Abstract
In human patients, disseminated candidiasis, a life-threatening disease for immunocompromised patients, is often associated with intestinal lesions. In this study, we demonstrate that immunosuppressed gnotobiotic (IGB) piglets orally inoculated with wild-type Candida albicans developed extensive intestinal lesions and disseminated infection. Severe ulceration of the ileal mucosa was observed overlying regions of colonization and necrosis of the gut-associated lymphoid tissue. Despite the high susceptibility of IGB piglets to many microbial pathogens, an avirulent mutant strain of C. albicans failed to produce intestinal lesions and exhibited poor dissemination, demonstrating that these effects required virulent organisms. It is likely that in IGB piglets, as in human patients, intestinal lesions provide the mechanism for escape of C. albicans from the gastrointestinal tract. Multinucleated giant cells containing fungal organisms were observed within lymph nodes and lymphatic vessels, and as with other pathogens, such cells could provide a mechanism for dissemination of C. albicans.
Candida albicans is an important opportunistic pathogen of immunocompromised individuals causing diseases that range from superficial mucocutaneous infections to life-threatening systemic candidiasis. As a result of modern medical interventions, the incidence of candidiasis has been increasing over the past decade (19). Populations at risk for serious disseminated disease include immunosuppressed patients, such as cancer patients, particularly leukemia patients, and transplant recipients. C. albicans also causes mucocutaneous infections, such as oropharyngeal candidiasis, which are particularly pronounced in AIDS patients and are typically recurrent (22).
In disseminated candidiasis, C. albicans is thought to originate from the gut (6, 28), where the organism is frequently found as a component of normal flora (5). Autopsy studies of cancer patients with fungal infections indicate that 20% of such patients exhibit lesions in the small or large intestine (9). Spontaneous perforation of the ileum is highly associated with systemic candidiasis in very-low-birth-weight infants, another susceptible population (1). Despite the association between intestinal lesions and systemic disease (9), gastrointestinal candidiasis has not been well studied. The interactions of C. albicans with the gastrointestinal mucosa are not well defined, and the mechanisms of invasion and systemic dissemination have not been identified.
In order to study candidiasis arising from the gastrointestinal tract, orally inoculated animals have been used. Oral inoculation of infant mice (10, 30), mice treated with antibiotics or immunosuppressive drugs (7, 8, 13), or immunodeficient mice (4, 20) has resulted in gastrointestinal colonization and some level of dissemination of the organism to internal organs. Invasion of the cardiac-atrial fold of the stomach was seen in infant mice, but intestinal lesions were not observed. Immunosuppressed rabbits were also susceptible to disseminated candidiasis following oral inoculation (38), but pathology of the gastrointestinal (GI) tract was not reported in this study. Thus, the interactions of C. albicans with the intestinal tract have not been studied in these models.
In this study, oral inoculation of a highly susceptible animal was used to develop an experimental model for gastrointestinal and disseminated candidiasis. Gnotobiotic (GB) infant piglets were chosen for this work, because these animals are susceptible to a variety of human enteric pathogens and suffer disease that closely resembles human disease. For example, rotaviruses (33), diarrheogenic Escherichia coli (12, 34, 35, 36), Shigella sp. (S. Tzipori, unpublished observations), Cryptosporidium parvum (37), and, recently, Enterocytozoon bieneusi (23) produce disease in the GB piglet with pathology that closely mimics what is seen in infected humans. In this study, we demonstrated that immunosuppression of newborn piglets orally inoculated with C. albicans led to the development of extensive mucocutaneous candidiasis, intestinal lesions, and disseminated disease. The intestinal lesions likely represent a significant portal of entry for the organism into the bloodstream. In addition, multinucleated giant cells (MNGC) containing C. albicans cells were observed and may contribute to dissemination. Despite the high susceptibility of the GB piglet to infection, development of GI tract lesions and dissemination of C. albicans required virulent organisms, because a mutant strain known to be avirulent following intravenous inoculation of mice (27) failed to produce lesions. Therefore, we conclude that in this animal model, intestinal lesions likely represent an important mechanism for hematogenous dissemination.
MATERIALS AND METHODS
Strains.
The wild-type strain used in this study was the clinical isolate strain SC5314 (11). Strain can36 (cph1/cph1 efg1/efg1 ura3/ura3), derived from SC5314, was the kind gift of G. Fink. This strain lacks two putative transcription factors, Cph1p and Efg1p, and is defective in filamentous growth and avirulent in the intravenously inoculated mouse model (27). Strain can36 was transformed with either pDBI52 (URA3+) or pHLB134 (URA3+ EFG1+) by lithium acetate transformation, generating CKY138 and HLC84, respectively.
