Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040.1
Keywords: pathology competencies, organ system pathology, musculoskeletal, bone, bone tumor, osteoid osteoma, children
Primary Objective
Objective MS1.1: Categories of Bone Tumors. Describe examples of bone forming, cartilage forming, and other common bone tumors including the clinicopathologic features, radiological features, treatment, and prognosis of each.
Competency 2: Organ System Pathology; Topic: MS: Musculoskeletal System; Learning Goal 1: Bone Neoplasia.
Patient Presentation
A 9-year-old previously healthy boy without recent illness presents to his pediatrician with severe left lower extremity pain for 4 months that seems worse at night and sometimes awakens him from sleep. Treating with ibuprofen tended to help him with the pain initially, but the pain persists. His soccer coach talked to him about his leg pain and said the associated limp could be due to shin splints or a bone bruise from overexertion or forgetting to wear shin guards during practice based on his experience with the other players. The boy says he wears his shin guards everyday, and the pain began before soccer season. Also, he says he does not have bruises on his shins. He denies any history of trauma to his leg. He denies any recent fever, malaise, cuts on his skin, or redness of the affected area on his left leg. His only concern is his left leg pain exacerbated by walking. His recent sports physical examinations have been routine without concerning findings, and he is up to date on all childhood vaccinations. His mother is concerned for her child and asks the pediatrician for further evaluation.
Diagnostic Findings, Part 1
The patient’s height is 4 feet, 5 inches, and he weighs 61 pounds. Vital signs are blood pressure 118/78 mmHg, heart rate 105 beats per minute, respiratory rate 20 breaths per minute, and temperature 99.5°F. There is tenderness to palpation with mild inflammation of the left anterior tibial diaphysis without warmth, erythema, or crepitus, on physical examination; no palpable deformity or mass of the affected left leg is visible. There is a normal range of motion of the left hip and knee. The skin is intact without evidence of abrasion, laceration, or puncture wound on the extremities. No rash is visible. A singular café au lait macule is evident on the patient’s right trunk, present since birth. Neurovascular exam of the affected lower extremity is unremarkable. No palpable lymph nodes are evident. His heart examination revealed normal S1, S2 sounds, regular rate and rhythm with no rubs, gallops, or murmurs. Lungs are clear to auscultation; no wheezing is evident.
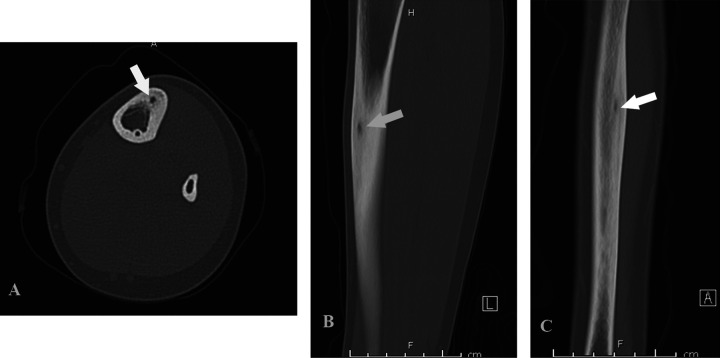
Complete blood count and markers of inflammation (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) are unremarkable. Conventional radiographs of the tibia and fibula are obtained (Figure 1). On the tibial diaphysis, there is a cortically based lesion. There is no associated periosteal reaction. Although the anteroposterior (AP) and lateral conventional radiographs of the lower extremity demonstrate a bone lesion without a nidus, in many cases for a patient with the same bone lesion, a nidus is visible. An AP radiograph from a different patient with the same bone lesion demonstrates a lytic lesion at the lateral aspect of the talus, with a subtle central sclerotic focus suggestive of a nidus (Figure 2).
Figure 1.
(A) Anteroposterior (AP), (B) Lateral. Radiograph of the lower extremity demonstrates thickening of the lateral cortex of the tibial diaphysis (arrow). There is suggestion of a small lytic focus within the cortex.
Figure 2.
Anteroposterior radiograph of the left lower extremity demonstrates a lytic lesion at the lateral aspect of the talus, with a subtle central sclerotic focus suggestive of a nidus (arrow). Mild sclerosis is noted surrounding the lytic lesion.
Further imaging with computed tomography (CT) and magnetic resonance imaging (MRI; Figures 3 and 4) are obtained for the patient in the clinical vignette. Computed tomography images of the affected left leg demonstrate a cortically based tibial diaphysis lesion measuring approximately 1 cm. No central nidus is visible. On MRI, the lesion is T1 dark and T2 bright. There is intramedullary and periosteal edema.
Figure 3.
(A) Axial, (B) Sagittal, (C) Coronal. Computed tomography images of the lower extremity demonstrate a cortically based lytic lesion with surrounding sclerotic reactive bone (arrow). No central sclerotic focus is noted to suggest a nidus. There is no periosteal reaction.
Figure 4.
