Abstract
mTORC1 (mechanistic target of rapamycin complex 1) serves as a molecular hub and intracellular energy sensor that regulate various cellular processes. Emerging evidence points to mTORC1 signaling as a critical regulator of cardiovascular function with implications for cardiovascular disease. Here, we show that selective disruption of mTORC1, through conditional Raptor gene deletion, in endothelial or smooth muscle cells alter vascular function. Endothelial cell-specific Raptor deletion results in reduced relaxation responses evoked by acetylcholine in the aorta, but not in the mesenteric artery. Of note, endothelial-specific Raptor deletion did not affect endothelial-independent vasorelaxation nor the contractile responses of the aorta or mesenteric artery. Interestingly, endothelial Raptor haploinsufficiency did not alter vascular endothelial function, but attenuated the endothelial dysfunction evoked by angiotensin II. Smooth muscle cell-specific conditional deletion of Raptor reduces both endothelial- and smooth muscle-dependent relaxation responses as well as receptor-dependent and -independent contractility in the aorta. This was associated with activation of autophagy signaling. Notably, the changes in vascular function evoked by endothelial and smooth muscle Raptor deletion were independent of changes in blood pressure and heart rate. Together, these data suggest that vascular mTORC1 signaling is a critical regulator of vascular endothelial and smooth muscle function. mTORC1 signaling may represent a potential target for the treatment of vascular diseases associated with altered mTORC1 activity.
Keywords: vascular function, endothelial, smooth muscle, mTORC1, angiotensin II, autophagy
Graphical Abstract
Introduction
mTORC1 (mechanistic target of rapamycin complex 1) is a master regulator of the cellular processes involved in metabolism, growth, proliferation and survival1. mTORC1 serves as an intracellular sensor that integrates a myriad of intracellular and extracellular signals such as PI3K (phosphoinositol-3 kinase), growth factors, hormones, amino acids and glucose. The mTORC1 complex consists of a conserved catalytic subunit (mTOR), the regulatory associated subunit Raptor (regulatory associated protein of mTOR), and multiple accessory proteins. Various downstream signaling pathways are modulated by mTORC1 including the S6K (S6 kinase) and its downstream effector, the ribosomal S6 protein, that regulate different cellular processes including gene expression, protein and lipid synthesis, autophagy and mitochondrial metabolism and biogenesis1.
Recently, the mTORC1 signaling pathway has emerged as an important mechanism for cardiovascular regulation2. For instance, activation of mTORC1 pathway in aortic rings with leucine or an adenoviral vector expressing a constitutively active S6K reduces endothelial-dependent vasorelaxation3. mTORC1 seems to integrate vascular-related signaling arising from oxidative stress, pro-inflammatory cytokines, and the renin-angiotensin system all of which can lead to vascular dysfunction4, 5. In a recent report, we showed that mTORC1 activation in endothelial cells increases ROS (reactive oxygen species) generation and expression of genes that promote a pro-oxidant environment3. Blockade of ROS signaling with Tempol reversed the endothelial dysfunction evoked by mTORC1 activation indicating a crucial interaction between mTORC1 and ROS signaling. Moreover, inhibition of mTORC1 signaling with rapamycin has been shown to improve the endothelial dysfunction induced by angiotensin II (Ang-II)6. However, the contribution of mTORC1 signaling in various cell types of the vasculature to the regulation of vascular function remains unclear.
In this study, we tested the hypothesis that mTORC1 signaling in endothelial and smooth muscle cells is required for the regulation of vascular function and that disruption of mTORC1 signaling affects vascular function. Using tamoxifen-inducible endothelial- and smooth muscle-specific Cre drivers, we generated mice lacking the Raptor gene selectively in endothelial or smooth muscle cells. Our data show that loss of Raptor in endothelial or smooth muscle cells lead to vascular dysfunction in a manner independent of changes in blood pressure. We further show that vascular rings isolated from endothelial Raptor haplo-insufficient mice are partially protected against the deleterious vascular endothelial effects evoked by Ang-II.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Animals
We generated two separate mouse models bearing either endothelial- or smooth muscle-specific disruption of mTORC1 signaling. To conditionally disrupt mTORC1 signaling in endothelial cells (TekCreERT2/RaptorF/F) we crossed RaptorF/F female mice (B6.CG-RPTORM1.1DMSA/J; Jackson Labs, stock #013188) with tamoxifen inducible Tie-2 promoter Cre recombinase (Tek-CreERT2) male mice (Strain: B6.Cg-Tg(Tek-Cre/ERT2)1Arnd/ArndCnrm; European Mouse Mutant Archive; stock #EM:00715). To disrupt mTORC1 signaling specifically in smooth muscle cells (SMCreERT2/RaptorF/F), we crossed RaptorF/F female mice (B6.CG-RPTORM1.1DMSA/J; Jackson Labs, stock #013188) with tamoxifen inducible smMHC promoter Cre recombinase (smMHC-CreERT2) male mice (Strain: B6.FVB-Tg(Myh11-Cre/ERT2)/1Soff/J; Jackson Labs; stock #019079) to generate smooth muscle specific deletion of Raptor. To visualize Cre recombinase we crossed the SMCreERT2 mice with ROSA (td-Tomato) reporter transgenic mouse line that has stop codon flanked by loxP sites preceding the start position of a td-Tomato locus (Stopfl/fl-tdTomato). Cre recombination removes the stop site, leading to the expression of the fluorescent td-Tomato protein. All mice were maintained on the B6 background for this study.
Tamoxifen (Sigma) dissolved in corn oil (vehicle) was administered intraperitoneally (75mg/kg) daily for 5 consecutive days to 8–12 weeks old male mice. Molecular and physiological analyses were performed 2- or 4-week post-Tamoxifen treatment as indicated. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Iowa and adhere to animal care guidelines of the National Institutes of Health.
Radiotelemetry Blood Pressure
Blood pressure, heart rate and activity were measured in conscious mice using radiotelemetry (PA-C10; Data Sciences International) as described previously7, 8. Telemetry catheters were inserted into the left carotid artery and telemeter units were placed in a subcutaneous pocket on the right flank. After 10 days of recovery, blood pressure, heart rate and activity were measured at baseline over a 4 consecutive day period prior to Tamoxifen treatment. The same parameters were then recorded for 3 consecutive days around 7, 14, and 28 days post-tamoxifen administration.
