Abstract
Background
Autophagy is a catabolic mechanism that involves lysosomal-dependent degradation of unnecessary intracellular components and responsible for normal cellular homeostasis. Autophagy pathway and its key participant ATG5/LC3 are associated with several pathologies such as diabetes mellitus and its complications.
Methods
Levels and expression of autophagy key components ATG5 and LC3B were analyzed in both human model and murine tissues. One hundred and twenty human subjects were divided into four groups: Healthy (control), diabetes mellitus without complications, diabetic nephropathy, and diabetic retinopathy. Additionally, we used kidneys from WT healthy and diabetic nephropathy mice. Lysate derived from human peripheral blood mononuclear cells and murine renal cortex lysates were subjected to western blot and immunohistochemical analysis.
Results
Western blot and immunohistochemical analysis demonstrate that ATG5 protein levels were significantly decreased in diabetes mellitus, diabetic nephropathy (DN), and diabetic retinopathy patients versus healthy controls and in DN mice compared to healthy mice (0.65 ± 0.04; 1.15 ± 0.13 A.U. units, respectively). Quantification of staining area (%) of ATG5 mice tissue expression also decreased in DN versus healthy mice (4.42 ± 1.08%; 10.87 ± 1.01%, respectively).
LC3B levels and expression
Significant reduction in peripheral blood mononuclear cells in diabetic patients (with or without complications) vs. healthy controls. Renal LC3B levels were lower in DN versus healthy mice (0.36 ± 0.03; 0.68 ± 0.07 A.U. units). Renal LC3B staining quantification revealed significant reduction in DN versus healthy mice (1.7 ± 0.23%; 8.56 ± 1.7%).
Conclusion
We conclude that ATG5, as well as LC3B, are down regulated in diabetic patients with or without complications. This diminution contributes to deficiencies in the autophagy process.
Keywords: Diabetes Mellitus; diabetic nephropathy; autophagy; Atg5 gene; LC3B; Key Indexing Terms: Diabetic Nephropathy, Retinopathy, Autophagy, Atg5, and LC3B
Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by chronic hyperglycemia, resulting from insulin deficiency, insulin resistance, or both. 1 Chronic hyperglycemia is associated with disruption and alterations in carbohydrate, lipid, and protein metabolism.2,3 Hyperglycemia is responsible for increased production of reactive oxygen species (ROS) with cellular damage and alters the protective basal autophagy, especially in the kidney. 4 Hyperglycemia is commonly responsible for the acute, short functional changes and the long-term complications of diabetes that cause irreversible damage to the heart, kidneys, and eyes.5–9 40% of diabetic patients are likely to develop microvascular and macro vascular complications over a period of 20 years.5–7
Microvascular complications include diabetic retinopathy (DR) that may lead to blindness. 5 DR is a very common long-term complication of DM. The frequent proliferative DR is characterized by growth of small abnormal blood vessels, increased synthesis of vascular endothelial growth factor (VEGF), vitreous hemorrhages which culminate in retinal detachment, and loss of vision.8–12
DN is characterized by hyper filtration and increased glomerular filtration rate (GFR) and irreversible structural changes including thickening of glomerular and tubular basement membranes, glomerular hypertrophy, mesangial expansion, and an accumulation of extracellular matrix (ECM) materials with diffuse glomerulosclerosis.5,6,13 These early changes are associated with a microalbuminuria and normal plasma creatinine, with progression to proteinuria, decreased GFR, arterial hypertension, and progressive elevation in plasma creatinine, and finally end stage renal disease (ESRD) development.5,6
Numerous risk factors are associated with the development and progression of DN such as the degree of hyperglycemia, extended duration of diabetes, HbA1C, arterial hypertension, obesity, and hyperlipidemia, with most being modifiable by appropriate treatment. Other contributing factors, such as genetic factors including Haptoglobin genotype, cannot be modified. 14 Among the risk factors mentioned above, hyperglycemia was identified as a factor of great importance in the development and progression of DN.
Hyperglycemia-mediated renal proximal convoluted tubule cell (PCT) damage (Proximal tubule theory) and podocytes (Glomerular theory) are triggered by increased production of free radicals, accumulation of AGEs, activation of protein kinase C, and the renin-angiotensin-aldosterone system (RAAS). These structural changes in the PCT and glomerular cells (podocytes) result in tubule-interstitial fibrosis and glomerulosclerosis leading to ESRD.15–18
Autophagy is a protective pathway involved in the normal homeostasis of glomeruli and tubules under metabolic stress and has an important role in human health and diseases such as DM. Impairment of autophagy is implicated in various inflammatory diseases, and particularly in the pathogenesis and progression of DN and DR.13,19,20 Hyperglycemia-induced alterations in intracellular metabolism and cellular events, especially PCT and podocytes, increased oxidative stress and endoplasmic reticulum stress. 21
Autophagy degrade damaged proteins and macromolecules, through a lysosome-dependent mechanism classified as macro-autophagy, a well-coordinated multi-step process regulated by autophagy-related gene (Atg) products. Initially, a phagophore forms around cytoplasmic components that are then sequestered by a double membrane, which forms the autophagosome. The autophagosome subsequently fuses with the lysosome to form an autolysosome; the enclosed contents are then degraded.