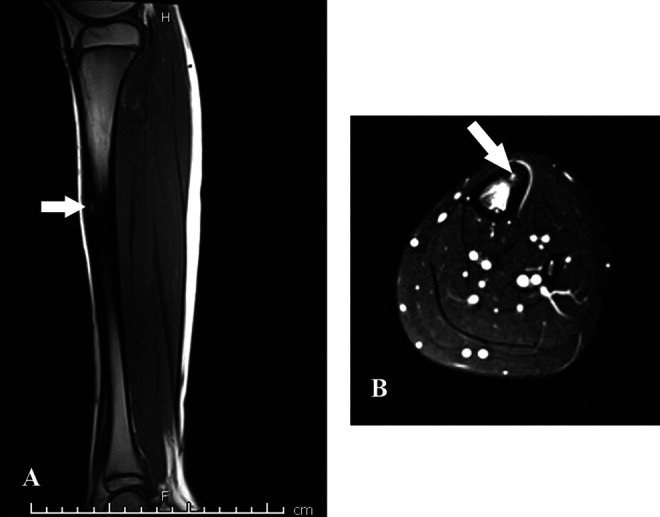
(A) T1 sag, (B) Axial T2 fat saturated. Magnetic resonance imaging of the lower extremity demonstrates significant osseous and periosteal edema at the tibial diaphysis. Cortical thickening is noted at the anterior tibial cortex, with a small T1 hypointense and T2 hyperintense lesion (arrow). There is no soft tissue component.
Questions/Discussion Points, Part 1
What Is in the Differential Diagnosis for Childhood Bone Lesion of the Diaphysis?
The differential diagnosis of childhood bone lesion of the diaphysis includes osteoid osteoma, osteomyelitis, Brodie abscess, stress fracture, pathologic fracture, and ossifying fibroma.
Radiographs are considered as an initial diagnostic step when a patient presents with symptoms concerning for bone fracture, tumor, or infection. The bone lesion location facilitates diagnostic evaluation as presentations generally follow patterns based on the patient’s age and growth plate status, the involved bone, and region of the bone (outer surface of the bone, cortex, medullary cavity, diaphysis, metaphysis, and epiphysis). 2 Approximately 50% of all stress fractures of the lower extremity in children occur in the tibia. 3 Around 50% of osteoid osteomas and 80% of subacute osteomyelitis (Brodie’s abscess) arise in the femur or tibia. 4,5 Additionally, boys are affected by osteoid osteoma and osteomyelitis twice as often as girls. 6,7 Bone tumors are usually classified based on the normal bone cell or matrix they secrete. 4 Benign bone tumors are more common than their malignant counterparts, especially in children and most bone neoplasms are osteolytic. 8 The most common benign bone tumors are osteochondroma, nonossifying fibroma, and osteoid osteoma 9 ; an osteoid osteoma represents 10% of all benign bone tumors. 10
Discuss the Benign Bone Tumor Classifications as It Relates to the Substance That They Produce
The long bones are formed by endochondral ossification, which requires a cartilaginous model to be secreted initially. The tibial nutrient artery enters its respective long bone periosteal collar through a foramen, permitting blood access to the bone marrow. The major cellular constituents of bone arise from mesenchymal cells such as osteoblasts and chondrocytes. Primary bone tumors develop from bone and cartilage forming resident cells, and the tumors are classified by the matrix they secrete, summarized in Table 1. 4,10 -12 Benign bone-forming tumors include osteoid osteoma and osteoblastoma. Cartilage-forming tumors are osteochondroma, chondroma (enchondroma and juxtacortical chondroma), chondroblastoma, and chondromyxoid fibroma. Additionally, fibrous, cystic, and vascular bone lesions also present in childhood. Vascular lesions of lower extremity bone in childhood that leads to localized osteolysis include epithelioid hemangioma, Kaposiform hemangioendothelioma, and angiosarcoma. 13
Table 1.
Benign Bone Lesion Classification Based on Matrix Secreted.
| Matrix | Bone tumor type | Age (year) | Long bone region | Common locations |
|---|---|---|---|---|
| Osteoid forming tumors | Osteoid osteoma | 10-20 4 | Metaphysis,*, 4 diaphysis | Proximal femur,* spine, tibia, 10 proximal tibia, distal femur, proximal humerus, phalanges 11 |
| Osteoblastoma | 10-20 4 | Metaphysis 10,12 | Posterior elements of the spine 4 or sacrum, femur, tibia 12 | |
| Cartilage forming tumors | Osteochondroma | 10-30 4 | Metaphysis 4 | Femur, tibia, pelvis, scapular, ribs 4 |
| Enchondroma | 20-50 4 | Metaphysis 4 | Tubular bones of the hands and feet 4 | |
| Juxtacortical chondroma | Children and adults 11 | Metaphysis, diaphysis 11 | Proximal humerus, femur, long bones of the hands and feet 11 | |
| Chondroblastoma |
10-20 4 | Epiphyses 4 | Humerus, femur, tibia, patella, talus, calcaneus 10,11 | |
| Chondromyxoid fibroma | 20-30 4 | Metaphysis 10 | Proximal tibia, pelvis, 4 distal femur 10 | |
| Fibrous lesions | Ossifying Fibroma | 0-5 11 | Diaphysis 11 | Tibia, fibula 11 |
| Nonossifying fibroma | Teenagers 11 | Metaphysis 11,12 | Femur, tibia 11 | |
| Cystic and vascular lesions | Unicameral bone cyst | 0-20 11 | Metaphysis, metadiaphysis 11 | Proximal humerus, femur, tibia 11 |
| Aneurysmal bone cyst | 10-20 4 | Metaphysis 11 | Proximal tibia, distal femur, 4 posterior spinal elements 11 |
* Most common site for lesion.