Vascular Function
Thoracic aortas (3mm length) and second-order mesenteric arteries (2mm length) were dissected and mounted on a wire myograph (DMT Model 610M). Preload for aortic rings was set at ~0.5g and starting tension (IC90) was applied to all mesenteric rings as previously described3, 9. Aortic and mesenteric rings were sub-maximally contracted with PGF2α (prostaglandin F2α Lutalyse, Zoetis, provided by University of Iowa Pharmacy) or U-46619 (Cayman Chemical) and cumulative response curves to acetylcholine (1nM-100μM) and SNP (sodium nitroprusside, 1nM-100μM) were assessed. Additionally, cumulative contractile responses to phenylephrine (1nM-100μM), PGF2α (Prostaglandin F2α, 1μM-100μM), U-46619 (1nM-10μM) and KCl depolarization (100mM) were assessed.
A subset of aortic rings were infected in 24hr culture (DMEM:F12 media) with Ad-S6KCA (adenovirus expressing a constitutively active S6K), or Ad-GFP (adenovirus expressing a green fluorescent protein, used as control) as previously described 3. A separate subset of aortic rings was incubated with 100nM Ang-II or PBS (Vehicle) for 24hr. Data are expressed as means ± SEM and reported as percentage relaxation (%), maximal force generated (g), or maximum tension generated (mN/mm).
Cell Sorting
Thoracic aortas were isolated and digested in an endothelial cell digestion buffer: MCDB-131 complete media, collagenase A (1mg/mL), collagenase B (1mg/mL), and DNase1 (100μg/mL) at 37°C for 20 minutes. Cell suspension was subjected to cell sorting using a neonatal cardiac endothelial cell isolation kit (Miltenyi Biotech #130–104-183) and an autoMACS cell sorter (Miltenyi Biotech). Cell fractions were then processed for molecular analysis.
Western Blotting
Aortic rings and endothelial positive and negative cell separations were homogenized in RIPA buffer (#R0278; Sigma) and a protease inhibitor cocktail (#11836170001; Roche). Lysates were subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes and probed for Raptor (1:1000; Cell Signaling #2280), eNOS (endothelial nitric oxide; 1:1000; BD Biosciences #610297), phospho-eNOS (Ser1177; 1:1000; Cell Signaling #9571), phospho-S6 (1:5000; Cell Signaling #5364), total S6 (1:1000; Cell Signaling #2217), total S6K (1:1000; Cell Signaling #2708), NRF2 (nuclear factor erythroid 2–related factor 2; 1:1000; Abcam #ab62352), p62 (1:1000; Cell Signaling #5114), LC3A (1:1000; Cell Signaling #4599), and β-actin (1:50000; Proteintech #60008). Protein detection was performed using HRP-conjugated anti-rabbit (1:10000; Cell Signaling #7074) or anti-mouse (1:10000; Cell Signaling #7076) secondary antibodies and visualized either on film with an ECL Prime chemiluminescent kit (GE Healthcare) or imaged on a Sapphire Biomolecular Imager (Azure Biosystems). Densitometry was calculated with ImageJ software.
Immunofluorescence
Aortic rings of SMCreERT2/Stopfl/fl-tdTomato mice were mounted in optimal cutting temperature (OCT) solution and then cryo-sectioned at 10μm thickness. Aortic rings sections were fixed in 4% PFA for 20 minutes at room temperature. Immunohistochemistry was performed on aortic ring sections to detect von Willebrand factor (vWF; 1:1000; Abcam, ab8822). Confocal microscopy (Zeiss LSM710) was used to visualize images.
Statistical Analysis
All data are presented as means ± SEM. Data were analyzed and graphed using GraphPad Prism 7 or 8 software. Group comparisons were performed using a One-way ANOVA or unpaired t-test where appropriate. Hemodynamic and vascular function analyses were performed using a Two-way ANOVA with or without repeated measures and a Bonferroni (2 groups) or Tukey’s (3 groups or more) post-hoc test. Statistical significance was accepted with p<0.05.
Results
Tamoxifen inducible endothelial-specific deletion of Raptor
To test the role of mTORC1 signaling in the regulation of vascular endothelial function, we generated a tamoxifen-inducible mouse model of endothelial-specific deletion of Raptor, a critical subunit of the mTORC1 signaling complex. To this end, we crossed RaptorF/F female mice with a tamoxifen-inducible, endothelial-specific Cre recombinase (TekCreERT2/RaptorF/F) male mouse. We began by validating our model by isolating endothelial cell fractions from pooled aortas of tamoxifen-treated RaptorF/F (control) and tamoxifen-inducible TekCreERT2/RaptorF/F conditional knockout male mice. Magnetically sorted endothelial cell-positive and -negative fractions were then subjected to western blot analysis to confirm knockdown of Raptor expression in TekCreERT2/RaptorF/F in endothelial cells with no change in Raptor expression in stromal fractions (p<0.05 via one-way ANOVA; Figure 1A). Four weeks after tamoxifen treatment body weight was not different in tamoxifen-inducible endothelial-specific conditional Raptor knockout mice relative to wildtype controls (Figure S1A in the online-only Data Supplement) indicating that interfering with endothelial mTORC1 signaling does not affect the overall health of the mice.
