Abstract
Background:
Anxiety disorders are common, associated with significant burden of disease, and have high levels of treatment resistance. Low-dose ketamine has been extensively studied in treatment-resistant depression, with fewer reports in treatment-resistant anxiety disorders.
Aims:
This systematic review and meta-analysis collected efficacy, safety, and tolerability data for ketamine as a treatment for anxiety spectrum disorders.
Methods:
We conducted a systematic search for randomized controlled trials (RCTs) of acute ketamine treatment for patients with anxiety disorders. Open-label trials of ketamine maintenance therapy were also considered. Qualitative and, where possible, quantitative syntheses of findings were performed using Review Manager software (RevMan). Acute dose-response and maintenance treatment data were also collected.
Results:
There were six eligible acute RCTs – two in social anxiety disorder (SAD), three in post-traumatic stress disorder (PTSD), and one in obsessive-compulsive disorder (OCD). Four of the six showed significant improvement in anxiety rating scores in ketamine compared with control groups. Pooled analysis showed ketamine was associated with an increased likelihood of treatment response for SAD (odds ratio (OR): 28.94; 95% confidence interval [CI]: 3.45–242.57; p = 0.002) but not for PTSD (OR: 2.03; 95% CI: 0.67–6.15; p = 0.21). A dose-response profile was observed for ketamine and changes in SAD symptoms, with doses ⩾0.5 mg/kg associated with greater reduction in anxiety rating scores than lower doses. Ketamine maintenance therapy was associated with sustained anxiolytic effects and improved social and/or work functioning.
Conclusion:
These preliminary analyses suggest that acute ketamine may be broadly effective across treatment-resistant anxiety spectrum disorders. These effects can be prolonged with maintenance treatment. Future studies will be needed to provide critical knowledge gaps around off-label use, side effects, and potential risks for abuse in clinical settings.
Keywords: Ketamine, treatment-resistant anxiety disorders, structured review, meta-analysis
Introduction
Anxiety disorders – including specific phobia, social anxiety disorder (SAD), generalized anxiety disorder (GAD), panic disorder, and agoraphobia 1 – are among the most prevalent psychiatric conditions, affecting an estimated 284 million people worldwide. 2 Burden of disease can be significant, and despite psychotherapy and drug treatment options, treatment resistance or relapse is a frequent problem.3,4
Over 20 years ago, ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist with anesthetic and analgesic properties, was reported to have rapid-onset antidepressant activity in treatment-resistant (TR) major depression (TRD) 5 and subsequently in TR bipolar depression. 6 Since then, these findings have been widely replicated,7–10 with the major focus on treating depressive disorders. Until a nasal spray formulation of esketamine for TRD was approved in the United States in 2019, 11 use of ketamine for TRD was off-label.
In contrast to TRD, relatively little clinical research has been published on the use of ketamine in treatment-resistant anxiety disorders. This is not because TR anxiety is a trivial or insignificant clinical problem; remission rates in clinical trials may be as low as 25–35%, 4 and relapse rates post-remission may be 30% after 10 years. 12 Possible reasons for the relatively small volume of published research in TR anxiety could be due to the lack of consistency in defining treatment resistance for anxiety disorders, 13 lack of interest from pharmaceutical sponsors in developing new anti-anxiety drugs, and/or the absence of regulatory guidance on how to develop drugs for TR anxiety.
Established pharmacotherapies for anxiety disorders include drugs that interact with monoamine and GABA-A systems. 4 Antidepressants have significant limitations, including slow onset of action, high rates of nonresponse, and they may worsen anxiety acutely. Benzodiazepines are not recommended for long-term use in some anxiety disorders, due to concerns about their potential for abuse, tolerance, and withdrawal, and they are ineffective in some anxiety spectrum disorders. 14 There are some compelling reasons why drugs interacting with the glutamate system could be therapeutically effective. Glutamate is involved in fear extinction. 15 Glutamate regulates neuropeptides involved in the stress response and may be linked to the development of anxiety disorders. 16 Glutamate also has important role in synaptic and neural plasticity linked to anxiety disorders. 17 Ketamine is a noncompetitive antagonist at NMDA glutamatergic receptors. However, it has a much more complex pharmacological properties, interacting with opioid, monoamine, and nicotinic and muscarinic cholinergic receptors, 18 and having a number of active metabolites with additional pharmacologies. 19
There have been a number of published clinical trials of ketamine in TR anxiety over the last eight years, and the purpose of this systematic review and meta-analysis was to assess the current evidence base for ketamine efficacy, safety, and tolerability in a number of anxiety disorders, based on data from randomized controlled trials (RCTs). It is important to highlight a recent narrative review on this topic, 14 which also includes data from case reports and case series, open-label clinical trials, and evaluation of anxiety ratings in patients with bipolar disorder or major depression. We also included data from studies in patients with obsessive-compulsive disorder (OCD) and post-traumatic stress disorder (PTSD) as anxiety spectrum disorders. While OCD and PTSD were reclassified from anxiety disorders to their own categories in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), 1 anxiety is a significant component of these disorders.