Based on the Clinical Presentation and Imaging What Is the Most Likely Diagnosis?
Based on the patient’s age, history of nocturnal pain relieved by ibuprofen, and CT findings, the most likely diagnosis is an osteoid osteoma. The lack of periosteal reaction is essential in ruling out osteomyelitis, a clinically significant concern due to associated morbidity.
What Long Bone Location Is the Most Common Presentation for an Osteoid Osteoma?
Although the proximal femur is the most common site for an osteoid osteoma, 11 they can be present in any bone. 10 A CT of a patient with an osteoid osteoma of the proximal femur is demonstrated in Figure 5.
Figure 5.
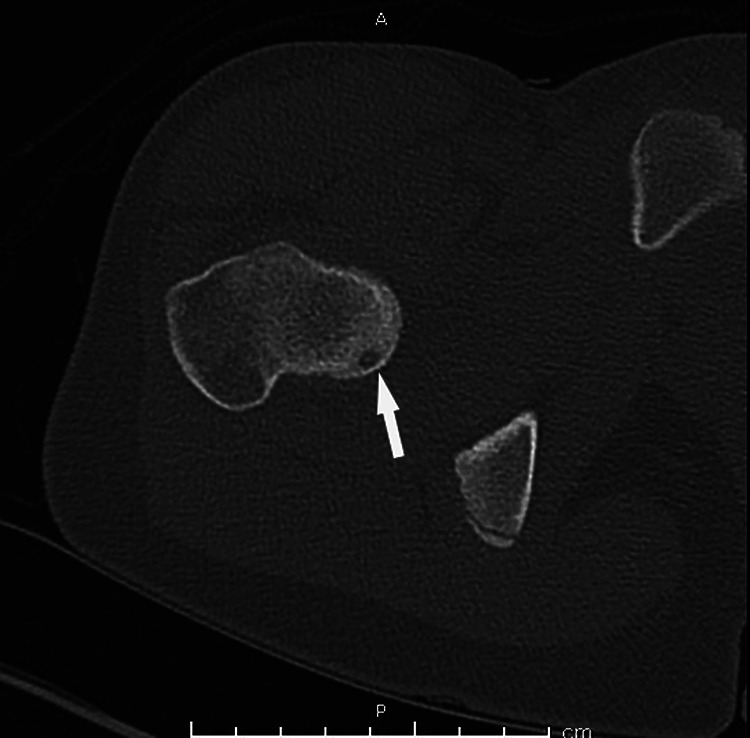
Computed tomography image of the right proximal femur reveals a cortically based lesion with central nidus with ill-defined surrounding sclerosis (arrow). There is no periosteal reaction.
Discuss the Typical Imaging Modalities Used to Diagnose Benign Bone Forming Neoplasms and Fracture
The combination of imaging modalities such as conventional radiography, bone scintigraphy, CT, and sometimes MRI are generally sufficient to diagnose osteoid osteoma and osteoblastoma. 7,14 The imaging modality of choice has been CT for osteoid osteoma and osteoblastoma. 7,14 A conventional radiograph of a cortical osteoid osteoma typically reveals an oval osteolytic nidus less than 2 cm in diameter surrounded by an area of reactive sclerotic bone plus or minus periosteal osteogenesis. 7 In contrast to osteoid osteoma, osteoblastoma radiographically appears wider in diameter (>2 cm) with decreased surrounding sclerosis and enhanced cortical thickening. 14 A study by Park et al found that 28.6% of osteoid osteoma cases showed no abnormal findings on conventional radiographs despite clinical indications. 15 Additionally, intramedullary or paraspinal osteoid osteoma or osteoblastoma may be challenging to diagnose due to the limitations of radiographs requiring other imaging modalities such as bone scintigraphy, CT, or MRI. 7
Nuclear medicine scans or bone scintigraphy is 100% sensitive but has low specificity for osteoid osteoma and has high sensitivity but low specificity for osteoblastoma. 7,14,16 The affinity of technetium Tc-99m-labeled diphosphonates for osteoblastic deposition of bone and remodeling activity favors bone scintigraphy over conventional radiographs for localizing osteoid osteoma and osteoblastoma. Computed tomography scan provides added specificity in osteoid osteoma cases that are anatomically difficult to uncover by conventional radiography, such as intra-articular lesions. 14 A previous study by Hosalkar et al demonstrated that board-certified musculoskeletal attending radiologists with at least 5 years’ experience correctly diagnosed osteoid osteoma by CT in 67% of scans compared to only 3% with MRI scans. 6 The features of osteoblastoma by MRI are equivocal due to the surrounding inflammation and significant marrow edema. 14
The evaluation of long bone diaphysis stress fracture by radiography is also limited. The first sign of stress fracture on conventional radiograph is the “grey cortex” sign due to the presence of a cortical microcrack or lucency in the bone and osteoclastic resorption. 17 The chronicity of a stress fracture is important radiographically as callus formation and periosteal thickening rules in bone tumor or osteomyelitis until proven otherwise. Magnetic resonance is the imaging modality of choice for stress fractures and has almost 100% sensitivity. 17 Additionally, nonossifying fibroma only presents with periosteal reaction when a fracture is present, which is excluded by MRI in this patient. 8
Based on the clinical picture, radiographic appearance of the left tibia shows periosteal thickening of the diaphysis, which is inconclusive, requiring further workup. Computed tomography scan and MRI demonstrate an intracortical oval-shaped osteolytic lesion located in the left anterolateral tibia diaphysis, less than 2 cm in diameter. The surrounding bone is sclerotic with minimal periosteal thickening supporting the diagnosis of osteoid osteoma over osteomyelitis and stress fracture. Further evaluation by histology of the affected area is needed if there is diagnostic doubt of a benign or malignant tumor based on imaging 18 or concern for osteomyelitis.