Several ATG proteins regulate autophagosome formation.13,20–23 The initiation of hyperglycemia-induced autophagy is regulated by the phosphorylation of ATG1 via the intracellular mammalian target of rapamycin (mTORC1) and adenosine monophosphate (AMP)-activated protein kinase (AMPK). Two ubiquitin-like conjugation systems, namely, the ATG12 and ATG5 with ATG16L1 tetrameric complex and the microtubule-associated protein 1 light chain 3 (LC3)/ATG8, are required for autophagosomal elongation.22,23
The conjugation of LC3-I, the mammalian homolog of Atg8, to Atg7 and Atg3, to form LC3-II, is a critical step in autophagosome formation. The Atg12-Atg5-Atg16 complex regulates this LC3 conjugation reaction positively. Atg5, Atg16, Atg7, Atg8, and Atg10 have a significant role in the autophagy process, specifically in autophagosome formation.23–27
We opted for ATG5/LC3 proteins because they are the most important ATGs involved in the autophagosome formation. A number of previous studies have shown that alterations in ATG5 protein levels induce pathological conditions by influencing the level of autophagy pathway.26,27 Thus, this study was designed to determine the role of the alteration of ATG5 and LC3 levels and Atg5 gene expression in DM with and without vascular complications.
Methods
Human study design
The study included 141 subjects, age >18 years old. 50 healthy subjects (Control) were recruited from the medical staff at the nephrology and ophthalmology divisions. 91 patients were randomly recruited from the ophthalmology (n = 30, DR) and nephrology (n = 61 DN and DM) outpatient clinics at Baruch Padeh Medical Center Poriya, Israel. Recruitment was conducted according to the following inclusion criteria: subjects diagnosed with type 1 or 2 diabetes mellitus; patients diagnosed with diabetic nephropathy were classified as part of the DN group (n = 30) and were treated with their chronic medications and followed by the nephrologist. The eGFR was calculated according to the NKF equation used to calculate GFR values. Specifically, eGFR =141 x (SCr/κ)α x0.993Age x 1.018 [if female], where SCr (standardized serum creatinine) = mg/dL, κ = 0.7 (females) or 0.9 (males), α = −0.329 (females) or −0.411 (males), and Age = years. The rest were classified as DM patients (n = 31). Ophthalmologist diagnosed DR using fundoscopy and was under conservative treated by their physician. The Helsinki Committee of Baruch Padeh Poriya Medical Center and the Israel Ministry of Health approved human study design; participation was voluntary and subjects signed informed written consent forms. Age and plasma creatinine were collected from patients file at Klalit Health services.
Subjects were divided into the following groups: healthy subjects (control); subjects with diabetes mellitus without complications (DM); diabetic nephropathy patients (DN); and diabetic retinopathy patients (DR), n = 30 for each group. In cases of pregnancy, malignancy, any other systemic kidney diseases, and subjects with incomplete laboratory data or those receiving dialysis treatment were all excluded from the study.
Subjects' demographic data were gathered: Age, geographic residence, gender, diabetic status, co-morbidities, and medications. In addition, we gathered clinical data of laboratory parameters: blood glucose, serum levels of creatinine, and HbA1c concentration. The data were recorded directly from the patients or from their medical files.
DN mice model
DBA/2J mice were selected due to their high susceptibility to develop diabetic nephropathy. 28 Mice were divided randomly into two study groups: Control group (wild type) mice without any treatment (n = 23, females); Diabetic mice group (n = 23, females). DM mice group: DM was induced in 8-week-old DBA/2J mice (n = 71) by intraperitoneal (IP) injection, once daily, for five consecutive days, of freshly prepared streptozotocin (STZ) (35 mg/kg body weight, dissolved in 10 mM citrate buffer pH 4.5). STZ is a chemical toxin for pancreatic β-cells and is a widely used model for diabetes induction. As we published in previous study, DM mice group demonstrate histological features of DN. 28
Isolation of peripheral blood mononuclear cells (PBMCs) of human subjects: Given the limitations surrounding experiments in renal or retinal samples from human subjects, we chose to examine alterations in ATG5 protein levels in the PBMCs of human subjects. Previous studies demonstrated that PBMCs are good candidates for investigating proteins involved in autophagy.28,29 Separation of PBMCs from whole blood was performed using the BD Vacutainer® CPT™ Cell Preparation Tube (Health Care Equipment and Supplies Ltd, Stains, UK).
Protein extraction
Proteins were extracted from PBMCs pellets using NP-40 lysis with Phosphatase Inhibitor Cocktail (Sigma Aldrich) in a ratio of 1:100 (cocktail solution/lysis buffer) and phenylmethylsulfonyl fluoride (PMSF) (Sigma Aldrich). Proteins were kept at −80 C for western blot analysis.
