Abstract
Background:
Low levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the low physiologic range, surrogate markers for reduced liver metabolic function, are associated with cerebral hypometabolism, impairment in neurotransmitter production and synaptic maintenance, and a higher prevalence of dementia. It is unknown whether a prospective association exists between low liver enzyme levels and incident dementia.
Objective:
To determine whether low levels of ALT and AST are associated with higher risk of incident dementia.
Methods:
Plasma ALT and AST were measured on 10,100 study participants (mean age 63.2 years, 55% female, 22% black) in 1996–1998. Dementia was ascertained from comprehensive neuropsychological assessments, annual contact, and medical record surveillance. Cox proportional hazards regression was used to estimate the association.
Results:
During a median follow-up of 18.3 years (maximum 21.9 years), 1,857 individuals developed dementia. Adjusted for demographic factors, incidence rates of dementia were higher at the lower levels of ALT and AST. Compared to the second quintile, ALT values <10th percentile were associated with a higher risk of dementia (hazard ratio [HR] 1.34, 95% CI 1.08–1.65). The corresponding HR was 1.22 (0.99–1.51) for AST.
Conclusion:
Plasma aminotransferases <10th percentile of the physiologic range at mid-life, particularly ALT, were associated with greater long-term risk of dementia, advocating for attention to the putative role of hepatic function in the pathogenesis of dementia.
Keywords: ALT, AST, Liver hypometabolism, Dementia, Alzheimer’s disease
Introduction
Dementia affects 5.8 million Americans aged 65 and older and 50 million people worldwide [1]. The global prevalence of dementia is projected to triple by 2050 due to population aging [2]. The lack of effective treatment for dementia highlights the critical importance of identifying markers that may have predictive value years before dementia onset, can track disease progression, and unveil underlying pathophysiological processes involved in the development of dementia. Emerging clinical and preclinical evidence suggests that early metabolic signaling alterations may contribute to risk of dementia yet these associations are not well characterized in populations of generally healthy individuals [3, 4]. Thus, investigation into liver functions that regulate systemic metabolism may offer insights to address this gap in knowledge.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are widely used in clinical practice to detect or monitor liver damage [5]. Depending on reference populations and laboratories, ALT values below 19–33 U/L and AST values below 20–40 U/L are usually defined as a normal physiologic range [5], without specific signs or symptoms, characteristic liver pathology or laboratory findings indicative of ‘low-normal’ levels of ALT and AST [6, 7]. Nevertheless, ‘low-normal’ levels of ALT or AST among older adults are reported to be associated with frailty, sarcopenia and the risk of mortality [8–14]. Moreover, a recent report documented lower levels of ALT and AST in patients with a dementia diagnosis compared to the levels in cognitively intact individuals, as well as reduced brain glucose metabolism and increased amyloid-β deposition among the former [15]. The authors proposed that liver hypometabolism, indexed by low levels of ALT and AST, plays a role in the pathogenesis of dementia [15]. Because the analyses were cross-sectional reverse causation cannot be ruled out, however. In this context, the aim of our study was to evaluate whether low levels of ALT and AST, even those considered to be in the physiologic range are associated with higher risk of dementia over the course of 20 years of follow-up in the population-based Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study population
The ARIC study enrolled 15,792 participants aged 45–64 years from four communities in the US: Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland [16]. Visit 1 was conducted in 1987–1989, with subsequent follow-up examinations conducted in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), 2011–2013 (visit 5 ARIC Neurocognitive Study [ARIC-NCS]), 2016–2017 (visit 6), and 2018–2019 (visit 7). We used the visit 4 examination as the baseline due to the availability of liver enzyme measures. Of the 11,656 cohort members who participated in visit 4 we excluded those who self-reported as non-white or non-black (n=31), black participants from Minneapolis and Washington County due to the small numbers (n=38), those with missing information on liver enzyme measures (n=169), and those with missing values for other covariates included into the basic model specified in Statistical Evaluation section (n=559). Participants with a diagnosis of dementia before visit 4 (n=8) were also excluded from the analytic set, as well as those with prevalent chronic liver disease or cirrhosis (n=37) identified by hospital discharge diagnoses (International Classification of Diseases, Tenth Revision Clinical Modification [ICD-10-CM] codes K70-K77). In order to minimize confounding by alcoholic fatty liver disease and undiagnosed advanced liver disease, we excluded from the analyses individuals with baseline AST:ALT ratio ≥2 (n=783). The final analytic sample included 10,100 participants. The Institutional Review Boards of all ARIC study sites approved the study protocol, and all cohort participants have given a written informed consent at each examination.
Liver Enzyme Measurements
Plasma ALT and AST were measured at the Baylor College of Medicine in 2010 with an Olympus AU400e automated chemistry analyzer (Center Valley, PA) in plasma specimens collected from exam visit 4 and stored at −70°C [17]. Following examination of the distributional properties of the liver enzyme measures we winsorized values at the top 99.5th percentile.
Characterization of Dementia
The ARIC study administered 3 cognitive tests at visits 2 and 4, and a comprehensive neuropsychological battery was implemented at ARIC-NCS visits 5 and 6. Three dementia variables have been defined. Level 1 dementia was defined for cohort participants who completed in-person neuropsychological assessments at ARIC-NCS visits 5 or 6 and had 1) a low Mini-Mental State Examination (MMSE) score (<21 for whites or <19 for blacks) or 2) Functional Activities Questionnaire (FAQ) >5 or Clinical Dementia Rating (CDR) sum of boxes >3, at least two cognitive domain Z score ≤−1.5, and a decline rate in general cognitive performance score >0.055/year. Inconsistent diagnosis or probable cases were adjudicated by an expert panel [18, 19]. Among participants who did not complete an in-person visit examination, level 2 dementia was determined by using the education-adjusted Telephone Interview for Cognitive Status–Modified (TICSm), or informant ratings for the CDR and FAQ, or the Eight-item Interview to Differentiate Aging and Dementia (AD8) or Six-Item Screener (SIS). Level 3 dementia included all level 1 and level 2 dementia diagnoses plus dementia cases identified through ICD-9 codes from discharge hospitalization records and death certificates [19]. Each participant was followed up for incident dementia up to June 30, 2017.