Media and growth conditions.
C. albicans strains were inoculated into YPD (1% yeast extract, 2% Bacto Peptone, 2% glucose) and grown overnight to saturation at 37°C. The cultures were centrifuged and washed three times with 1× phosphate-buffered saline (PBS). The concentration of the inoculum was determined by hemocytometer counts and confirmed by dilution plating on YPD.
Experimental animals.
The derivation and maintenance of GB piglets have been described previously (23). Briefly, 20 piglets derived from five litters were divided into three groups, one for each C. albicans strain, with each strain except strain HLC84 tested at least twice in animals from separate litters. Piglets were inoculated 24 h postdelivery, when it was clear that they were healthy and drinking well. The inoculum consisted of 109 cells in a 5-ml total volume delivered orally with a 16-gauge feeding needle (Popper & Sons, New Hyde Park, N.Y.). Piglets were housed individually and observed regularly for clinical signs of candidiasis (i.e., thrush and ocular lesions), diarrhea, and morbidity. When severe illness developed, or at approximately 3 weeks postinoculation, piglets were euthanized by intravenous injection of euthanasia solution (Beuthanasia-D Special; Schering-Plough Animal Health Corp., Kenilworth, N.J.).
Immunosuppression.
Eighteen of the 20 newborn GB piglets were immunosuppressed, beginning when they were 1 day old and continuing for the duration of the experiment as described previously (23). Briefly, once a day, piglets received 25 mg of intramuscular methylprednisolone sodium succinate per kg of body weight (Solu-Medrol; Pharmacia & Upjohn, Kalamazoo, Mich.) and 15 mg of oral cyclosporine per kg of body weight (Sandimmune Oral Solution; Sandoz Pharmaceuticals Corp., East Hanover, N.J.) for the duration of the study.
Necropsy procedure.
The piglets were examined for gross pathological changes, and samples were taken from the oral and ocular regions, including eyelids and cornea. Animals were dissected aseptically. The GI tract was removed and further dissected into the esophagus, stomach, duodenum, jejunum, ileum, cecum, spiral colon, and distal colon. The following internal organs were also removed: kidney, liver, spleen, heart, lungs, and mesenteric lymph nodes. After fixation in 10% neutral buffered formalin, tissues were sectioned at 5 μm and stained with hematoxylin and eosin for histopathological examination by light microscopy. Tissues were also sectioned at 5 μm for immunohistochemistry (IHC) by using the polyclonal antibody described below and a standard immunoperoxidase technique (18). Microscopic photography was performed with an Olympus BX40 microscope with an Olympus PM20 automatic photographic system (Olympus America, Melville, N.Y.).
Tissue samples were also taken for quantitation of C. albicans. Organ pieces were minced with a scalpel, diluted 10-fold (wt/wt) in 1× PBS, and homogenized with a handheld tissue tearer (BioSpec Products, Bartlesville, Okla.). Dilutions of organ homogenates were plated on YPD agar plus streptomycin and ampicillin (each at 100 μg/ml), and the numbers of CFU per gram (wet weight) were determined.
Generation of polyclonal antiserum against C. albicans.
Rabbit antiserum was produced to perform IHC on paraffin sections. C. albicans strain SC5314 cells grown at 37°C in YPD were washed three times with 1× PBS, resuspended in 2% neutral formalin (vol/vol), and incubated overnight at room temperature. The cells were then washed extensively in 1× PBS and resuspended at a concentration of 109/ml. Two young adult female New Zealand White rabbits (Millbrook Farms, Amherst, Mass.) were used for polyclonal antibody production as previously described (15). Briefly, rabbits were inoculated four or five times at 4-week intervals with 109 CFU of killed C. albicans mixed with incomplete Freund's adjuvant. Blood was obtained at euthanasia, and serum was prepared by standard methods (15).
RESULTS
Intestinal candidiasis in immunosuppressed GB infant piglets.
Oral inoculation of two GB piglets with 109 CFU of a wild-type C. albicans strain, SC5314 (11), led to C. albicans colonization throughout the GI tract, as shown by quantitative culture of organ homogenates (data not shown). In addition, both piglets developed mild oral thrush by day 10 after inoculation. However, histologic examination by IHC of the intestinal tract and internal organs failed to detect lesions or organisms in tissue. Except for a few organisms detected in kidney homogenates, none were recovered from homogenates of other internal organs. Therefore, although C. albicans colonized the GI tract of GB piglets, only a few organisms disseminated to the kidney, with no apparent lesions.
