Abstract
Itch, or pruritus, is the uncomfortable sensation underlying the desire to scratch. Itch is a very common complaint in the general population that can result from dermatologic, systemic (eg, renal, hepatobiliary, endocrine), paraneoplastic, neuropathic, and psychogenic etiologies. Chronic itch is associated with significant sleep disturbances and profoundly reduces overall quality of life. Certain populations, including elderly and African Americans, are at increased risk of experiencing heightened burden of itch. Because of the variable clinical presentation and wide-ranging etiologies, itch presents a challenge for clinicians. The initial evaluation should include a complete blood count, with differential, hepatic, renal, and thyroid function testing along with diabetes screening. Further testing should be guided by history and physical examination findings. There should be a heightened concern for underlying malignancy in individuals older than 60 years of age who have a history of liver disease and diffuse itch less than 12 months of duration. For individuals with chronic pruritus of unknown origin, increased blood eosinophils may serve as a biomarker of T helper cell type 2 polarization and response to immunomodulator therapies. In this first part of a 2-part continuing medical education series, we describe the broader epidemiology and specific conditions associated with itch and the clinical presentation and diagnostic workup for patients with itch.
Keywords: clinical features, diagnostic workup, epidemiology, itch, pruritus
EPIDEMIOLOGY OF ITCH
Key points
Itch is a highly prevalent symptom in the general population, especially among the elderly.
African Americans are at increased risk of experiencing chronic pruritus and associated comorbidities and have more-severe reductions in multiple quality of life domains.
Prevalence
Itch, or pruritus, is a common symptom that leads to more than 7 million ambulatory visits annually in the United States. It is among the 50 most prevalent conditions worldwide.1,2 The estimated lifetime prevalence of chronic pruritus (itch lasting >6 weeks) ranges between 8% and 25.5%, as reported by several European population-based studies,3–6 whereas the 12-month cumulative-incidence of chronic pruritus is approximately 7%.5
Impact on quality of life
Itch can be as debilitating as chronic pain.7,8 Patients with chronic pruritus had lower reported overall health-related quality of life than patients with a history of a stroke.8 Patients with itch often experience sleep disturbances,9 mood disorders,10,11 and negative psychosocial impact,3,12 culminating in a significant overall reduction in quality of life.
Age
Chronic itch is especially common among the elderly, affecting approximately 11.5%−25% of the elderly, especially those older than 85 years of age.13 Multiple factors contribute to itch in older patients. Older individuals are at increased risk of xerosis and neuropathy as well as the systemic and psychiatric diseases associated with pruritus.14,15 Age-related physiologic changes, including the progressive loss of skin barrier function and functional loss of pain-mediating fibers, culminate in the central disinhibition of itch among the elderly.16 Calcium-channel blockers and hydrochlorothiazide may be associated with inflammatory pruritic skin conditions in the elderly.17,18 Evidence is lacking, however, on the role of other medications as culprits of pruritus in the elderly, making the benefit of discontinuing medications unclear.15,19 Older individuals are also more likely to have itch driven by age-related immuno-senescence or a shift toward T helper cell type 2-mediated cytokine response.20–22
Sex
Gender differences exist in the subjective experience of itch. Women are more likely to present with itch that worsens with psychosomatic factors, neuropathic symptoms, and secondary scratch lesions.23,24 Men who report itch are older and more likely to have comorbid systemic diseases.23 Women also experience pruritic disorders that are associated with pregnancy, with approximately 18%−20% of pregnant women experiencing pruritus during gestation.25,26 For example, intrahepatic cholestasis of pregnancy can cause itch in the second to third trimesters of pregnancy.27
Race and ethnicity
Patients who seek ambulatory care for itch are more likely to be African American or Asian.1 Black patients are more likely to be diagnosed with a variety of pruritic inflammatory skin diseases.28–34 In addition to genetic and immunologic factors, this is thought to be due, in part, to the structural properties of black skin, leading to increased transepidermal water loss, decreased ceramide levels, and lower pH in the stratum corneum.29 Individuals of non-White race were also associated with a more-negative impact of chronic itch on their quality of life, even after adjusting for socioeconomic status.35 African Americans reported heightened mental distress from chronic itch, with postinflammatory hyperpigmentation from chronic itch as a significant contributor to this negative emotional impact.36
The clinical presentation of itch can also vary between racial and ethnic groups. For example, African American patients are more likely to experience more-severe atopic dermatitis than White patients.29,34 Similarly, itch related to primary biliary cholangitis is more severe among African Americans and Hispanic patients than among Caucasians.37 Black patients are also at heightened risk of being diagnosed with systemic disorders associated with itch, including end-stage renal disease and HIV-related pruritic dermatoses.38 In a study of HIV-positive patients at a large tertiary center, African American HIV-positive patients were at increased risk of pruritic disorders compared with White patients.39
ETIOLOGIES OF ITCH
Key points
The common nondermatologic causes of itch include renal, hepatobiliary, oncologic, neuropathic, and psychogeneic etiologies.
The risk factors that suggest that itch is associated with an underlying malignancy include itch with a duration of less than 12 months, age greater than 60 years, male sex, and history of liver disease and tobacco use.
HIV-positive patients are at increased risk of experiencing pruritic dermatoses, such as lichen simplex chronicus, prurigo nodularis, and scabies.
Various inflammatory, neoplastic, genetic, infestation or infectious, and autoimmune dermatologic diseases can cause itch (Table I). Approximately 50% of patients with primary diagnoses of dermatologic problems reported pruritus, with 25% reporting severe itch.40 Itch is also a common symptom associated with numerous nondermatologic conditions and may arise in the context of various systemic, neuropathic, and psychogenic etiologies (Fig 1).
Table I.
Examples of dermatologic causes of itch
| Types | Examples |
|---|---|
| Inflammatory | Atopic dermatitis, contact dermatitis, psoriasis, lichen planus, urticaria, pityriasis rubra pilaris, prurigo nodularis, dermal hypersensitivity reaction, Grover disease, granuloma annulare, and primary cutaneous amyloidosis |
| Infections or infestations | Bacterial, viral, fungal, and parasitic infections (eg, scabies and shistosomal dermatitis) |
| Neoplastic | Cutaneous T-cell lymphoma and nonmelanoma skin cancer |
| Autoimmune | Bullous pemphigoid, dermatitis herpetiformis, dermatomyositis, and cutaneous lupus erythematosus |
| Genetic | Darier disease, Hailey-Hailey disease, epidermolysis bullosa pruriginosa, Sjögren-Larsson syndrome, and porphyria cutanea tarda |
| Fibrosis-related | Scar-related pruritus, keloids, and sarcoidosis |
Fig 1.
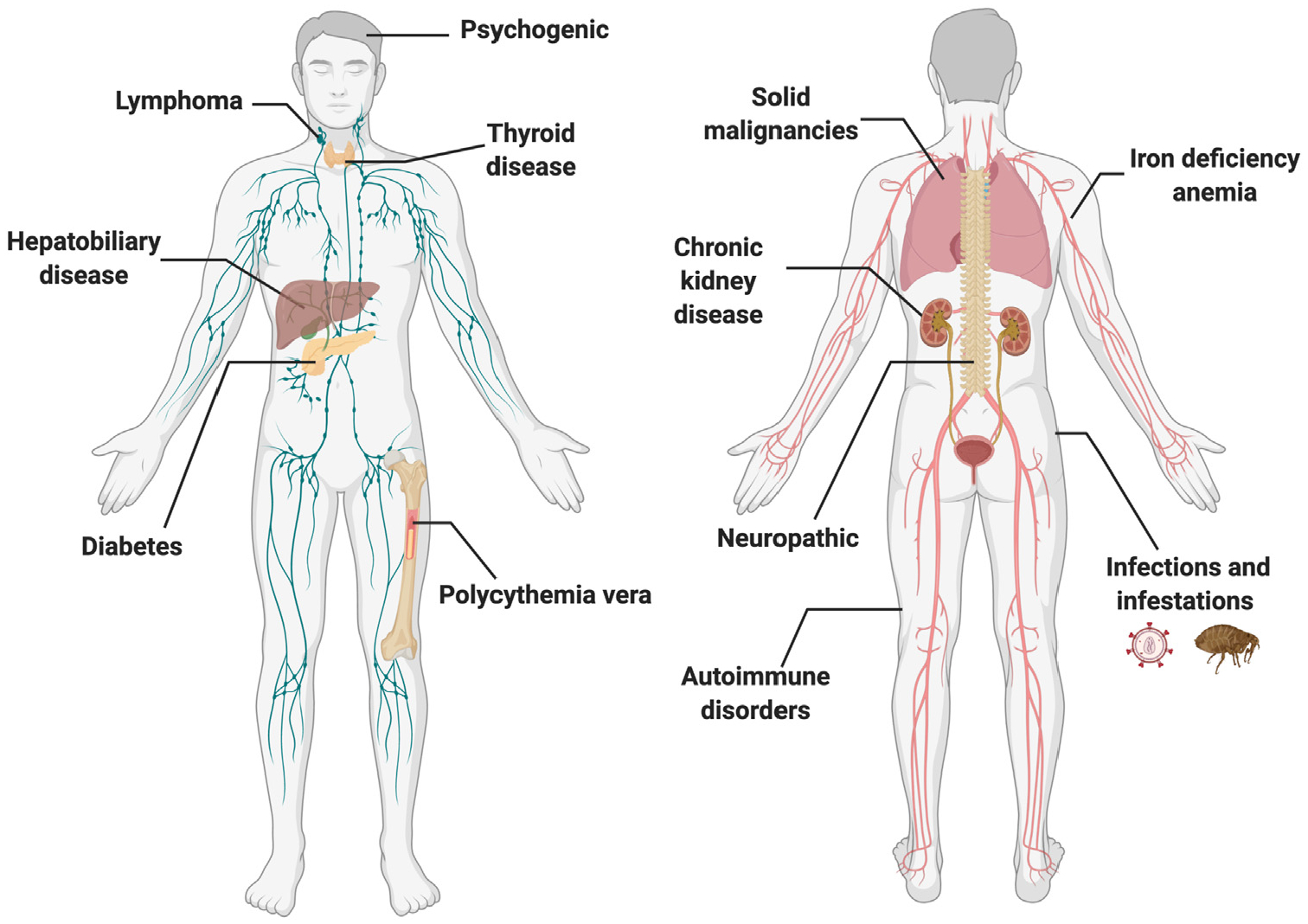
Itch can arise in the context of various systemic, neuropathic, and psychogenic etiologies.