We used level 3 dementia (with dementia determination available for all cohort participants) as the outcome for our primary analysis and confirmed the main results with level 1 dementia (deemed the most accurate but only available for 5,384 cohort participants who came at scheduled visits 5), accounting for attrition and missing diagnoses.
Covariates
Information about date of birth, sex, race-center, and education was collected at visit 1 by interview. Education level was categorized as 1) less than high school; 2) completed high school or vocational school; and 3) any college, graduate, or professional school. Apolipoprotein E (APOE) ε4 (0 or ≥1 allele) was genotyped using the TaqMan assay (Applied Biosystems, Foster City, CA) [20]. Participants’ lifestyle and clinical characteristics collected at each examination were updated at visit 4. Smoking and habitual alcohol use were self-reported as current, former, and never. Body mass index (BMI) was calculated using weight in kilograms divided by the square of height in meters. Sitting arm blood pressures were measured after a 5-minute rest using a standardized Hawksley random-zero sphygmomanometer [21]. Two measures were taken for each individual and the average was recorded. Total and high-density lipoprotein cholesterols were measured using automated enzymatic methods [22, 23]. Diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, self-reported diagnosis of diabetes by a physician, or using antidiabetic medications. Use of medications for hypertension, dyslipidemia, and diabetes in the previous two weeks was self-reported by the participants and validated by medication containers brought to the ARIC clinic.
Statistical Evaluation
ALT and AST were categorized as quintiles. The first quintile was subdivided into a <10th percentile and a 10th-20th percentiles to allow for observed non-linearity of associations at low plasma aminotransferase levels. Participant characteristics were compared across the six categories of ALT and AST at visit 4 and summarized as means (standard deviation) for continuous variables and counts (proportions) for categorical variables.
We estimated age, sex, race-center adjusted incidence rates of dementia over the full spectrum of plasma aminotransferases using Poisson regression with robust standard errors. ALT and AST were modeled as continuous variables with linear splines at 10th, 20th, 40th, and 60th percentiles (with a linear association at higher levels).
Multivariable Cox proportional hazards regression was used to quantify the prospective association of plasma aminotransferases with incident dementia. ALT and AST were examined individually according to the aforementioned categories, with the second quintile serving as the distribution-based referent. The basic analytic model was adjusted for age, sex, race-center, education, and APOE ε4 genotype. Model 2 was additionally adjusted for alcohol use and diabetes. Model 3 incorporated all other covariates described above.
Consistency of the associations of ALT and AST categories with incident dementia across subgroups was determined via analyses stratified by age, sex, race, APOE ε4 genotype, alcohol use, and diabetes. We tested interaction terms using the likelihood ratio test and the Bonferroni adjustment was applied to correct for multiple testing.
To confirm the robustness of our results, several sensitivity analyses were performed. To reduce the effect of diurnal variation, blood draws were standardized to occur between 7:30 am and 10:30 am throughout the study. We conducted a sensitivity analysis to assess the effect fasting time by restricting the analytic sample to 9,640 (out of 10,100 in total) cohort participants with fasting times of 8 hours and longer. Second, to account for a potential confounding effect by alcohol use or body weight, we additionally included in our adjustment set the following: total weekly alcohol consumption (drinks/week) for current drinkers; years since cessation of alcohol use (<5, 5-<10, ≥10 years), cumulative drinking years before cessation (<10, 10-<20, ≥20 years), and total weekly alcohol consumption before cessation (<8, 8-<15, ≥15 drinks/week) among former drinkers. BMI was modelled either continuously or categorically (underweight <18.5 kg/m2, normal weight 18.5–<25 kg/m2, overweight 25–<30 kg/m2, and obese ≥30 kg/m2). Third, we included 783 individuals with AST:ALT ratio ≥2. Finally, we repeated the primary analyses using level 1 dementia as the outcome. In this analysis, cohort attrition due to death and non-death drop-out associated with plasma aminotransferase levels and cognitive function was addressed analytically using stabilized inverse probability weighting. Logistic regression was used to estimate the probability of death and non-death drop-out separately, with covariates selected per a priori knowledge, including all variables mentioned above plus dementia status, a composite factor score of global cognition [24], comorbidities associated with attrition (coronary heart disease, heart failure, stroke), health information collected through annual follow-up interviews (an indicator of a report by proxy, self-reported poor health, number of hospitalizations), and interactions between variables.
All analyses were performed with Stata version 14.0 (StataCorp LLC, College Station, Texas). A p-value <0.05 was considered nominally statistically significant.
Results
Of 10,100 cohort participants free of dementia at baseline, 55.0% were women, 21.5% were black, and the mean age was 63.2 (SD 5.7) years. The median value of ALT was 16.7 U/L with an interquartile interval of 13.4–22.1 U/L. The corresponding estimates for AST were 21.9, 18.7–25.1 U/L. As shown in Table 1, mean age, BMI, blood pressure levels, lipid profiles, and proportion of never smoked cigarettes were comparable by categorized levels of ALT. Participants at lower levels of ALT were more likely to be female, black, less educated, and carriers of the APOE ε4 allele; in addition, they were less likely to be current alcohol consumers. A J-shaped association was observed for prevalent diabetes. A largely consistent pattern was seen across categorized levels of AST, with a few exceptions (Supplemental Table 1). A lower prevalence of APOE ε4 genotype but much higher prevalence of diabetes were observed in the group below the 10th percentile of AST.
Table 1.