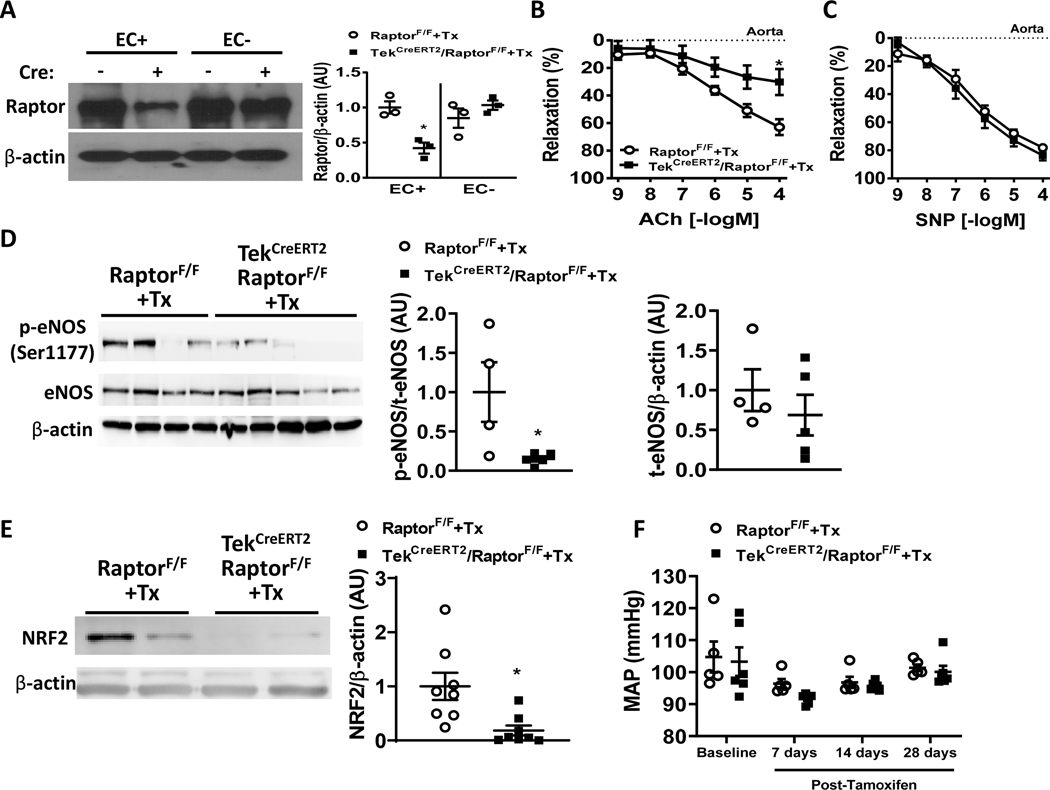
Figure 1 – Conditional endothelial-specific Raptor deletion impairs endothelial relaxation responses.
(A) Validation of endothelial-specific Raptor knockout in aortas from tamoxifen-treated control (RaptorF/F) and endothelial-specific conditional Raptor knockout (TekCreERT2/RaptorF/F) male mice. Representative western blot images of magnetically sorted endothelial (EC+) and stromal fractions (EC-) probed for Raptor and β-actin (n=3 independent experiments; *p<0.05 via one-way ANOVA). (B-C) Vasorelaxation responses induced by endothelial-dependent ACh (acetylcholine; B) and endothelial-independent SNP (sodium nitroprusside; C) of aortic rings of tamoxifen-treated RaptorF/F and TekCreERT2/RaptorF/F male mice (n=7–8/group) (*p<0.05 via two-way ANOVA with repeated measures). (D-E) Representative western blot images of aortic lysates isolated from tamoxifen-treated control (RaptorF/F) and endothelial-specific conditional Raptor knockout (TekCreERT2/RaptorF/F) male mice probed for phospho-eNOS (Ser1177), eNOS (*p<0.05 via unpaired t-test; D) and NRF2 (*p<0.05 via unpaired t-test; E). β-actin was used as loading control. (F) Comparison of mean arterial pressure (MAP) between TekCreERT2/RaptorF/F and RaptorF/F control male mice at baseline and after tamoxifen treatment.
Endothelial-specific Raptor deletion impairs vascular endothelial function
We then tested the effect of endothelial deletion of Raptor on aortic and mesenteric vascular function. Surprisingly, aortic rings isolated from tamoxifen-inducible endothelial-specific conditional Raptor knockout male mice have reduced acetylcholine-mediated endothelial vasorelaxation responses compared to tamoxifen-treated controls 4-week post-Tamoxifen administration (pinteraction<0.01 via two-way ANOVA with repeated measures; Figure 1B). This reduction in acetylcholine-induced vasorelaxation was evident as early as 2-week post Tamoxifen administration (p<0.05 via one-way ANOVA; Figure S1B). No differences were noted in the endothelial-independent vasorelaxation responses evoked by SNP (Figure 1C and Figure S1C). These data indicate that the detrimental effects of disrupting endothelial Raptor are restricted to the endothelium. Interestingly, this finding seems to be unique to aortic function as there was no effect of endothelial Raptor conditional knockout on resistance mesenteric artery relaxation responses (Figure S1D–E). The reduced aortic endothelial dependent acetylcholine-induced relaxation responses of TekCreERT2/RaptorF/F male mice suggest reduced NO (nitric oxide) availability. Interestingly, we found a significant decrease in the ratio of phospho-eNOS (Ser1177) to total eNOS protein in TekCreERT2/RaptorF/F male mice compared to tamoxifen-treated wildtypes (p<0.05 via unpaired t-test; Figure 1D) with no change in total eNOS expression. NRF2 is a transcription factor known to modulate antioxidant enzyme and NO availability10. Aortas isolated from TekCreERT2/RaptorF/F male mice demonstrated a significant reduction in NRF2 expression compared to tamoxifen-treated controls (p<0.05 via unpaired t-test; Figure 1E). Taken together, these data suggest that loss of Raptor in endothelial cells results in reduced NO and NRF2 signaling may account for the reduced endothelial-dependent relaxation responses in the aorta of TekCreERT2/RaptorF/F mice. Surprisingly, endothelial deletion of Raptor did not affect arterial pressure (Figure 1F and Figure S2A–B) or heart rate (Figure S2C). Physical activity was also unchanged in TekCreERT2/RaptorF/F mice compared to controls (Figure S2D). These data suggest that the reduced endothelial-dependent vasorelaxation of the aorta are not caused by blood pressure changes.
In addition to relaxation responses, we also investigated contractile responses to tamoxifen-inducible endothelial-specific Raptor deletion in male mice. Tamoxifen-inducible endothelial-specific Raptor conditional deletion did not affect contractile responses in aortic or mesenteric arterial rings to PE (phenylephrine)-, PGF2α-, U-46619, or KCl-induced (100mM) depolarization responses (Figure S3A–F).
Restoration of mTORC1 signaling rescues the endothelial dysfunction evoked by loss of Raptor
To verify that the reduction in acetylcholine-mediated vasorelaxation responses in TekCreERT2/RaptorF/F male mice was mTORC1 signaling dependent, we rescued S6K signaling, downstream of Raptor, using Ad-S6KCA (an adenovirus expressing a constitutively active S6K) as previously described3. Aortic rings isolated from tamoxifen-treated TekCreERT2/RaptorF/F mice were infected, in culture (DMEM:F12; 24hrs), with Ad-S6KCA or Ad-GFP (control) before they were subjected to wire myography. Efficacy of adenoviral infection was demonstrated by increased expression of the total S6K in aortic lysates and restoration of mTORC1 signaling was demonstrated by increased phospho-S6/S6 levels in lysates of aortas subjected to adenoviral infection (p<0.05 via unpaired t-test; Figure S4).