A cortically based osteoid osteoma stimulates a significant amount of sclerosis, 19 which explains why a nidus may not always be apparent radiographically. Computed tomography imaging for best visualization of the nidus is required. 19 A CT from a patient with an osteoid osteoma of the anterior tibia with a tiny punctate central sclerotic focus representing a nidus is seen in Figure 6.
Figure 6.
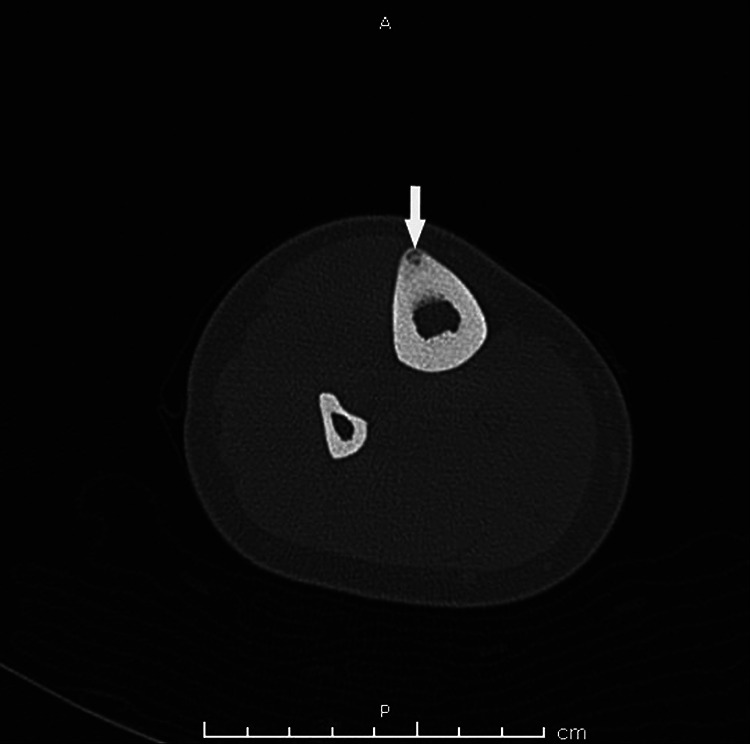
Computed tomography image of the right tibia and fibula. At the anterior tibia, there is a small cortically based lesion with surrounding sclerosis. A tiny punctate central sclerotic focus represents a nidus (arrow). There is no periosteal reaction.
Discuss How Staphylococcus aureus Osteomyelitis Is Differentiated Clinically From Osteoid Osteoma
Within the medullary cavity and proximal to the epiphysis at the extremely vascular metaphysis, congestion of vessels occurs especially when bacteria such as Staphylococcus aureus adhere to the surrounding bone extracellular matrix at these localities. Bacterial adhesins attach to bone extracellular matrix elements such as fibrinogen and fibronectin, which bridges S aureus and osteoblasts. 20,21 Most cases of osteomyelitis occur in the metaphysis within the medullary cavity due to the sinusoidal blood flow architecture at this region of the long bone. 22 The diffusion of S aureus via lamellar Haversian channels to subperiosteal elements permits intramedullary bacterial seeding in the diaphysis or epiphysis. 23 The abscess is lucent on conventional radiograph and is not cortically located. Stress fracture and hematogenous spread of bacteria may cause osteomyelitis within the tibial diaphysis without skin barrier breakage. Imaging, labs, or biopsy are needed to clinically differentiate osteomyelitis from osteoid osteoma.
Radiographic findings and MRI may be misleading in the case of subacute osteomyelitis. Markers of inflammation such as CRP and ESR are the most sensitive markers for osteomyelitis, which are negative in this patient. 22 Surgical biopsy is required when there is any question that the lesion is subacute osteomyelitis (Brodie’s abscess) or bone neoplasm. 16
Diagnostic Findings, Part 2
A bone resection is performed due to the absence of a nidus on imaging in this patient (Figures 7, 8, 9, and 10).
Figure 7.