Systemic causes of itch
Renal.
Itch is a common manifestation of advanced chronic kidney disease, with 40%−90% of hemodialysis patients experiencing chronic pruritus.41–43 The itch with chronic kidney disease is related to uremic neuropathy, systemic inflammation, and increased μ-opioid receptor activity along with decreased κ-opioid activity.44 Secondary hyperparathyroidism due to chronic kidney disease has also been postulated as a cause of generalized pruritus with an unclear mechanism, suggested by small cohort studies that observed improvement of itch following parathyroidectomy.45–47
Hepatobiliary.
Cholestasis from conditions affecting the hepatobiliary system is a common culprit of itch. These include both primary and secondary causes of biliary obstruction that lead to a systemic accumulation of bile acid, including primary biliary cholangitis, primary sclerosing cholangitis, intrahepatic cholestasis of pregnancy, viral hepatitis, and cirrhosis.48–50 Cholestatic itch arises from a complex interplay between bile acids, lysophosphatidic acid, bilirubin, and increased μ-opioid receptor activity.49 Recent studies have suggested that bilirubin induces pruritus through the activation of Mas-related G-protein coupled receptor member X4 receptors on sensory neurons.51 Cholestatic pruritus can be uniquely characterized by the presence of itch that initially affects the palms and soles, becoming more generalized with disease progression.
Endocrine.
Pruritus is more prevalent in diabetic patients than in healthy controls (26.3% vs 14.6%, respectively). Patients with diabetes are predisposed to conditions associated with itch, including superficial mycotic infections, neuropathy, excoriation disorder, and pruritus of the scalp and vulva.52–55 Itch in diabetes may be secondary to the detrimental effect of increased glucose on cutaneous nerve fibers, representing a sequela of diabetic polyneuropathy. Uncontrolled hyperthyroidism causes itch in a subset of patients, possibly due to reduced itch threshold due to increased body temperature, vasodilation, and kinin activation.56,57 Hypothyroidism is less frequently associated with itch, but it is associated with xerosis.57
Rheumatologic.
Itch is a common symptom of various rheumatologic diseases, due to downstream effects of variable immune activation.58 Pruritus occurs in approximately half of patients with systemic sclerosis who often have accompanying xerosis.59 Itch is also a common symptom of dermatomyositis, with 50.8% of patients with dermatomyositis reporting moderate-to-severe itch60 and itch severity correlating with the degree of skin involvement.61 The other autoimmune diseases featuring varying degrees of itch include Sjögren syndrome and both cutaneous and systemic lupus erythematosus.58,62
Hematologic or oncologic.
Itch can be a prodrome of malignancy, often preceding other signs and symptoms. Although the exact pathophysiology is not known, malignancy-related pruritus may result from a local inflammatory reaction to the tumor or as a paraneoplastic phenomenon. Itch is particularly common in hematologic malignancies,48 with prevalence estimates as high as 30% among patients with Hodgkin lymphoma,63 15% among patients with non-Hodgkin lymphoma,64 and 67% among patients with polycythemia vera.65,66 Patients with polycythemia vera often present with aquagenic pruritus, evoked by contact with water of any temperature.65,66 Other hematologic conditions can also present with generalized pruritus, with eczematous, urticarial, or lichenified skin findings, including hypereosinophilic syndrome, defined as 2 or more separate examinations of absolute eosinophil count >1.5 × 109/L in the peripheral blood in the course of 1 month.67,68
Itch is also associated with cutaneous lymphomas and other dermatologic cancers.69–72 Among solid tumors, there is a significant association between itch and cancers of the hepatobiliary system. Although pruritus is thought to be an uncommon symptom in other solid malignancies, there have been case reports of itch occurring in patients with non-small–cell lung carcinoma,73 insulinoma,74 gastric carcinoid tumors,75 and other solid malignancies.76,77
A longitudinal Danish study demonstrated that rates of both hematologic and various solid cancers were higher than expected in patients with pruritus.78 The incidence ratio of cancers was the most increased compared to the general population within the first 3 months of pruritus diagnosis and remained elevated during the first 12 months. Another study has also suggested that patients with chronic itch but without primary dermatologic findings are at increased risk of an underlying malignancy. This increased risk was especially associated with age older than 60 years, male sex, and history of liver disease and tobacco use.79 Racial differences have been observed in the association between itch and certain malignancies. Notably, Black pruritic patients may have greater odds of hematologic malignancies, whereas White pruritic patients may be at increased risk of liver and skin malignancies.69
Other systemic etiologies.
Itch can occur as an iatrogenic adverse effect of many drugs, suggesting that it is important that physicians across specialties remain vigilant. The common culprits of drug-induced pruritus include immune checkpoint inhibitors80,81; agents targeting epidermal growth factor receptor, B-Raf proto-oncogene, cytotoxic T-lymphocyte-associated protein 4, and programmed cell death protein 1/ programmed cell death-ligand 182–84; opioids; and chloroquine and other antimalarials.85
Although their exact pathophysiology is yet to be explored, the other potential etiologies of itch may include iron-deficiency anemia, exposure to heavy metal, vitamin deficiency, HIV, and other viral infections. One study found that 13.6% of men and 7.4% of women with iron-deficiency anemia presented with itching, which was significantly increased compared with controls.86 Elevated blood levels of heavy metals, including cadmium and lead, are also associated with chronic itch.87 Low levels of vitamin D were observed in patients with chronic pruritic skin conditions, including atopic dermatitis, psoriasis, and chronic urticaria,88–90 whereas low levels of vitamin B12 were noted in patients with generalized itch from various systemic causes.91 Oral or topical vitamin supplements had modest positive effects in reducing pruritus in limited studies, although definitive studies are lacking on the association of vitamin deficiencies with the development of chronic itch.92–95
Itch is also commonly reported in patients with viral infections, particularly among those with HIV. Pruritus is a significant cause of comorbidity among HIV-positive patients, of whom 13%−45% experience chronic itch.39,96,97 Many HIV-positive patients have concomitant pruritic disorders, including lichen simplex chronicus, prurigo nodularis, scabies, seborrheic dermatitis, mycosis fungoides, and psoriasis.39,48,98–100 Patients with advanced HIV are also at risk for eosinophilic folliculitis, an intensely pruritic eruption of follicular papules and pustules in the setting of elevated eosinophils.98,101
Neuropathic causes of itch
Itch can arise from neural dysregulation, either from excess stimulation of the peripheral sensory nerves or from the loss of the central inhibition of the itch pathway.102 Neuropathic pruritus is estimated to comprise 8% of all cases of chronic pruritus.103 Commonly recognized causes of neuropathic itch often have distinct dermatomal localizations (Fig 2), but can become generalized.
Fig 2.
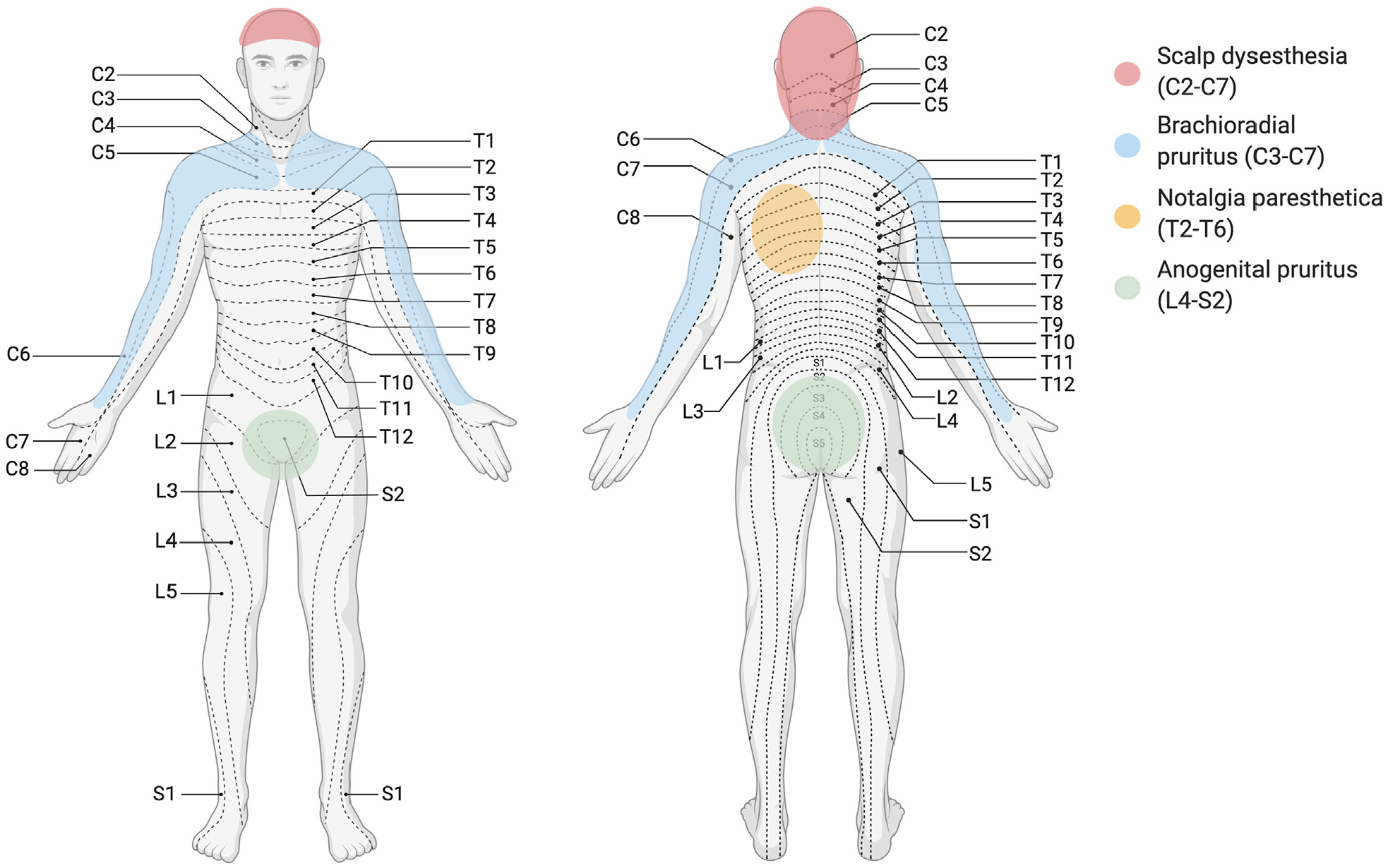
Neuropathic etiologies of itch. C, Cervical; L, Lumbar; T, Thoracic.
