To the Editor,
Inflammation plays an important role in the development of major depression (MD) and is a critical disease modification factor that promotes susceptibility to MD.1,2 Recent reports demonstrated that the altered levels of serum various cytokines and C-reactive protein (CRP) in MD patients may represent a homeostatic mechanism that enhances the inflammatory process during MD.3–5 Another report demonstrated that the variation of the patterns of cytokines is associated with the phenotype of major depression. 6 One of the most common approaches to evaluate peripheral inflammation is to measure the serum levels of CRP. CRP plays a key role in the human inflammation process and can provide a proxy estimate for inflammatory activity. 7 A systematic review and meta-analysis showed that CRP is a useful biomarker for investigating acute inflammatory processes in MD. 8 Vegetative symptoms, but not cognitive symptoms of MD, were associated with higher CRP.9,10 The aim of the present study was to compare the psychiatric symptoms between MD patients with a higher level of high-sensitivity CRP (hsCRP) and a lower level of hsCRP in the serum. This study included 40 patients who met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for MD [male/female: 17/23, age: 56 (47–65; interquartile range) years]. We used the Montgomery Asberg Depression Rating Scale (MADRS) to evaluate depression severity. Blood samples were quickly separated using a centrifuge (2000 g, 15 min, 4°C) and stored at −80°C until assay. We found that the serum hsCRP levels were not normally distributed in the histogram. We then classified 20 MD patients [male/female: 6/14, age: 55.5 (48–67) years] above the median (337 ng/m) as the higher hsCRP group and 20 MD patients [male/female: 10/10, age: 55 (46–62.5) years] below the median as the lower hsCRP group (Figure 1). The number of the depressive episodes were 1.5 (1–2) for the higher hsCRP group, and 2 (1–2) for the lower hsCRP group, respectively. The imipramine equivalence of dosage of antidepressants were 75 (37.5–225) mg/day for the higher hsCRP group, and 113 (89–150) mg/day for the lower hsCRP group, respectively. No differences were found between both groups regarding these factors. Mann-Whitney U test and Spearman’s rank correlation were used for statistical analysis. Statistical significance was defined as p < 0.05. There was no significant difference in the total MADRS scores between the higher hsCRP group and the lower hsCRP group [12 (8–15) versus 13 (7.5–19), p = 0.29]. Regarding the MADRS score for each item, only reduced appetite showed a significant difference [0 (0–2) versus 2 (1–3), p = 0.024] (Table 1). The body mass index did not differ between the groups [25 (22–28) versus 23 (19–26) kg/m2]. A recent report demonstrated that poor appetite and low food intake are associated with inflammation in older hospitalized patients. 11 In addition, inflammation is associated with hedonic inferences about food stimuli using a functional magnetic resonance imaging (fMRI) scan. 12 The results of our study were not in agreement with the previous findings that higher CRP levels were associated with vegetative symptoms and reduced food intake. The discrepancy between the results of the present study and those of recent studies9,10,12 remains unknown. Many factors may be involved in the regulation of appetite in MD. The limitations of this study include its small sample size, mild to moderate severity of MD, and the influence of antidepressants in the present study. There were no differences in the dosage of antidepressants to imipramine equivalence between the groups as we mentioned. The most serious problem in the present study is a lack of control group. In conclusion, reduced appetite might be associated with lower hsCRP levels in MD patients. Comparing serum hsCRP levels with those with healthy subjects might lead to a rational interpretation of this preliminary result.
Figure 1.
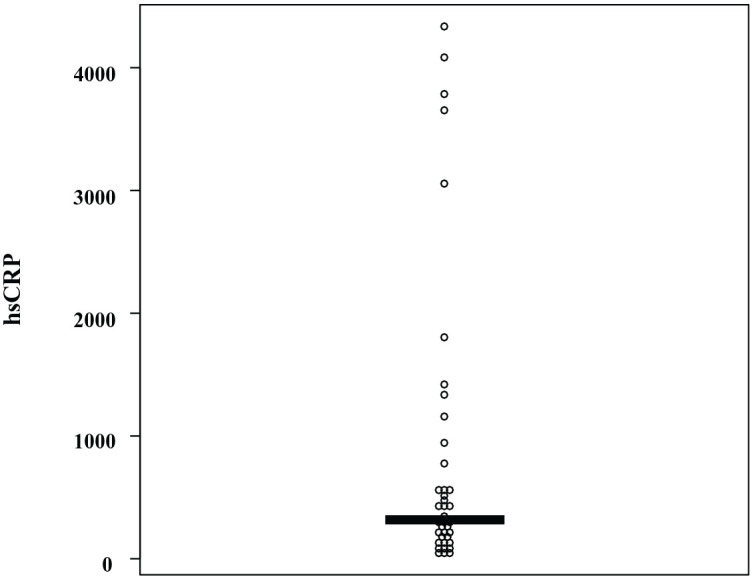
Distribution of serum levels of hsCRP in MD patients.