Characteristics of participant at Atherosclerosis Risk in Communities (ARIC) Study Visit 4 (1996–1998) and incidence rate (95% confidence interval) of dementia by categories of alanine aminotransferase (N=10,100)
| Alanine aminotransferase (U/L) | ||||||
|---|---|---|---|---|---|---|
| 10th percentile (5–10) | 10–20th percentile (11–12) | 2nd quintile (13–14) | 3rd quintile (15–17) | 4th quintile (18–22) | 5th quintile (≥23) | |
| N | 644 | 1281 | 1649 | 2279 | 1981 | 2266 |
| Age, years, mean (SD) | 63.2 (5.8) | 63.6 (5.9) | 63.6 (5.6) | 63.6 (5.6) | 63.2 (5.6) | 62.4 (5.5) |
| Female, N (%) | 486 (75.5) | 891 (69.6) | 1058 (64.2) | 1293 (56.7) | 940 (47.5) | 885 (39.1) |
| Race-Center, N (%) | ||||||
| NC/white | 164 (25.5) | 335 (26.2) | 381 (23.1) | 484 (21.2) | 452 (22.8) | 470 (20.7) |
| NC/black | 27 (4.2) | 43 (3.4) | 36 (2.2) | 51 (2.2) | 33 (1.7) | 36 (1.6) |
| MI/black | 191 (29.7) | 297 (23.2) | 353 (21.4) | 411 (18.0) | 330 (16.7) | 364 (16.1) |
| MN/white | 101 (15.7) | 291 (22.7) | 442 (26.8) | 690 (30.3) | 593 (29.9) | 714 (31.5) |
| MD/white | 161 (25.0) | 315 (24.6) | 437 (26.5) | 643 (28.2) | 573 (28.9) | 682 (30.1) |
| Education, N (%) | ||||||
| <High school | 152 (23.6) | 270 (21.1) | 329 (20.0) | 423 (18.6) | 373 (18.8) | 376 (16.6) |
| High school or vocational school | 294 (45.7) | 537 (41.9) | 697 (42.3) | 974 (42.7) | 820 (41.4) | 947 (41.8) |
| College, graduate, or professional school | 198 (30.7) | 474 (37.0) | 623 (37.8) | 882 (38.7) | 788 (39.8) | 943 (41.6) |
| APOE e4 status, N (%) | 221 (34.3) | 407 (31.8) | 508 (30.8) | 670 (29.4) | 593 (29.9) | 632 (27.9) |
| Alcohol Use, N (%) | ||||||
| Current | 225 (35.4) | 567 (44.6) | 784 (47.8) | 1132 (49.8) | 1009 (51.2) | 1250 (55.4) |
| Former | 225 (35.4) | 398 (31.3) | 495 (30.2) | 664 (29.2) | 584 (29.6) | 646 (28.6) |
| Never | 186 (29.2) | 305 (24.0) | 362 (22.1) | 475 (20.9) | 377 (19.1) | 360 (16.0) |
| Smoking, N (%) | ||||||
| Current | 154 (24.3) | 264 (20.8) | 278 (17.0) | 322 (14.2) | 219 (11.1) | 227 (10.1) |
| Former | 237 (37.3) | 474 (37.3) | 669 (40.8) | 975 (42.9) | 942 (47.8) | 1125 (49.9) |
| Never | 244 (38.4) | 532 (41.9) | 693 (42.3) | 974 (42.9) | 810 (41.1) | 903 (40.0) |
| BMI, kg/m2, mean (SD) | 28.8 (6.7) | 28.5 (6.1) | 28.3 (5.7) | 28.6 (5.4) | 29.1 (5.4) | 29.8 (5.1) |
| Underweight, N (%) | 8 (1.2) | 12 (0.9) | 11 (0.7) | 12 (0.5) | 19 (1.0) | 5 (0.2) |
| SBP, mmHg, mean (SD) | 127.2 (19.8) | 126.9 (19.8) | 127.5 (19.0) | 127.3 (18.6) | 127.6 (18.6) | 128.0 (18.0) |
| DBP, mmHg, mean (SD) | 69.7 (11.2) | 69.9 (10.5) | 70.4 (10.1) | 70.9 (10.2) | 71.4 (9.7) | 72.4 (10.2) |
| Hypertension, N (%) | 328 (51.3) | 588 (46.0) | 778 (47.4) | 1055 (46.5) | 913 (46.3) | 1103 (48.8) |
| Total cholesterol, mmol/L, mean (SD) | 5.1 (1.0) | 5.2 (1.0) | 5.2 (0.9) | 5.2 (0.9) | 5.2 (1.0) | 5.2 (1.0) |
| HDL, mmol/L, mean (SD) | 1.4 (0.4) | 1.3 (0.4) | 1.3 (0.4) | 1.3 (0.4) | 1.2 (0.4) | 1.2 (0.4) |
| Diabetes, N (%) | 111 (17.3) | 179 (14.1) | 210 (12.8) | 359 (15.8) | 357 (18.1) | 522 (23.1) |
| Hypoglycemia, N (%) | 3 (0.5) | 1 (0.1) | 4 (0.2) | 5 (0.2) | 6 (0.3) | 4 (0.2) |
| Incidence rate of Dementia * | 12.6 (10.7–14.9) | 12.1 (10.7–13.6) | 11.4 (10.3–12.7) | 11.6 (10.6–12.6) | 12.2 (11.1–13.3) | 10.6 (9.7–11.7) |
Values are Count (Proportion), mean (SD), or incidence rate (95% confidence interval)
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol
Dementia was ascertained by an expert adjudication panel at scheduled visits, telephone or informant interviews, or ICD-9 codes
Underweight was defined as BMI <18.5 kg/m2. Hypoglycemia was defined as fasting glucose <70 mg/dL
Per 1,000 person-year, estimated by Poisson regression with continuous variables centered at the mean
During a median follow up of 18.3 years (maximum of 21.9 years) 1,857 incident cases of dementia were ascertained and documented. The unadjusted incidence was 11.6 per 1,000 person-years in the entire study population, ranging from 10.6 per 1,000 person-years in the top quintile level of ALT to 12.6 per 1,000 person-years in the bottom <10th percentile of ALT (Table 1). A modestly higher unadjusted incidence than the population average was observed for <10th percentile level of AST as well (11.9 per 1,000 person-years). Likewise, after adjusting for age, sex, race-center a higher incidence of dementia was observed at levels of ALT <12 U/L (Figure 1 A). A similar pattern was seen for AST (Figure 1 B).