Methods
The protocol for this review was registered prospectively with PROSPERO (international database of prospectively registered systematic reviews): CRD42020190282. For the meta-analysis, we selected prospective acute treatment RCTs. The intervention of interest was ketamine therapy (oral, subcutaneous, intramuscular, or intravenous) compared to a control (psychoactive or inactive) given via the same route of administration. Case reports or case series were excluded. Participants in the reviewed trials needed to meet the following inclusion criteria: adults with DSM-51-diagnosed anxiety spectrum disorders, including SAD, GAD, specific phobia, panic disorder, agoraphobia, separation anxiety disorder, PTSD, or OCD. MEDLINE (Ovid), EMBASE (Ovid), PsycINFO (Ovid), and Cochrane Library databases were systematically searched using keywords (ketamine AND (anxiety OR anxi* OR ‘general* anxiety’ OR GAD OR ‘social anxiety’ OR panic OR agoraphobia OR separation OR OCD OR PTSD OR phobia OR ‘simple phobia’ OR ‘specific phobia’)) and keyword mapping to indexed subject headings. Google Scholar was also searched using keywords, and the first 200 hits reviewed. Full details of the search strategy and number of hits per database can be found in Supplementary Data. Potential studies were reviewed independently by two researchers to assess for eligibility. Reference lists of eligible papers were also manually searched for additional papers, and trial authors were contacted for further information where necessary. Studies meeting the inclusion criteria were compiled using Review Manager (RevMan), Version 5.3. 20 Data were extracted independently by two researchers using standardized tables, and risk of bias assessments made using the Cochrane risk-of-bias tool for randomized trials.
As this was a structured review and meta-analysis of studies that had previously received ethics committee approval, no additional ethics approval was required.
A narrative synthesis of the findings of the included studies was provided. Where appropriate, efficacy and safety results were pooled using a random-effects meta-analysis with odds ratios for binary outcomes, along with 95% confidence intervals. Statistical heterogeneity was assessed using chi-square and I-squared tests.
We also included data from any published sources on acute dose-response and responses to maintenance treatment, for any disorder, presented as percent change-from-baseline.
Analyses are presented in three sections: (1) acute efficacy, safety, and tolerability responses in controlled studies; (2) acute dose-response data; and (3) maintenance treatment.
Results
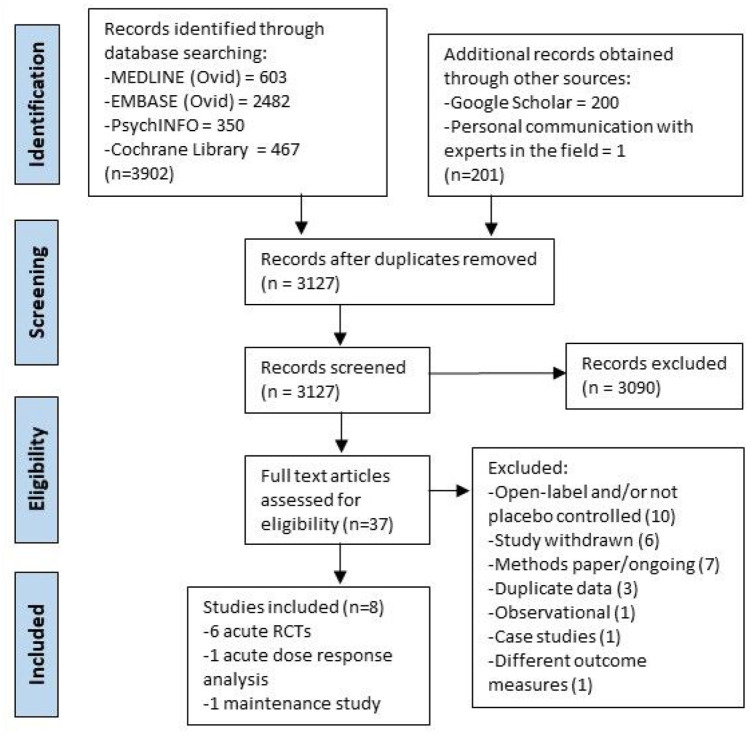
From 3,127 records screened by title and/or abstract, 37 were assessed as potentially eligible, and eight were included in the review (Figure 1). The eight studies selected include six double-blind acute treatment RCTs (five crossover and one parallel), one acute dose-response analysis of both open-label and double-blind efficacy data, and one open-label maintenance therapy trial.
Figure 1.
PRISMA flow diagram.