Western blot analysis
In order to evaluate ATG5 and LC3 protein levels in human subjects and in mouse kidneys, protein-extract samples from human PBMCs and mouse kidney tissue lysate were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
These were transferred to polyvinylidene difluoride (PDVF) membranes using a Trans-Blot Turbo System (Bio-Rad) blocked with 5% dry skim milk (Bio-Rad) in Tris-buffered saline with Tween 20 (TBST) at room temperature, and then washed and incubated with primary antibodies at 4oC overnight. TBST was used to wash the membranes followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature followed by TBST washes. Bands were visualized using Clarity Enhanced chemiluminescence (ECL) (Bio-Rad, CA, USA) and were analyzed using Bio-Rad image Lab software.
Immunohistochemistry (IHC)
Xylene and graded alcohol were used for deparaffinization. Sections were then subjected to citrate buffer 30% pH 6, (ZyToMED systems, Berlin, Germany) for antigen retrieval. 3% H2O2 was used to block non-specific antibody binding followed by incubation with primary antibodies (diluted in blocking solution (CAS-block, Invitrogen) at 4°C, overnight). HRP-conjugated anti-rabbit (NICHIREI Biosciences INC.) were used as secondary antibody. Immunoreactive signals were developed upon incubation with 3,3-diaminobenzidine (DAB) (Thermo Scientific). Slides were counter-stained with hematoxylin, dehydrated, and mounted with quick hardening mounting (Sigma). Images were captured with Axiocam 503 color (ZEISS) and analyzed with ZEISS ZEN software.
Immunohistochemistry and western blot antibodies
Primary antibodies used were as follows: anti-β-actin (Sigma), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam), anti-APG5L/ATG5 (Abcam), and anti LC3A/B (D3U4C) XP® Rabbit mAb (Cell Signaling, Israel). Secondary antibody used was goat anti-rabbit (Jackson Immunoresearch).
Statistical analysis
Results are presented as mean ± standard error of the means (SEM) and all experiments were independently repeated at least three times. Student’s t-test and ANOVA were performed to compare between study groups, using Graph Pad Prism version 5.00 for Windows (Graph Pad Software, La Jolla, California USA) with a p value <0.05 considered statistically significant.
Results
Demographic and clinical characteristics of human subjects
A total of 120 subjects (61% females and 59% males) who were outpatients at the Baruch Padeh Medical Center were recruited to the study after meeting the inclusion criteria. Age range and means of subjects from all groups were 57.3±7.2 years healthy controls; 61.2 ± 13.7 years DM patients; 65.1 ± 11.2 years DN patients; and 67.6± 7.8 years DR patients. A majority of the patients were diagnosed with type 2 DM (82 patients diagnosed with type 2 DM, and 8 with type 1 DM). Hemoglobin A1C (HbA1C), a gold standard test for monitoring glycemic control in patients with DM, was higher in DN patients versus those with DM and DR (8.18 ± 2.04, 7.95 ±1.67, and 7.86 ± 1.53, respectively). The overall mean estimated GFR (eGFR) using MDRD formula was 53.32 ± 14.4 mL/min/1.73 m2 (range 35–82 mL/min/1.73 m2) with reduced GFR levels in all of the DN patients (Table 1). eGFR was not available in the sheets of DM and DR patients.
Table 1.
Demographic and Clinical Characteristics of Study subjects.
| Group | Healthy control | DM | DN | DR | Remarks |
|---|---|---|---|---|---|
| Parameter | |||||
| n | 50 | 31 | 30 | 30 | |
| Male/female (n/n) ratio | 19/31 0.61 |
16/15 1.07 |
11/19 0.58 |
11/19 0.58 |
|
| Mean age (years) [range] | 57 ± 7 [24–78] | 61 ± 14*** [31–84] | 65 ± 11*** [36–85] | 68 ± 8*** [54–84] | |
| DM type T1DM:T2DM | N/A | 5:25† | 0:30 | 1:25† | |
| Hb1Ac (%) [range] | N/A | 7.38 ± 1.96 [1.2–11.5] | 8.19 ± 2.09 [5.2–12.6] | 7.45 ± 1.10 [5.8–9.4] | |
| Creatinine (mg/dl) | 0.8 ± 0.1 | 0.9 ± 0.3 | 1.9 ± 0.9***### | 1.7 ± 0.6**## | |
| eGFR (MDRD) (ml/min/1.73 m2) [range] |
101 ± 14 [82–128] | 86 ± 13** [58–112] | 67 ± 13***### [42–98] | 67 ± 12***## [52–89] |
Values presented as means ± standard deviation. Abbreviation: DM diabetes mellitus, DN diabetic nephropathy, DR diabetic retinopathy, HbA1c Hemoglobin A1c, GFR Glomerular filtration rate, MDRD Modification of Diet in Renal Disease study formula, N/A not available. ** p < 0.01 vs. healthy control, *** p < 0.001 vs. healthy control, ## p < 0.01 vs. DM, ### p < 0.001 vs. DM. †One patient of the DM group and four patients of the DR are missing for their DM type.