Figure 1.
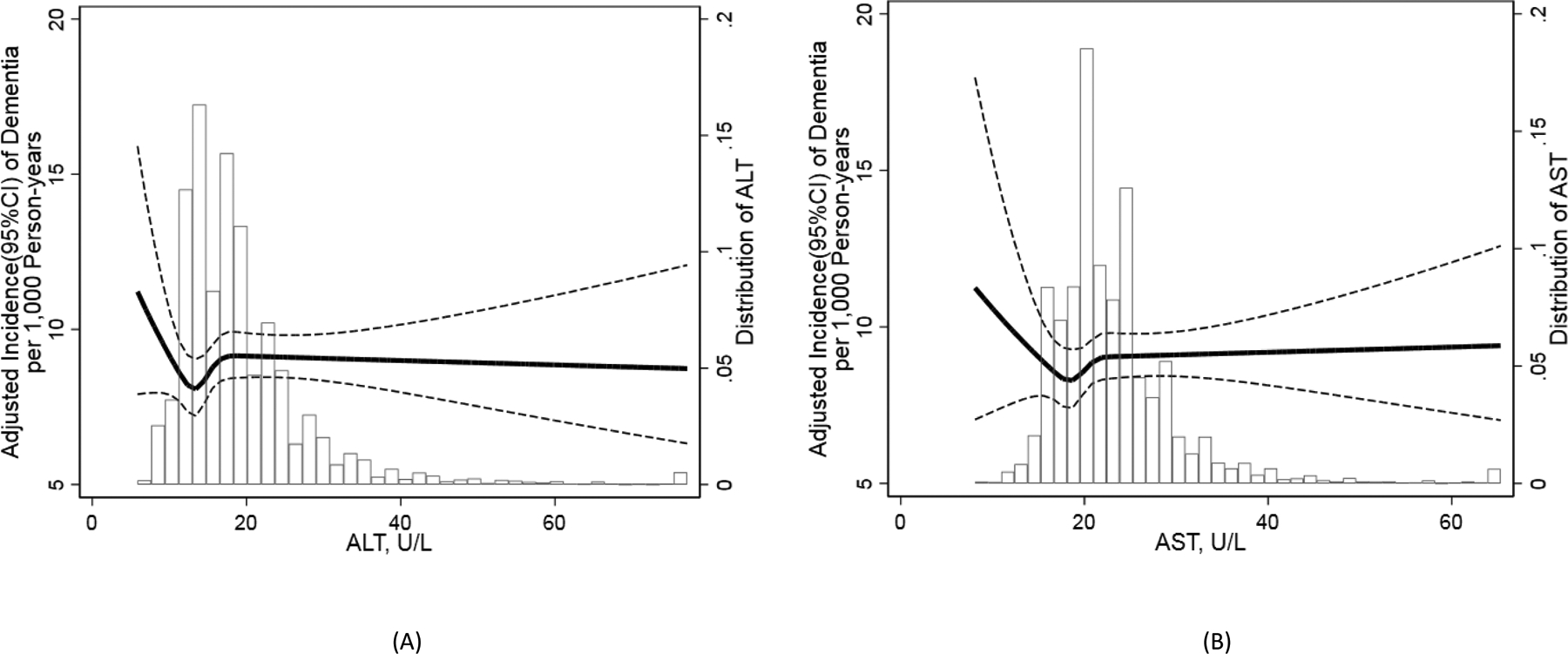
Age, sex, race-center adjusted incidence rates of dementia over full spectrum levels of alanine aminotransferase (A) and aspartate aminotransferase (B) measured at Atherosclerosis Risk in Communities (ARIC) Study Visit 4 (N=10,100). Alanine aminotransferase and aspartate aminotransferase were modelled continuously using linear splines, with knots at 10th, 20th, 40th, 60th percentiles. Dementia was ascertained by an expert adjudication panel at scheduled visits, telephone or informant interviews, or ICD-9 codes. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval.
Using multivariable Cox regression to quantify the association of plasma aminotransferases with time to incident dementia, ALT levels below the 10th percentile were associated with a 34% higher risk of dementia compared to the second quintile adjusting for age, sex, race-center, education, and APOE ε4 genotype (Model 1 in Table 2, hazard ratio [HR] 1.34, 95% CI 1.08–1.65). Additional adjustment for alcohol use and diabetes (Model 2, HR 1.28, 95% CI 1.04–1.59) and other vascular and metabolic risk factors (Model 3, HR 1.27, 95% CI 1.03–1.58) did not materially change the results. The top quintile level of ALT was associated with elevated dementia risk in model 1 (HR 1.19, 95% CI 1.02–1.39), but this was no longer statistically significant after accounting for vascular risk factors. A similar pattern was observed for the association of AST with incident dementia, but of smaller magnitude (Table 2).
Table 2.