Since immunosuppression is a well known predisposing factor for candidiasis, orally inoculated piglets were administered immunosuppressive agents as described in Materials and Methods. Piglets inoculated with wild-type strain SC5314 developed clinical signs of thrush and conjunctivitis beginning at 4 days postinoculation. The dorsal surface of the tongue showed small, white foci that progressed to larger white or yellow coalescing foci to form large plaques on the dorsal and ventral surfaces of the tongue and oropharyngeal mucosa (Fig. 1A). Most of these piglets also developed bilateral mild-to-moderate conjunctivitis and had corneal involvement initially consisting of focal opacity followed by ulceration of the corneal epithelium and keratitis.
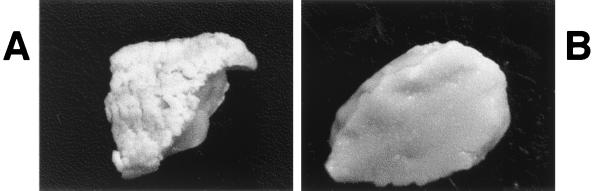
FIG. 1.
Gross lesions of mucocutaneous candidiasis. IGB piglets were orally inoculated with C. albicans and euthanized after morbidity was observed. Tongue tissue was dissected and photographed. (A) Inoculation with SC5314. Severe thrush with complete coverage by fungal plaques is shown (magnification, ×1). (B) Inoculation with CKY138. A normal surface with no evidence of thrush is shown (magnification, ×1).
Upon sacrifice, quantitative culture of organ homogenates showed that the GI tract was extensively colonized by C. albicans (Table 1). The tongue, esophagus, stomach, and colon exhibited the highest colonization levels. Thus, immunosuppressed GB (IGB) piglets were readily colonized by C. albicans in the GI tract and were susceptible to mucocutaneous candidiasis.
TABLE 1.
Quantitative culture of tissue from IGB piglets orally inoculated with C. albicans strains
| Tissue | No. of piglets positive/total (log mean CFU/g of tissue)
|
||
|---|---|---|---|
| SC5314a | CKY138 | HLC84 | |
| Tongue | 6/6 (7.0) | 6/6 (5.2) | 2/2 (6.8) |
| Esophagus | 6/6 (6.9) | 7/7 (6.0) | 2/2 (6.9) |
| Stomach | 5/5 (6.9) | 7/7 (5.0) | 2/2 (7.0) |
| Ileum | 3/3 (6.1) | 6/6 (6.0) | 2/2 (6.5) |
| Cecum | 3/3 (6.5) | 6/6 (6.7) | 2/2 (6.8) |
| Colon | 6/6 (7.0) | 7/7 (6.7) | 2/2 (6.9) |
| Kidney | 4/5 (4.4) | 4/7 (3.8) | 2/2 (4.3) |
| Liver | 4/5 (3.2) | 0/7 (NAb) | 1/2 (3.3) |
| Spleen | 4/5 (3.2) | 1/7 (2.0) | 1/2 (2.9) |
| Heart | 5/5 (3.0) | 2/7 (2.0) | 2/2 (2.8) |
| Lung | 5/5 (3.5) | 1/7 (2.0) | 2/2 (2.5) |
C. albicans strain.
NA, not applicable.
Histological and IHC analysis of tissues from IGB piglets orally inoculated with the wild-type strain demonstrated the presence of ileal lesions, which ranged from moderate submucosal mixed inflammation to segmental ulceration or complete obliteration of the mucosa overlying the gut-associated lymphoid tissue (GALT) (Fig. 2A). Lesions consisted of extensive necrosis and suppurative inflammation of the GALT and contained yeast cells and filamentous forms of C. albicans detected by IHC. Similar lesions were found in the mesenteric lymph nodes associated with the ileum. Foci of similar but less extensive invasive lesions were also seen in the cecum, spiral colon, and distal colon, with numerous organisms and cellular debris in the lumen (Fig. 2B). In contrast, the glandular and pyloric regions of the stomach, duodenum, and jujunum usually exhibited only casual association of organisms with surface enterocytes, with no evidence of invasion, ulceration, or inflammation. These results demonstrated the presence of extensive intestinal lesions in the orally inoculated IGB piglet.