Decreased ATG5 protein levels in PBMCs of human subjects
ATG5 levels were decreased significantly in DM patients, with and without complications (Figure 1(a)). Quantification of total western blot results present significant reduction in PBMCs ATG5 protein levels in diabetic patients (with or without complications) compared with the healthy controls (0.59 ± 0.07 A.U in DM patients, n = 20 p < 0.001, 0.67 ± 0.06 A.U in DN, n = 23 p < 0.01, 0.72 ± 0.06 A.U. in DR patients, n = 24 p < 0.05, vs. 0.96 ± 0.16 A.U. in healthy controls, n = 18) (Figure 1(b)). No substantial difference was found between ATG5 protein levels in DM patients compared with DN or DR patients.
Figure 1.
Alterations in ATG5 protein levels isolated from PBMCs of healthy controls and DM, DN, and DR patients. (a) Representative western blots of ATG5 protein level. PBMCs protein samples of one subject from each group were randomly selected and subjected to western blot analysis. Proteins were detected by immunoblotting using antibodies against ATG5 (ATG5-ATG12 conjugate) and GAPDH as a loading control. (b) Quantification of total western blot analysis. ATG5 protein levels were significantly higher in healthy controls versus DM patients with and without complications (AU, arbitrary units). The results represent the mean ± SEM. One-way ANOVA and subsequent Tukey test were used to derive the p value indicated on the graph *p < 0.05 **p < 0.01, ***p < 0.001.
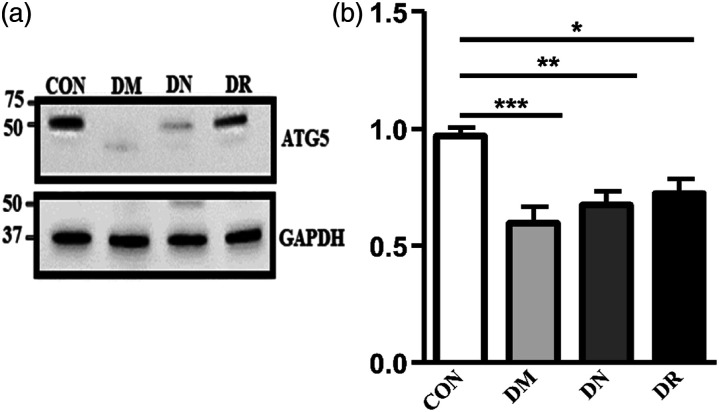
Decreased ATG5 protein levels in mouse kidney tissue lysate
Western blot analysis of ATG5 protein level in mouse kidneys was performed in order to examine ATG5 alteration in a tissue-specific manner during diabetic nephropathy. Similar to the results obtained from DN patients, ATG5 protein levels decreased in DN mouse kidneys compared with WT mice (Figure 2(a)). Quantification of total western blot results presented significant reduction in renal ATG5 with average levels decreased by nearly 2-fold in the DN mice (0.65± 0.04 AU, n = 7) vs. WT mice (1.15± 0.13 A. U, n = 7 p < 0. 01) (Figure 2(b)).
Figure 2.
Renal ATG5 levels and expression in WT and DN mice. (a) Representative western blot of ATG5 protein levels in randomly selected mouse renal lysates. Proteins were detected by immunoblotting using antibodies against ATG5 (ATG5-ATG12 conjugate) and GAPDH as a loading control. (b) Quantification of total Western blot. Renal ATG5 level was significantly reduced in DN renal lysate versus WT (AU, arbitrary units). The results represent the mean ± SEM. Unpaired student’s t-tests were performed to obtain the p values indicated on the graph **p < 0.01. (c) Renal section from randomly selected WT and DN mice. Images show decrease of ATG5 expression (brown) in the tubules of DN mice versus WT. (d) Quantification of total ATG5 staining. The percentage of the stained area of ATG5 is significantly decreased in the renal tubules of DN mice versus WT mice. Areas were measured using Image Pro software. All results are shown as mean ± SEM. and compared by unpaired Student’s t-test with p < 0.001 considered statistically significant.