Acute treatment
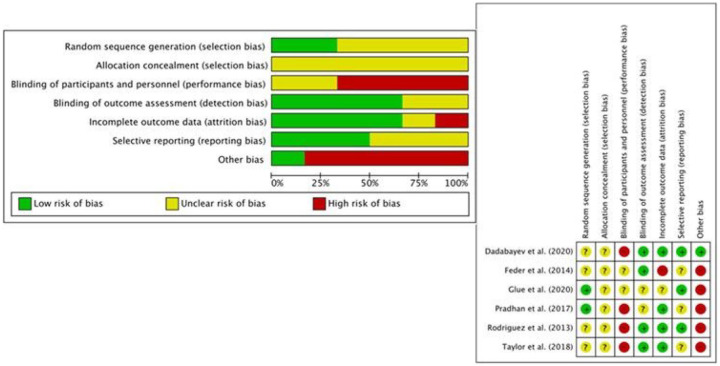
Six RCTs were identified that assessed ketamine as an acute treatment for anxiety disorders. Two papers included patients with SAD and GAD;21,22 three with PTSD;23–25 and one with OCD. 26 The Cochrane risk-of-bias assessment tool for randomized trials is shown in Figure 2. Performance bias arising from functional unblinding of participants and personnel was an issue due to the dissociative effects of ketamine, particularly in contrast to non-psychoactive (e.g. saline) placebo. ‘Other bias’ included the risks of carryover and period effects, so crossover designs were categorized as high risk, and parallel studies as low risk.
Figure 2.
Risk of bias in included acute treatment RCTs (n = 6).
SAD
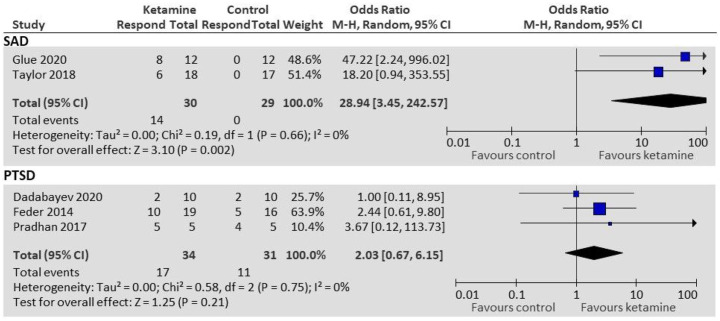
Glue et al. 21 and Taylor et al. 22 both treated patients with SAD in crossover RCTs. Glue et al. 21 (n = 12) used an ascending-dose ketamine schedule of 0.25, 0.5, and 1 mg/kg administered subcutaneously (SC) at weekly intervals, with a psychoactive placebo (midazolam 0.01 mg/kg SC) inserted randomly into the dosing schedule. Outcomes included anxiety ratings on the Fear Questionnaire (FQ) 27 and Hamilton Anxiety Rating Scale (HAM-A) 28 at 1, 2, 24, 72, and 168 hours post-dose. Ketamine demonstrated anxiolytic effects within 1 hour of administration, lasting up to a week. Taylor et al. 22 (n = 18) used a single dose 0.5 mg/kg intravenous (IV) ketamine infusion given over 40 minutes, with an inactive placebo (saline IV infusion over 40 minutes). Anxiety ratings, including scores from the Liebowitz Social Anxiety Scale (LSAS) 29 and Visual Analogue Scale (VAS), were taken pre-infusion, 3-h post-infusion, and 1, 2, 3, 5, 7, 10, and 14 days post-infusion. Results from both studies were pooled for a responder analysis. Treatment response was defined by Glue et al. 21 as >50% decrease in FQ or HAM-A from baseline; and by Taylor et al. 22 as >35% decrease in LSAS from baseline. Participants receiving ketamine were more likely to be responders than those receiving control (OR = 28.94; 95% CI: 3.45–242.57; p = 0.002; Figure 3).
Figure 3.
Responder analysis for patients with SAD and PTSD.
GAD
Both Glue et al. 21 and Taylor et al. 22 had study populations with high comorbidity of SAD and GAD. Insufficient data were available to allow pooled analysis for the GAD subgroup. Response data from Glue et al. 21 in patients with GAD (n = 10, all comorbid with SAD) showed a higher likelihood of treatment response after ketamine (defined as >50% decrease in FQ or HAM-A from baseline at 24 h; 6/10 vs 0/10 for control), which was statistically significant (OR: 30.33; 95% CI: 1.39–660.76; p = 0.03).
PTSD
Three studies investigated ketamine as a treatment for patients with PTSD.23–25 Feder et al. 24 treated 41 patients with chronic PTSD with ketamine 0.5 mg/kg or midazolam 0.045 mg/kg IV infusions given over 40 minutes. PTSD symptom scores were measured using the Impact of Event Scale-Revised (IES-R) 30 at pre-infusion (baseline), 48 h, 72 h, and 7 days post-infusion; and the Clinician-Administered PTSD Scale (CAPS) 31 at pre-infusion and after 7 days. The washout period between infusions was 2 weeks. Compared to midazolam, ketamine was associated with significantly greater improvement in PTSD symptom severity at 24 h based on mean IES-R scores (mean difference: 12.7; 95% CI: 2.5–22.8; p = 0.02).
Dadabayev et al. 23 conducted a randomized parallel-arm trial of ketamine (0.5 mg/kg IV over 40 minutes) compared to ketorolac (15 mg IV over 40 minutes) in participants with either chronic pain (CP), or comorbid PTSD and CP (PTSD + CP). IES-R ratings were measured at pre-infusion (baseline) and at 1- and 7-days post-infusion. Improvements in IES-R occurred at 24 h and 7 days in both the ketamine and ketorolac groups. Using raw data provided by the authors, we calculated no significant difference in IES-R score reduction between the PTSD + CP ketamine and PTSD + CP ketorolac groups at 24-h post-infusion: mean difference -0.34 (95% CI -11.95–11.27; p = 0.95).