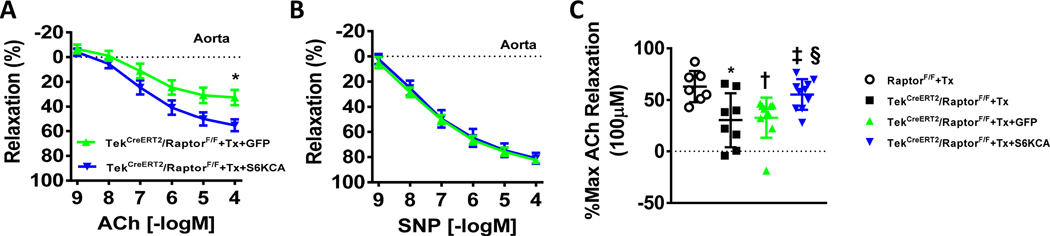
Adenoviral-mediated restoration of mTORC1 signaling in aortic rings of TekCreERT2/RaptorF/F conditional knockout male mice reversed the reduction in the relaxation evoked by acetylcholine (pinteraction<0.05 via two-way ANOVA with repeated measures; Figure 2A) without altering endothelial-independent SNP relaxation responses (Figure 2B). Maximal relaxation to acetylcholine (100μM) was reduced in TekCreERT2/RaptorF/F mice compared to tamoxifen-treated controls (p<0.05 via one-way ANOVA). This reduced maximal relaxation was maintained in culture with the Ad-GFP infection and was partially restored back to control levels after Ad-S6KCA infection (p<0.05 via one-way ANOVA; Figure 2C).
Figure 2 – Adenoviral-mediated rescue of mTORC1 signaling restores endothelial function in response to endothelial Raptor deletion.
(A-B) Vasorelaxation responses to ACh (acetylcholine; A) and SNP (sodium nitroprusside; B) of aortic rings isolated from tamoxifen-treated TekCreERT2/RaptorF/F conditional knockout male mice and infected in culture with Ad-GFP or Ad-S6KCA (DMEM:F12; 24hrs; n=10/group; *p<0.05 via two way ANOVA with repeated measures). (C) Maximal ACh relaxation responses (100μM) of aortic rings from indicated groups (*†p<0.05 vs RaptorF/F+Tx; ‡p<0.05 vs TekCreERT2/RaptorF/F+Tx; §p<0.05 vs TekCreERT2/RaptorF/F+Tx+GFP via one-way ANOVA).
Endothelial-specific Raptor haploinsufficiency attenuates Ang-II induced endothelial dysfunction
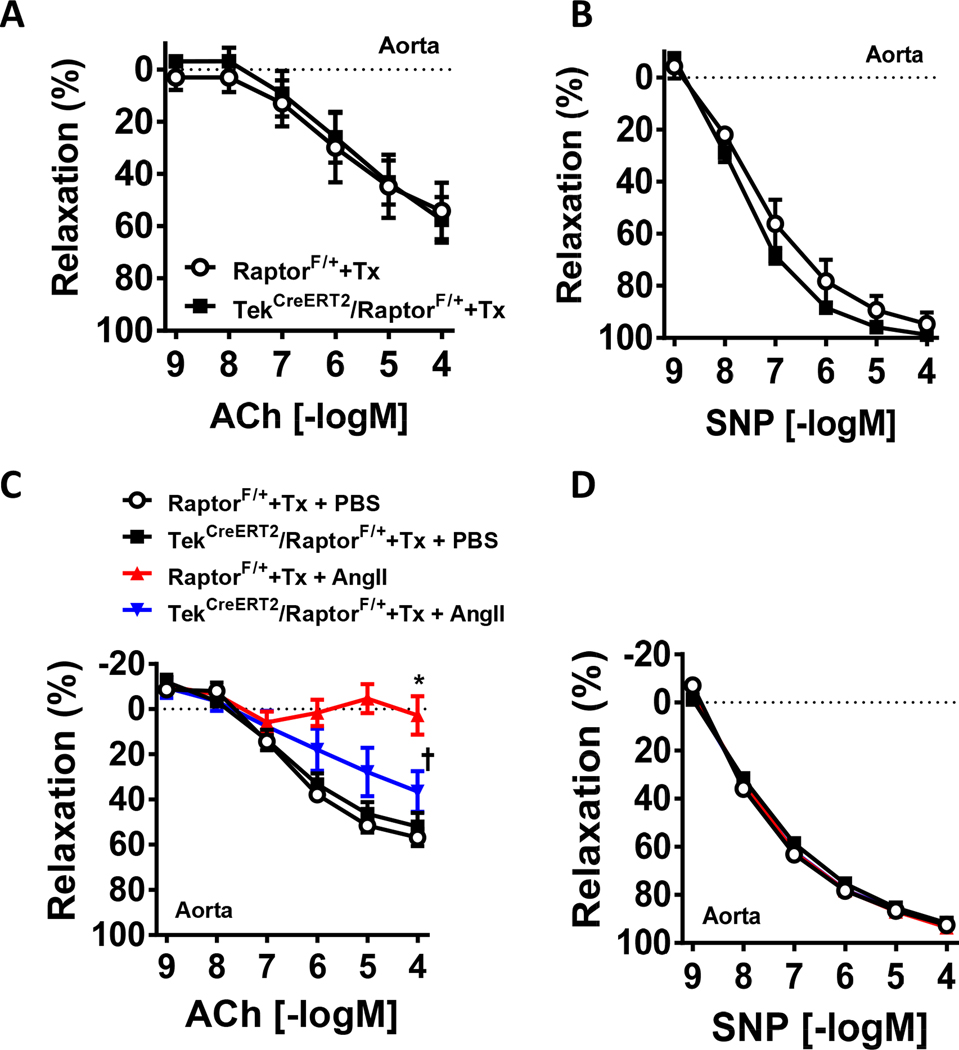
The reduced acetylcholine-mediated endothelial responses in tamoxifen-inducible endothelial homozygous Raptor deleted male mice led us to investigate whether Raptor haploinsufficiency interferes with endothelial function using heterozygous tamoxifen-inducible endothelial Raptor male mice (TekCreERT2/RaptorF/+). Interestingly, we found that heterozygous deletion of endothelial Raptor displayed normal endothelial function compared to tamoxifen-treated wild-type (RaptorF/+) controls (Figure 3A). No differences were found in SNP-induced smooth muscle vasorelaxation responses (Figure 3B). These data suggest that endothelial function is sensitive to the gene dosage of Raptor.