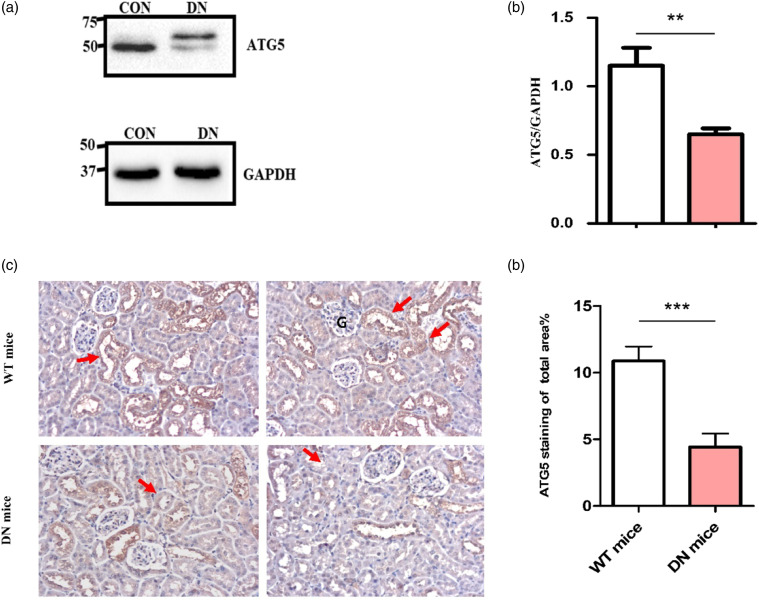
Decreased ATG5 protein expression in renal tissue of WT and DN mice
Renal tissue from WT and DN mice were subjected to IHC staining for ATG5 by using DAB staining. Corresponding to western blot analysis results, ATG5 expression in renal tubules of DN mice was lower than in WT mice (Figure 2(c)). Quantification of total renal tissue staining showed dramatic decrease (nearly a 2.5-fold) in the percentage of ATG5-stained areas in the tubules of DN mice (4.42 ± 1.08%) vs. WT mice (10.87 ± 1.01%) (Figure 2(d)).
Lower ATG5 levels is associated with lower level of autophagy in diabetes
To determine whether reduced levels of ATG5 may be associated with reduced basal autophagy among diabetic patients with and without complication, we examined the protein level of LC3B, a specific marker for autophagy.
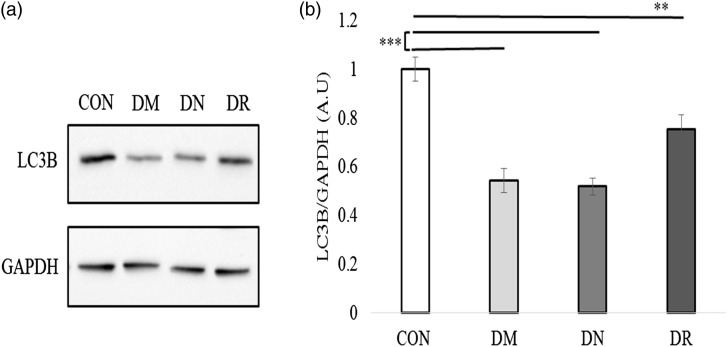
Low levels of LC3B in PBMCs of patients with DM and without complication
PBMCs lysates from DM, DN, and DR patients, as well as from healthy controls, were subjected to western blot analysis using antibodies against LC3B. Results indicated substantial reduction of LC3B levels in DM patients compared with healthy controls (Figure 3(a)). Quantification of total western blot analysis showed significant decrease of LC3B levels in DM patients with or without complications (0.44 ± 0.05 AU, n = 18 p < 0.001 DM patients; 0.42 ± 0.035 AU, n = 19 p < 0.001 DN patients; 0.48 ± 0.06 AU, n = 18 p < 0.001 DR patients and 0.81 ± 0.05 AU, n = 19 healthy controls) (Figure 3(b)). There was no significant difference between DM patients and DR or DN patients.
Figure 3.
Reduced LC3B protein levels in PBMCs of healthy controls, DM, DN, and DR. (a) Representative western blots of LC3B protein were randomly selected and represent one subject from the different groups. Proteins were detected by immunoblotting with antibodies against LC3B and GAPDH as a loading group. (b) Quantification of total Western blot. LC3B protein levels were significantly reduced in DM patients with and without complication versus healthy controls (AU, arbitrary units). Data are reported as mean ± SEM.one-way ANOVA analysis used to derive the p value indicated on the graph ***p < 0.001.