Pradhan et al. 25 compared ketamine 0.5 mg/kg IV infusion to normal saline infusion in the context of Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER) psychotherapy in 10 patients with chronic PTSD. This was a modified crossover design; participants were switched from placebo to ketamine after a sustained relapse. They found improvement in both groups, with no significant difference between the ketamine and saline groups for percentage change in PTSD symptom scores from baseline at any time point, using the CAPS and the PTSD Checklist (PCL). 32 Using first-phase data, mean change in CAPS score at 24-h post-infusion was −80.77% post-ketamine versus −72.21% post-saline, while mean change in PCL score was −85.24% post-ketamine versus −78.37% post-saline. A more sustained response to treatment following ketamine compared to saline was also noted, although this was not statistically significant (mean (SD) 33 (23) vs 25 (17) days; p = 0.55).
Results from all three studies were pooled for responder analysis. Treatment response was defined by Feder et al. 24 as at least 50% reduction in IES-R score from baseline to 24-h post-infusion, and this was able to be calculated for PTSD patients from Dadabayev 23 using raw data provided. Treatment response was defined by Pradhan 25 as a reduction of ⩾20 points from baseline in PCL and CAPS scores at 24 h, sustained for at least 7 days (data used from first phase only). There was no statistically significant difference in odds of treatment response between ketamine and control groups (OR: 2.03; 95% CI: 0.67–6.15; p = 0.21; Figure 3).
OCD
Rodriguez et al. 26 was the only eligible study of acute ketamine treatment in OCD patients. Fifteen participants with near-constant obsessions underwent a crossover trial of IV ketamine (0.5 mg/kg) or saline, given over 40 minutes. The Yale-Brown Obsessive-Compulsive Score (Y-BOCS) 33 and the OCD Visual Analogue Scale (OCD-VAS) 34 were used to measure OCD symptomatology. OCD-VAS was measured pre-infusion (baseline), mid-infusion, and at 90, 110, and 230 mins, and daily for 7 days post-infusion. Y-BOCS was measured pre-infusion and 1 week post-infusion. The washout period was 1 week. Based on analysis of first-phase OCD-VAS data only (due to carryover), a statistically significant benefit in reducing OCD symptom scores was reported in the ketamine group compared to the saline group mid-infusion (mean difference: −4.52 points; SE: 1.23; p < 0.005), at 230 minutes (mean difference: −3.84 points; SE: 1.59; p < 0.05), and at 7 days (mean difference −3.67 points; SE: 1.36; p < 0.05). A higher proportion of treatment response (defined as ⩾35% reduction in Y-BOCS score at 7 days) was also found in participants receiving ketamine compared to those receiving placebo (50% vs 0%; X 2 (1, n = 15) = 4.77, p < 0.05), again based on first-phase data.
Using published first-phase data, we calculated a statistically nonsignificant greater reduction in Y-BOCS score after ketamine treatment compared with control treatment at 7 days post-infusion: mean difference −5.79 (95% CI: −11.94 to 0.36; p = 0.07). Using additional unpublished data provided by the authors, we calculated a statistically nonsignificant greater reduction in OCD-VAS in the first-phase ketamine group compared to the first-phase control group at 7 days post-infusion: mean difference −2.50 (95% CI: −5.19 to 0.19; p = 0.07).
Safety and tolerability
CADSS
Compared with control, Clinician-Administered Dissociative States Scale (CADSS) 35 scores tended to increase after ketamine administration, and return to baseline by 120 minutes (Figure 4). This effect was greatest at 30–40 minutes post-dose21,26 and smaller at 60 minutes. 22 Of note, patients with comorbid PTSD and CP from Dadabayev et al. 23 reported minimal dissociative effects after ketamine, which the authors attributed to either distinct neurobiological processes related to comorbid CP, or possibly due to concomitant use of adjuvant pain medications and/or anticonvulsants prescribed for pain or PTSD.
Figure 4.
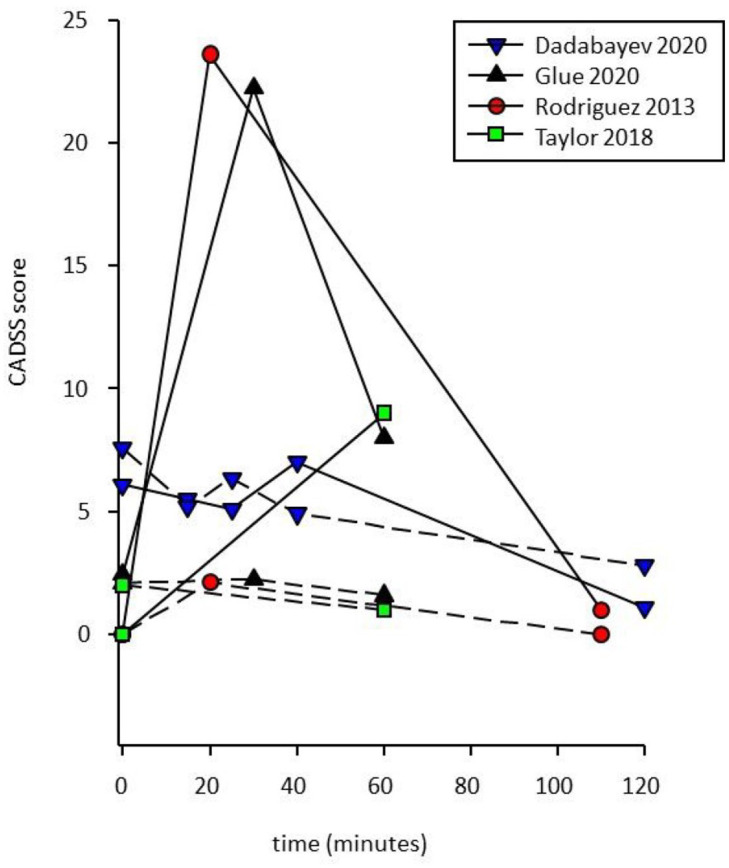
Effects of ketamine (solid lines) and control (dashed lines) on mean Clinician-Administered Dissociative States Scale (CADSS) scores over time.