Hazard ratios (95% confidence interval) of incident dementia by categories of alanine aminotransferase and aspartate aminotransferase measured at Atherosclerosis Risk in Communities (ARIC) Study Visit 4 (N=10,100)
| Alanine aminotransferase (U/L) | ||||||
|---|---|---|---|---|---|---|
| 10th percentile (5–10) | 10–20th percentile (11–12) | 2nd quintile (13–14) | 3rd quintile (15–17) | 4th quintile (18–22) | 5th quintile (≥23) | |
| N=644 | N=1281 | N=1649 | N=2279 | N=1981 | N=2266 | |
| Model 1 | 1.34 (1.08–1.65)* | 1.16 (0.98–1.38) | 1 (Ref.) | 1.05 (0.91–1.22) | 1.15 (0.98–1.34) | 1.19 (1.02–1.39)* |
| Model 2 | 1.28 (1.04–1.59)* | 1.14 (0.96–1.35) | 1 (Ref.) | 1.02 (0.88–1.18) | 1.10 (0.94–1.28) | 1.11 (0.95–1.30) |
| Model 3 | 1.27 (1.03–1.58)* | 1.12 (0.95–1.33) | 1 (Ref.) | 1.02 (0.88–1.19) | 1.11 (0.95–1.29) | 1.13 (0.97–1.32) |
| Aspartate aminotransferase (U/L) | ||||||
| 10th percentile (8–15) | 10–20th percentile (16–17) | 2nd quintile (18–19) | 3rd quintile (20–21) | 4th quintile (22–26) | 5th quintile (≥27) | |
| N=672 | N=1230 | N=1784 | N=1874 | N=2519 | N=2021 | |
| Model 1 | 1.22 (0.99–1.51) | 1.04 (0.87–1.24) | 1 (Ref.) | 1.01 (0.87–1.17) | 0.96 (0.83–1.11) | 1.13 (0.97–1.31) |
| Model 2 | 1.15 (0.93–1.43) | 1.02 (0.85–1.22) | 1 (Ref.) | 1.02 (0.87–1.18) | 0.96 (0.83–1.11) | 1.13 (0.97–1.31) |
| Model 3 | 1.14 (0.92–1.42) | 1.01 (0.85–1.21) | 1 (Ref.) | 1.03 (0.88–1.19) | 0.97 (0.84–1.11) | 1.15 (0.99–1.34) |
Dementia was ascertained by an expert adjudication panel at scheduled visits, telephone or informant interviews, or ICD-9 codes
Model 1 (Primary model): age, gender, race-center, education, APOE e4 status
Model 2: + alcohol use, diabetes
Model 3: + smoking, body mass index, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol
Statistically significance at P<0.05
In general, the associations of ALT and AST with incident dementia were consistent across all subgroups explored, although differences in the magnitude of the associations were observed in some instances (Table 3 and Supplemental Table 2). Specifically, we observed stronger associations of low levels of ALT and AST with dementia in white than in black cohort members. Men also showed a greater magnitude of association of low levels of AST with dementia than women. Restricting the analyses to fasting samples, additionally accounting for alcohol consumption, and for body weight did not materially alter our results, nor did the inclusion of individuals with an AST:ALT ratio ≥2 (Supplemental Tables 3–5). Finally, we confirmed an elevated risk of level 1 dementia in <10th percentile level of ALT after accounting for attrition and selection bias (Supplemental Table 6). However, the opposite risk pattern was observed for AST.
Table 3.
Subgroup hazard ratios (95% confidence interval) of incident dementia by categories of alanine aminotransferase measured at Atherosclerosis Risk in Communities (ARIC) Study Visit 4 (N=10,100)
| Alanine aminotransferase (U/L) | |||||||
|---|---|---|---|---|---|---|---|
| 10th percentile (5–10) | 10–20th percentile (11–12) | 2nd quintile (13–14) | 3rd quintile (15–17) | 4th quintile (18–22) | 5th quintile (≥23) | P value for interaction | |
| Age | 0.800 | ||||||
| <65 | 1.44 (1.02–2.04)* | 1.13 (0.84–1.51) | 1 (Ref.) | 1.11 (0.86–1.42) | 1.15 (0.90–1.48) | 1.11 (0.87–1.42) | |
| ≥65 | 1.31 (1.00–1.71) | 1.17 (0.95–1.45) | 1 (Ref.) | 1.01 (0.84–1.21) | 1.13 (0.93–1.36) | 1.24 (1.02–1.51)* | |
| Sex | 0.847 | ||||||
| Male | 1.32 (0.84–2.07) | 1.33 (0.98–1.79) | 1 (Ref.) | 1.07 (0.83–1.37) | 1.13 (0.89–1.44) | 1.20 (0.94–1.52) | |
| Female | 1.35 (1.05–1.72)* | 1.10 (0.89–1.35) | 1 (Ref.) | 1.04 (0.86–1.25) | 1.17 (0.96–1.43) | 1.18 (0.96–1.45) | |
| Race | 0.491 | ||||||
| White | 1.52 (1.17–1.97)* | 1.22 (0.99–1.51) | 1 (Ref.) | 1.09 (0.91–1.30) | 1.25 (1.05–1.49)* | 1.30 (1.09–1.56)* | |
| Black | 1.02 (0.70–1.48) | 1.00 (0.74–1.35) | 1 (Ref.) | 0.96 (0.73–1.27) | 0.89 (0.66–1.20) | 0.94 (0.69–1.28) | |
| APOE | 0.389 | ||||||
| No | 1.28 (0.95–1.71) | 0.97 (0.77–1.22) | 1 (Ref.) | 1.03 (0.84–1.25) | 1.12 (0.92–1.37) | 1.14 (0.94–1.40) | |
| Yes | 1.40 (1.02–1.91)* | 1.39 (1.08–1.78)* | 1 (Ref.) | 1.08 (0.86–1.35) | 1.18 (0.94–1.49) | 1.25 (0.98–1.59) | |
| Current alcohol use | - | ||||||
| No | 1.37 (1.06–1.78)* | 1.16 (0.94–1.44) | 1 (Ref.) | 1.10 (0.91–1.33) | 1.11 (0.91–1.35) | 1.28 (1.05–1.56)* | |
| Yes | 1.24 (0.85–1.83) | 1.18 (0.89–1.57) | 1 (Ref.) | 0.98 (0.77–1.24) | 1.21 (0.95–1.53) | 1.09 (0.86–1.39) | |
| Diabetes | - | ||||||
| No | 1.25 (0.98–1.59) | 1.16 (0.96–1.40) | 1 (Ref.) | 1.07 (0.91–1.26) | 1.15 (0.97–1.36) | 1.19 (1.00–1.41) | |
| Yes | 1.38 (0.87–2.18) | 1.04 (0.68–1.58) | 1 (Ref.) | 0.76 (0.53–1.11) | 0.90 (0.63–1.28) | 0.82 (0.58–1.16) | |
Dementia was ascertained by an expert adjudication panel at scheduled visits, telephone or informant interviews, or ICD-9 codes
All models were adjusted for age, gender, race-center, education, APOE e4 status. No interaction term was tested for alcohol use and diabetes with alanine aminotransferase categories.