Figure 3 – Tamoxifen-inducible endothelial Raptor haploinsufficiency attenuates Ang-II-induced endothelial dysfunction.
(A-B) Aortic relaxation responses of tamoxifen-treated TekCreERT2/RaptorF/+ and control (RaptorF/+) male mice to ACh (acetylcholine; A) and SNP (sodium nitroprusside; B) (n=4–5/group). (C-D) Aortic relaxation responses induced by ACh (C) and SNP (D) in tamoxifen-treated TekCreERT2/RaptorF/+ mice and control (RaptorF/+) male mice cultured for 24hrs with Ang-II (100nM) or PBS (n=12/group) (*p<0.05 vs RaptorF/++Tx+PBS via two-way ANOVA with repeated measures; †p<0.05 vs RaptorF/++Tx+Ang-II via two-way ANOVA with repeated measures).
We next sought to challenge vascular rings from TekCreERT2/RaptorF/+ mice with an activator of mTORC1 signaling. Ang-II has been previously demonstrated to activate mTORC1 signaling in endothelial cells and blockade of mTORC1 signaling with the rapalog, rapamycin, attenuates the endothelial dysfunction associated with Ang-II6. Incubation of vascular rings from tamoxifen-treated control male mice (RaptorF/+) with Ang-II resulted in a reduction in acetylcholine-mediated relaxation responses (pinteraction<0.0001 via two-way ANOVA with repeated measures; Figure 3C). Remarkably, vascular rings from tamoxifen-treated TekCreERT2/RaptorF/+ male mice incubated with Ang-II exhibited a partial attenuation of the reduced endothelial response induced by acetylcholine. As expected, there were no difference in SNP-induced smooth muscle-mediated relaxation responses (Figure 3D). Taken together, these data indicate that one copy of the Raptor allele is sufficient to maintain endothelial homeostasis and function, and this can also partially protect against the deleterious endothelial effects of Ang-II.
Smooth muscle Raptor knockout alter endothelial and smooth muscle relaxation
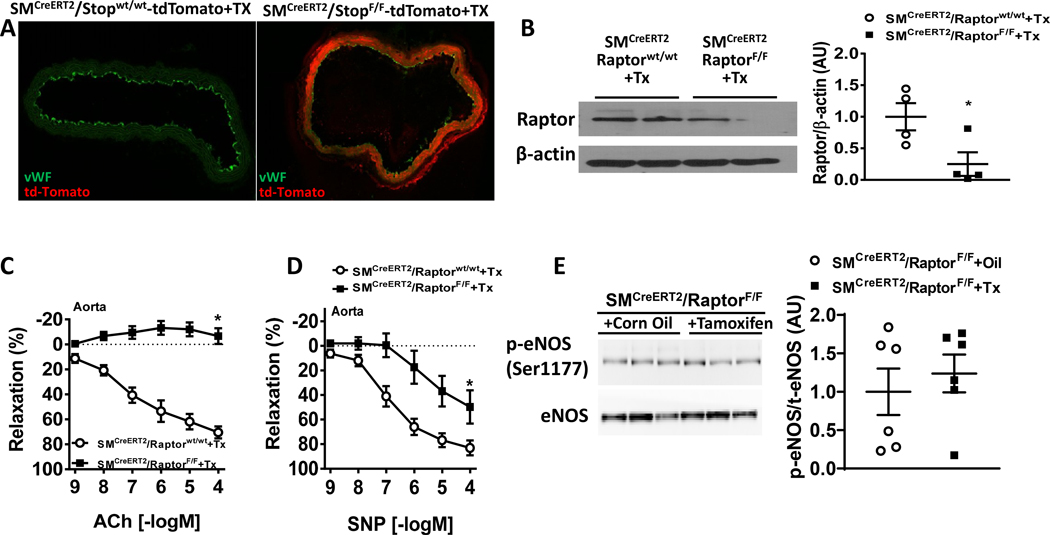
Next, we tested the role of Raptor signaling in the regulation of smooth muscle function by crossing RaptorF/F female mice with a smooth muscle specific Cre driver (smMHCCreERT2 referred to as SMCreERT2) male mice. We first validated the SMCreERT2 mouse model by visualizing Cre dependent tdTomato expression in the vascular smooth muscle layer of tamoxifen treated SMCreERT2/StopF/F-tdTomato reporter mice with tdTomato (red fluorescence) being present in aortic smooth muscle cells, but not endothelial cells (stained with vWF, Figure 4A). Next, we probed for Raptor expression in whole aortic and mesenteric lysates obtained from in SMCreERT2/RaptorF/F mice treated with tamoxifen compared to tamoxifen-treated wild-type male controls, SMCreERT2/RaptorF/F mice treated with tamoxifen displayed significant reduction in Raptor expression in both aorta (Figure 4B) and mesenteric artery (Figure S5A) associated with significant decrease in phospho-S6 levels (Figure S5B–C) indicative of inhibition of mTORC1 signaling. Similar to the endothelial-specific conditional Raptor knockout model, there was no effect on overall body weight measured 4-week post-Tamoxifen administration (Figure S5D).
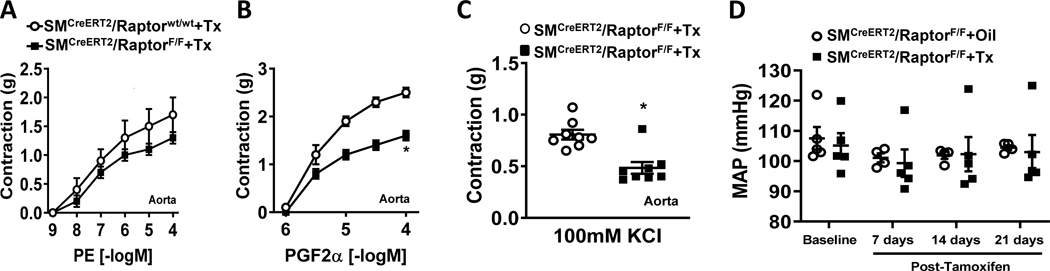
Figure 4 – Conditional smooth muscle-specific Raptor deletion alters aortic relaxation responses.