Side effects
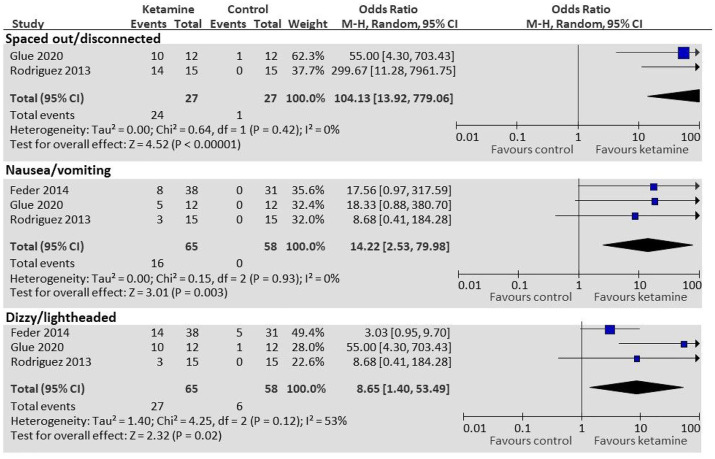
The most common side effects reported after administration of ketamine included feeling dissociated/spaced out, nausea and vomiting, dizziness or light-headedness, blurred vision, and fatigue. Using supplementary data from Feder et al. 24 and raw data from Rodriguez et al. 26 and Glue et al., 21 we performed a pooled analysis to evaluate the odds ratio of various side effects between ketamine and control groups (see Figure 5). Compared with control, patients given ketamine had statistically significantly greater odds of feeling spaced out or disconnected (OR: 104.13; 95% CI: 13.92–799.06; p < 0.0001), nausea/vomiting (OR: 14.22; 95% CI: 2.53–79.98; p = 0.003), and feeling dizzy/light-headed (OR: 8.65; 95% CI: 1.40–53.49; p = 0.02). Ketamine has sympathomimetic effects, and increases in heart rate and/or blood pressure in the ketamine group compared to the placebo group has been consistently reported.
Figure 5.
Side effects (ketamine compared to controls).
Acute dose response in SAD/GAD
There were two publications which reported acute ketamine dose-responses in change in anxiety rating scores: Glue et al. 21 with an ascending-dose randomized trial in patients with SAD; and Truppman Lattie et al. 36 with secondary analysis from this data set, which included Spielberger State Anxiety Inventory (SSAI) 37 scores. A dose-response profile was observed for the FQ, HAM-A, and SSAI rating scales (see Figure 6). Higher doses (0.5 and 1 mg/kg) were associated with greater reduction in anxiety symptoms than the lowest dose used (0.25 mg/kg), which overlapped with the midazolam control. The 0.5 mg/kg dose appeared to be associated with the greatest changes from baseline anxiety rating scores at 24 h, with minimal additional improvement at 1 mg/kg.
Figure 6.
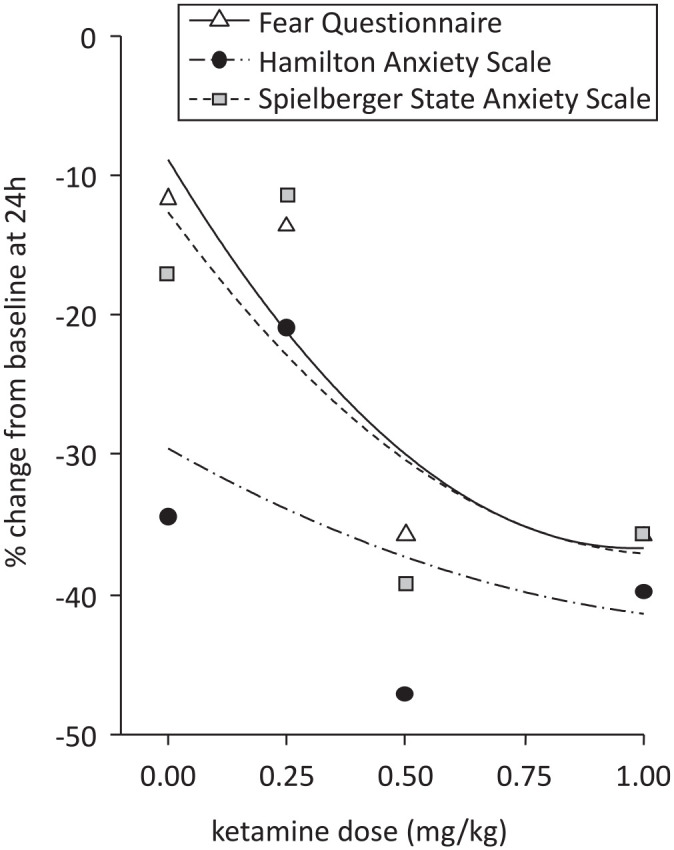
Relationship between acute ketamine dose and change in anxiety rating scores at 24 hours post-dose.