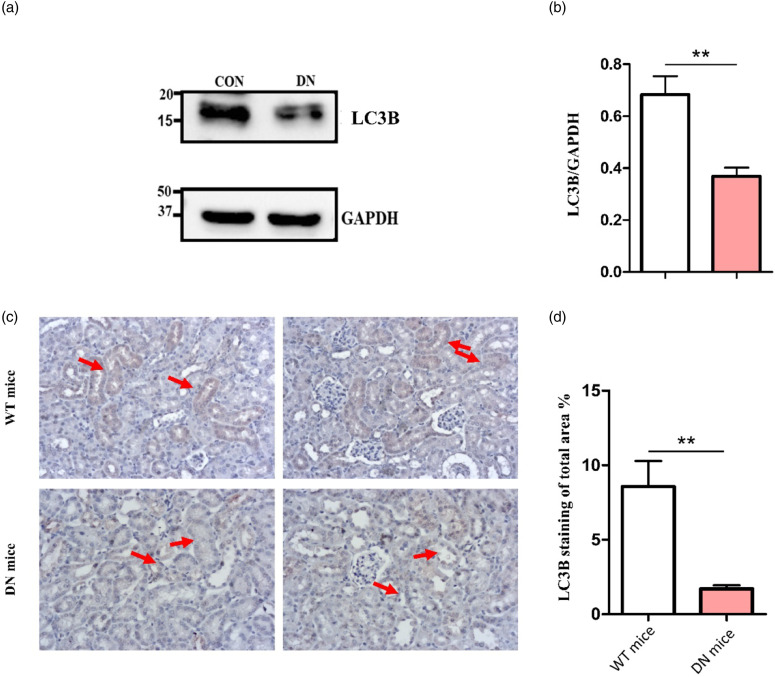
Low levels of LC3B in DN mouse kidneys
To support the western bolt results in the PBMC lysates of the patients and healthy controls, we analyzed the protein levels of LC3B in renal kidney lysates of WT and DN mice. We subjected the lysates to Western blot analysis, using antibodies against LC3B. Results indicated a nearly 2-fold decrease in renal LC3B levels in DN mice compared with WT (Figure 4(a)). Quantification showed significant differences between LC3B levels shown in WT mice (0.68 ± 0.07 AU n = 6) compared to DN mice (0.36 ± 0.03 AU, n = 6 p < 0.01) (Figure 4(b)).
Figure 4.
LC3B renal protein levels and expression in WT and DN mouse renal lysates. (a) Representative randomly selected western blots of LC3B protein. Proteins were detected by immunoblotting with antibodies against LC3B and GAPDH as a loading group. (b) Quantification of total Western blot. LC3B renal level was significantly reduced in DN renal lysate versus WT renal lysate (AU, arbitrary units). The results represent the mean ± SEM. Unpaired student’s t-tests were performed to obtain the p values indicated on the graph **p < 0.01. (c) Representative randomly selected LC3B immunostaining images of WT mice and DN. There was a significant decrease in LC3B expression (brown) in the tubules of DN mice versus WT. (d) Quantification of total LC3B staining. Quantification of LC3B-stained area percentage is markedly decreased in the renal tubules of DN mice versus WT mice. Areas were measured using Image Pro software. All results are shown as mean ± SEM and compared by unpaired Student’s t-test with p < 0.01 considered statistically significant.
Decreased renal LC3B expression in renal tissue of DN mice
Renal tissue IHC staining for LC3Bby using DAB staining showed a dramatic reduction in renal tubules. LC3B protein levels of DN mice versus WT mice (Figure 4(c)) demonstrated a nearly 5-fold decrease in the percentage of LC3B-stained areas in the tubules of DN mice (1.7 ± 0.23 %) vs. WT mice (8.56 ± 1.7 %) (Figure 4(d)).
Discussion
Autophagy is an important mechanism involved in the pathogenesis of diabetic nephropathy.13,18,19 Several studies have suggested that the autophagy pathway is crucial for cell survival, differentiation, development, and homeostasis under stress conditions such as hyperglycemia.20–22 In many cases, dysfunction in autophagy mechanisms that results in accumulation of damaged proteins and organelles has been linked with the loss of cell function, leading to diverse pathologies and disease such as DN and its progression to ESRD, and proliferative DR with blindness.13,23,24 Evidence suggests that enhancing autophagy in such diseases may have beneficial therapeutic effects in the future. 20
Previous studies have suggested that alterations in ATG5 protein levels induce pathological conditions by influencing the levels of autophagy pathway.23,24 In light of these reports, we aimed to underline the possible role of ATG5 in the pathogenesis of DN and DR.
Various studies have provided evidence suggesting that autophagy-related protein 5 (ATG5) has a crucial role in a variety of disease processes, but few studies have examined the possible role of ATG5 in the development of diabetes mellitus complications.24–27
Here, we report that patients with diabetes mellitus and without complications exhibit significantly decreased levels of ATG5 compared with healthy controls. Similar to this result, DN mice also showed a substantial reduction in kidney ATG5 protein levels compared with wild type mice. Low expression of ATG5 in DN mouse kidney tissue also supports the low ATG5 levels found in mouse kidney lysate. Retinas from diabetic mice were not available for measurement of ATG5 and LC3 proteins.
It is important to mention that we examined ATG5 protein levels in PBMCs of diabetic patients with or without complications (nephropathy or retinopathy) and compared them with healthy individuals. Since we could not obtain renal or retinal samples from these patients,27,28 we evaluated the alteration of ATG5 protein levels in their PBMCs. Our results show significant reduction of ATG5 levels and expression in kidneys of DN mice versus WT healthy mice. These finding resemble the results from human PBMCs and therefore support our interpretation. 28
Considering ATG5 dissociates from the membrane immediately before or after completion of autophagosome formation, it cannot serve as an autophagy marker and is unsuitable to measure changes in autophagy. Hence, we analyzed the protein levels of LC3B, one of the most frequently used biomarkers for autophagy. LC3B is a gold-standard marker for tracing changes in the autophagy process. It is specifically localized to autophagy structures throughout the process, from phagophore to lysosomal degradation.