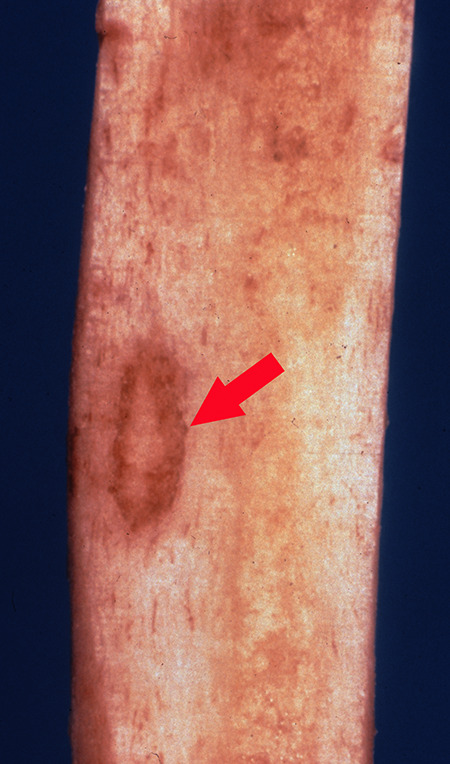
The section of cortical bone shows a nidus represented by a well demarcated red oval lesion surrounded by a hypervascular rim (arrow).
Figure 8.
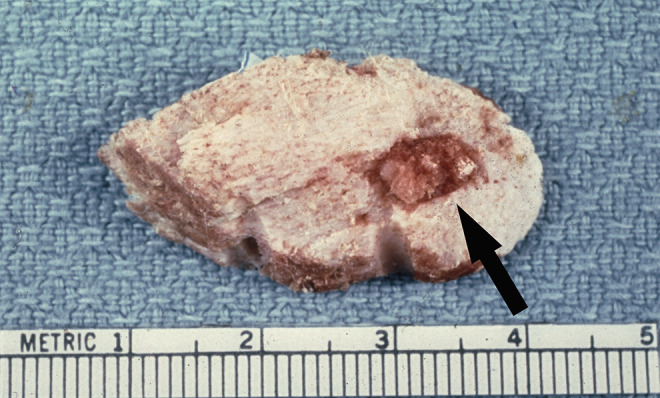
Femur. This femoral specimen shows an approximately one cm diameter ovoid nidus with surrounding white sclerotic bone is easily demarcated from the normal cortical bone (arrow).
Figure 9.
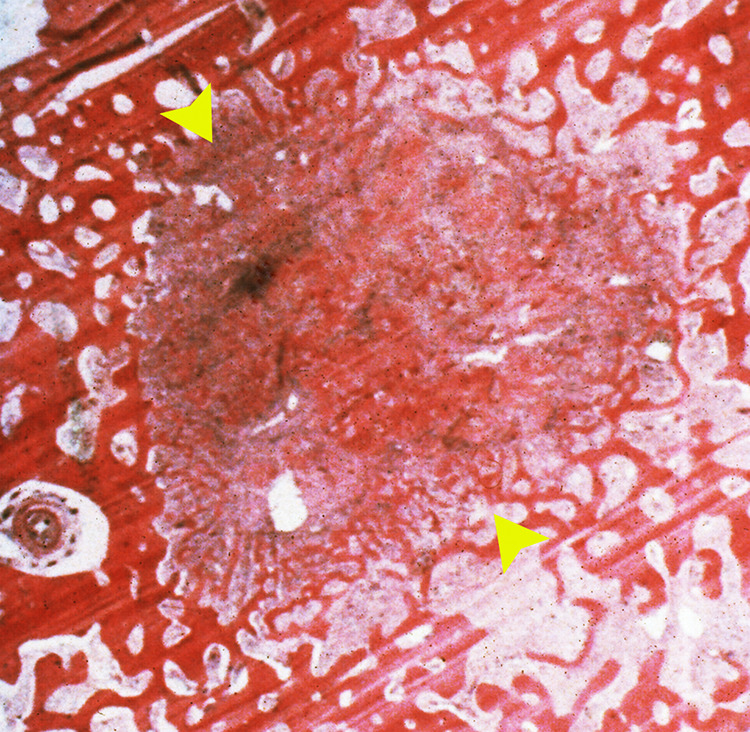
The nidus (arrowheads) is composed of woven bone in a loose fibrovascular stroma surrounded by sclerotic bone (H&E, intermediate magnification).
Figure 10.
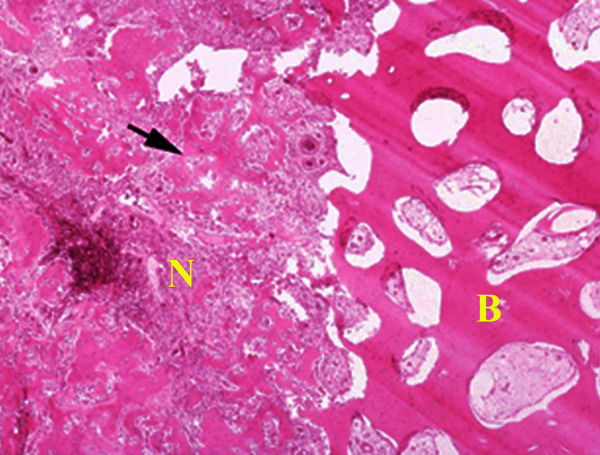
Section of nidus (N) showing central area of irregular woven bony trabeculae (arrow) rimmed with osteoblasts distributed in a loose fibrovascular stroma surrounded by a rim of reactive sclerotic bone (B) (H&E, intermediate magnification).
