Abstract
Objective
This study assesses the effectiveness of different interventions of knowledge transfer and behaviour modification to improve type 2 diabetes mellitus patients’ (T2DM) reported outcomes measures (PROMs) in the long-term. Design: open, community-based pragmatic, multicentre, controlled trial with random allocation by clusters to usual care (UC) or to one of the three interventions.
Participants
A total of 2334 patients with uncomplicated T2DM and 211 healthcare professionals were included of 32 primary care centres.
Setting
Primary Care Centers in Canary Islands (Spain).
Intervention
The intervention for patients (PTI) included an educational group programme, logs and a web-based platform for monitoring and automated short message service (SMS). The intervention for professionals (PFI) included an educational programme, a decision support tool embedded into the electronic clinical record and periodic feedback about patients’ results. A third group received both PTI and PFI (combined intervention, CBI).
Outcome measure
Cognitive-attitudinal, behavioural, affective and health-related quality of life (HQoL) variables.
Results
Compared with UC at 24 months, the PTI group significantly improved knowledge (p=0.005), self-empowerment (p=0.002), adherence to dietary recommendations (p<0.001) and distress (p=0.01). The PFI group improved at 24 months in distress (p=0.03) and at 12 months there were improvements in depression (p=0.003), anxiety (p=0.05), HQoL (p=0.005) and self-empowerment (p<0.001). The CBI group improved at 24 months in self-empowerment (p=0.008) and adherence to dietary recommendations (p=0.004) and at 12 months in knowledge (p=0.008), depression (p=0.006), anxiety (p=0.003), distress (p=0.01), HQoL (p<0.001) and neuropathic symptoms (p=0.02). Statistically significant improvements were also observed at 24 months in the proportion of patients who quit smoking for PTI and CBI (41.5% in PTI and 42.3% in CBI vs 21.2% in the UC group).
Conclusions
Assessed interventions to improve PROMs in T2DM attain effectiveness for knowledge, self-empowerment, distress, diet adherence and tobacco cessation. PTI produced the most lasting benefits.
Trial registration number
ClinicalTrials.gov NCT01657227 (6 August 2012) https://clinicaltrials.gov/ct2/show/NCT01657227.
Keywords: primary care, diabetes & endocrinology, quality in health care, health informatics
Strengths and limitations of this study.
The INDICA study provides randomised evidence on the effectiveness of complex interventions to improve outcomes in patients with type 2 diabetes mellitus, with a longer follow-up than previous studies.
All relevant stakeholders in the community are involved in the INDICA study (patients and family caregivers and primary care professionals).
The trial included a large sample of patients with type 2 diabetes regardless of their baseline HbA1c level, reinforcing the external validity of the results.
The INDICA interventions with information and communication technology-based components favour applicability and access, in a cost-effective manner, to a growing number of patients.
A limitation in the use of patient-reported outcome measures is the absence of well-established empirically derived minimum clinically significant differences
Introduction
Many patients with type 2 diabetes mellitus (T2DM) do not achieve the recommended treatment goals for glycaemic control.1 This might be due to inappropriate healthcare access and/or clinical management. Moreover, psychological and emotional aspects, such as knowledge of the disease or diabetes-related distress, are also important issues for an appropriate self-management and glycaemic control.2 3 Previous research has shown the value of patient-reported outcome measures (PROMs) to monitor these variables in diabetes,4 which contribute to patient empowerment and patient-centred care.5 PROMs are generally assessed with standardised, validated questionnaires aimed to measure patients’ perception of their health status, perceived level of impairment, disability or health-related quality of life.6
Interventions that aim to empower people with chronic illnesses and specifically diabetes have included distinct strategies such as educational programmes, websites, support phone calls, text messages and other technological resources,4 7–10 in order to improve patients’ diabetes knowledge, self-management, psychological outcomes and health status. However, the results obtained have been mixed, with a considerable number of studies showing no effect of the interventions.8–11 The INDICA study is a pragmatic, cluster-randomised controlled trial with 2 years follow-up that assesses the effectiveness and cost-effectiveness of multicomponent interventions for knowledge transfer and behaviour modification of patients with T2DM, their families and healthcare professionals (physicians and nurses) in a large number of Primary Care Health Practices (PHCP). These interventions combine conventional group educational and training activities with different information and communication technology (ICT)-based interventions to guide the decisions of the main actors involved in the management of T2DM.12 The intervention for patients (PTI) included an educational group programme led by trained nurses, consisting of eight face-to-face sessions (one every 3 months over 2 years); continuous self-monitoring by means of logs and a web-based platform; and tailored automated SMS to provide continuous support to patients and to reinforce self-care and lifestyle changes. The intervention for professionals (PFI) included an educational programme to update their diabetes knowledge, a decision support tool embedded into the electronic clinical record (ECR) with recommendations based on the best available scientific knowledge, adapted to the specific needs of every patient, and periodic feedback about patients’ results.