Table 1.
Scores for each criterion of the MADRS between both groups.
| Higher hsCRP group (n = 20) |
Lower hsCRP group (n = 20) |
p value | |
|---|---|---|---|
| Apparent sadness | 2 [1–2] | 2 [2–2] | 0.15 |
| Reported sadness | 2 [1–2.5] | 2 [1–3] | 0.47 |
| Inner tension | 2 [1–2] | 1 [1–2] | 0.87 |
| Reduced sleep | 2 [2–4] | 2 [2–4] | 1.0 |
| Reduced appetite | 0 [0–2] | 2 [1–3] | 0.024 |
| Concentration difficulty | 2 [2–2] | 2 [2–3] | 0.49 |
| Lassitude | 1 [1–2] | 2 [1–2] | 0.35 |
| Inability to feel | 2 [1–2.5] | 2 [1–4] | 0.17 |
| Pessimistic thoughts | 1 [1–2] | 2 [1–3] | 0.26 |
| Suicidal thoughts | 1 [1–2] | 1 [1–3] | 0.92 |
hsCRP, high-sensitivity C-reactive protein; MADRS, Montgomery Asberg Depression Rating Scale.
Data are expressed as median [interquartile range].
Statistically significant differences (p < 0.05) are in bold.
Acknowledgments
The authors thank Ms. Tomomi Ishiba for her assistance with blood sampling and paperwork.
Footnotes
Author contributions: NO and TH were involved in the clinical investigations. NO and RY drafted the manuscript. NO performed a statistical analysis of the data. AI, TH, and RY conducted the literature review and corrections. All authors have read and approved the final manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the Institute of Health, Labor, and Welfare in Japan to RY (18K07576).
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics statement: We obtained a written informed consent from all the participants. This study was approved by the Ethics Committee of the University of Occupational and Environmental Health Kitakyushu, Japan (approval number: UOHECRB21-057) and followed the tenets of Helsinki Declaration.
ORCID iD: Reiji Yoshimura  https://orcid.org/0000-0002-7637-5576
https://orcid.org/0000-0002-7637-5576
Availability of data and materials: The data sets used and/or analyzed during the current case report are available from the corresponding author upon reasonable request.
Contributor Information
Naomichi Okamoto, Medical Center for Dementia, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan; Department of Psychiatry, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan.
Takashi Hoshikawa, Department of Psychiatry, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan.
Atsuko Ikenouchi, Medical Center for Dementia, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan; Department of Psychiatry, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan.
Reiji Yoshimura, Department of Psychiatry, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu 8078555, Fukuoka, Japan; Medical Center for Dementia, University Hospital, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan.
References
- 1. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron 2020; 107: 234–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Troubat R, Barone P, Leman S, et al. Neuroinflammation and depression: a review. Eur J Neurosci 2021; 53: 151–171. [DOI] [PubMed] [Google Scholar]
- 3. Anjum S, Qusar S, Shahriar M, et al. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol 2020; 10: 2045125320916655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Primo de Carvalho Alves L, Sica da Rocha N. Different cytokine patterns associate with melancholia severity among inpatients with major depressive disorder. Ther Adv Psychopharmacol 2020; 10: 2045125320937921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das R, Emon MPZ, Shahriar M, et al. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res 2021; 295: 113568. [DOI] [PubMed] [Google Scholar]
- 6. Nishuty NL, Khandoker MH, Karmoker JR, et al. Evaluation of serum interleukin-6 and C-reactive protein levels in drug-naïve major depressive disorder patients. Cureus 2019; 11: e3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowe GDO. Circulating inflammatory markers and risks of cardiovascular and non-cardiovascular disease. J Thromb Haemost 2005; 3: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 8. Osimo EF, Baxter LJ, Lewis G, et al. Prevalence of low-grade inflammation in depression: a systematic review and meta-Analysis of CRP levels. Psychol Med 2019; 49: 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duivis HE, Vogelzangs N, Kupper N, et al. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 2013; 38: 1573–1585. [DOI] [PubMed] [Google Scholar]
- 10. Chamberlain SR, Cavanagh J, de Boer P, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry 2019; 214: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sieske L, Janssen G, Babel N, et al. Inflammation, appetite and food intake in older hospitalized patients. Nutrients 2019; 11: 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cosgrove KT, Burrows K, Avery JA, et al. Appetite change profiles in depression exhibit differential relationships between systemic inflammation and activity in reward and interoceptive neurocircuitry. Brain Behav Immun 2020; 83: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]