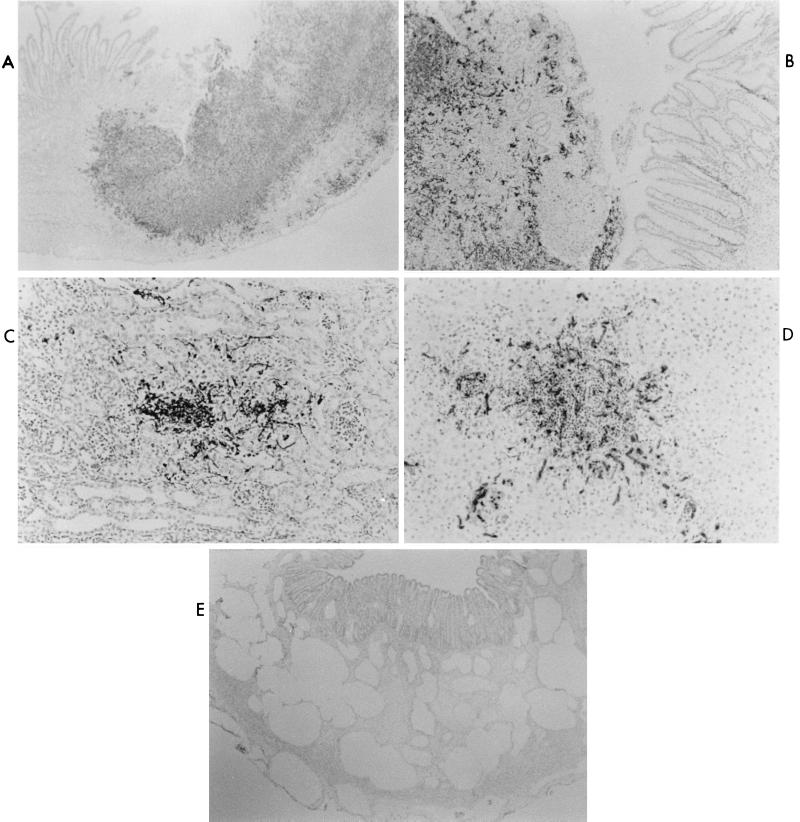
FIG. 2.
Microscopic lesions in IGB piglets orally inoculated with C. albicans. IGB piglets were orally inoculated with C. albicans and euthanized after morbidity was observed. IHC analysis of fixed tissue with antiserum directed against surface antigens of C. albicans was performed as described in Materials and Methods. (A) Ileum of IGB piglet inoculated with SC5314. Segmental colonization of the ileum with mucosal ulceration and transmural necrosis and inflammation is shown (magnification, ×40). (B) Colon of IGB piglet inoculated with SC5314. Segmental colonization of the colon with ulceration, necrosis, and inflammation is shown (×40). (C) Kidney of IGB piglet inoculated with SC5314. Focus of colonization with mild inflammation is shown (×200). (D) Liver of IGB piglet inoculated with SC5314. Focus of colonization with marked inflammation is shown (×200). (E) Large intestine of IGB piglet inoculated with CKY138. Pneumatosis intestinalis of the colon with large cysts in the gut wall is shown (×40).
In addition to GI tract lesions, multifocal lesions containing organisms were observed in the kidney, lung, liver, spleen, and heart (Fig. 2C and D), demonstrating the presence of disseminated disease. Organisms were also cultured from homogenates of these organs (Table 1). Thus, in this animal model, as in many human patients, disseminated candidiasis accompanied the extensive lesions in the intestinal tract.
In tissues from orally inoculated IGB piglets, a few MNGC containing fungal organisms were seen in the parenchyma and in the lymphatic vessels of the mesenteric lymphoid tissue (Fig. 3). MNGC are a common feature of granulomas that develop in response to certain inflammatory reactions. The presence of MNGC containing C. albicans cells in the orally inoculated IGB piglet suggests that MNGC may provide a vehicle for dissemination of C. albicans into the bloodstream and localization in internal organs.
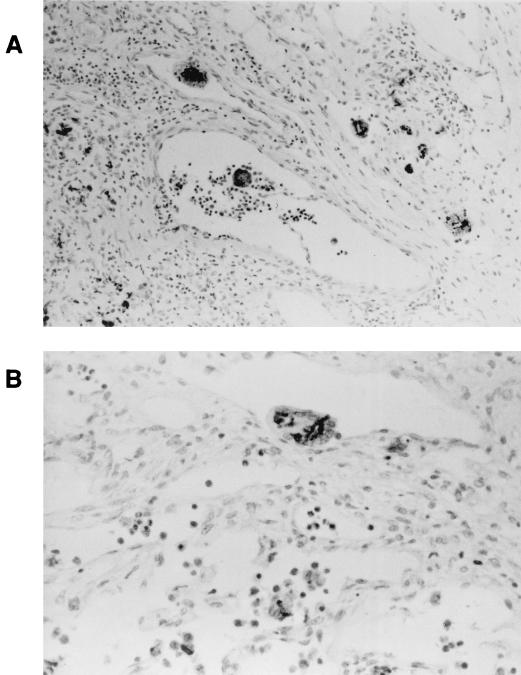
FIG. 3.