The results on the effectiveness of these interventions on clinical outcomes can be seen in Ramallo-Fariña et al. 13 and the cost-effectiveness evaluation can be reviewed in García-Pérez et al.14 The aim of this article is to report the effect of the INDICA interventions on a set of PROMs assessed in the trial: cognitive-attitudinal (knowledge, empowerment), behavioural (adherence to the dietary recommendation, medication and tobacco use), affective (anxiety, depression, distress) and health-related quality of life dimensions. These outcomes are commonly targeted for most diabetes interventions because of their association with critical, longer term outcomes, such as functional capacity,15 complications,16–18 mortality,19 healthcare costs20 and quality of life.21
Methods
Trial design
The INDICA study is an open, community-based pragmatic, multicentre, controlled trial with random allocation by clusters to usual care (UC) or one of three multicomponent interventions of knowledge transfer and behaviour modification. One intervention was aimed at patient and family members (PTI); another intervention was aimed at primary care healthcare professionals (physicians and nurses) (PFI) and the third intervention combined the other two (CBI). In the control group, both patients/families and physicians/nurses received the usual activities provided by the PHCP. The full study protocol has been published before.12
Study participants
The INDICA study included adults aged 18–65 years who had been diagnosed with T2DM at least 1 year before, did not have any diabetes-related complications, and used a mobile phone regularly.12 Family Care Units (FCU) in each PHCP, comprised of a family physician and a nurse, were the recruitment unit. All PHCPs included had to have at least eight FCUs and the availability of appropriate facilities to provide educational group sessions. FCUs planning or awaiting placement changes among PHCP in the first 6 months after the project began were excluded.
Setting and recruitment
PHCPs were randomly selected in the islands of Tenerife, Gran Canaria, Lanzarote, and La Palma (Canary Islands, Spain). Moreover, FCUs were randomly selected from all consenting FCUs at each PHCP. The ECRs of all potentially eligible patients in selected FCUs were screened to verify inclusion and exclusion criteria.
Patient and public involvement
Patients were actively involved in the design of the trial. Two associations of patients with T2DM in the Canary Islands were included from the beginning of the study as part of the research team, with an active participation in the design of the interventions and selection of the outcomes measured. In the same way, primary care professionals and clinical management staff participated in the elaboration of the protocol. The patients and professionals included in the study could express their satisfaction with the interventions through a questionnaire, as well as through focus groups and in-depth interviews that will be the objective of another publication. Finally, we established a commitment with patients and healthcare professionals to share the results with them in an easy-to-understand way.
Random assignment
Randomisation was applied at different levels. First, three different strata were created according to the geographic areas in the more populated islands (Tenerife and Gran Canaria). Second, four PHCP (clusters) were randomly allocated to every geographical stratum and block permutation was used to assign PHCPs to the study arms; the PHCP being the sampling unit. La Palma and Lanzarote (less populated islands) were geographically divided into four zones with only one eligible PHCP available in each zone randomly assigned to one of the study arms. In every island, all arms were equally distributed. Six FCUs were randomly selected, from all those consenting to participate in each PHCP. From all patients fulfilling inclusion criteria and consenting to participate in each PHCP, 15 were randomly selected per FCU. Exceptionally, more than six FCUs or more than 15 patients per FCU were selected, to try to recruit 90 patients in every PHCP. However, it was not possible to attain this objective of 90 patients in all PHCPs as there were insufficient patients in all FCU selected that complied with the inclusion and exclusion criteria.
FCU and patient randomisation were performed by simple generation from a list of random numbers.
Interventions
Patient interventions
Patients recruited to the PTI and CBI groups received a complex intervention of knowledge transfer and behaviour modification, informed by conceptual frameworks of behavioural change.16 The intervention combined: (1) an eight-session, conventional, group educational programme given by a nurse specialised in diabetes; (2) monitoring of physical activity, diet, drug adherence, mood, blood pressure and blood glucose readings by daily use of paper workbooks, complemented by weekly access to a website platform to upload paper workbook data; and (3) continuous, personalised feedback by semiautomated mobile phone messages (SMS), modified according to the website information.
Interventions for primary care professionals
Primary care professionals recruited to the PFI and CBI groups received a complex intervention of knowledge transfer and decision support, informed by the determinants of behaviour change suggested by Michie et al 22 for its design and implementation. The intervention included: (1) an educational and interactive group programme of two sessions to update clinical management information and promote patient-centred care; (2) an automated decision aid tool, based on a Clinical Practice Guide (CPG) for T2DM embedded into the ECR; and (3) monthly computerised graphic feedback, which displayed a set of processes and outcome indicators for all patients with T2DM of the corresponding FCU compared with other FCU in their setting and the FCU with the best results. Both interventions were applied during the 2 years follow-up.