Brachioradial pruritus most commonly affects middle-aged women of lighter skin types and worsens with exposure to sunlight.102,104 It typically presents initially with localized itch or a tingling or burning sensation along either proximal upper extremities and shoulders along the C3-C7 dermatomes, often with accompanying degenerative changes noted in the respective cervical spine.102,105–107 Brachioradial pruritus can become generalized, in a phenomena related to central neural sensitization.108
Notalgia paresthetica presents with localized, unilateral pruritus of the area medial to the scapula on the mid-to-upper back. It originates from nerve entrapment of spinal nerves that arise from T2 to T6.102 Itch localization often correlates with radiologic findings of the vertebrae109,110 as well as with reduced intraepidermal nerve fiber density in the skin likely as a results of chronic scratching.109
Scalp dysesthesia presents with an uncomfortable sensation of the scalp. Although the healthy scalp normally has decreased sensitivity of C-fibers to itch,111 scalp dysesthesia can result from degenerative changes at C2-C7 levels.112,113 Similarly, anogenital pruritus is associated with degenerative changes of the lower spine at L4-S2 levels.114
The other neurologic conditions associated with itch include trigeminal trophic syndrome, cerebrovascular events, brain infections (eg, encephalitis, Creutzfeldt-Jakob disease), and small fiber neuropathies.102
Psychogenic causes of itch
Itch is commonly reported among patients with anxiety and depression, although the pathophysiology still needs further investigation. Itch severity correlates with the level of depressive symptoms.115 Because of its detrimental effects on sleep and quality of life,116,117 chronic pruritus leads to increased psychiatric burden of disease118 and higher odds of suicidal ideation.11 Itch also is often reported in patients with primary psychodermatologic conditions, including somatic symptom disorder, dermatitis artefacta, obsessive-compulsive disorder, delusional infestation, excoriation disorder, and Morgellons disease. Excoriation disorder is associated with type 2 diabetes mellitus, anxiety, and depression.55 Chronic itch can be a manifestation of an underlying substance use disorder, including opioids, cocaine, and methylenedioxymethamphetamine.119
CLINICAL PRESENTATION, EVALUATION, AND DIAGNOSTIC WORKUP
Key points
The initial goal of evaluating patients with itch is to determine whether there is primary skin eruption or lesion.
All patients with chronic itch without primary dermatologic findings should receive a screening laboratory workup consisting of complete blood count with differential, hepatic, renal, and thyroid function testing as well as diabetes screening, Further testing should be guided by history, review of systems, and findings from the physical examination.
For individuals with chronic pruritus of unknown origin, increased blood eosinophils is a biomarker of T helper cell type 2 polarization and response to immunomodulator therapy.
Itch can vary drastically in clinical presentation (Fig 3). It can present with inflamed or diseased skin, suggestive of primary dermatologic disorder, or with noninflamed skin, suggestive of a nondermatologic cause.120 As a caveat, pruritic skin conditions rarely occur without primary skin lesions, as in the case of nonbullous pemphigoid in the elderly or “invisible” mycosis fungoides.121–123 Secondary scratch lesions (eg, excoriations) can occur with or without primary skin lesions. Itch can be broadly categorized by clinical presentations and underlying etiologies, as presented in Fig 4.
Fig 3.
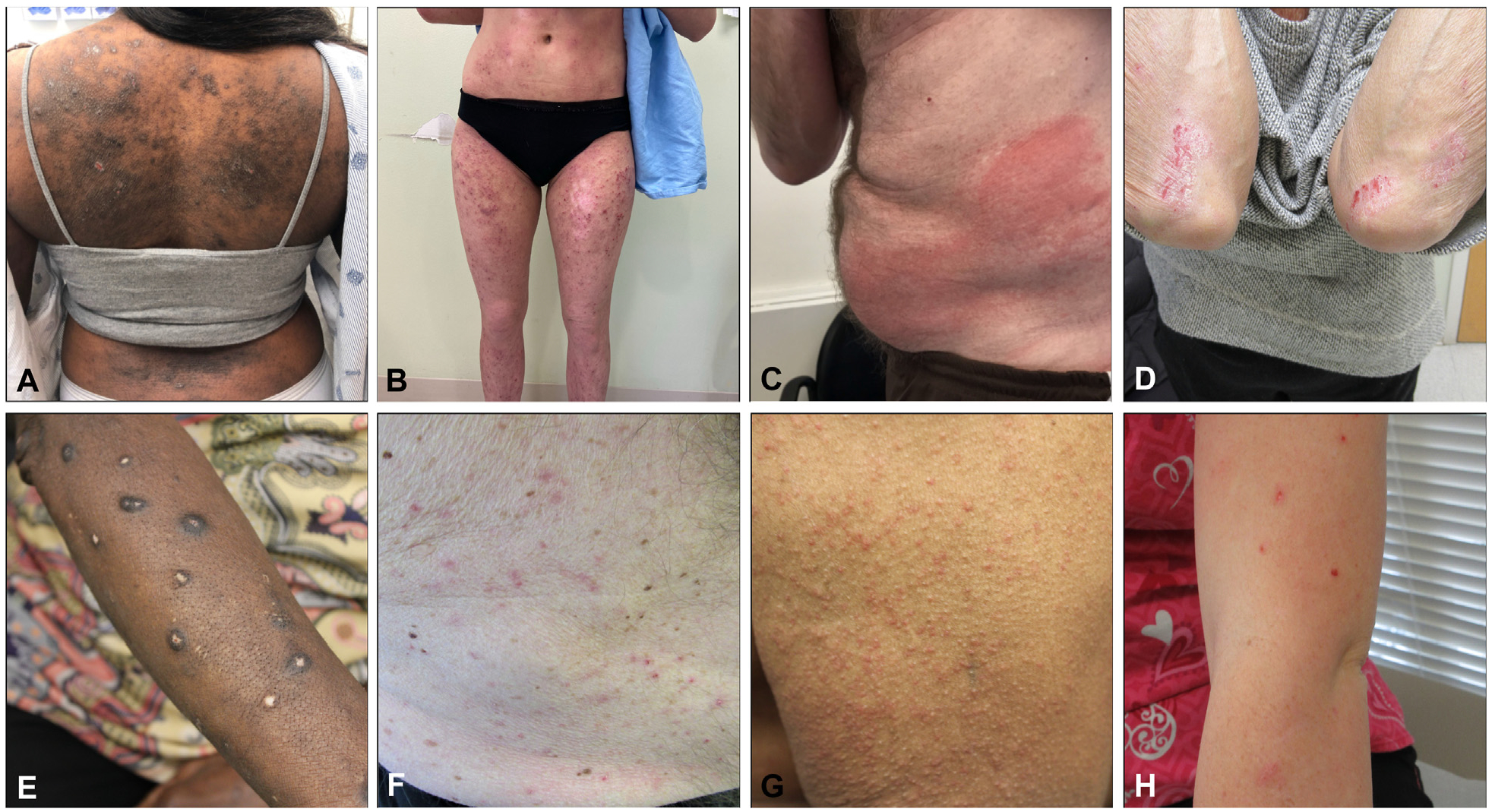
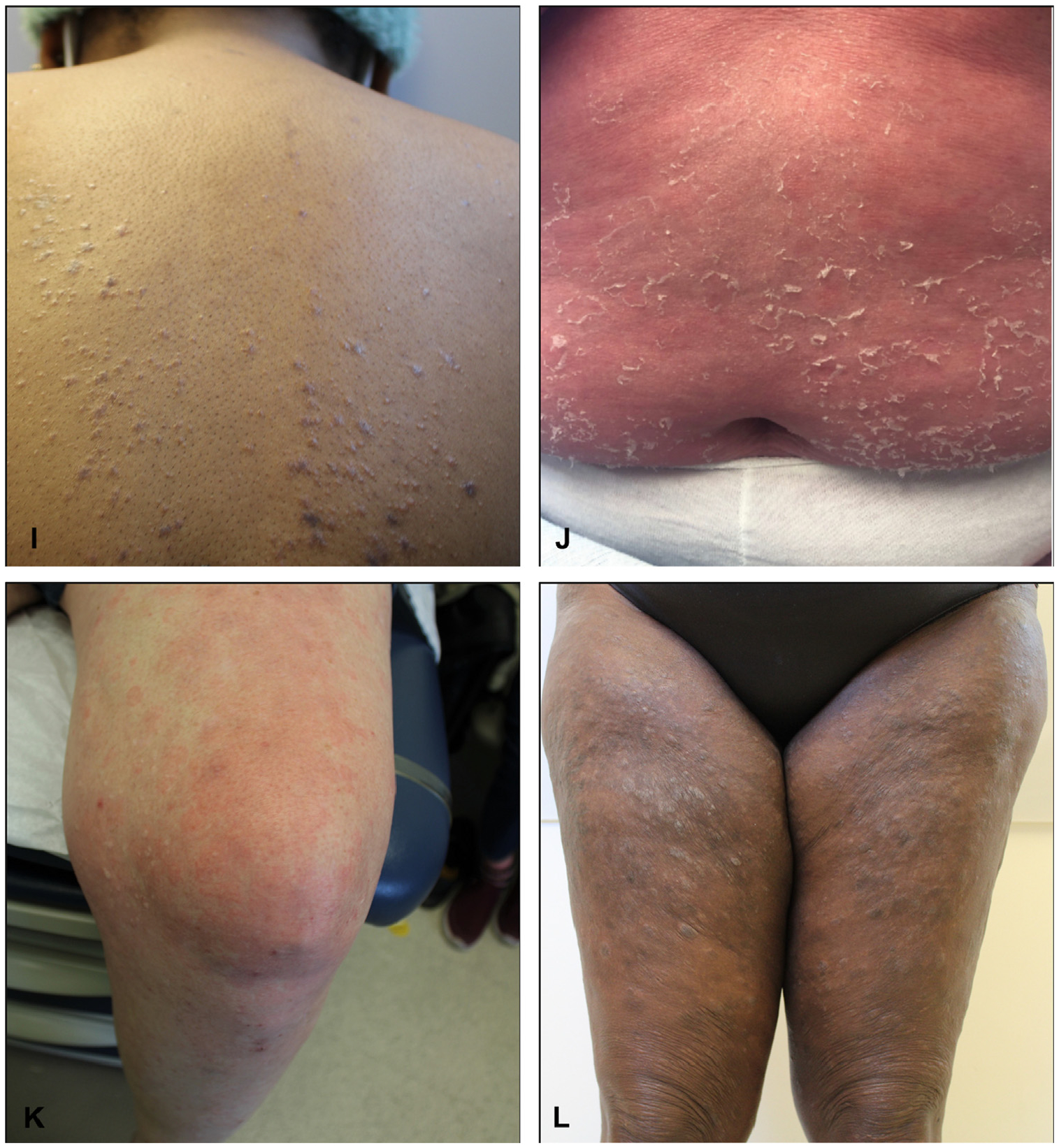
Various clinical presentations of itch. A and B, Atopic dermatitis. C, Contact dermatitis. D, Psoriasis. E, Prurigo nodularis. F, Grover disease. G, Scabies. H, Dermal hypersensitivity reaction, often referred to as “itchy red bump disease.” I, Lichen planus. J, Pityriasis rubra pilaris. K, Chronic urticaria. L, Mycosis fungoides.