Statistically significance at P<0.05
Discussion
We evaluated the prospective association between plasma aminotransferase levels and the long-term risk of dementia among 10,100 middle-aged adults who were cognitively intact, free of chronic liver disease, and had a AST:ALT ratio ≤2 (to exclude alcoholic fatty liver disease and undiagnosed advanced liver disease) at baseline. Over the course of ~20 years, 1,857 cases of dementia accrued. Compared to the second quintile of ALT, a lower level of ALT (<10th percentile) was robustly associated with elevated risk of dementia, even in the fully adjusted analytic model accounting for demographic, lifestyle, vascular and metabolic risk factors. A similar pattern was seen for low levels of AST, although of smaller magnitude. The aforementioned associations were largely consistent in the subgroups explored. Our analyses considered exposure to hepatotoxic agents (e.g., habitual alcohol use and pharmacologic agents), as well as hospitalizations for non-alcoholic fatty liver disease in order to avoid potential confounding by those factors.
While a previous study identified lower levels of liver enzymes in individuals with dementia [15], our study appears to be the first to report this association in a prospective manner, years before dementia is clinically manifest. Given that hepatic metabolite profiles are altered in the presence of dementia [25, 26], identifying the temporality of this association is key to understanding the role of hepatic metabolic dysfunction in influencing the risk of dementia. Although dementias are considered metabolic disorders, most attention has been focused on brain metabolism as opposed to metabolomics in peripheral samples. Work on amyloid precursor protein/presenilin 1 (APP/PS1) mouse models described metabolic impairments in the liver, kidney and heart that affect energy metabolism, metabolism of amino acids, lipid homeostasis, oxidative stress, and hyperammonemia [27]. PS1 also interacts with glutamate transporter 1, interfering with glutamate homeostasis at the synapse. ALT and AST levels positively correlate with plasma glutamate levels, and glutamate dysregulation has been shown to precede cognitive deficits [28]. Other animal work suggests that hepatic metabolic dysregulation is associated with amyloid pathology progression. For example, metabolic dysregulation in the liver was the initial organ-specific impairment observed during amyloid pathology progression in APP/PS1 mice [29].
It has further been shown that the levels of amyloid-β in the brain reflect a balance between amyloid-β production and its clearance [30] and that amyloid-β homeostasis intersects multiple systems, including the liver [31]. Bassendine et al. assembled several lines of evidence suggesting that amyloid-β produced peripherally contributes to brain amyloid-β, that the liver is the source of brain deposits of amyloid-β, that efflux of amyloid-β to peripheral blood accounts for ~50% of total brain amyloid-β clearance in humans, and that the liver contributes to clearance of circulating amyloid-β in the peripheral circulation [31]. Reports based on murine models document uptake, metabolization and excretion of large doses of amyloid-β by the liver [32] and in vitro work suggests that reduced hepatic degradation of amyloid-β could influence deposition of amyloid-β in the brain [33].
Other potential mechanisms speak to the plausibility of our results. Reduced liver synthesis and metabolic function, indexed by low levels of plasma aminotransferases, may contribute to or correlate with cerebral hypometabolism, which is reported to occur preceding the onset of dementia [3, 15]. As the key enzyme catalyzing the reactions from alanine and α-ketoglutarate to form pyruvate, ALT is placed at the initial step in gluconeogenesis [34]. Consequently, a reduction in ALT levels is associated with lower availability of pyruvate and may be associated with reduced gluconeogenesis in the liver and thus lower levels of glucose available as energy source to various tissues [35]. Further, we observed a higher prevalence of diabetes in the bottom decile of liver enzymes. Diabetes has been well-established as a risk factor for dementia and is associated with brain insulin resistance and impaired glucose uptake [3, 36], further linking liver hypometabolism and energy metabolism disorder in the brain.
Both ALT and AST facilitate the production of glutamate, an excitatory neurotransmitter required for the maintenance of synapses [37]. Reduced levels of ALT or AST could lead to lower levels of glutamate, a metabolite related to memory performance [15]. Moreover, low plasma aminotransferase levels are reported to be associated with poor nutritional intake and pyridoxine (vitamin B6) deficiency [12]. The active form of pyridoxine, Pyridoxal 5′-phosphate, is a coenzyme in neurotransmitter synthesis and catalyzes transamination reactions that are essential for providing amino acids as a substrate for gluconeogenesis [12, 38]. Although requiring further elucidation, reduced levels of ALT are reportedly associated with greater structural cerebral atrophy, amyloid-β deposition, and altered neurodegenerative biomarkers in cerebrospinal fluid associated with dementia [15].
Although plasma aminotransferase levels decrease with age and dementia is strongly associated with age [8, 39], our results are unlikely to be confounded by age since the plasma aminotransferases were measured years before the onset of dementia, and analytic results stratified by age were consistent with those of the primary analysis. Moreover, the inverse association of plasma aminotransferase levels with age may be an indicator of age-associated frailty [8, 11–14] and manifestations of frailty were observed to be more prevalent at low levels of plasma aminotransferases among the members of this cohort (data not shown). Frailty has been reported to interact with cognitive impairment in accelerating the development of dementia [40]. Future studies clarifying the role of liver function in the bi-directional relationship between frailty and dementia are needed.
The association of AST with dementia was weaker than that of ALT, which merits comments. ALT is predominantly generated in the liver whereas AST can be synthesized in the liver, skeletal muscle, heart, brain, and other tissues. The greater specificity to hepatic origin of ALT adds support to the hypothesized role of liver function in the development of dementia. The greater proportion of women and of black members of the cohort in the lower range of plasma aminotransferase levels is consistent with previously observed lower ALT levels among women and black study participants compared to their counterparts [11]. It is possible that the use of uniform thresholds for plasma aminotransferase categories may blur variation by population groups in reduced and low-normal plasma aminotransferases in what is considered the physiologic range.