(A) Evidence of smooth muscle-specific Cre recombinase (presence of red fluorescent td-Tomato) in the aorta of SMCreERT2/Stopfl/fl-tdTomato reporter mice. (B) Representative western blot images and quantification of Raptor in aortas from SMCreERT2/RaptorF/F and tamoxifen treated control male mice (*p<0.05 via unpaired t-test). β-actin was used as loading control. (C-D) Relaxation responses to ACh (acetylcholine; C) and SNP (sodium nitroprusside; D) of aortic rings of SMCreERT2/RaptorF/F male mice and controls (n=8/group; *p<0.05 via two-way ANOVA with repeated measures). (E) Representative western blot images and quantification of phospho-eNOS (Ser1177) and eNOS in aortas from SMCreERT2/RaptorF/F male mice treated with tamoxifen or corn oil (vehicle). *P<0.05 vs controls via t-test (B) or two-way ANOVA with repeated measures (C-D).
We then characterized aortic and mesenteric relaxation function in response to smooth muscle-specific conditional Raptor knockout male mice. Surprisingly, aortic endothelial-mediated responses to acetylcholine was reduced compared to tamoxifen-treated wild-type male controls (pinteraction<0.0001 via two-way ANOVA with repeated measures; Figure 4C). SNP-induced smooth muscle relaxation was also reduced in mice lacking Raptor in the smooth muscle (pinteraction<0.001 via two-way ANOVA with repeated measures; Figure 4D). However, there was no effects of smooth muscle Raptor knockout on mesenteric artery relaxation responses (Figure S6A–B) similar to endothelial specific Raptor knockout mice. Additionally, we found no change in phospho-eNOS levels suggesting NO signaling is intact in these vascular segments (Figure 4E).
Smooth muscle Raptor knockout affect aortic contractility
We also investigated the contractile function in mice lacking the Raptor gene in smooth muscle. Aortic contraction to phenylephrine was intact in smooth muscle Raptor null male mice compared to tamoxifen treated controls (Figure 5A). Interestingly, smooth muscle Raptor deletion caused a significant reduction in aortic contractile responses to PGF2α (pinteraction<0.0001 via two-way ANOVA with repeated measures; Figure 5B) and KCl-induced depolarization (100mM; p<0.05 via unpaired t-test; Figure 5C). We found no effect of smooth muscle Raptor knockout on contractile functions in mesenteric arterial rings (Figure S6C–E). The changes in aortic vascular function evoked by smooth muscle Raptor deletion are independent of alterations in hemodynamic parameters as we found no differences in arterial pressure (Figure 5D and Figure S7A–B) or heart rate (Figure S7C). Activity was also not altered by smooth muscle Raptor deletion (Figure S7D).
Figure 5 – Conditional smooth muscle-specific Raptor deletion alters differentially the aortic contractile responses.
(A-C) Contractile responses to PE (phenylephrine; A), PGF2α (Prostaglandin F2α, B) and 100mM KCl (C) of aortic arterial rings from SMCreERT2/RaptorF/F male mice and controls (n=8/group; *p<0.05 via two-way ANOVA with repeated measures or unpaired t-test). (D) Comparison of mean arterial pressure (MAP) between SMCreERT2/RaptorF/F male mice and controls at baseline and after tamoxifen treatment.
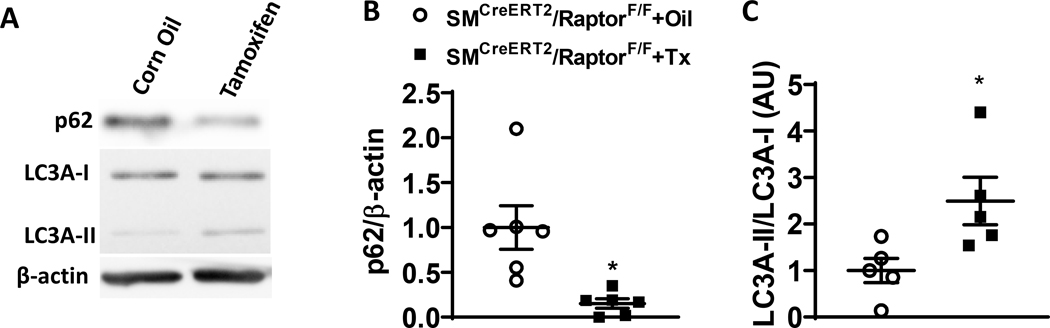
Smooth muscle Raptor knockout activate vascular autophagy
mTORC1 is a negative regulator of autophagy11 and the reduced contractile function in aortas of smooth muscle Raptor deleted mice suggests alterations in autophagy signaling. Consistent with such possibility, smooth muscle Raptor gene deletion decreased expression of autophagy markers p62 (p<0.05 via unpaired t-test, Figure 6) and increased the ratio of LC3A-II/LC3A-I (p<0.05 via unpaired t-test, Figure 6) in the aorta of smooth muscle Raptor deleted mice compared to controls. These data indicate that selective Raptor deletion in smooth muscle may alter aortic vascular function through activation of autophagy flux.
Figure 6 – Conditional smooth muscle-specific Raptor deletion alters vascular autophagic signaling.
(A-C) Representative western blot images (A) and quantification data of p62/β-actin (B) and LC3A-II/LC3A-I (C) in the aorta of smooth muscle-specific conditional Raptor knockout (SMCreERT2/RaptorF/F) mice treated with tamoxifen or vehicle (corn oil) (*p<0.05 via unpaired t-test).
Discussion
In this study, we show that mTORC1 signaling in endothelial and smooth muscle cells is necessary for the regulation of vascular function. Indeed, we demonstrate that endothelial cell-specific disruption of mTORC1 through deletion of Raptor results in impaired aortic endothelial-mediated vasorelaxation responses with reduced NO activity and NRF2 expression. Furthermore, haploinsufficiency of endothelial Raptor attenuates the endothelial dysfunction associated with the vascular actions of Ang-II suggesting a critical role of mTORC1 in mediating the vascular effects of Ang-II. We also demonstrate that deletion of Raptor in smooth muscle cells results in significant impairment in both endothelial- and smooth muscle-mediated aortic vasorelaxation as well as reducing contractility to receptor-dependent and -independent activation of the smooth muscle contractile apparatus. This was associated with activation of autophagic signaling in the aorta. Notably, the vascular changes evoked by endothelial and smooth muscle Raptor deletion are independent from changes in body weight, arterial pressure and heart rate. Taken together, these results demonstrate the importance of mTORC1 signaling pathway for the regulation of both endothelial and smooth muscle vascular function.