Maintenance treatment, SAD and GAD
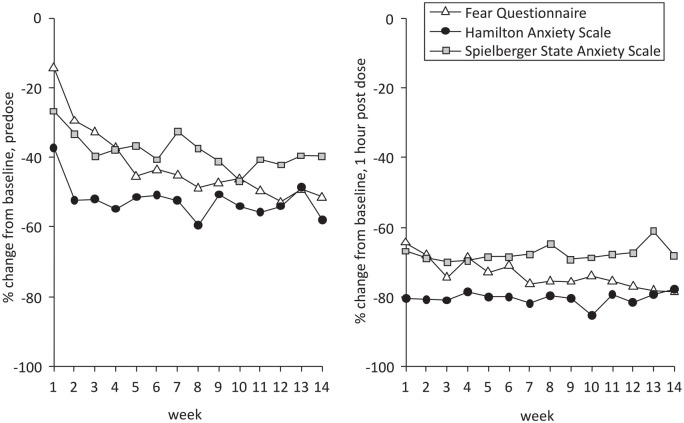
There were two publications reporting on mood responses to ketamine maintenance therapy. Glue et al. 38 reported an open-label study of ketamine maintenance treatment (1 mg/kg subcutaneous injection once or twice weekly) in 20 patients with SAD and/or GAD who had experienced a treatment response to ketamine in the double blind study 21 or in an earlier open-label, proof-of-concept, ascending-dose study. 39 Each week, anxiety rating scales (including FQ and HAM-A) were administered pre-infusion, and at 1 h and 2 h post-ketamine. Eighteen of the 20 patients reported that ketamine maintenance treatment helped improve their social and/or work functioning. Truppman Lattie et al. 36 carried out further analysis on data from these patients, including SSAI scores. Figure 7 shows mean change in FQ, HAM-A, and SSAI anxiety rating scores over 14 weeks’ maintenance treatment. For the HAM-A and SSAI, improvement from baseline in predose anxiety scores approached asymptote around 3–4 weeks after the start of maintenance treatment, while improvement as measured by the FQ appeared to occur later, approaching asymptote at around 7–8 weeks (Figure 7, left panel). There were further ~50% reductions in anxiety scale scores by 1 hour after dosing (Figure 7, right panel), which occurred consistently for 14 weeks.
Figure 7.
Change in anxiety rating score over time during ketamine maintenance therapy.
Discussion
This is the first systematic review and meta-analysis of controlled acute ketamine studies in anxiety spectrum disorders, and builds on the earlier thorough narrative review on this subject by Banov et al. 14 This review includes data from a range of disorders – including SAD, GAD, PTSD, and OCD – providing insight into the potential treatment efficacy, safety, and tolerability of ketamine for a range of anxiety spectrum diagnoses. Other strengths include the consideration of both acute and maintenance ketamine treatment, acute dose-response data, and the inclusion of unpublished data from a number of centers.
Of the six included randomized acute treatment trials, four (including patients with SAD/GAD, PTSD, and OCD) reported evidence of significant benefit from ketamine compared to placebo, as measured by change in symptom rating scores at varying time points post-ketamine. Two studies found no significant difference between ketamine and controls (saline and ketorolac) in terms of reduction in PTSD scores post-infusion.23,25 Within individual studies, results also varied between different rating scales used (e.g. FQ vs HAM-A for SAD), likely reflecting psychometric differences.
Based on pooled analysis, we found that compared to controls, ketamine was associated with a statistically significant increased likelihood of acute treatment response for SAD (28.94; 95% CI: 3.45–242.57; p = 0.002). Responder analysis of patients with GAD (who all had comorbid SAD) was also statistically significantly greater for ketamine (OR: 30.33; 95% CI: 1.39–660.76, p = 0.03). For PTSD, based on pooled treatment response data from three studies, this difference was not significant (OR: 2.03; 95% CI: 0.67–6.15; p = 0.21). However, differences in study design (e.g. the presence of CP, use of other medications, 23 or provision of psychotherapy 25 could have influenced response rates in control arms. Other evidence of anxiolytic effects include Feder et al’.s 24 study in patients with PTSD, which reported greater reduction in IES-R scores at 24 hours after ketamine treatment compared with control, and Rodriguez et al’.s 26 study in patients with OCD, which reported greater reductions in OCD-VAS scores after ketamine compared with control in the first period of this crossover study. Based on these findings, we predict that ketamine may have broad anxiolytic effects across anxiety spectrum disorders.