Questions/Discussion Points, Part 2
Describe the Gross and Histologic Findings From a Patient With Osteoid Osteoma as Depicted in Figures 7, 8, 9, and 10
A bone resection from a patient with osteoid osteoma is shown to demonstrate how an osteoid osteoma appears on gross exam. While en bloc surgical resection of an osteoid osteoma in the long bone was historically performed, a more conservative approach is now utilized by clinicians. En bloc resection is intact removal of the entire lesion, which increases morbidity. However, en bloc resection is a standard of care for an osteoid osteoma in the hand; curettage is another. 24,25
Within the section of cortical bone, there is a well-demarcated round to oval red-brown lesion (arrow) that represents the nidus surrounded by hemorrhage and sclerotic bone (Figures 7 and 8). Figure 9 shows a 1-cm diameter nidus composed of woven bone in a loose fibrovascular stroma surrounded by thickened sclerotic bone on histological examination. Figure 10 demonstrates the nidus consisting of irregular woven bony trabeculae rimmed with osteoblasts distributed in a loose fibrovascular stroma surrounded by a rim of reactive sclerotic bone.
What Is the Diagnosis Based on the Above Findings?
Based on the size of the lesion less than 2 cm and the pathologic features, the diagnosis is osteoid osteoma. Morphologically identical lesions but 2 cm or more in diameter defines an osteoblastoma. 4
Discuss the Gross and Histomorphologic Features of Osteoid Osteoma
The usual gross appearance of osteoid osteoma on biopsy is a round or oval central nidus (the neoplasm) hyperemic with red to pink hue due to the vascular trabeculae. 7,16 The surrounding reactive osteosclerosis is white and well-delineated from the nidus. 7 The neoplasm is softer than the surrounding dense bone. The histological distinction between osteoid osteoma and osteoblastoma may not be possible without imaging and size to confirm the diagnosis. 10,16 Current treatment strategies such as CT-guided radiofrequency ablation for osteoid osteoma rarely leave intact specimens for histopathological evaluation. 7
The characteristic appearance of osteoid osteoma on histology is a well-circumscribed nidus of immature woven bone with variable mineralization and interconnecting trabeculae. 7 The trabeculae may be thin or thick with a bordering singular layer of osteoblasts. 4 Additionally, many osteoclasts are located within the neoplasm. 12 The lack of cellular pleomorphism, small tumor size, and discrete margins guides the diagnosis of osteoid osteoma, ruling out any concern for malignancy. 4 By comparison, osteosarcoma is a malignant tumor of undifferentiated cells producing osteoid matrix. Furthermore, osteosarcoma is generally located in the proximal tibial metaphysis, not diaphysis. The radiographic hallmarks of osteosarcoma are a Codman triangle shadow and “sunburst” appearance due to the destruction of the surrounding periosteum, which is not observed on imaging in this patient.
Discuss the Possible Treatments of Osteoid Osteoma and Associated Outcomes
Benign tumors have a staging system. Stage 1 tumors are latent and commonly asymptomatic. 10 They can progress but generally self-resolve. 10 An osteoid osteoma is typically a stage 1 lesion, requiring only symptomatic relief with Non-Steroidal Anti-Inflammatory Drugs NSAIDs (ibuprofen or naproxen) until the tumor spontaneously regresses, generally within 2 to 3 years. 7,10 Surgical resection of the entire osteoid osteoma has been the only curative treatment for decades and is warranted if the patient fails treatment with NSAIDs. 10 Still tibial diaphysis bone defect status post-surgery requires counseling the patient to limit physical activities and weight-bearing. 7 If the tumor approximates the tibia epiphyseal growth plates, limb-length differences or osteoarthritis are possible without surgical intervention. 7 The bur-down technique better controls excessive removal of sclerotic bone following curettage of the nidus. 10 Osteoblastoma by comparison can be stage 1 or 2. 10 Stage 2 lesions are generally active and do not resolve on their own, commonly requiring surgical intervention. 10 Stage 3 benign bone lesions are destructive and treatment is generally en bloc resection.