Fig 4.
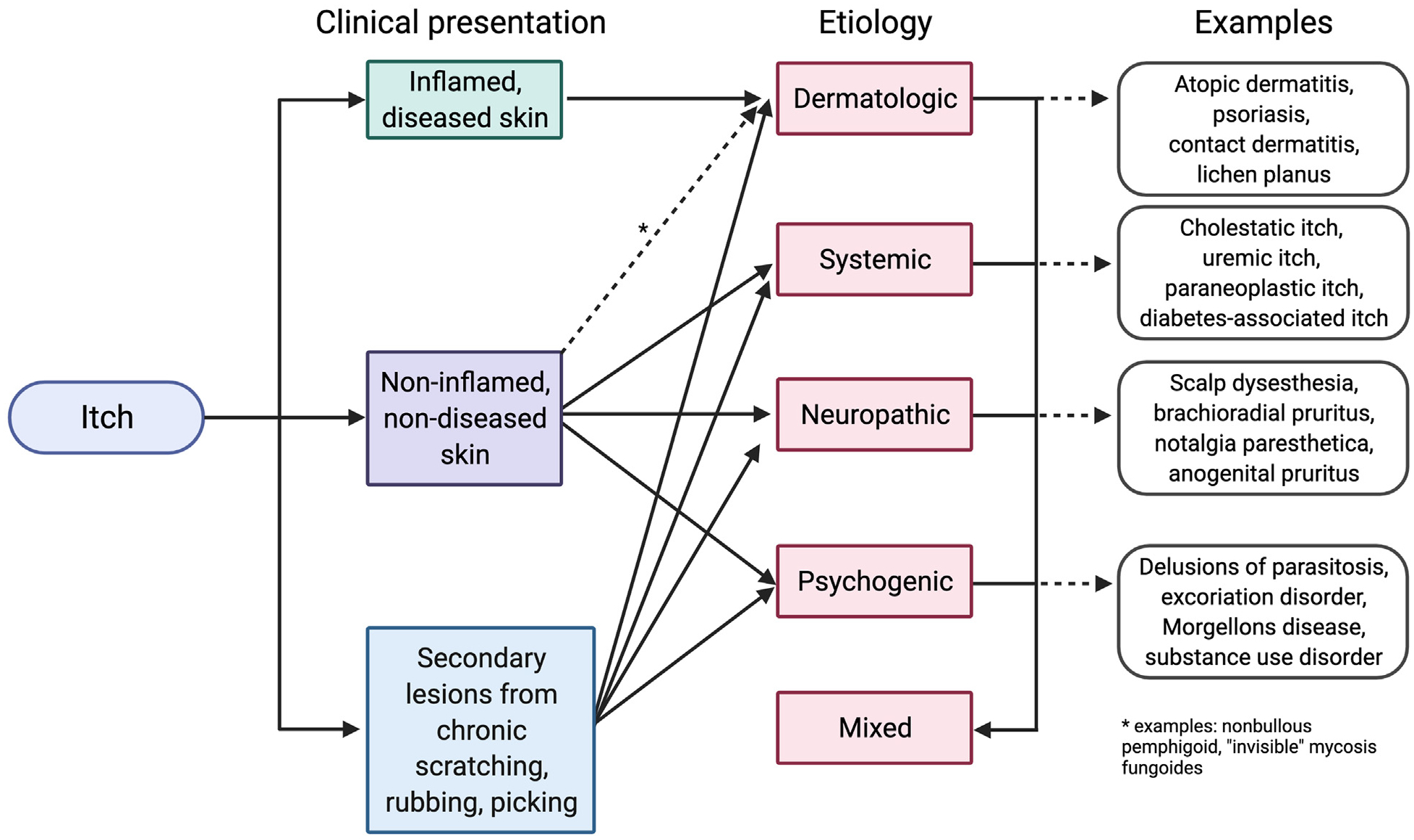
General approach to itch based on clinical presentation and underlying etiology. These are not absolute categorizations, but represent a general schema.
There are several well-validated tools that evaluate itch severity, including the itch numerical rating scale, pruritus grading system, peak pruritus rating scale, worst itch numerical rating scale, and verbal itch rating scale, which are convenient to use in daily practice. Impact on quality of life can also be assessed through the 5D pruritus scale,124 ItchyQoL,125 Skindex-29,126 Patient-Reported Outcomes Measurement Information System Itch Questionnaire,127 dermatological life quality index, Pittsburgh sleep quality index, Beck Depression Inventory, and hospital anxiety and depression scale, although many of these tools are better suited for clinical trials. In particular, the ItchyQoL has been validated across many European languages128,129 and is one of the most comprehensive tools to capture multiple domains of itch-related quality of life disruptions.125
The first step in evaluating a patient presenting with itch involves determining whether the itch is attributed to a dermatologic cause. This requires a comprehensive history and a thorough physical examination (Table II). Duration of symptoms, episodic nature, severity, and itch localization can provide important clues to the pathogenesis of the itch. For example, nocturnal worsening of itch is observed in most types of itch but may be associated with increased activity of mites at nighttime in patients with scabies. Exogenous triggers or exacerbating factors, such as occupational irritants, water, and heat or sweat, should be identified, as exposure to the indicated triggers can lead to contact dermatitis, aquagenic pruritus, or cholinergic urticaria, respectively. Additional factors that warrant consideration are included in Table II. A thorough assessment of medical history (eg, thyroid, liver, and renal disease; HIV infection; malignancy; psychiatric diagnoses; history of atopy; and/or neck or back pain) and constitutional signs (eg, fevers, chills, night sweats, unintended weight loss, fatigue, heat intolerance) may suggest underlying etiologic factors.
Table II.
Initial history and physical examination for the assessment of itch
| History | Physical examination |
|---|---|
| Duration | Skin examination |
| Localization of pruritus | Inflamed skin vs noninflamed skin |
| Timing and direction | Primary skin lesion |
| Worsen at night? | Secondary skin lesion (eg, excoriations) |
| Intermittent? Constant? Worsening? | Examples of other skin examination findings |
| Triggers | Dermatographism |
| Environmental exposures | Signs of atopy |
| Contact with water | Hyperlinear, thickened palms |
| Heat, exercise, sweating | Infraorbital Dennie-Morgan folds |
| Household members affected? | Track-like burrows between fingers |
| Pregnancy status | Erosions and scales between toes |
| ROS | Stigmata of liver disease |
| Constitutional B symptoms | Jaundice |
| Pain, paresthesia, heat intolerance | Ascites |
| Past medical history | Palmar erythema |
| History of atopic triad | Gynecomastia |
| History of neck or back pain | Thyroid examination |
| Recent medication change | Lymphadenopathy |
| Allergic history | Cachexia |
| Social history | Assessment of itch and its burden |
| Risk factors for communicable disease (HIV, hepatitis C, etc) | Itch NRS |
| Scale of 0–10, 0 (“no itch”) and 10 (“the worst imaginable itch”) | |
| Occupational exposure | |
| Risk factors of malabsorption | WI-NRS for 24 h |
| History of substance abuse | Scale of 0–10 |
| Travel history | Verbal rating scale of itch on a scale of 0–4 |
| Risk factors for infestation | No itch (0), mild itch (1), moderate itch (2), severe itch (3), very severe itch (4) |
NRS, Numeric rating scale; ROS, review of systems; WI-NRS, worst itch numeric rating scale.
Clinical characteristics of itch offers important clues to the etiology of itch (Fig 5). A thorough skin examination for any primary dermatologic lesion necessitates the identification of a primary lesion (if present) and/or characterization of secondary skin changes due to scratching, rubbing, and picking. Physical manifestations of nondermatologic conditions also should be investigated. Jaundice, ascites, palmar erythema, spider hemangiomas, or gynecomastia, may suggest a hepatobiliary cause; whereas, lymphadenopathy and signs of cachexia or wasting may point toward an underlying malignancy.