Duration of fieldwork
Fieldwork took place between February 2013 and October 2016. The first year and the following 2 years were devoted to recruitment of patients and healthcare providers, and intervention and follow-up, respectively. As interventions were maintained over time, intervention and follow-up periods overlapped.
Outcomes
Cognitive-attitudinal outcomes
To assess potential changes in patient knowledge about T2DM and its self-management, we developed a specific instrument created in the context of this project, Diabetes Knowledge Test (DIATEK), which consisted of 30 questions. Each item has four response options and only one correct answer. Items examined risk factors for disease development and deterioration, objective values for biochemical parameters; recommendations on nutrition, physical activity, drug use and self-management. The total score, obtained by adding all correct responses, and ranging from 0 to 30, was later rescaled from 0 to 10.
The Diabetes Empowerment Scale-Short Form (DES-SF)23 is a validated questionnaire designed to evaluate psychosocial self-efficacy in diabetes. DES-SF is the short form of the original DES, which includes eight items (need for change, developing a plan, overcoming barriers, asking for support, supporting oneself, coping with emotion, motivating oneself and making diabetes care choices appropriate for one’s priorities and circumstances) with responses on a five-point Likert scale and an overall range from eight to 40, according to increasing patient empowerment.
Behavioural outcomes
The Mediterranean Diet Adherence Screener (MEDAS)24 is a validated questionnaire to assess dietary recommendation adherence, which consists of 14 targets for food consumption, rated with one point for each target attained. According to the final score, patients are classified as having low (0–6 points), moderate (7–10) or high adherence (11–14 points) to the Mediterranean diet.
The Morisky Medication Adherence Scale (MGLS)25 assesses drug-treatment adherence, by means of a validated four-item self-report instrument and a final score ranging from 0 to 4. Patients are considered adherent, only if they obtain four points.
Smoking status was monitored from baseline and during follow-up, to check for potential cessation throughout the study.
Affective outcomes
The State Trait Anxiety Inventory (STAI-S)26 is a validated patient-reporting questionnaire that includes two non-dependent scales; the applied state-anxiety scale (STAI State) and the trait-anxiety scale (STAI Trait). It assesses transient emotional state or condition as characterised by subjective feelings of tension and apprehension that can fluctuate in time and intensity. The STAI-S includes 20 items, with each item scored on a four-point Likert scale. Anxiety is defined by a cut-off point≥30.
The Beck Depression Inventory II (BDI-II)27 consists of 21 items scored on a four-point scale from 0 (‘not at all’) to 3 (‘most of the time’). The items assess depression symptoms in the last 2 weeks. All item scores are added to a maximum score of 63. A BDI-II score of ≥14 indicates mild depressive symptoms.
The Diabetes Distress Scale (DDS2)28 is a validated two-item diabetes distress-screening instrument that asks respondents to rate, on a six-point scale, the degree of distress caused by the two following items: (1) feeling overwhelmed by the demands of living with diabetes and (2) feeling that I am often failing with my diabetes regimen. High diabetes distress can be identified by an average score ≥3 or more, low distress by scores under 2, and moderate distress by the scores in between.
Health-related quality of life and symptoms
The Audit of Diabetes-Dependent Quality of life (ADDQoL-19)29 is a specific health-related quality of life (HRQoL) questionnaire for diabetes. It assesses 19 domains, each with its impact and importance index to provide an integrated score for each domain. The sum of the score in each domain forms the global score (range: −9 to 3). The lower the score, the worse the quality of life.
The Michigan Neuropathy Screening Instrument (MNSI)30 is an instrument that measures the incidence of distal diabetic peripheral polyneuropathy. It is composed of 15 self-administered items, in which the abnormal responses are added. A score of seven or more is considered abnormal.
Satisfaction
An ad-hoc self-completed questionnaire Patient Satisfaction with INDICA (INDICA-SATP) was developed to measure satisfaction with each component of the interventions in PTI and CBI groups. It was measured in the 24-month follow-up in patients who, having attended the group educational programme, also used the web platform or received the semiautomated mobile phone messages. Satisfaction with each component was valued from 0 to 10 points, with 10 reflecting maximum satisfaction.
All information, including demographic data, overall and personal health history, diabetes health status, current medications, smoking status and risk factors for complications, was obtained in a face to face interview at baseline and at 3, 6, 12, 18 and 24 months of follow-up. Similarly, all self-administered questionnaires (ADDQoL-19, BDI-II, DES-SF, DDS2, DIATEK, MEDAS, STAI-S, MGLS and MNSI) were distributed and collected at baseline, and at 12 and 24 months follow-up. ADDQol-19, MEDAS and MGLS were also applied at 6 and 18 months.