We recently implicated mTORC1 signaling in the regulation of vascular endothelial function. Activation of mTORC1 signaling via the branched chain amino-acid leucine or an adenoviral construct encoding a constitutively active S6-kinase (Ad-S6KCA) resulted in deficits in endothelial-mediated relaxation to acetylcholine without altering smooth muscle relaxation or contractile responses3. This is in agreement with a study that used a cocktail of branched chained-amino acids to activate the mTORC1 signaling pathway which caused endothelial dysfunction12. Together, these studies suggest that activation of the vascular mTORC1 signaling above a critical threshold level leads to detrimental effects on vascular endothelial function. Strikingly, here we show that conditional endothelial-specific disruption of mTORC1 also results in endothelial dysfunction without altering smooth muscle relaxation or contractile responses. This outcome mirror the vascular changes evoked by activation of mTORC1 signaling in cultured vascular rings we reported previously3. Altogether, these findings indicate that either gain or loss of function of mTORC1 signaling results in vascular endothelial dysfunction.
Consistent with our data, blockade of the mTORC1 pathway with rapamycin has been associated with decreased vascular endothelial function. Moreover, sirolimus (rapamycin) treatment leads to hypertension in heart transplant patients13 and sirolimus eluting stents have been demonstrated to result in coronary artery endothelial dysfunction14. Similarly, rapamycin infusion causes elevated arterial pressure and aortic endothelial dysfunction in wild-type mice15. Thus, mTORC1 signaling levels above or below these triggering thresholds produce vascular endothelial dysfunction suggesting a physiological range that vascular mTORC1 signaling oscillates within to maintain vascular endothelial homeostasis. Further supporting this concept, we found that mice with endothelial Raptor haploinsufficiency (TekCreERT2/RaptorF/+; heterozygous deletion) did not alter vascular endothelial or smooth muscle relaxation function suggesting the relaxation responses are sensitive to the gene dosage and one copy of Raptor is enough to maintain vascular homeostasis. These findings are in contrast with a recent report which showed that heterozygous deletion of endothelial Raptor resulted in reduced vascular endothelial function of mesenteric arteries16. The reason for this discrepancy is currently unclear but may involve differences in the efficacy of Tie2 Cre drivers used for endothelial specific deletion of Raptor (i.e. tamoxifen-inducible versus constitutively expressed) as well as the timing of Raptor deletion (adulthood versus embryonic).
We and others have previously demonstrated a paradoxical increase in expression of eNOS, a critical enzyme in the generation of NO, in response to vascular mTORC1 activation3, 12. However, the reduced aortic endothelial-dependent vasorelaxation responses to endothelial Raptor gene deletion was associated with decreased levels of phospho-eNOS protein in whole aortic lysates isolated from TekCreERT2/RaptorF/F male mice compared to tamoxifen-treated controls suggesting that endothelial Raptor deletion alone is not sufficient to alter NO signaling. NRF2 has been shown to modulate eNOS signaling through alterations in antioxidant enzyme status10. We found a significant decrease in expression of NRF2 in aortas from TekCreERT2/RaptorF/F male mice suggesting that altered vascular oxidant stress may be involved in the vascular endothelial dysfunction associated with reduced mTORC1 signaling. Additional analyses using in vivo functional studies and cultured endothelial cells will be necessary to further explore further the link between Raptor/mTORC1 and NRF2 and eNOS in the endothelium.
In our previous report, we demonstrated using cultured vascular rings that activation of mTORC1 signaling above a critical threshold with the Ad-S6KCA infection (downstream of Raptor) causes endothelial dysfunction while maintaining smooth muscle relaxation and contractile responses3. Our similar findings of reduced endothelial-dependent relaxation in endothelial Raptor deleted mice led us to use Ad-S6KCA infection to restore mTORC1 signaling back to normal physiological levels. Remarkably, Ad-S6KCA infection rescued the decreased acetylcholine responses back to control levels further demonstrating the critical role of mTORC1 signaling in the regulation of vascular endothelial function. In addition, this further supports the concept of an upper and lower critical threshold level that mTORC1 signaling oscillates within to maintain endothelial homeostasis and function.
mTORC1 integrates a variety of vascular signals including those arising from ROS, pro-inflammatory cytokines, adipokines, and the renin-angiotensin system. Ang-II activates mTORC1 signaling in endothelial6 and smooth muscle cells17. A study by Kim and colleagues demonstrated that Ang-II induced endothelial dysfunction could be ameliorated with rapamycin pre-treatment through a mechanism that involve eNOS signaling. In this study, we found that mice with endothelial Raptor haploinsufficiency were partially protected against the endothelial dysfunction associated with acute Ang-II incubation. These data suggest that mTORC1 signaling is at least partially involved in the deleterious effects of Ang-II on the endothelium. This is in contrast with the recent study by Yao and colleagues that showed no effect of heterozygous deletion of endothelial Raptor on the development of endothelial dysfunction evoked by chronic Ang-II infusion16. Differences are likely due to conditional versus constitutive Raptor deletion and differential techniques to induce Ang-II mediated endothelial dysfunction.
mTORC1 signaling in smooth muscle contributes to several cellular processes such as differentiation18, migration19, proliferation20, and autophagy21. Using a tamoxifen-inducible smooth muscle specific Cre driver, we deleted Raptor in smooth muscle cells and demonstrated reduced relaxation and contractile function in aortic but not mesenteric vascular rings. This suggest that the contribution of smooth muscle mTORC1 to vascular function is vascular bed specific. However, the size of the vessel may have contributed to the differential vascular effects induced by Raptor deletion in smooth muscle cells.
The reduced contractile responses to receptor-dependent and -independent stimuli suggests alterations in autophagy signaling. Indeed, dysregulation of autophagic flux has been implicated in a variety of vascular-related diseases as well as smooth muscle phenotypic diversity and in response to vascular injury22. Increased indices of autophagy has been demonstrated in the vasculature such as endothelial cells in response to altered flow/shear stress23, obesity associated endothelial dysfunction24 and in smooth muscle cells through modulation of a variety of cellular functions25. Several mechanisms have been implicated in the dysregulation of vascular autophagy including elevation in generation of reactive oxygen species and a decrease in NO bioavailability26. Measurement of the autophagic markers p62 and the ratio of LC3A-II/LC3A-I revealed significant alterations in expression of these markers suggesting that Raptor deletion and subsequent mTORC1 disruption induce autophagy in these vascular segments which may contribute to the vascular dysfunction associated with Raptor deletion in vascular smooth muscle cells. Further functional studies are warranted to better understand the role of autophagy in the vascular defects evoked by loss of Raptor and disruption of mTORC1 signaling in smooth muscle cells.