Fig 5.
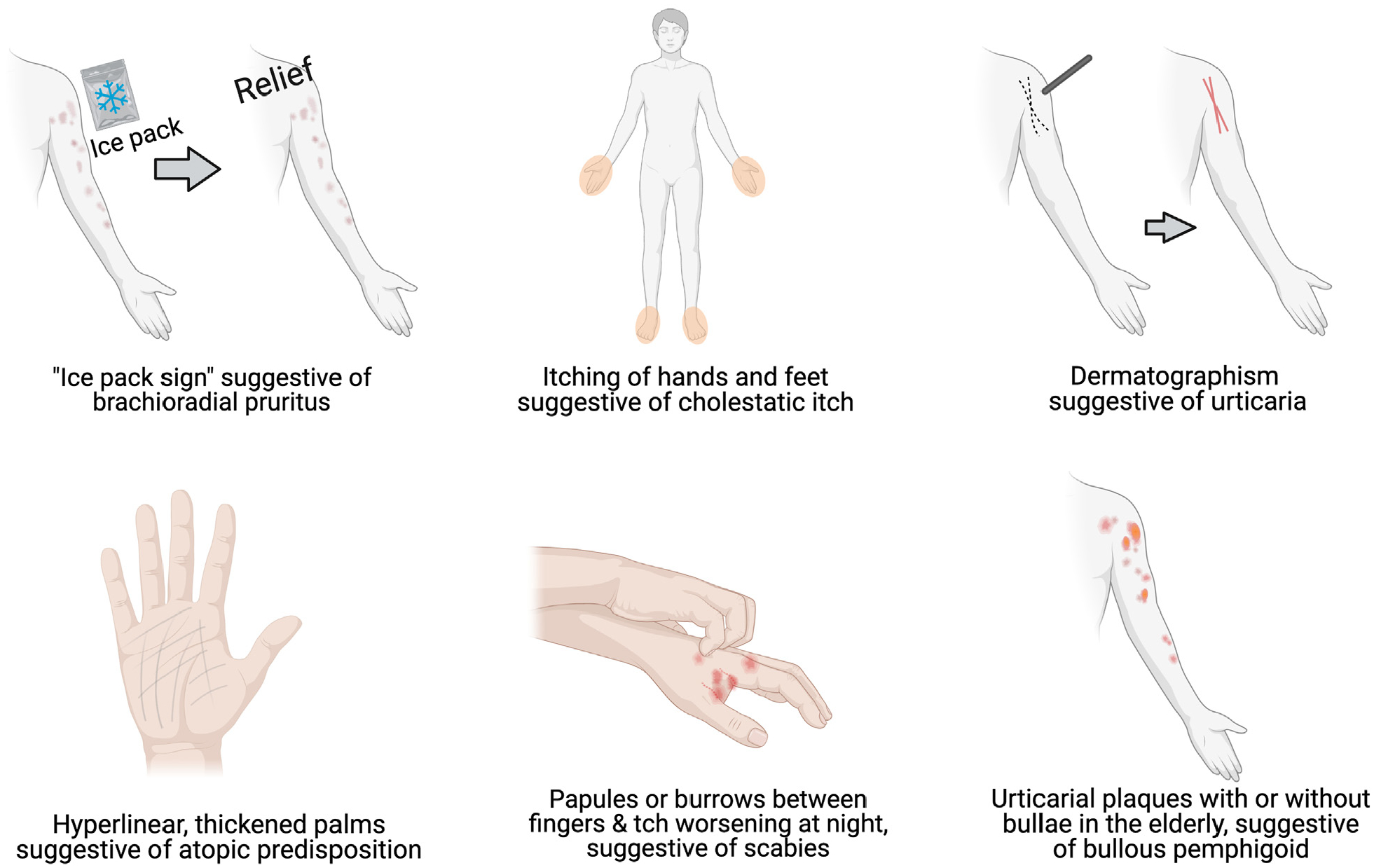
Bedside clinical clues suggestive of etiologies of itch.
A suggested diagnostic algorithm for the workup of chronic itch is shown in Fig 6. Patients who present with pruritus in the absence of primary dermatologic lesions or eruptions should be screened for nondermatologic causes. The initial tests may include complete blood count with differential, liver function tests, kidney function tests, hemoglobin A1c or fasting glucose, and thyroid function tests. The additional tests to be considered based on a focused history and review of systems include HIV serology, hepatitis serologies, iron studies, and stool ova and parasites. Testing for heavy metal levels and vitamin D or B12 levels in the blood is also a potential consideration. A biopsy with hematoxylin-eosin staining and direct immunoflouresence staining may be considered, even in the absence of primary skin findings, for the elderly patients who may present with nonbullous pemphigoid or “invisible” mycosis fungoides.
Fig 6.
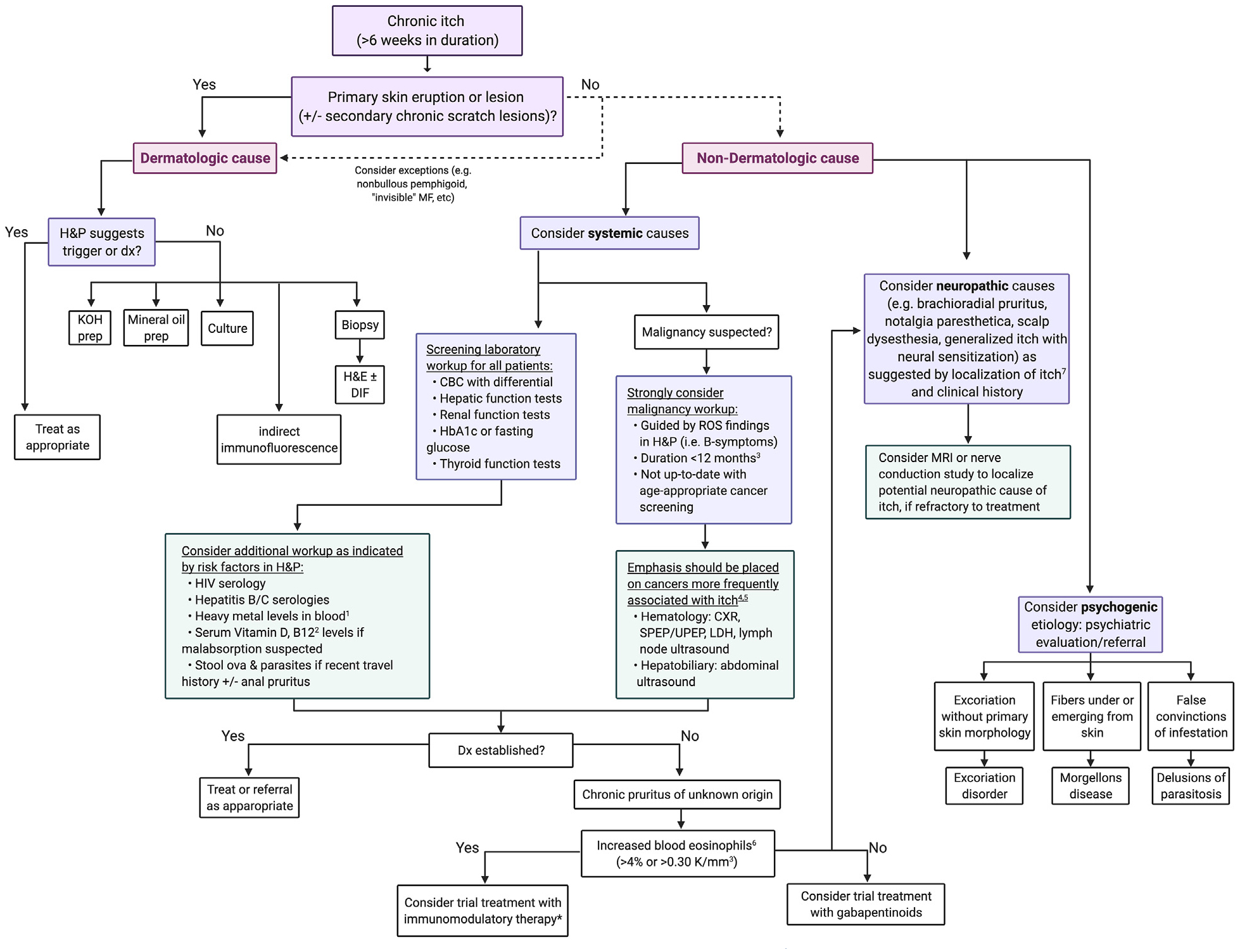
Diagnostic workup algorithm of chronic itch.20,69,70,78,87,91,107 *Although the consideration of trial treatment with immunomodulary therapy needs further exploration, patients with elevated IgE may also be responsive to immunodulatory therapy.130 CBC, Complete blood count; CXR, chest x-ray; DIF, direct immunofluorescence; Dx, diagnosis; H&E, hematoxylin and eosin; H&P, history and physical examination; HbA1c, hemoglobin A1c; KOH, potassium hydroxide; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; ROS, review of systems; SPEP, serum protein electrophoresis; UPEP, urine protein electrophoresis.
Clinicians should consider a workup for occult malignancy based on findings of history, physical examination, and the results of the basic screening laboratory tests. There should be an especially lower threshold for malignancy workup for patients within the first 3 months up to 12 months of pruritus diagnosis,78 or among those who are not up-to-date with age-appropriate cancer screenings. In this context, a higher index of suspicion should be maintained for hematologic cancers63–66 and cancers of the hepatobiliary system,69–71 which are most frequently associated with itch. Such oncologic causes of itch may be ruled out through further imaging and laboratory studies, including chest x-ray, serum protein electrophoresis or urine protein electrophoresis, and ultrasound.