Contemporary surgical treatments for osteoid osteoma include percutaneous CT-guided radiofrequency ablation in the outpatient setting. 7,26 These new techniques offer a low recurrence rate of osteoid osteoma and decreased hospital cost and inpatient stay. 26 Computed tomography scan in this patient (Figure 3) shows a superficial bone tumor that is accessible, making surgical resection a good option and radiofrequency ablation a good to excellent option. 16 An osteoid osteoma has a low risk of malignant potential. 4
Identify Why Non-Steroidal Anti-Inflammatory Drug (NSAID) Pharmacotherapy Works for Osteoid Osteoma but Not Osteoblastoma
The mainstay pharmacotherapy for osteoid osteoma are NSAIDs. While a reduction in cyclooxygenase pathway end-products somehow relieves pain in patients with osteoid osteoma, the specific pathogenesis of osteoid osteoma is currently unknown. 7 The increased osteoblastic expression of cyclooxygenase-2 enzyme and elevated levels of prostaglandin E2 and prostacyclin are found within the osteolytic nidus in osteoid osteoma. 7,9 The contributory pain in osteoid osteoma is due to unmyelinated nerve fibers within the neoplastic nidus and peripherally, at the junction between the nidus and sclerotic bone. 9 The increased prostaglandin synthesis leads to vasodilation and compression of proximal nerves fibers via tissue edema, which induces pain, and is reversible after surgical removal of the lesion. 9,27
Whereas pain fiber modulation occurs by prostaglandin E2 induced vasodilation of the surrounding vasculature within the osteoid osteoma nidus, the same has yet to be documented for osteoblastoma. 14 Osteoblastoma has a progressive growth tendency but a decreased bone reaction compared to osteoid osteoma. 12 Thus, local pain in osteoblastoma is thought to be secondary to increased pressure on adjacent structures due to neoplastic expansion. 14 Additionally, posterior vertebral involvement of osteoblastoma and proximity to spinal nerve roots leads to different symptoms than osteoid osteoma such as paresthesia and paraparesis and sometimes dull pain. 14
Teaching Points
Osteoid osteoma is a benign bone-forming tumor that typically affects children in the second decade and more commonly affects boys than girls.
Osteoid osteoma involves the bone cortex and most frequently the metaphysis of the femur, or tibia, but any bone may be affected by the benign lesion.
The most common benign bone tumors are osteoid osteoma, osteochondroma, and nonossifying fibroma, which frequently affect the femur and tibial metaphysis.
The radiographic appearance of osteoid osteoma may be nonspecific, requiring a CT scan to further characterize the lesion and facilitate clinical decision-making.
Magnetic resonance is the imaging modality of choice for osteomyelitis when conventional radiographs are inconclusive, but CT is the gold standard for the diagnosis of osteoid osteoma.
Osteoid osteoma and osteoblastoma histologically are the same, requiring imaging to confirm the diagnosis based on morphometry and respective lesion size of <2 cm and >2 cm.
Single cortical lesions of the tibia are unlikely to be Staphylococcus aureus osteomyelitis based on intramedullary seeding within the metaphysis.
Histopathological examination of osteoid osteoma shows prominent osteoblasts that rim thin or thick trabeculae with variable mineralization of a central nidus surrounded by sclerotic bone.
Osteoid osteoma-induced nocturnal pain typically responds to NSAID therapy due to increased prostaglandin E2, and the tumor can spontaneously heal.
Osteoblastoma does not usually respond to NSAID therapy and is typically located in the axial skeleton: spine or sacrum.
Computed tomography–guided radiofrequency ablation or curettage are treatment options for osteoid osteoma when NSAID therapy fails or when long-term salicylate use is not possible.
Footnotes
Authors’ Note: Figures 7, 8, 9, and 10 were obtained during the scope of US government employment for Dr Conran.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jonathan Light  https://orcid.org/0000-0002-3557-2274
https://orcid.org/0000-0002-3557-2274
Richard M. Conran  https://orcid.org/0000-0002-4053-1784
https://orcid.org/0000-0002-4053-1784
References
- 1. Knollmann-Ritschel BEC, Regula DP, Borowitz MJ, Conran R, Prystowsky MB. Pathology competencies for medical education and educational cases. Acad Pathol. 2017;4. doi:10.1177/2374289517715040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reith J. Bone and joints. In: Goldblum J, Lamps L, McKenney J, Myers J, eds. Rosai and Ackerman’s Surgical Pathology. 11th ed. Elsevier; 2018:1740–1809. [Google Scholar]
- 3. Sanderlin BW, Raspa RF. Common stress fractures. Am Fam Physician. 2003;68:1527–1532. [PubMed] [Google Scholar]
- 4. Horvai A. Bones, joints, and soft tissue tumors. In: Kumar V, Abbas A, Aster JC, Turner JR, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2021:1171–1216. [Google Scholar]