We used immunohistochemistry on mouse tissue sections as well as western blot analysis of mice kidney lysate and human PBMCs lysate to asses LC3B levels and expression. 28 Here, we report significant reduction in LC3B protein levels in both diabetic patients with and without complication and in diabetic mice. Based on these results, we infer that ATG5 aberrant levels affect the LC3-II conjugation system. We suggest that reduction in ATG5 levels may lead to significant impairment in the conversion of LC3-I to LC3-II in patients and mice. Our results coincide with several clinical studies that have been published, showing that Atg5, Atg8, and LC3 mRNA level were found to be largely reduced in the renal tissue of DN patients.29,30
Autophagy is required to maintain normal cellular homeostasis in both podocytes and proximal tubular cells. Various studies have assessed the impact of diabetes on the reno-protective autophagy processes in proximal tubular cells exposed to hyperglycemia.31,32 Similar to results obtained in our DN mice model, autophagy has been shown to be significantly decreased in the kidneys of streptozotocin-induced diabetic mice. Our IHC analysis revealed that the proximal tubular cells in renal sections of DN mice express lower levels of autophagy that were confirmed by lower expression of both LC3B and ATG5 proteins compared with wild type mice. Given that no differences were demonstrated in patients with DM and DM with nephropathy, the results imply that ATG5-mediated autophagy is altered in patients with DM, relative to controls without DM.
In addition to diabetic nephropathy, an emerging role of autophagy in pathogenesis of DR has been reported. 11 Hyperglycemia enhances inflammation, advanced glycation end products (AGEs), and oxidative stress in the retina and its capillary cells. 33 ,34 Blood vessels and neurons of diabetic patients are imperatively damaged by inflammation, ROS, and endoplasmic reticulum (ER) stress. 35
A vast amount of evidence has demonstrated that oxidative stress is involved in retinal cell damage and subsequent development of DR pathogenesis. So, a large body of evidence has revealed that abnormal autophagy has important impacts on the pathobiology of retinal diseases such as diabetic retinopathy. 35
We believe that our data suggest that there is a link between ATG5-LC3B dysregulation and diabetes mellitus. These results correlate with previous studies, which indicated that alterations in ATG5/LC3 expression are implicated in many pathological conditions, including diabetes mellitus.29,30
Conclusions
In our study, the ATG5 and LC3 protein levels decreased in diabetic patients with and without complications, leading to lower level of autophagy. Given the prevalence of DM worldwide, the relationship between this condition and autophagy has received much attention in recent years. A link has not been made between ATG5/LC3b and diabetes complications and therefore they are not candidate markers for DN/DR. Our study strength was the use of new pathways such as autophagy and its key proteins ATG5 and LC3 in the diabetes mellitus and its vascular complications. Our findings may be translated into clinical practice approach and may lead to further studies to address diabetes mellitus and its vascular complications by selective modulation of ATG5/LC3 expression. The new anti-diabetes drugs, Sodium–glucose cotransporter 2 inhibitors, normalize not only glucose metabolism and suppress oxidative stress and inflammation in the kidneys of diabetic mice, but possess also other pleiotropic effects. 36
A key weakness of the study is the use of PBMCs to examine changes in ATG5 protein that not necessarily reflect alterations in cells predisposed to diabetes complications. However, we were forced into such an approach, given the practical difficulties encountered in investigating human retinal and renal cells. Furthermore, not all patients’ blood samples were analyzed, again due to methodological reasons. However, from the control group, we have analyzed ∼36% of the group, and from the DM, DR, and DN, the percentages were >55% of each group of patients, and the patients selection was blinded for each group.
Acknowledgments
We thank all patients who participated in this study. Dr. Offir Ertracht, Research Institute, The Galilee Medical Center, Nahariya Israel for revising the manuscript.
Footnotes
Author contributions: NN and FN wrote the paper; RA, ID and HT were involved in the protocol development, study design, supervision of the data collection, critical revision, reviewing of the report, and quality checking. EF and AA reviewed the paper. ID performed the statistical analysis. RA and AA contributed to the data collection. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Ministry of Development of the Negev and the Galilee.