If no specific systemic disease process is identified, patients may be given the diagnosis of chronic pruritus of unknown origin (CPUO). A retrospective study at the Johns Hopkins Itch Center found that the patients with CPUO can be subclassified by the presence or absence of increased number of blood eosinophils (>4%, or >0.30 K/mm3).20 Those with increased eosinophils had better therapeutic response to immunomodulatory therapies. Conversely, CPUO patients without increased eosinophils were more likely to have spinal disorder history and have better response to neuromodulator therapies such as gabapentin. Elevated serum immunoglobulin E levels observed in patients with CPUO may also suggest response to immunomodulatory therapies.21 Although the value of using immunoglobulin E as a therapeutic biomarker in CPUO needs to be explored further, there are reports of dupilumab-associated reduction in immunoglobulin E levels and itch severity in patients with atopic dermatitis.130 In patients with concurrent history of neck or back pain, additional consideration should be given for neural sensitization as an etiologic factor in their itch.
Conflicts of interest
Dr Kwatra is an advisory board member or consultant for AbbVie, Celldex Therapeutics, Incyte Corporation, Galderma, Pfizer Inc, Regeneron Pharmaceuticals, and Menlo Therapeutics; is an investigator or has received grant funding from Galderma SA, Kiniksa Pharmaceuticals, Pfizer Inc, and Sanofi; is a recipient of a Dermatology Foundation Medical Dermatology Career Development Award and has received grant funding from the Skin of Color Society; and is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under the award number K23AR077073. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Authors Roh, Choi, and Sutaria have no conflicts of interest to declare.
Abbreviation used:
- CPUO
chronic pruritus of unknown origin
Footnotes
Reprints not available from the authors.
Disclosures
None.
REFERENCES
- 1.Shive M, Linos E, Berger T, Wehner M, Chren MM. Itch as a patient-reported symptom in ambulatory care visits in the United States. J Am Acad Dermatol. 2013;69(4):550–556. 10.1016/j.jaad.2013.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6): 1527–1534. 10.1038/jid.2013.446 [DOI] [PubMed] [Google Scholar]
- 3.Dalgard F, Svensson A, Holm J, Sundby J. Self-reported skin morbidity among adults: associations with quality of life and general health in a Norwegian survey. J Investig Dermatol Symp Proc. 2004;9(2):120–125. 10.1046/j.1087-0024.2003.09111.x [DOI] [PubMed] [Google Scholar]
- 4.Ständer S, Schafer I, Phan NQ, et al. Prevalence of chronic pruritus in Germany: results of a cross-sectional study in a sample working population of 11,730. Dermatology. 2010; 221(3):229–235. 10.1159/000319862 [DOI] [PubMed] [Google Scholar]
- 5.Matterne U, Apfelbacher CJ, Vogelgsang L, Loerbroks A, Weisshaar E. Incidence and determinants of chronic pruritus: a population-based cohort study. Acta Derm Venereol. 2013; 93(5):532–537. 10.2340/00015555-1572 [DOI] [PubMed] [Google Scholar]
- 6.Matterne U, Apfelbacher CJ, Loerbroks A, et al. Prevalence, correlates and characteristics of chronic pruritus: a population-based cross-sectional study. Acta Derm Venereol. 2011;91(6):674–679. 10.2340/00015555-1159 [DOI] [PubMed] [Google Scholar]
- 7.Kini SP, DeLong LK, Veledar E, McKenzi-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10): 1153–1156. 10.1001/archdermatol.2011.178 [DOI] [PubMed] [Google Scholar]
- 8.Whang KA, Khanna R, Williams KA, Mahadevan V, Semenov Y, Swatra SG. Health-related QOL and economic burden of chronic pruritus. J Invest Dermatol. 2021;141(4):754–760.e1. 10.1016/j.jid.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 9.Jensen P, Zachariae C, Skov L, Zachariae R. Sleep disturbance in psoriasis: a case-controlled study. Br J Dermatol. 2018; 179(6):1376–1384. 10.1111/bjd.16702 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Yamazaki S, Hayashino Y, et al. Association between frequency of pruritic symptoms and perceived psychological stress: a Japanese population-based study. Arch Dermatol. 2009;145(12):1384–1388. 10.1001/archdermatol.2009.290 [DOI] [PubMed] [Google Scholar]
- 11.Dalgard FJ, Svensson Å, Halvorsen JA, et al. Itch and mental health in dermatological patients across Europe: a cross-sectional study in 13 countries. J Invest Dermatol. 2020;140(3): 568–573. 10.1016/j.jid.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 12.Erturk IE, Arican O, Omurlu IK, Sut N. Effect of the pruritus on the quality of life: a preliminary study. Ann Dermatol. 2012;24(4):406–412. 10.5021/ad.2012.24.4.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yalçin B, Tamer E, Toy GG, Oztas P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45(6):672–676. 10.1111/j.1365-4632.2005.02607.x [DOI] [PubMed] [Google Scholar]
- 14.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769–781. 10.1097/JGP.0b013e3181ad4f5a [DOI] [PubMed] [Google Scholar]
- 15.Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310(22):2443–2450. 10.1001/jama.2013.282023 [DOI] [PubMed] [Google Scholar]
- 16.Namer B Age related changes in human C-fiber function. Neurosci Lett. 2010;470(3):185–187. 10.1016/j.neulet.2009.07.023 [DOI] [PubMed] [Google Scholar]
- 17.Joly P, Benoit-Corven C, Baricault S, et al. Chronic eczematous eruptions of the elderly are associated with chronic exposure to calcium channel blockers: results from a case-control study. J Invest Dermatol. 2007;127(12): 2766–2771. 10.1038/sj.jid.5701018 [DOI] [PubMed] [Google Scholar]
- 18.Pétavy-Catala C, Martin L, Fontès V, Lorette G, Vaillant L. Hydrochlorothiazide-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2001;81(3): 209. 10.1080/000155501750376348 [DOI] [PubMed] [Google Scholar]
- 19.Reich A, Ständer S, Szepietowski JC. Drug-induced pruritus: a review. Acta Derm Venereol. 2009;89(3): 236–244. 10.2340/00015555-0650 [DOI] [PubMed] [Google Scholar]
- 20.Roh YS, Khanna R, Patel SP, et al. Circulating blood eosinophils as a biomarker for variable clinical presentation and therapeutic response in patients with chronic pruritus of unknown origin. J Allergy Clin Immunol Pract. 2021;9(6): 2513–2516. 10.1016/j.jaip.2021.01.034 [DOI] [PubMed] [Google Scholar]
- 21.Dehner C, Chen L, Kim B, Rosman IS. Chronic itch of unknown origin is associated with an enhanced Th2 skin immune profile. Preprint. Am J Dermatopathol 2021. 10.1097/dad.0000000000001902 [DOI] [PubMed] [Google Scholar]
- 22.Xu AZ, Tripathi SV, Kau AL, Schaffer A, Kim BS. Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. J Am Acad Dermatol. 2016;74(5): 1017–1020. 10.1016/j.jaad.2015.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ständer S, Stumpf A, Osada N, Wilp S, Chatzigeorgakidis E, Pfleiderer B. Gender differences in chronic pruritus: women present different morbidity, more scratch lesions and higher burden. Br J Dermatol. 2013;168(6):1273–1280. 10.1111/bjd.12267 [DOI] [PubMed] [Google Scholar]
- 24.Kursewicz C, Fowler E, Rosen J, et al. Sex differences in the perception of itch and quality of life in patients with chronic pruritus in the United States. Itch. 2020;5(3):e41. 10.1097/itx.0000000000000041 [DOI] [Google Scholar]
- 25.Bergman H, Melamed N, Koren G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59(12):1290–1294. [PMC free article] [PubMed] [Google Scholar]
- 26.Weisshaar E, Diepgen TL, Luger TA, Seeliger S, Witteler R, Stander S. Pruritus in pregnancy and childhood—do we really consider all relevant differential diagnoses? Eur J Dermatol. 2005;15(5):320–331. [PubMed] [Google Scholar]
- 27.Piechota J, Jelski W. Intrahepatic cholestasis in pregnancy: review of the literature. J Clin Med. 2020;9(5):1361. 10.3390/jcm9051361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whang KA, Khanna R, Thomas J, Aguh C, Kwatra SG. Racial and gender differences in the presentation of pruritus. Medicines (Basel). 2019;6(4):98. 10.3390/medicines6040098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McColl M, Boozalis E, Aguh C, Eseonu A, Okoye GA, Kwatra SG. Pruritus in Black skin: unique molecular characteristics and clinical features. J Natl Med Assoc. 2021; 113(1):30–38. 10.1016/j.jnma.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Huang AH, Swatra SG, Khanna R, Semenov YR, Okoye GA, Sweren RJ. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111(6):633–639. 10.1016/j.jnma.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 31.Boozalis E, Tang O, Patel, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79(4):714–719.e3. 10.1016/j.jaad.2018.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. 10.1038/jid.2010.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutaria N, Parthasarathy V, Roh YS, et al. Itch in skin of color: a multicenter cross-sectional study. Preprint. Br J Dermatol. 2021. 10.1111/bjd.20403 [DOI] [PubMed] [Google Scholar]
- 34.Wongvibulsin S, Sutaria N, Kannan S, et al. Transcriptomic analysis of atopic dermatitis in African Americans is characterized by Th2/Th17-centered cutaneous immune activation. Sci Rep. 2021;11(1):1–11. 10.1038/s41598-021-90105-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014; 150(6):613–620. 10.1001/jamadermatol.2013.7696 [DOI] [PubMed] [Google Scholar]