- 5. van der Naald N, Smeeing DPJ, Houwert RM, Hietbrink F, Govaert GAM, van der Velde D. Brodie’s abscess: a systematic review of reported cases. J Bone Jt Infect. 2019;4:33–39. doi:10.7150/jbji.31843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hosalkar HS, Garg S, Moroz L, Pollack A, Dormans JP. The diagnostic accuracy of MRI versus CT imaging for osteoid osteoma in children [published correction appears in Clin Orthop Relat Res. 2005 Jul; 286]. Clin Orthop Relat Res. 2005:171–177. doi:10.1097/01.blo.0000151426.55933.be [DOI] [PubMed] [Google Scholar]
- 7. Noordin S, Allana S, Hilal K, et al. Osteoid osteoma: contemporary management. Orthop Rev (Pavia). 2018;10:7496. doi:10.4081/or.2018.7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Woude HJ, Smithuis R. Differential diagnosis of bone tumors. The radiology assistant. 2010. Accessed March 16, 2021. https://radiologyassistant.nl/musculoskeletal/bone-tumors/differential-diagnosis
- 9. Boscainos PJ, Cousins GR, Kulshreshtha R, Oliver TB, Papagelopoulos PJ. Osteoid osteoma. Orthopedics. 2013;36:792–800. doi:10.3928/01477447-20130920-10 [DOI] [PubMed] [Google Scholar]
- 10. Bernthal N, Burke Z, Blumstein G, Greig D, Shah A. Musculoskeletal oncology. In: McMahon P, Skinner H, eds. Current Diagnosis & Treatment in Orthopedics. 6th ed. McGraw-Hill; 2021. [Google Scholar]
- 11. Tis J. Nonmalignant bone lesions in children and adolescents. In: Phillips W, Torchia M, eds. UpToDate. 2021. Accessed March 13, 2021. https://www.uptodate.com/contents/nonmalignant-bone-lesions-in-children-and-adolescents
- 12. Garcia R, Demicco E, Klein M, Schiller A. Bones, joints and soft tissue. In: Strayer D, Rubin E, Saffitz J, Schiller A, eds. Rubin’s Pathology: Clinical Pathologic Foundations of Medicine. 7th ed. Wolters Kluwer Health; 2015:1305–1380. [Google Scholar]
- 13. Bruder E, Perez-Atayde AR, Jundt G, et al. Vascular lesions of bone in children, adolescents, and young adults. A clinicopathologic reappraisal and application of the ISSVA classification. Virchows Arch. 2009;454:161–179. doi:10.1007/s00428-008-0709-3 [DOI] [PubMed] [Google Scholar]
- 14. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19:678–689. doi:10.5435/00124635-201111000-00004 [DOI] [PubMed] [Google Scholar]
- 15. Park JH, Pahk K, Kim S, Lee SH, Song SH, Choe JG. Radionuclide imaging in the diagnosis of osteoid osteoma. Oncol Lett. 2015;10:1131–1134. doi:10.3892/ol.2015.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlur C, Bachy M, Wajfisz A, Ducou le Pointe H, Josset P, Vialle R. Osteoid osteoma mimicking Brodie’s abscess in a 13-year-old girl. Pediatr Int. 2013;55:e29–e31. doi:10.1111/ped.12056 [DOI] [PubMed] [Google Scholar]
- 17. Marshall RA, Mandell JC, Weaver MJ, Ferrone M, Sodickson A, Khurana B. Imaging features and management of stress, atypical, and pathologic fractures. Radiographics. 2018;38:2173–2192. doi:10.1148/rg.2018180073 [DOI] [PubMed] [Google Scholar]
- 18. Hornicek F, McCarville B, Agaram N. Bone tumors: diagnosis and biopsy techniques. In: DeLaney T, Pappo A, Shah S, eds. UpToDate. 2021. Accessed March 14, 2021. https://www.uptodate.com/contents/bone-tumors-diagnosis-and-biopsy-techniques
- 19. Soheili A, Momeni MG, Tehranzadeh J. Tumors. In: Tehranzadeh J, ed. Basic Musculoskeletal Imaging, Second Edition. McGraw-Hill Education; 2021. http://accessmedicine.mhmedical.com/content.aspx?aid=1181069469 [Google Scholar]
- 20. Crosby HA, Kwiecinski J, Horswill AR. Staphylococcus aureus aggregation and coagulation mechanisms, and their function in host-pathogen interactions. Adv Appl Microbiol. 2016;96:1–41. doi:10.1016/bs.aambs.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. Osteoblast: relationship and consequences in osteomyelitis. Front Cell Infect Microbiol. 2015;5:85. doi:10.3389/fcimb.2015.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dartnell J, Ramachandran M, Katchburian M. Haematogenous acute and subacute paediatric osteomyelitis: a systematic review of the literature. J Bone Joint Surg Br. 2012;94:584–595. doi:10.1302/0301-620X.94B5.28523 [DOI] [PubMed] [Google Scholar]
- 23. McCarville MB, Chen JY, Coleman JL, et al. Distinguishing osteomyelitis from Ewing sarcoma on radiography and MRI. AJR Am J Roentgenol. 2015;205:640–651. doi:10.2214/AJR.15.14341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu EH, Stone N, Alowami SO, Thoma A. Proximal phalanx osteoid osteoma: a case report and literature review. Plast Reconstr Surg Glob Open. 2017;5:e1332. doi:10.1097/GOX.0000000000001332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamdi MF, Tarhouni L, Daghfous M, Bergaoui N, Baccari S. Osteoid osteoma of the phalanx and metacarpal bone: report of 17 cases. Musculoskelet Surg. 2015;99:61–65. doi:10.1007/s12306-014-0337-9 [DOI] [PubMed] [Google Scholar]
- 26. De Filippo M, Russo U, Papapietro VR, et al. Radiofrequency ablation of osteoid osteoma. Acta Biomed. 2018;89:175–185. doi:10.23750/abm.v89i1-S.7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katolik LI. Osteoid osteoma of the scaphoid presenting with radiocarpal arthritis: a case report. Hand (N Y). 2009;4:187–190. doi:10.1007/s11552-008-9159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]