Our study has implications for research and public health. Whereas clinical interest in ALT and AST is mostly focused on their elevation as markers of hepatocyte damage, we examined low levels of ALT as an aminotransferase surrogate marker for loss of liver metabolic function, brain amyloid-β clearance capacity, vitamin deficiency, or lower glutamine levels among middle aged individuals free of manifest liver disease. Aminotransferase activity is a highly regulated trait, as indicted by genome-wide association studies coupled with metabolomics on the role of aminotransferase genes (e.g., GOT, GPT) in the regulation of amino acid and lipid metabolism [41]. If our results are replicated, low levels of ALT in what is deemed normal physiologic range may index reduced liver metabolism and associated health effects. Our results support the arguments that hepatic functionality should be considered for further insights into brain health and amyloid-β balance [31, 42].
Our results should be interpreted with consideration of several limitations. First, we lack biomarkers to discern the character of impairment in hepatic metabolic function. It is thus unclear what forms of liver dysfunction are indexed by the low levels of ALT and AST in free-living adults. Second, the natural history of dementia is protracted and the time of diagnosis of dementia may not accurately reflect disease inception. As a result, estimated associations with time to dementia may be underestimated. Third, dementia subsumes heterogeneous etiologic processes, whereas an etiologic classification of dementia was not available in this study. Fourth, although this study included samples of the residents of four geographic areas in the US, aged 54–73 years, generalization of our results to other populations should be done with caution. Indeed, in contrast to studies conducted among older adults, a null association of low liver enzymes with mortality was reported among younger adults [11, 43]. Lastly, some key variables such as sarcopenia were not measured and could potentially introduce residual confounding.
Conclusions
Low levels of plasma aminotransferases <10th percentile of the physiologic range at mid-life, particularly ALT, were associated with greater long-term risk of dementia. These results advocate for attention to the putative role of hepatic function in the pathogenesis of dementia.
Supplementary Material
Acknowledgement
We thank the staff and participants of the ARIC study for their important contributions. The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U012U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. P. Palta is supported by grant R00AG052830 from the National Institute on Aging. A. Thomas is supported by T32HL007055.
Footnotes
Conflict of Interest/Disclosure Statement
The authors declare no conflicts of interest.
References
- [1].World Health Organization https://www.who.int/news-room/fact-sheets/detail/dementia, Accessed May 8. [Google Scholar]
- [2].Yaffe K (2018) Modifiable Risk Factors and Prevention of Dementia: What Is the Latest Evidence? JAMA Internal Medicine 178, 281–282. [DOI] [PubMed] [Google Scholar]
- [3].Clarke JR, Ribeiro FC, Frozza RL, De Felice FG, Lourenco MV (2018) Metabolic Dysfunction in Alzheimer’s Disease: From Basic Neurobiology to Clinical Approaches. J Alzheimers Dis 64, S405–s426. [DOI] [PubMed] [Google Scholar]
- [4].Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. The Lancet Neurology 10, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kwo PY, Cohen SM, Lim JK (2017) ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. American Journal of Gastroenterology 112. [DOI] [PubMed] [Google Scholar]
- [6].Goessling W, Massaro JM, Vasan RS, D’Agostino RB Sr., Ellison RC, Fox CS (2008) Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 135, 1935–1944.e1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC (2008) Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 47, 1363–1370. [DOI] [PubMed] [Google Scholar]
- [8].Le Couteur DG, Blyth FM, Creasey HM, Handelsman DJ, Naganathan V, Sambrook PN, Seibel MJ, Waite LM, Cumming RG (2010) The association of alanine transaminase with aging, frailty, and mortality. The journals of gerontology. Series A, Biological sciences and medical sciences 65, 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Elinav E, Ackerman Z, Maaravi Y, Ben-Dov IZ, Ein-Mor E, Stessman J (2006) Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc 54, 1719–1724. [DOI] [PubMed] [Google Scholar]
- [10].Koehler EM, Sanna D, Hansen BE, van Rooij FJ, Heeringa J, Hofman A, Tiemeier H, Stricker BH, Schouten JN, Janssen HL (2014) Serum liver enzymes are associated with all-cause mortality in an elderly population. Liver Int 34, 296–304. [DOI] [PubMed] [Google Scholar]
- [11].Ruhl CE, Everhart JE (2013) The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol 178, 1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vespasiani-Gentilucci U, De Vincentis A, Ferrucci L, Bandinelli S, Antonelli Incalzi R, Picardi A (2018) Low Alanine Aminotransferase Levels in the Elderly Population: Frailty, Disability, Sarcopenia, and Reduced Survival. J Gerontol A Biol Sci Med Sci 73, 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oh CM, Won YJ, Cho H, Lee JK, Park BY, Jun JK, Koh DH, Ki M, Jung KW, Oh IH (2016) Alanine aminotransferase and gamma-glutamyl transferase have different dose-response relationships with risk of mortality by age. Liver Int 36, 126–135. [DOI] [PubMed] [Google Scholar]
- [14].Yamazaki H, Kamitani T, Matsui T, Yamamoto Y, Fukuhara S (2019) Association of low alanine aminotransferase with loss of independence or death: A 5-year population-based cohort study. J Gastroenterol Hepatol 34, 1793–1799. [DOI] [PubMed] [Google Scholar]
- [15].Nho K, Kueider-Paisley A, Ahmad S, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmuller G, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, van Duijn C, Saykin AJ, Kaddurah-Daouk R (2019) Association of Altered Liver Enzymes With Alzheimer Disease Diagnosis, Cognition, Neuroimaging Measures, and Cerebrospinal Fluid Biomarkers. JAMA Netw Open 2, e197978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].(1989) The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 129, 687–702. [PubMed] [Google Scholar]
- [17].Alonso A, Misialek JR, Amiin MA, Hoogeveen RC, Chen LY, Agarwal SK, Loehr LR, Soliman EZ, Selvin E (2014) Circulating levels of liver enzymes and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities cohort. Heart (British Cardiac Society) 100, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Atherosclerosis Risk in Communities Neurocognitive Study. Visit 6 Manual 17. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/ARIC%20MOP17%20170405.pdf, [Google Scholar]
- [19].Atherosclerosis Risk in Communities Neurocognitive Study. STATUS61 Derived Variable Dictionary. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/ARIC%20STATUS61%20Derived%20Variable%20Dictionary%20190927.pdf, [Google Scholar]
- [20].Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider AL, Hengrui S, Alonso A, Coresh J, Albert MS, Mosley TH Jr. (2016) Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Atherosclerosis Risk in Colmlunities study Protocol Manual 11: Sitting Blood Pressure. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Sitting_Blood_Pressure_and_Postural_Changes_in_Blood_Pressure_and_Heart_Rate.4_11.pdf.