- 36.Shaw FM, Luk KM, Chen KH, Wrenn G, Chen SC. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77(1): 63–69. 10.1016/j.jaad.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 37.Peters MG, Di Bisceglie AM, Kowdley K, et al. Differences between Caucasian, African American, and Hispanic patients with primary biliary cirrhosis in the United States. Hepatology. 2007;46(3):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nzerue CM, Demissochew H, Tucker JK. Race and kidney disease: role of social and environmental factors. J Natl Med Assoc. 2002;94(suppl 8):28S–38S. [PMC free article] [PubMed] [Google Scholar]
- 39.Bender AM, Tang O, Khanna R, Stander S, Kang S, Swatra S. Racial differences in dermatologic conditions associated with HIV: a cross-sectional study of 4679 patients in an urban tertiary care center. J Am Acad Dermatol. 2020; 82(5):1117–1123. 10.1016/j.jaad.2019.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhoeven EWM, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156(6):1346–1349. 10.1111/j.1365-2133.2007.07916.x [DOI] [PubMed] [Google Scholar]
- 41.Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35(4):383–391. 10.1016/j.semnephrol.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Sang Y, Yang M, Chen X, Tang W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: a meta-analysis of cross-sectional studies. Medicine (Baltimore). 2018;97(21):e10633. 10.1097/md.0000000000010633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ponticelli C, Bencini PL. Pruritus in dialysis patients: a neglected problem. Nephrol Dial Transpl. 1995;10(12): 2174–2176. 10.1093/ndt/10.12.2174 [DOI] [PubMed] [Google Scholar]
- 44.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transpl. 2010;25(4):1251–1257. 10.1093/ndt/gfp588 [DOI] [PubMed] [Google Scholar]
- 45.Massry SG, Popovtzer MM, Coburn JW, Makoff DL, Maxwell MH, Kleeman CR. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia: disappearance of itching after subtotal parathyroidectomy. N Engl J Med. 1968;279(13):697–700. 10.1056/nejm196809262791308 [DOI] [PubMed] [Google Scholar]
- 46.Chou FF, Ho JC, Huang SC, Sheen-Chen SM. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2000;190(1):65–70. 10.1016/s1072-7515(99)00212-4 [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Lu Y, Feng S, Zhan Z, Shen H. Evaluation of laboratory parameters and symptoms after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Ren Fail. 2019;41(1):921–929. 10.1080/0886022x.2019.1666724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weisshaar E, Dalgard F. Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol. 2009;89(4): 339–350. 10.2340/00015555-0662 [DOI] [PubMed] [Google Scholar]
- 49.Patel SP, Vasavda C, Ho B, Meixiong J, Dong Z, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371–1378. 10.1016/j.jaad.2019.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunchorntavakul C, Reddy KR. Pruritus in chronic cholestatic liver disease. Clin Liver Dis. 2012;16(2):331–346. 10.1016/j.cld.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 51.Meixiong J, Vasavda C, Green D, et al. Identification of a bilirubin receptor that may mediate a component of cholestatic itch. Elife. 2019;8(e44116). 10.7554/eLife.44116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neilly JB, Martin A, Simpson N, MacCuish AC. Pruritus in diabetes mellitus: Investigation of prevalence and correlation with diabetes control. Diabetes Care. 1986;9(3): 273–275. 10.2337/diacare.9.3.273 [DOI] [PubMed] [Google Scholar]
- 53.Diabetes Scribner M. and pruritus of the scalp. JAMA. 1977; 237(15):1559. [PubMed] [Google Scholar]
- 54.Stawiski MA, Voorhees JJ. Cutaneous signs of diabetes mellitus. Cutis. 1976;18(3):415–421. [PubMed] [Google Scholar]
- 55.Kwon C, Sutaria N, Khanna R, et al. Epidemiology and comorbidities of excoriation disorder: a retrospective case-control study. J Clin Med. 2020;9(9):2703. 10.3390/jcm9092703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caravati CM Jr, Richardson DR, Wood BT, Cawley EP. Cutaneous manifestations of hyperthyroidism. South Med J. 1969;62(9):1127–1130. 10.1097/00007611-196909000-00020 [DOI] [PubMed] [Google Scholar]
- 57.Krajnik M, Zylicz Z. Understanding pruritus in systemic disease. J Pain Symptom Manage. 2001;21(2):151–168. 10.1016/s0885-3924(00)00256-6 [DOI] [PubMed] [Google Scholar]
- 58.Yahya A, Gideon PS. Characterizing pruritus in autoimmune connective tissue diseases. J Drugs Dermatol. 2019;18(10): 995–998. [PubMed] [Google Scholar]
- 59.Razykov I, Levis B, Hudson M, Baron M, Thombs BD. Prevalence and clinical correlates of pruritus in patients with systemic sclerosis: an updated analysis of 959 patients. Rheumatology (Oxford). 2013;52(11):2056–2061. 10.1093/rheumatology/ket275 [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Zeidi M, Bonciani D, et al. Itch in dermatomyositis: the role of increased skin interleukin-31. Br J Dermatol. 2018; 179(3):669–678. 10.1111/bjd.16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson ES, Feng R, Okawa J, Werth VP. Improvement in the cutaneous disease activity of patients with dermatomyositis is associated with a better quality of life. Br J Dermatol. 2015; 172(1):169–174. 10.1111/bjd.13167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valdes-Rodriguez R, Rowe B, Lee HG, et al. Chronic pruritus in primary Sjögren’s syndrome: characteristics and effect on quality of life. Acta Derm Venereol. 2017;97(3):385–386. 10.2340/00015555-2524 [DOI] [PubMed] [Google Scholar]
- 63.Gobbi PG, Attardo-Parrinello G, Lattanzio G, Rizzo SC, Ascari E. Severe pruritus should be a B-symptom in Hodgkin’s disease. Cancer. 1983;51(10):1934–1936. [DOI] [PubMed] [Google Scholar]
- 64.Kumar SS, Kuruvilla M, Pai GS, Dinesh M. Cutaneous manifestations of non-Hodgkin’s lymphoma. Indian J Dermatol Venereol Leprol. 2003;69(1):12–15. [PubMed] [Google Scholar]
- 65.Steinman HK, Greaves MW. Aquagenic pruritus. J Am Acad Dermatol. 1985;13(1):91–96. 10.1016/s0190-9622(85)70149-1 [DOI] [PubMed] [Google Scholar]
- 66.Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88(8):665–669. 10.1002/ajh.23474 [DOI] [PubMed] [Google Scholar]
- 67.Leiferman KM, Gleich GJ, Peters MS. Dermatologic manifestations of the hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):415–441. 10.1016/j.iac.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 68.Valent P, Klion AD, Horny H-P, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012; 130(3):607–612.e9. 10.1016/j.jaci.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larson VA, Tang O, Ständer S, Kang S, Kwatra SG. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80(4):931–937. 10.1016/j.jaad.2018.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fett N, Haynes K, Propert KJ, Margolis DJ. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014; 70(4):651–658. 10.1016/j.jaad.2013.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Yosipovitch G. New insights into the pathophysiology and treatment of chronic itch in patients with end-stage renal disease, chronic liver disease, and lymphoma. Int J Dermatol. 2010;49(1):1–11. 10.1111/j.1365-4632.2009.04249.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012; 148(12):1422–1423. 10.1001/archdermatol.2012.3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campanella N, Moraca A, Pergolini M, et al. Paraneoplastic syndromes in 68 cases of resectable non-small cell lung carcinoma: can they help in early detection? Med Oncol. 1999; 16(2):129–133. 10.1007/bf02785846 [DOI] [PubMed] [Google Scholar]
- 74.King NK, Siriwardana HP, Coyne JD, Siriwardena AK. Intractable pruritus associated with insulinoma in the absence of multiple endocrine neoplasia: a novel paraneoplastic phenomenon. Scand J Gastroenterol. 2003; 38(6):678–680. 10.1080/00365520310001950 [DOI] [PubMed] [Google Scholar]
- 75.Ojeda IC, Calderon JC, Plaza K, Vanegas E, Cherrez A, Cano J. Urticaria as initial finding of a patient with carcinoid tumor. World Allergy Organ J. 2015;8(1):1–4. 10.1186/s40413-015-0083-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Etter L, Myers SA. Pruritus in systemic disease: mechanisms and management. Dermatol Clin. 2002;20(3):459–472. 10.1016/s0733-8635(02)00011-6 [DOI] [PubMed] [Google Scholar]
- 77.Cormia FE. Pruritus, an uncommon but important symptom of systemic carcinoma. Arch Dermatol. 1965;92(1):36–39. [PubMed] [Google Scholar]
- 78.Johannesdottir SA, Farkas DK, Vinding GR, et al. Cancer incidence among patients with a hospital diagnosis of pruritus: a nationwide Danish cohort study. Br J Dermatol. 2014;171(4):839–846. 10.1111/bjd.13157 [DOI] [PubMed] [Google Scholar]
- 79.Fett N, Haynes K, Propert KJ, Margolis DJ. Predictors of malignancy development in patients with chronic pruritus. J Dermatol Sci. 2016;82(2):123–128. 10.1016/j.jdermsci.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 80.Kaul S, Kaffenberger BH, Choi JN, Kwatra SG. Cutaneous adverse reactions of anticancer agents. Dermatol Clin. 2019;37(4):555–568. 10.1016/j.det.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 81.Le TK, Kaul S, Cappelli LC, Naidoo J, Semenov YR, Kwatra SG. Cutaneous adverse events of immune checkpoint inhibitor therapy: Incidence and types of reactive dermatoses. J Dermatolog Treat. 2021;Accepted(In Press):1–5. 10.1080/09546634.2021.1898529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fischer A, Rosen AC, Ensslin CJ, Wu S, Lacouture ME. Pruritus to anticancer agents targeting the EGFR, BRAF, and CTLA-4. Dermatol Ther. 2013;26(2):135–148. 10.1111/dth.12027 [DOI] [PubMed] [Google Scholar]