MNGC containing C. albicans. IGB piglets were inoculated orally with HLC84 [cph1/cph1 efg1/efg1 (EFG1+)], a strain that exhibits a wild-type phenotype. Tissue was processed as described in the legend to Fig. 2. (A) Mesenteric lymph node. Several MNGC containing organisms are present in lymphatic vessels (magnification, ×200). (B) Mesenteric lymph node. An MNGC containing several organisms in a lymphatic vessel is shown (×400).
A mutant strain of C. albicans defective in regulation of filamentous growth fails to produce GI tract lesions and exhibits poor dissemination.
Because IGB piglets are highly susceptible to infection by a variety of pathogens, we sought to demonstrate that the lesions and dissemination observed above required virulent C. albicans. For this purpose, a mutant strain that had been previously shown to be avirulent following intravenous inoculation of mice (27) was used. This strain, CKY138 (cph1/cph1 efg1/efg1), lacks the putative transcription factors Efg1p and Cph1p and is extremely defective in filamentous growth under standard laboratory conditions (27).
In IGB piglets, mutant strain CKY138 (cph1/cph1 efg1/efg1) was observed to colonize the GI tract (Table 1) and cause mild mucosal disease. Five of eight piglets developed grossly visible thrush, although the extent and distribution of lesions were reduced (Fig. 1B). The development of clinical signs of thrush was also delayed compared to that in the wild-type strain (9 days versus 4 days). Upon sacrifice, quantitative culture of organ homogenates demonstrated that colonization levels for the esophagus and GI tract ranged from 105 to 106 CFU/g (Table 1), very similar to the levels observed with wild-type C. albicans. However, in most of the piglets, no organisms were detected in the liver, spleen, heart, or lungs (Table 1). Approximately half of the piglets were colonized in the kidney, but the level of colonization was usually low. Thus, despite the fact that CKY138 colonized the lower GI tract at close to wild-type levels, dissemination was significantly attenuated.
In IGB piglets orally inoculated with CKY138 (cph1/cph1 efg1/efg1), infection of the GI tract was limited to superficial colonization of the mucosa, except for two piglets in which the ileal GALT was involved. In these piglets, the GALT lesions consisted of limited necrosis and mixed inflammation with predominantly yeast-form organisms. These lesions were much less severe than in infection with the wild-type strain (data not shown). Organisms were also present in the mesenteric lymph nodes of all piglets infected with CKY138. These results demonstrate that a mutant strain with low virulence in the intravenous mouse model similarly exhibited reduced virulence in the IGB piglet model. Neither the extensive lesions in the intestinal tract nor extensive systemic disease was observed with this mutant strain.
Histological analysis of mesenteric lymphoid tissue demonstrated that MNGC containing organisms were commonly seen within the parenchyma and in surrounding lymphatics. These results suggest that MNGC may commonly form during infection with C. albicans.
Finally, three of the eight IGB piglets orally inoculated with CKY138 developed pneumatosis intestinalis with severe mesenteric edema and gas-filled bullae surrounding the cecum and spiral colon. No organisms were found associated with these lesions (Fig. 2E). Although similar lesions were not observed histologically in piglets inoculated with wild-type organisms, gas in the bowel wall was observed in one such piglet, whose tissues were not examined due to death of the animal prior to euthanasia.