List of abbreviations and definitions: DM = Diabetes Mellitus DN = Diabetic Nephropathy DR = Diabetes Retinopathy GFR = Glomerular Filtratio Rate ER = Endoplasmic reticulum
Ethics statement: The studies involving human participants were reviewed and approved by the ethics committee of Baruch Padeh Poriya Medical Center. The patients/participants provided their written informed consent to participate in this study. The study was undertaken in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
ORCID iD
Farid Nakhoul https://orcid.org/0000-0001-8657-1530
References
- 1.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 2014; 220(2): T1–T23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doshi Simit M, Friedman AN. Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol 2017; 12: 1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genuth S. Classification and diagnosis of diabetes mellitus. Med Clin North Am 1982; 66(6): 1191–1207. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet P, Alberti KG, Magliano DJ, et al. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 2016; 12: 616–622. [DOI] [PubMed] [Google Scholar]
- 5.Nakhoul FM, Miller-Lotan R, Awaad H, et al. Hypothesis - Haptoglobin genotype and diabetic nephropathy. Nat Clin Pract Nephrol 2007. [DOI] [PubMed] [Google Scholar]
- 6.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2011; 29(3): 116–122. [Google Scholar]
- 7.Lim Andy KH. Diabetic nephropathy – Complications and treatment. Int J Nephrol Renovasc Dis 2014; 7: 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang S, Cai Guang Y, Chen XM, et al. Clinical and pathological factors associated with progression of diabetic nephropathy. Nephrology 2017; 22: 14–19. [DOI] [PubMed] [Google Scholar]
- 9.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376: 124–136. [DOI] [PubMed] [Google Scholar]
- 10.Judith L, O’Leary Olivia E, Stitt Alan W. The pathology associated with diabetic retinopathy. Vis Res 2017; 139: 7–14. [DOI] [PubMed] [Google Scholar]
- 11.Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic Med 2013; 30: 640–650. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Cheung CMG, Larsen M, et al. Diabetic retinopathy. Nat Rev Dis Prim 2016; 2: 16012. [DOI] [PubMed] [Google Scholar]
- 13.Sahajpal NS, Goel RK, Chaubey A, et al. Pathological perturbations in diabetic retinopathy: hyperglycemia, AGEs, oxidative stress and inflammatory pathways. Curr Protein Pept Sci 2018; 20(1): 92–110. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol 2015; 224: R15–R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy AP, Asleh R, Blum S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signaling 2010; 12: 293–304. [DOI] [PubMed] [Google Scholar]
- 16.Carranza K, Veron D, Cercado A, et al. Aspectos celulares y moleculares de la nefropatía diabética, rol del VEGF-A. Nefrologia 2015; 35(2): 131–138.26300505 [Google Scholar]
- 17.Lin YC, Chang YH, Yang SY, et al. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc 2018; 117: 662–675. [DOI] [PubMed] [Google Scholar]
- 18.Nakhoul R, Nakhoul F, Nakhoul N. Diabetic nephropathy from RAAS to autophagy: the era for new players. J Clin Exp Nephrol 2017; 2(3). [Google Scholar]
- 19.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 2010; 6(6): 319–330. [DOI] [PubMed] [Google Scholar]
- 20.Kume S, Yamahara K, Yasuda M, et al. Autophagy: emerging therapeutic target for diabetic nephropathy. Semin Nephrol 2014; 34: 9–16. [DOI] [PubMed] [Google Scholar]
- 21.Takabatake Y, Kimura T, Takahashi A, et al. Autophagy and the kidney: health and disease. Nephrol Dial Transplant 2014; 29: 1639–1647. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Livingston M, Liu Z, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci 2018; 75: 669–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi AMK, Ryter SW, Beth L. Autophagy in human health and disease. N Engl J Med 2013; 368(7): 651–662. [DOI] [PubMed] [Google Scholar]
- 24.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 2010; 221: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamahara K, Yasuda M, Kume S, et al. The role of autophagy in the pathogenesis of diabetic nephropathy. J Diabetes Res 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakawa S, Honda S, Yamaguchi H, et al. Molecular mechanisms and physiological roles of Atg5/Atg7-independent alternative autophagy. Proc Jpn Acad Ser B Phys Biol Sci 2017; 93: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walczak M, Martens S. Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 2013; 9(3): 424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Z, Fujita H, Jin J, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 2005; 54(9): 2628–2637. [DOI] [PubMed] [Google Scholar]
- 29.Alizadeh S, Mazloom H, Sadeghi A, et al. Evidence for the link between defective autophagy and inflammation in peripheral blood mononuclear cells of type 2 diabetic patients. J Physiol Biochem 2018; 74(3): 369–379. [DOI] [PubMed] [Google Scholar]
- 30.Fang L, Zhou Y, Cao H, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia induced podocyte injury. PLoS One 2013; 8(4): e60546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W-J, Huang W-F, Ye L, et al. The activity and role of autophagy in the pathogenesis of diabetic nephropathy. Eur Rev Med Pharmacol Sci 2018; 22: 3182–3189. [DOI] [PubMed] [Google Scholar]
- 32.Dong D, Fan TT, Ji YH. Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes. Int Urol Nephrol 2019; 51(4): 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets 2019; 23(7): 579–591. [DOI] [PubMed] [Google Scholar]
- 34.Kitada M, Ogura Y, Monno I, et al. Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr Diab Rep 2017; 17. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharya D, Mukhopadhyay M, Bhattacharyya M, et al. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J 2018; 17: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehdashtian E, Mehrzadi S, Yousefi B, et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci 2018; 193: 20–33. [DOI] [PubMed] [Google Scholar]