K27me3 expression in 119 RT-S and found complete loss in 19% of tumours (9/10 MPNST, 7/77 UPS, 5/25 AS, 1/5 leiomyosarcoma, and 1/2 osteosarcoma). These results document that H3K27me3 loss is not specific to radiation-associated MPNST and but can be seen in other histologic types of RT-S.
Limitations to our study include its retrospective nature and inclusion of only cases with material available for MYC amplification testing. Although all the cases were reviewed at our institution at the time of diagnosis, not all slides were available for review for this study. This may have resulted in subclassification of RT-AS per grade, which has been recently reported to have prognostic value.
In conclusion, our results support the role of MYC as an adverse prognostic factor in RT-AS suggesting genetic heterogeneity among these tumours. At present, the pathogenetic alterations underpinning RT-AS lacking MYC amplification remain unclear, and further studies are needed to elucidate the pathogenesis of these tumors and identify potential actionable targets.
ACKNOWLEDGEMENTS
This work was supported in part by a Cancer Center Support Grant of the National Institute of Health/National Cancer Institute (P30 CA008748); Cycle for Survival (CRA), Angiosarcoma Awareness (CRA), P50 CA140146 (CRA), P50 CA217694 (CRA).
Footnotes
The authors declare no conflict of interest
REFERENCES
- 1.Strobbe LJ, Peterse HL, van Tinteren H et al. Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome. An unforseen sequela. Breast Cancer Res Treat. 1998; 47: 101–9. [DOI] [PubMed] [Google Scholar]
- 2.Billings SD, McKenney JK, Folpe AL et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004; 28: 781–8. [DOI] [PubMed] [Google Scholar]
- 3.Marchal C, Weber B, de Lafontan B et al. Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys. 1999; 44: 113–9. [DOI] [PubMed] [Google Scholar]
- 4.Guo T, Zhang L, Chang NE et al. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011; 50: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mentzel T, Schildhaus HU, Palmedo G et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012; 25: 75–85. [DOI] [PubMed] [Google Scholar]
- 6.Italiano A, Thomas R, Breen M et al. The miR-17–92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer. 2012; 51: 569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginter PS, Mosquera JM, MacDonald TY et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014; 45: 709–16. [DOI] [PubMed] [Google Scholar]
- 8.Manner J, Radlwimmer B, Hohenberger P et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010; 176:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraga-Guedes C, Andre S, Mastropasqua MG et al. Angiosarcoma and atypical vascular lesions of the breast: diagnostic and prognostic role of MYC gene amplification and protein expression. Breast Cancer Res Treat. 2015; 151: 131–40. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016; 29: 4–13. [DOI] [PubMed] [Google Scholar]
- 11.Prieto-Granada CN, Wiesner T, Messina JL et al. Loss of H3K27me3 Expression Is a Highly Sensitive Marker for Sporadic and Radiation-induced MPNST. Am J Surg Pathol. 2016; 40: 479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mentzel T, Kiss K. Reduced H3K27me3 expression in radiation-associated angiosarcoma of the breast. Virchows Arch. 2018; 472: 361–8. [DOI] [PubMed] [Google Scholar]
- 13.Cheng DT, Mitchell TN, Zehir A et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015; 17: 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross DS, Zehir A, Cheng DT et al. Next-Generation Assessment of Human Epidermal Growth Factor Receptor 2 (ERBB2) Amplification Status: Clinical Validation in the Context of a Hybrid Capture-Based, Comprehensive Solid Tumor Genomic Profiling Assay. J Mol Diagn. 2017; 19: 244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002; 2: 764–76. [DOI] [PubMed] [Google Scholar]
- 16.Cornejo KM, Deng A, Wu H et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015; 46: 868–75. [DOI] [PubMed] [Google Scholar]
- 17.Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005; 29: 983–96. [PubMed] [Google Scholar]
- 18.Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008; 32: 943–50. [DOI] [PubMed] [Google Scholar]
- 19.Lae M, Lebel A, Hamel-Viard F et al. Can c-myc amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother. 2015; 19: 168–74. [DOI] [PubMed] [Google Scholar]
- 20.Mito JK, Qian X, Jo VY et al. MYC expression has limited utility in the distinction of undifferentiated radiation-associated sarcomas from sporadic sarcomas and sarcomatoid carcinoma. Histopathology. 2020; 77: 667–72. [DOI] [PubMed] [Google Scholar]
- 21.Kacker C, Marx A, Mossinger K et al. High frequency of MYC gene amplification is a common feature of radiation-induced sarcomas. Further results from EORTC STBSG TL 01/01. Genes Chromosomes Cancer. 2013; 52: 93–8. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento AF, Raut CP, Fletcher CD. Primary angiosarcoma of the breast: clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol. 2008; 32: 1896–904. [DOI] [PubMed] [Google Scholar]
- 23.Corradini AG, Asioli S, Morandi L et al. Post-radiotherapy vascular lesions of the breast: immunohistochemical and molecular features of 74 cases with long-term follow-up and literature review. Histopathology. 2020; 77: 293–302. [DOI] [PubMed] [Google Scholar]
- 24.Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997; 31: 189–95. [DOI] [PubMed] [Google Scholar]
- 25.Mito JK, Mitra D, Barysauskas CM et al. A Comparison of Outcomes and Prognostic Features for Radiation-Associated Angiosarcoma of the Breast and Other Radiation-Associated Sarcomas. Int J Radiat Oncol Biol Phys. 2019; 104: 425–435. [DOI] [PubMed] [Google Scholar]
- 26.Rombouts AJM, Huising J, Hugen N et al. Assessment of Radiotherapy-Associated Angiosarcoma After Breast Cancer Treatment in a Dutch Population-Based Study. JAMA Oncol. 2019; 5: 267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panse G, Mito JK, Ingram DR et al. Radiation-associated sarcomas other than malignant peripheral nerve sheath tumours demonstrate loss of histone H3K27 trimethylation. Histopathology. 2021; 78: 321–6. [DOI] [PubMed] [Google Scholar]
- 28.Asano N, Yoshida A, Ichikawa H et al. Immunohistochemistry for trimethylated H3K27 in the diagnosis of malignant peripheral nerve sheath tumours. Histopathology. 2017; 70: 385–93. [DOI] [PubMed] [Google Scholar]