We also report that ketamine demonstrates an acute dose-response profile, with doses of 0.5 mg/kg and above associated with greater improvement in anxiety ratings than lower doses. A similar dose-response was described for ketamine’s antidepressant effects. 40 As a maintenance treatment, ketamine was associated with sustained anxiolytic effects as well as improved social and/or work functioning. 38 A recent midazolam-controlled study of repeat ketamine dosing in patients with chronic PTSD reported reduced PTSD symptom severity after repeated ketamine infusions. 41 If ketamine is to be used clinically for treatment of other anxiety disorders, it will almost certainly require repeat dosing, and it will be important to identify optimal doses and dosing frequency for each indication.
Ketamine was reported to be generally safe and well tolerated. The dissociative and sympathomimetic effects of ketamine are well recognized. Dissociative symptoms may reduce in intensity after repeat dosing;38,42 and interestingly, patients who had comorbid CP and PTSD appeared to be more resistant to the dissociative effects of ketamine. 23 Other side effects commonly associated with ketamine dosing include dizziness/lightheadedness and nausea/vomiting. Dissociation and dizziness are of relatively brief duration and can be managed by having patients resting comfortably for the first hour after dosing. Pre-dosing patients with ondansetron may reduce the risk of nausea and vomiting.
This review has a number of limitations, including the small number of eligible studies and differences in study protocols (e.g. psychoactive vs inactive controls, different dosing regimens, and different routes of administration), which could potentially limit comparability between studies. Second, many studies had small numbers of participants (n < 20 in four of the six RCTs). Four studies had a crossover design (vs one modified crossover and one parallel study), which allows participants to act as their own control and thereby reduces the number of participants required to power the study. In order to preserve power, no adjustments for multiple measures were made in this meta-analysis. Crossover trials do have limitations, specifically with carryover effects, as noted above. In this analysis, only parallel group data from the first dosing period were used. Third, effective blinding could have been affected due to the psychogenic and dissociative nature of ketamine, even when psychoactive controls (e.g. midazolam) were used. This means participants and personnel could be unblinded. Finally, comorbidity is both a limitation and advantage of this review. Many of the studies included patients with multiple psychiatric and/or physical diagnoses. This increases clinical heterogeneity but also more accurately reflects the real-world population, as anxiety disorders are known to be associated with high levels of comorbidity. 3 Another limitation relating to this whole area of research is the lack of accepted operational definitions for TR anxiety disorders. The conclusions of a recent systematic review 13 of this topic are promising but would need to be more widely accepted by professional bodies and regulators to assist with this issue.
In conclusion, this review suggests that ketamine may be a safe and broadly effective anxiolytic for patients with anxiety spectrum disorders, including treatment refractory cases. In contrast to the extensive published data for TRD, the evidence base of controlled trials of ketamine in patients with TRA is very limited. The findings from this review must be considered as tentative due to relatively small sample sizes and lack of long-term data around safety and efficacy. Of note, while ketamine may be helpful to some patients, off-label use should employ clinical decision-making that includes discussion of the limitations of the existing knowledge base. 43 Further RCTs in this area, including parallel arm studies, should be encouraged, given the significant burden of disease and high rates of treatment resistance associated with these disorders worldwide.
Supplemental Material
Supplemental material, sj-docx-1-tpp-10.1177_20451253211056743 for Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders by Elizabeth Whittaker, Alisher R. Dadabayev, Sonalee A. Joshi and Paul Glue in Therapeutic Advances in Psychopharmacology
Acknowledgments
Drs Caroline Rodriguez and Pavithra Mukunda provided excellent feedback and advice on an earlier draft of this manuscript.
Footnotes
Author contributions: Drs Whittaker and Glue decided on the review topic. Dr Whittaker collected and analyzed data, and wrote the first draft of the manuscript. All authors reviewed and approved the final draft of the manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Glue has a contract with Douglas Pharmaceuticals to develop novel ketamine formulations. Within the last 3 years, Dr Glue has participated in an advisory board for Janssen Pharma. No other authors have disclosures.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Paul Glue  https://orcid.org/0000-0002-7305-2800
https://orcid.org/0000-0002-7305-2800
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Elizabeth Whittaker, Department of Psychological Medicine, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand.
Alisher R. Dadabayev, Anesthesiology Service, Ralph H. Johnson VA Medical Center, Charleston, SC, USA
Sonalee A. Joshi, Department of Psychology, University of Michigan, Ann Arbor, MI, USA
Paul Glue, Hazel Buckland Chair, Psychological Medicine, School of Medical Sciences, University of Otago, PO Box 913, Dunedin 9054, New Zealand.