To demonstrate that the reduced virulence observed with CKY138 (cph1/cph1 efg1/efg1) reflected the absence of Efg1p and Cph1p, studies were conducted with strain HLC84 [cph1/cph1 efg1/efg1 (EFG1+)], which carries a wild-type copy of the EFG1 gene. Introduction of wild-type EFG1 restores the ability of the double-null mutant to form hyphae at 37°C in serum (27) and restores the virulence of the double-null mutant in mice (P. J. Riggle and C. A. Kumamoto, unpublished observations). Only two piglets were inoculated with HLC84, but both developed mucocutaneous and disseminated infections. Both piglets developed significant thrush lesions by 4 days postinoculation, and one piglet had severe corneal involvement. Histologic examination also showed invasion of the ileal GALT and mesenteric lymph nodes and dissemination to internal organs (data not shown). Colonization levels for the tongue and esophagus approached 107 CFU/g, while those in the GI tract ranged from 106 to 107 CFU/g and those in the internal organs ranged from 102 to 104 CFU/g (Table 1). Again, the kidney yielded the highest numbers of colonies. These levels of colonization were similar to those of the wild-type strain and were 10- to 100-fold greater than those of strain CKY138 (cph1/cph1 efg1/efg1). Thus, this strain produced clinical signs and pathologic lesions similar in time course and severity to those of the wild-type strain of C. albicans, demonstrating that the reduced virulence of CKY138 was due to the absence of Efg1p.
DISCUSSION
In this study, we demonstrated extensive intestinal lesions associated with systemic candidiasis in a highly susceptible animal model. In the GI tract, mucosal invasion by C. albicans and proliferation within the GALT were extensive in segments of the ileum and occasionally in the large intestine. The mucosal surfaces overlying these areas of necrosis and inflammation in the GALT were either severely ulcerated or completely absent and were often replaced by a pseudomembrane of cellular debris and fungal organisms.
Although intestinal candidiasis is not commonly diagnosed, human cases of infection have been reported and studied (9, 14, 21, 24, 31). In addition, an association between invasive enteritis and candidemia has been noted (14). In leukemia patients, the lungs and bowel were the organs most commonly involved in patients suffering from severe candidiasis (3). Autopsy studies of infants and children infected with Candida demonstrate that the small intestine is one of the most common sites of infection (24), and spontaneous perforation of the intestine in very-low-birth-weight infants is associated with systemic candidiasis (1). Thus, the events observed in IGB piglets resemble events that occur in human patients.
In human patients, systemic dissemination of C. albicans is believed to occur by dissemination from the GI tract (6, 28), and gastrointestinal disease frequently precedes systemic disease (9). In IGB piglets too, invasion of the gut mucosa occurs long before dissemination is apparent (data not shown). However, the mechanism of translocation from the GI tract is unclear. The results obtained in IGB piglets suggest that organisms invading the gut and proliferating in the GALT could spread to adjacent mesenteric lymph nodes, where they would continue to multiply and then spread through the lymphatics into the bloodstream and internal organs. In IGB piglets, C. albicans organisms were observed in large numbers in the adjacent mesenteric lymph nodes.
Studies with dogs have demonstrated that a major mechanism for clearance of C. albicans from the portal vein is phagocytosis by Kupffer cells in the liver (32). This mechanism may clear low levels of organisms that cross the GI tract by persorption. Persorption of C. albicans in a healthy human has been demonstrated (25). In this study, the avirulent strain CKY138 did not produce lesions in the GI tract. In the absence of lesions, the mutant organisms may utilize only low-efficiency mechanisms such as persorption to cross the GI tract and hence may be readily eliminated by phagocytic cells. The mutant organisms may also be more effectively killed by phagocytic cells (27). These effects may account for the poor dissemination observed with the mutant strain. The results support the hypothesis that virulent organisms create lesions and significantly damage the GI tract, allowing them to escape the GI tract, overwhelm the host defense mechanisms, and become hematogenously disseminated.
In infected lymph nodes, MNGC containing C. albicans were seen scattered throughout the parenchyma and in the surrounding lymphatic vessels. Interestingly, we found that MNGC were more common in IGB piglets infected with the mutant strain CKY138 than in those infected with the wild-type strain. This observation is consistent with the finding that the cph1/cph1 efg1/efg1 double mutant organisms were defective in escaping from macrophage-like cells in tissue culture (27).
MNGC originate from fusion of monocytes or macrophages, but the exact mechanism of their formation is unclear. They are a characteristic feature of several infectious diseases such as tuberculosis, Crohn's disease, some viral infections, and some fungal infections, including candidiasis in humans (29). MNGC have also been observed in hyperplastic GALT of a patient with AIDS (26). MNGC containing C. albicans may contribute to dissemination by delivering organisms directly into the bloodstream. The organisms within these giant cells appeared to be morphologically intact and presumably were viable.
Pneumatosis intestinalis was observed in the ileum and colon of several piglets. Despite the presence of gas in the gut wall, C. albicans antigen was not detected in association with the lesions. Similar conditions have been observed in human patients. For example, pneumatosis intestinalis was observed in association with necrotizing enterocolitis attributed to candidiasis in a patient with AIDS (2). Gas in the bowel wall was detected in a patient suffering from unusually severe infection with Candida glabrata (16). Emphysematous pyelonephritis and cystitis, diseases which may have a similar mechanism, have also been reported in humans (17).