- 83.Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. 10.1016/j.currproblcancer.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 84.Sibaud V Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. 10.1007/s40257-017-0336-3 [DOI] [PubMed] [Google Scholar]
- 85.Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol. 1989;37(5):539–540. 10.1007/bf00558141 [DOI] [PubMed] [Google Scholar]
- 86.Takkunen H Iron deficiency in the Finnish adult population. An epidemiological survey from 1967 to 1972 inclusive. Scand J Haematol Suppl. 1976;25:1–91. [PubMed] [Google Scholar]
- 87.Patel SP, Khanna R, Belzberg M, Kang S, Kwatra SG. Elevated blood cadmium and lead levels in chronic pruritic dermatoses. J Invest Dermatol. 2020;140(1):238–241. 10.1016/j.jid.2019.06.130 [DOI] [PubMed] [Google Scholar]
- 88.Umar M, Sastry KS, Ali FA, Al-Khulaifi M, Wang E, Chouchane AI. Vitamin D and the pathophysiology of inflammatory skin diseases. Skin Pharmacol Physiol. 2018; 31(2):74–86. 10.1159/000485132 [DOI] [PubMed] [Google Scholar]
- 89.Goetz DW. Idiopathic itch, rash, and urticaria/angioedema merit serum vitamin D evaluation: a descriptive case series. W V Med J. 2011;107(1):14–20. [PubMed] [Google Scholar]
- 90.Tuchinda P, Kulthanan K, Chularojanamontri L, Arunkajohnsak S, Sriussadaporn S. Relationship between vitamin D and chronic spontaneous urticaria: a systematic review. Clin Transl Allergy. 2018;8(1):51. 10.1186/s13601-018-0234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polat M, Oztas P, Ilhan MN, Yalçin B, Alli N. Generalized pruritus: a prospective study concerning etiology. Am J Clin Dermatol. 2008;9(1):39–44. 10.2165/00128071-200809010-00004 [DOI] [PubMed] [Google Scholar]
- 92.Jaffary F, Faghihi G, Mokhtarian A, Hosseini SM. Effects of oral vitamin E on treatment of atopic dermatitis: a randomized controlled trial. J Res Med Sci. 2015;20(11): 1053–1057. 10.4103/1735-1995.172815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung KE, Woo YR, Lee JS, et al. Effect of topical vitamin D on chronic kidney disease-associated pruritus: an open-label pilot study. J Dermatol. 2015;42(8):800–803. 10.1111/1346-8138.12895 [DOI] [PubMed] [Google Scholar]
- 94.Katayama I, Miyazaki Y, Nishioka K. Topical vitamin D3 (tacalcitol) for steroid-resistant prurigo. Br J Dermatol. 1996; 135(2):237–240. [PubMed] [Google Scholar]
- 95.Efficacy Kircik L. and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis. J Drugs Dermatol. 2009;8(suppl 8):S9–S16. [PubMed] [Google Scholar]
- 96.Kaushik SB, Cerci FB, Miracle J, et al. Chronic pruritus in HIV-positive patients in the southeastern United States: its prevalence and effect on quality of life. J Am Acad Dermatol. 2014;70(4):659–664. 10.1016/j.jaad.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 97.Blanes M, Belinchon I, Portilla J, Betlloch I, Reus S, Sanchez-Paya J. Pruritus in HIV-infected patients in the era of combination antiretroviral therapy: a study of its prevalence and causes. Int J STD AIDS. 2012;23(4):255–257. 10.1258/ijsa.2009.009189 [DOI] [PubMed] [Google Scholar]
- 98.Rodwell GE, Berger TG. Pruritus and cutaneous inflammatory conditions in HIV disease. Clin Dermatol. 2000;18(4):479–484. 10.1016/s0738-081x(99)00143-1 [DOI] [PubMed] [Google Scholar]
- 99.Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140(2):480–483.e4. 10.1016/j.jid.2019.07.697 [DOI] [PubMed] [Google Scholar]
- 100.Dlova NC, Mosam A. Inflammatory noninfectious dermatoses of HIV. Dermatol Clin. 2006;24(4):439–448. 10.1016/j.det.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 101.Eisman S Pruritic papular eruption in HIV. Dermatol Clin. 2006;24(4):449–457. 10.1016/j.det.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 102.Steinhoff M, Schmelz M, Szabó IL, Oaklander AL. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018;17(8):709–720. 10.1016/s1474-4422(18)30217-5 [DOI] [PubMed] [Google Scholar]
- 103.Stumpf A, Ständer S. Neuropathic itch: diagnosis and management. Dermatol Ther. 2013;26(2):104–109. 10.1111/dth.12028 [DOI] [PubMed] [Google Scholar]
- 104.Pinto AC, Wachholz PA, Masuda PY, Martelli AC. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91(4):549–551. 10.1590/abd1806-4841.201644767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mirzoyev SA, Davis MD. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169(5): 1007–1015. 10.1111/bjd.12483 [DOI] [PubMed] [Google Scholar]
- 106.Weinberg BD, Amans M, Deviren S, Berger T, Shah V. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4(7):640–644. 10.1016/j.jdcr.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magnetic resonance tomography study. J Am Acad Dermatol. 2011; 65(4):756–762. 10.1016/j.jaad.2010.07.036 [DOI] [PubMed] [Google Scholar]
- 108.Kwatra SG, Stander S, Bernhard JD, Weisshaar E, Yosipovitch G. Brachioradial pruritus: a trigger for generalization of itch. J Am Acad Dermatol. 2013;68(5):870–873. 10.1016/j.jaad.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 109.Huesmann T, Cunha PR, Osada N, et al. Notalgia paraesthetica: a descriptive two-cohort study of 65 patients from Brazil and Germany. Acta Derm Venereol. 2012;92(5): 535–540. 10.2340/00015555-1344 [DOI] [PubMed] [Google Scholar]
- 110.Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52(6):1085–1087. 10.1016/j.jaad.2005.01.138 [DOI] [PubMed] [Google Scholar]
- 111.Bin Saif GA, Alajroush A, McMichael A, et al. Aberrant C nerve fibre function of the healthy scalp. Br J Dermatol. 2012;167(3): 485–489. 10.1111/j.1365-2133.2012.11070.x [DOI] [PubMed] [Google Scholar]
- 112.Thornsberry LA, English JC III. Scalp dysesthesia related to cervical spine disease. JAMA Dermatol. 2013;149(2):200–203. 10.1001/jamadermatol.2013.914 [DOI] [PubMed] [Google Scholar]
- 113.Lee JJ, Morillo-Hernandez C, Agarwal V, Standaert CJ, English JC III. Cervical spine imaging and treatment outcomes in scalp dysesthesia: a retrospective cohort study. Preprint. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohen AD, Vander T, Medvendovsky E, et al. Neuropathic scrotal pruritus: anogenital pruritus is a symptom of lumbosacral radiculopathy. J Am Acad Dermatol. 2005;52(1): 61–66. 10.1016/j.jaad.2004.04.039 [DOI] [PubMed] [Google Scholar]
- 115.Gupta MA, Gupta AK, Schork NJ, Ellis CN. Depression modulates pruritus perception: a study of pruritus in psoriasis, atopic dermatitis, and chronic idiopathic urticaria. Psychosom Med. 1994;56(1):36–40. 10.1097/00006842-199401000-00005 [DOI] [PubMed] [Google Scholar]
- 116.Kaaz K, Szepietowski JC, Matusiak Ł. Sleep quality among adult patients with chronic dermatoses. Postepy Dermatol Alergol. 2019;36(6):659–666. 10.5114/ada.2019.84007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel SP, Khanna R, Choi J, et al. Sleep disturbance in adults with chronic pruritic dermatoses is associated with increased C-reactive protein levels. J Am Acad Dermatol. 2021;84(2):265–272. 10.1016/j.jaad.2020.08.059 [DOI] [PubMed] [Google Scholar]
- 118.Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Stander S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol. 2006;31(6): 762–767. 10.1111/j.1365-2230.2006.02211.x [DOI] [PubMed] [Google Scholar]
- 119.Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148–155. 10.1016/j.jaad.2020.08.117 [DOI] [PubMed] [Google Scholar]
- 120.Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87(4):291–294. 10.2340/00015555-0305 [DOI] [PubMed] [Google Scholar]
- 121.DeenDeen K, O’Brien B, Wu J. Invisible mycosis fungoides: not to be missed in chronic pruritus. Dermatol Ther (Heidelb). 2015;5(3):213–216. 10.1007/s13555-015-0083-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bakker CV, Terra JB, Pas HH, Jonkman MF. Bullous pemphigoid as pruritus in the elderly: a common presentation. JAMA Dermatol. 2013;149(8):950–953. 10.1001/jamadermatol.2013.756 [DOI] [PubMed] [Google Scholar]
- 123.Meijer JM, Diercks GF, de Lang EW, Pas HH, Jonkman MF. Assessment of diagnostic strategy for early recognition of bullous and nonbullous variants of pemphigoid. JAMA Dermatol. 2019;155(2):158–165. 10.1001/jamadermatol.2018.4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593. 10.1111/j.1365-2133.2009.09586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59(2):234–244. 10.1016/j.jaad.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 126.Chren MM, Lasek RJ, Quinn LM, Covinsky KE. Convergent and discriminant validity of a generic and a disease-specific instrument to measure quality of life in patients with skin disease. J Invest Dermatol. 1997;108(1):103–107. 10.1111/1523-1747.ep12285650 [DOI] [PubMed] [Google Scholar]
- 127.Silverberg JI, Lai JS, Kantor RW, et al. Development, validation, and interpretation of the PROMIS itch questionnaire: a patient-reported outcome measure for the quality of life impact of itch. J Invest Dermatol. 2020;140(5):986–994. 10.1016/j.jid.2019.08.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krause K, Kessler B, Weller K, et al. German version of ItchyQoL: validation and initial clinical findings. Acta Derm Venereol. 2013;93(5):562–568. 10.2340/00015555-1544 [DOI] [PubMed] [Google Scholar]
- 129.Zeidler C, Steinke S, Riepe C, et al. Cross-European validation of the ItchyQoL in pruritic dermatoses. J Eur Acad Dermatol Venereol. 2019;33(2):391–397. 10.1111/jdv.15225 [DOI] [PubMed] [Google Scholar]
- 130.Olesen CM, Holm JG, Norreslet LB, Serup JV, Thomsen SF, Agner T. Treatment of atopic dermatitis with dupilumab: experience from a tertiary referral centre. J Eur Acad Dermatol Venereol. 2019;33(8):1562–1568. 10.1111/jdv.15609 [DOI] [PubMed] [Google Scholar]