References
- 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 2. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry 2006; 11: 805–814. [DOI] [PubMed] [Google Scholar]
- 4. Roy-Byrne P. Treatment-refractory anxiety; definition, risk factors, and treatment challenges. Dialogues Clin Neurosci 2015; 17: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 6. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcantoni WS, Akoumba BS, Wassef M, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009–January 2019. J Affect Dis 2000; 277: 831–841. [DOI] [PubMed] [Google Scholar]
- 8. McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry 2021; 178: 383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 2015; 172: 950–966. [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Hackett M, Carter G, et al. Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2016; 19: pyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J, Farchione T, Potter A, et al. Esketamine for treatment-resistant depression – first FDA-approved antidepressant in a new class. N Engl J Med 2019; 381: 1–4. [DOI] [PubMed] [Google Scholar]
- 12. Bruce SE, Yonkers KA, Otto MW, et al. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry 2005; 162: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bokma WA, Wetzer GA, Gehrels JB, et al. Aligning the many definitions of treatment resistance in anxiety disorders: a systematic review. Depress Anxiety 2019; 36: 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banov MD, Young JR, Dunn T, et al. Efficacy and safety of ketamine in the management of anxiety and anxiety spectrum disorders: a review of the literature. CNS Spectr 2020; 25: 331–342. [DOI] [PubMed] [Google Scholar]
- 15. Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry 2002; 52: 998–1007. [DOI] [PubMed] [Google Scholar]
- 16. Kormos V, Gaszner B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 2013; 47: 401–419. [DOI] [PubMed] [Google Scholar]
- 17. McEwen BS, Eiland L, Hunter RG, et al. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012; 62: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mion G, Villevieille T. Ketamine pharmacology: an update. CNS Neurosci Ther 2013; 19: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 2018; 70: 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Review Manager (RevMan) , version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 21. Glue P, Neehoff S, Sabadel A, et al. Effects of ketamine in patients with treatment-refractory generalized anxiety and social anxiety disorders: exploratory double-blind psychoactive-controlled replication study. J Psychopharmacol 2020; 34: 267–272. [DOI] [PubMed] [Google Scholar]
- 22. Taylor JH, Landeros-Weisenberger A, Coughlin C, et al. Ketamine for social anxiety disorder: a randomized, placebo-controlled crossover trial. Neuropsychopharmacology 2018; 43: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dadabayev AR, Joshi SA, Reda MH, et al. Low dose ketamine infusion for comorbid posttraumatic stress disorder and chronic pain: a randomized double-blind clinical trial. Chronic Stress 2020; 4: 2470547020981670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feder A, Parides MK, Murrough JW, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 2014; 71: 681–688. [DOI] [PubMed] [Google Scholar]
- 25. Pradhan B, Wainer I, Moaddel R, et al. Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER) psychotherapy prolong the therapeutic effects of single ketamine infusion on post-traumatic stress disorder and comorbid depression: a pilot randomized, placebo-controlled, crossover clinical trial. Asia Pac J Clin Trials Nerv Syst Dis 2017; 2: 80–90. [Google Scholar]
- 26. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 2013; 38: 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marks IM, Mathews AM. Brief standard self-rating for phobic patients. Behav Res Ther 1979; 17: 263–267. [DOI] [PubMed] [Google Scholar]
- 28. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50–55. [DOI] [PubMed] [Google Scholar]
- 29. Liebowitz M. Social phobia. Mod Probl Pharmacopsychiatry 1987; 22: 141–173. [DOI] [PubMed] [Google Scholar]
- 30. Weiss DS, Marmar CR. The Impact of Event Scale–Revised. In: Wilson J, Keane TM. (eds) Assessing psychological trauma and PTSD. New York: Guilford Press, 1996, pp. 399–411. [Google Scholar]
- 31. Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001; 13: 132–156. [DOI] [PubMed] [Google Scholar]
- 32. Weathers F, Litz B, Herman D, et al. The PTSD Checklist-civilian version (PCL-C). Boston, MA: National Center for PTSD, 1994. [Google Scholar]
- 33. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez CI, Kegeles LS, Flood P, et al. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2011; 72: 567–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress 1998; 11: 125–136. [DOI] [PubMed] [Google Scholar]
- 36. Truppman Lattie D, Nehoff H, Neehoff S, et al. Anxiolytic effects of acute and maintenance ketamine, as assessed by the Fear Questionnaire subscales and the Spielberger State Anxiety Rating Scale. J Psychopharmacol 2021; 35: 137–141. [DOI] [PubMed] [Google Scholar]
- 37. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologist Press, 1983. [Google Scholar]
- 38. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalized anxiety and social anxiety disorders. J Psychopharmacol 2018; 32: 663–667. [DOI] [PubMed] [Google Scholar]
- 39. Glue P, Medlicott NJ, Harland S, et al. Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol 2017; 31: 1302–1305. [DOI] [PubMed] [Google Scholar]
- 40. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 2020; 25: 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry 2021; 178: 193–202. [DOI] [PubMed] [Google Scholar]
- 42. Phillips JL, Norris S, Talbot J, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry 2020; 176: 401–409. [DOI] [PubMed] [Google Scholar]
- 43. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 2017; 74: 399–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpp-10.1177_20451253211056743 for Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders by Elizabeth Whittaker, Alisher R. Dadabayev, Sonalee A. Joshi and Paul Glue in Therapeutic Advances in Psychopharmacology