The newborn piglet is able to mount an immune response, but the level of the response is suboptimal and increases gradually over the first 4 weeks of life. Therefore, it was not surprising that immunocompetent GB piglets developed only transient mild signs of superficial candidiasis with limited dissemination. The combination of daily oral cyclosporine and parenteral methylprednisolone induces a state of moderate immunosuppression in newborn piglets, as determined by marked reduction in the proliferative response of mesenteric lymph node and peripheral blood lymphocytes to stimulation with concanavalin A and lipopolysaccharide mitogens (23). This immunosuppressive regime led to the establishment of severe candidiasis in IGB piglets inoculated with wild-type strain SC5314. The immunosuppressive regimen appeared to mimic T-cell immunodeficiency and, as in AIDS patients, predisposed the piglets to extensive mucocutaneous disease.
In summary, the IGB piglet exhibited severe forms of mucocutaneous invasion, intestinal candidiasis, and systemic dissemination. The locally invasive form of candidiasis that most commonly afflicts patients with immunodeficiencies includes ulcerations of the respiratory, intestinal, and genitourinary tracts. The most serious form, disseminated candidiasis, includes intraparenchymal lesions in all major internal organs. The IGB piglet model exhibited many of the features of candidiasis seen in humans. Features such as MNGC formation in mesenteric lymph nodes, corneal lesions, and pneumatosis intestinalis were also observed in this model. We believe the IGB piglet model will facilitate future studies of the expression and role of virulence factors in vivo and facilitate preclinical evaluation of therapeutic agents against C. albicans infection.
ACKNOWLEDGMENTS
We thank Honorine Ward, Andrew Camilli, and Matt Waldor for helpful discussion and Ralph Isberg for discussion and comments on the manuscript. We are grateful to Gerry Fink for strains and plasmids. We also thank Jessica Brisben, Melissa Paris, and Susan Chapman for assistance with animal care and Barry Stein and Cheryl Sibley for assistance with histology and immunohistochemistry. The use of resources and facilities of the Division of Infectious Disease at Tufts University School of Veterinary Medicine is greatly appreciated.
This work was supported in part by National Institute of Allergy and Infectious Diseases grants K08 AI01407 (to K.A.A.) and R01 AI38591 (to C.A.K.).
REFERENCES
- 1.Adderson E E, Pappin A, Pavia A T. Spontaneous intestinal perforation in premature infants: a distinct clinical entity associated with systemic candidiasis. J Pediatr Surg. 1998;33:1463–1467. doi: 10.1016/s0022-3468(98)90475-4. [DOI] [PubMed] [Google Scholar]
- 2.Balthazar E J, Stern J. Necrotizing Candida enterocolitis in AIDS: CT features. J Comput Assisted Tomogr. 1994;18:298–300. doi: 10.1097/00004728-199403000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Bodey G P. Fungal infections complicating acute leukemia. J Chronic Dis. 1966;19:667–687. doi: 10.1016/0021-9681(66)90066-x. [DOI] [PubMed] [Google Scholar]
- 4.Cantorna M T, Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990;58:1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen R, Roth J F, Delgado E, Ahearn D G, Kalser M H. Fungal flora of the normal human small and large intestine. N Engl J Med. 1968;279:340–344. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- 6.Cole G T, Halawa A A, Anaissie E J. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis. 1996;22(Suppl. 2):S73–S88. doi: 10.1093/clinids/22.supplement_2.s73. [DOI] [PubMed] [Google Scholar]
- 7.Cole G T, Lynn K T, Seshan K R, Pope L M. Gastrointestinal and systemic candidosis in immunocompromised mice. J Med Vet Mycol. 1989;27:363–380. doi: 10.1080/02681218980000491. [DOI] [PubMed] [Google Scholar]
- 8.Ekenna O, Sherertz R J. Factors affecting colonization and dissemination of Candida albicans from the gastrointestinal tract of mice. Infect Immun. 1987;55:1558–1563. doi: 10.1128/iai.55.7.1558-1563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eras P, Goldstein M J, Sherlock P. Candida infection of the gastrointestinal tract. Medicine. 1972;51:367–379. doi: 10.1097/00005792-197209000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Field L H, Pope L M, Cole G T, Guentzel M N, Berry L J. Persistence and spread of Candida albicans after intragastric inoculation of infant mice. Infect Immun. 