Perspectives
Our study demonstrates a critical role of mTORC1 signaling in endothelial and smooth muscle cells in the control of vascular function. These findings suggest that alterations in mTORC1 signaling may have detrimental implications for cardiovascular health in disease conditions that affect mTORC1 signaling such as diabetes, obesity and hypertension. Thus, further understanding of the relevance of mTORC1 signaling for vascular regulation under physiological and disease conditions is warranted to determine the value of targeting this pathway for therapeutic interventions to alleviate cardiovascular disease.
Supplementary Material
Novelty and Significance
What Is New?
Conditional endothelial cells-specific disruption of mTORC1 signaling alter vascular endothelial-mediated relaxation response in the aorta.
Reduction in endothelial mTORC1 signaling R attenuate the endothelial dysfunction evoked by Ang II.
Smooth muscle cell-specific conditional disruption of mTORC1 signaling reduces aortic endothelial- and smooth muscle-dependent relaxation responses and receptor-dependent and -independent contractility.
What Is Relevant?
Vascular mTORC1 signaling is a critical regulator of vascular endothelial and smooth muscle reactivity and function.
mTORC1 signaling may represent a potential therapeutic target for the treatment of vascular diseases.
Summary
We show that conditional endothelial cell-specific disruption of mTORC1 through deletion of Raptor results in impaired aortic endothelial-mediated vasorelaxation responses. Haploinsufficiency in endothelial Raptor attenuates the endothelial dysfunction associated with the vascular actions of Ang-II. We also show that deletion of Raptor in smooth muscle cells impairs aortic endothelial- and smooth muscle-mediated aortic vasorelaxation as well reducing contractility to receptor-dependent and -independent activation. These results highlight the importance of mTORC1 signaling pathway for the regulation of both endothelial and smooth muscle vascular function.
Acknowledgements
The Flow Cytometry Facility that helped with the cell sorting data is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center.
Sources of Funding
This work was supported by NIH grant HL084207, VA grant BX004249, AHA grants 14EIA18860041 and 16POST30830004, the University of Iowa Fraternal Order of Eagles Diabetes Research Center, and the University of Iowa Center for Hypertension Research.
Footnotes
Disclosure
None.
References
- 1.Saxton RA and Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sciarretta S, Forte M, Frati G and Sadoshima J. New Insights Into the Role of mTOR Signaling in the Cardiovascular System. Circ Res. 2018;122:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reho JJ, Guo DF and Rahmouni K. Mechanistic Target of Rapamycin Complex 1 Signaling Modulates Vascular Endothelial Function Through Reactive Oxygen Species. J Am Heart Assoc. 2019;8:e010662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reho JJ and Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci (Lond). 2017;131:1689–1700. [DOI] [PubMed] [Google Scholar]
- 5.Cabandugama PK, Gardner MJ and Sowers JR. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med Clin North Am. 2017;101:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JA, Jang HJ, Martinez-Lemus LA and Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302:E201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer AM, Guo DF and Rahmouni K. Prolonged treatment with angiotensin 1–7 improves endothelial function in diet-induced obesity. Journal of hypertension. 2013;31:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reho JJ, Guo DF, Morgan DA and Rahmouni K. Smooth Muscle Cell-Specific Disruption of the BBSome Causes Vascular Dysfunction. Hypertension (Dallas, Tex : 1979). 2019;74:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reho JJ, Zheng X, Benjamin JE and Fisher SA. Neural programming of mesenteric and renal arteries. Am J Physiol Heart Circ Physiol. 2014;307:H563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McSweeney SR, Warabi E and Siow RC. Nrf2 as an Endothelial Mechanosensitive Transcription Factor: Going With the Flow. Hypertension (Dallas, Tex : 1979). 2016;67:20–9. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Kundu M, Viollet B and Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature cell biology. 2011;13:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhenyukh O, Gonzalez-Amor M, Rodrigues-Diez RR, Esteban V, Ruiz-Ortega M, Salaices M, Mas S, Briones AM and Egido J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J Cell Mol Med. 2018;22:4948–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL 2nd, and Kobashigawa J. Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation. 2004;110:3858–65. [DOI] [PubMed] [Google Scholar]
- 14.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JM, van Essen D, de Feyter PJ and Serruys PW. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:166–70. [DOI] [PubMed] [Google Scholar]
- 15.Long C, Cook LG, Hamilton SL, Wu GY and Mitchell BM. FK506 binding protein 12/12.6 depletion increases endothelial nitric oxide synthase threonine 495 phosphorylation and blood pressure. Hypertension. 2007;49:569–76. [DOI] [PubMed] [Google Scholar]
- 16.Yao L, He J, Li B, Yan M, Wang H, Tan L, Liu M, Lv X, Lv H, Zhang X, Chen C, Wang D, Yu Y, Huang Y, Zhu Y and Ai D. Regulation of YAP by Mammalian Target of Rapamycin Complex 1 in Endothelial Cells Controls Blood Pressure Through COX-2/mPGES-1/PGE2 Cascade. Hypertension. 2019:HYPERTENSIONAHA11912834. [DOI] [PubMed] [Google Scholar]
- 17.Hafizi S, Wang X, Chester AH, Yacoub MH and Proud CG. ANG II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;287:H1232–8. [DOI] [PubMed] [Google Scholar]
- 18.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ and Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–17. [DOI] [PubMed] [Google Scholar]
- 19.Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB and Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marx SO, Jayaraman T, Go LO and Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–7. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Park JK, Seo JH, Ryu HS, Lim KS, Jeong MH, Kang DH and Kang SW. A rapamycin derivative, biolimus, preferentially activates autophagy in vascular smooth muscle cells. Sci Rep. 2018;8:16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salabei JK and Hill BG. Implications of autophagy for vascular smooth muscle cell function and plasticity. Free Radic Biol Med. 2013;65:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE and Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Canadian journal of physiology and pharmacology. 2014;92:605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ait-Aissa K, Nguyen QM, Gabani M, Kassan A, Kumar S, Choi SK, Gonzalez AA, Khataei T, Sahyoun AM, Chen C and Kassan M. MicroRNAs and obesity-induced endothelial dysfunction: key paradigms in molecular therapy. Cardiovascular diabetology. 2020;19:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai S, Hu XQ, Peng DQ, Zhou SH and Zheng XL. The roles of autophagy in vascular smooth muscle cells. International journal of cardiology. 2016;211:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Hughes WE, Beyer AM and Gutterman DD. Vascular autophagy in health and disease. Basic research in cardiology. 2020;115:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.