- [22].Warnick GR, Benderson J, Albers JJ (1982) Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem 28, 1379–1388. [PubMed] [Google Scholar]
- [23].Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW (1983) Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clinical Chemistry 29, 1075. [PubMed] [Google Scholar]
- [24].Gross AL, Power MC, Albert MS, Deal JA, Gottesman RF, Griswold M, Wruck LM, Mosley TH Jr., Coresh J, Sharrett AR, Bandeen-Roche K (2015) Application of Latent Variable Methods to the Study of Cognitive Decline When Tests Change over Time. Epidemiology 26, 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Craft S (2009) The Role of Metabolic Disorders in Alzheimer Disease and Vascular Dementia: Two Roads Converged. Archives of Neurology 66, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Toledo JB, Arnold M, Kastenmuller G, Chang R, Baillie RA, Han X, Thambisetty M, Tenenbaum JD, Suhre K, Thompson JW, John-Williams LS, MahmoudianDehkordi S, Rotroff DM, Jack JR, Motsinger-Reif A, Risacher SL, Blach C, Lucas JE, Massaro T, Louie G, Zhu H, Dallmann G, Klavins K, Koal T, Kim S, Nho K, Shen L, Casanova R, Varma S, Legido-Quigley C, Moseley MA, Zhu K, Henrion MYR, van der Lee SJ, Harms AC, Demirkan A, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Saykin AJ, Weiner MW, Doraiswamy PM, Kaddurah-Daouk R (2017) Metabolic network failures in Alzheimer’s disease: A biochemical road map. Alzheimers Dement 13, 965–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].González-Domínguez R, García-Barrera T, Vitorica J, Gómez-Ariza JL (2015) High throughput multiorgan metabolomics in the APP/PS1 mouse model of Alzheimer’s disease. Electrophoresis 36, 2237–2249. [DOI] [PubMed] [Google Scholar]
- [28].Zoltowska KM, Maesako M, Meier J, Berezovska O (2018) Novel interaction between Alzheimer’s disease-related protein presenilin 1 and glutamate transporter 1. Scientific reports 8, 8718–8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zheng H, Cai A, Shu Q, Niu Y, Xu P, Li C, Lin L, Gao H (2019) Tissue-Specific Metabolomics Analysis Identifies the Liver as a Major Organ of Metabolic Disorders in Amyloid Precursor Protein/Presenilin 1 Mice of Alzheimer’s Disease. J Proteome Res 18, 1218–1227. [DOI] [PubMed] [Google Scholar]
- [30].Sutcliffe JG, Hedlund PB, Thomas EA, Bloom FE, Hilbush BS (2011) Peripheral reduction of β-amyloid is sufficient to reduce brain β-amyloid: implications for Alzheimer’s disease. J Neurosci Res 89, 808–814. [DOI] [PubMed] [Google Scholar]
- [31].Bassendine MF, Taylor-Robinson SD, Fertleman M, Khan M, Neely D (2020) Is Alzheimer’s Disease a Liver Disease of the Brain? J Alzheimers Dis 75, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT 3rd, Derdeyn CP, Bateman RJ (2014) Amyloid-β efflux from the central nervous system into the plasma. Ann Neurol 76, 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maarouf CL, Walker JE, Sue LI, Dugger BN, Beach TG, Serrano GE (2018) Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS One 13, e0203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qian K, Zhong S, Xie K, Yu D, Yang R, Gong DW (2015) Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab Res Rev 31, 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reis HJ, Guatimosim C, Paquet M, Santos M, Ribeiro FM, Kummer A, Schenatto G, Salgado JV, Vieira LB, Teixeira AL, Palotas A (2009) Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Curr Med Chem 16, 796–840. [DOI] [PubMed] [Google Scholar]
- [36].De Felice FG, Lourenco MV, Ferreira ST (2014) How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement 10, S26–32. [DOI] [PubMed] [Google Scholar]
- [37].Rui L (2014) Energy metabolism in the liver. Compr Physiol 4, 177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yarlagadda A, Clayton AH (2007) Blood brain barrier: the role of pyridoxine. Psychiatry (Edgmont (Pa.: Township)) 4, 58–60. [PMC free article] [PubMed] [Google Scholar]
- [39].Vespasiani-Gentilucci U, Gallo P, Piccinocchi G, Piccinocchi R, Schena E, Galati G, De Vincentis A, Dell’Unto C, Picardi A (2014) Determinants of alanine aminotransferase levels in a large population from Southern Italy: relationship between alanine aminotransferase and age. Dig Liver Dis 46, 909–915. [DOI] [PubMed] [Google Scholar]
- [40].Robertson DA, Savva GM, Kenny RA (2013) Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev 12, 840–851. [DOI] [PubMed] [Google Scholar]
- [41].Sookoian S, Pirola CJ (2015) Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World journal of gastroenterology 21, 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zolezzi JM, Bastías-Candia S, Santos MJ, Inestrosa NC (2014) Alzheimer’s disease: relevant molecular and physiopathological events affecting amyloid-β brain balance and the putative role of PPARs. Front Aging Neurosci 6, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I (2004) Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ (Clinical research ed.) 328, 983–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