1981;31:783–791. doi: 10.1128/iai.31.2.783-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis D H, Collins J E, Duimstra J R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guentzel M N, Herrera C. Effects of compromising agents on candidosis in mice with persistent infections initiated in infancy. Infect Immun. 1982;35:222–228. doi: 10.1128/iai.35.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta T P, Ehrinpreis M N. Candida-associated diarrhea in hospitalized patients. Gastroenterology. 1990;98:780–785. doi: 10.1016/0016-5085(90)90303-i. [DOI] [PubMed] [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 16.Hickey W F, Sommerville L H, Schoen F J. Disseminated Candida glabrata: report of a uniquely severe infection and a literature review. Am J Clin Pathol. 1983;80:724–727. doi: 10.1093/ajcp/80.5.724. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand T S, Nibble L, Frie U, Schindler R. Bilateral emphysematous pyelonephritis caused by Candida infection. Am J Kidney Dis. 1999;33:E10. doi: 10.1016/s0272-6386(99)70331-8. [DOI] [PubMed] [Google Scholar]
- 18.Hrapchak R. Theory and practice of histotechnology. Columbus, Ohio: Battelle Press; 1980. [Google Scholar]
- 19.Jarvis W R. Epidemiology of nosocomial fungal infections with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 20.Jensen J, Vazquez-Torres A, Balish E. Poly(I · C)-induced interferons enhance susceptibility of SCID mice to systemic candidiasis. Infect Immun. 1992;60:4549–4557. doi: 10.1128/iai.60.11.4549-4557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi S N, Garvin P J, Sunwoo Y C. Candidiasis of the duodenum and jejunum. Gastroenterology. 1981;80:829–833. [PubMed] [Google Scholar]
- 22.Klein R S, Harris C A, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 23.Kondova I, Mansfield K, Buckholt M A, Stein B, Widmer G, Carville A, Lackner A, Tzipori S. Transmission and serial propagation of Enterocytozoon bieneusi from humans and rhesus macaques in gnotobiotic piglets. Infect Immun. 1998;66:5515–5519. doi: 10.1128/iai.66.11.5515-5519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozinn P J, Taschdjian C L. Enteric candidiasis: diagnosis and clinical considerations. Pediatrics. 1962;30:71–85. [PubMed] [Google Scholar]
- 25.Krause W, Matheis H, Wulf K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969;i:598–599. doi: 10.1016/s0140-6736(69)91534-7. [DOI] [PubMed] [Google Scholar]
- 26.Lewin-Smith M, Wahl S M, Orenstein J M. Human immunodeficiency virus-rich multinucleated giant cells in the colon: a case report with transmission electron microscopy, immunohistochemistry and in situ hybridization. Mod Pathol. 1999;12:75–81. [PubMed] [Google Scholar]
- 27.Lo H-J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 28.Louria D B, Stiff D P, Bennett B. Disseminated moniliasis in the adult. Medicine. 1962;41:307–337. [Google Scholar]
- 29.Luna M. Pathology of infectious diseases. Stamford, Conn: Appleton & Lange; 1997. pp. 953–963. [Google Scholar]
- 30.Pope L M, Cole G T, Guentzel M N, Berry L J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979;25:702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott R J, Harris M, Banerjee S S. Fungal infections of the small and large intestine. J Clin Pathol. 1992;45:806–811. doi: 10.1136/jcp.45.9.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone H H, Kolb L D, Currie C A, Geheber C E, Cuzzell J Z. Candida sepsis: pathogenesis and principles of treatment. Ann Surg. 1974;179:697–711. doi: 10.1097/00000658-197405000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theil K W, Saif L J, Moorhead P D, Whitmoyer R E. Porcine rotavirus-like virus (group B rotavirus): characterization and pathogenicity for gnotobiotic pigs. J Clin Microbiol. 1985;21:340–345. doi: 10.1128/jcm.21.3.340-345.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzipori S, Gibson R, Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic (EPEC) and enterohemorrhagic (EHEC) Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989;57:1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzipori S, Robins-Browne R, Gonis G, Hayes J, Withers M, McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985;26:570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzipori S, Wachsmuth I, Chapman C, Birden R, Brittingham J, Jackson C, Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic pigs. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 37.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diag Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh T J, Pizzo P A. Experimental gastrointestinal and disseminated candidiasis in immunocompromised animals. Eur J Epidemiol. 1992;8:477–483. doi: 10.1007/BF00158585. [DOI] [PubMed] [Google Scholar]