Two other questionnaires were included in the trial registry and the published protocol,12 the International Physical Activity Questionnaire and a scale developed for this project to assess patients’ attitudinal changes regarding lifestyles (INDICA-LSQ). However, the data quality checking identified many inconsistent or meaningless responses to these questionnaires, which indicates that patients did not correctly understand the instructions. Therefore, we decided to exclude them from the analyses.
Statistical analysis
Multilevel mixed models including the baseline value of dependent variables and time elapsed since diagnosis (in years) as covariates were implemented for all PROMs. First level variables are those corresponding to each measurement along follow-up (repeated time measurements). The second level includes patient variables (the baseline value of dependent variables and time elapsed since diagnosis) and third level variables correspond to PHCP in which patients are grouped (the variable arm to which PHCP was assigned is included in this level). The effect that identifies the intervention arm has been considered fixed for the different PHCP, while the intercept has been considered random. The model also included an interaction term between arm and month, which allows for differences in the intervention effect between follow-up assessments.31 The intraclass correlation coefficient (ICC) was obtained for each model for the PHCP and by patient according to their PHCP. The adjusted estimated mean was calculated for each follow-up moment compared with baseline; and its statistical significance was calculated by means of the model already set out. The relative improvement for each follow-up was obtained as the ratio between the adjusted difference in mean between the intervention and control group and the mean of the control group.
A logistic regression model was implemented to compare the proportion of patients who quit smoking at each follow-up, by intervention arm. Only basal smokers were included in the analysis.
Analysis was performed on an intention-to-treat basis, that is, participants were analysed in the group to which they were randomised. Missing values were treated by means of multiple imputation procedures,32 with results based on 100 imputed datasets (missing values from all follow-up visits were imputed). Analysis under multiple imputation is valid for randomly missing data.33 We compared the results of imputed and non-imputed data. All the analyses were conducted using STATA V.15.0.34 Differences were considered statistically significant if p<0.05.
Results
Study participants
A total of 2334 patients and 211 healthcare professionals were included. Figure 1 shows the flowchart with cluster randomisation of patients for each intervention, attendance at educational/training sessions of patients and professionals and the number of PROMs questionnaires received for each follow-up assessment. The patients’ baseline characteristic according to the intervention assignment can be seen in Ramallo-Fariña et al.13 Mean age of the whole population was 55.7±7.1 years, with 51.9% women. Mean baseline HbA1c was 7.3%/56 mmol/mol. From baseline, 49.4% of patients started with HbA1c levels within the accepted therapeutic goal (≤7%/53 mmol/mol). There were no statistically significant differences among groups in terms of their baseline characteristics.
Figure 1.
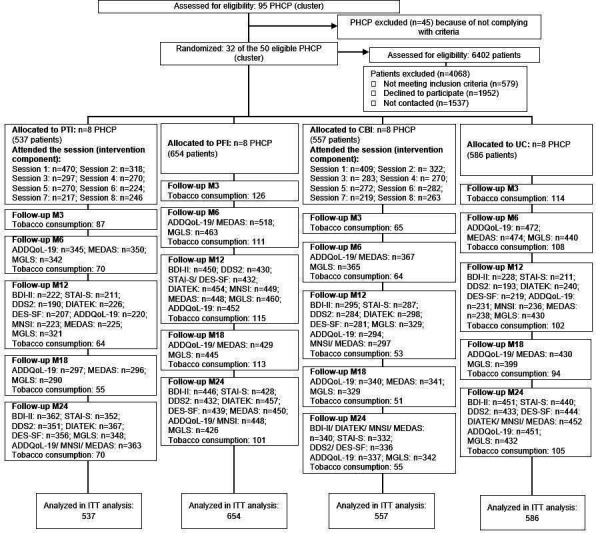
Consolidated Standards of Reporting Trials flow diagram. ADDQoL, Audit of Diabetes-Dependent Quality of life; BDI-II, Beck Depression Inventory II; CBI, combined intervention for patients and professionals; DDS2, Diabetes Distress Scale; DES-SF, Diabetes Empowerment Scale-Short Form; MEDAS, Mediterranean Diet Adherence Screener; MGLS, Morisky Medication Adherence Scale; MNSI, Michigan Neuropathy Screening Instrument; PFI, intervention for professionals; PHCP, Primary Care Health Practices; PTI, intervention for patients; STAI-S, State Trait Anxiety Inventory.
Intention-to-treat results (ITT), reported below, were very similar to those obtained with non-imputed data. Only three discrepancies were observed that will be discussed in the corresponding outcome section. Results at all time points are shown in table 1 (intergroup differences), tables 2 and 3 (intragroup changes).
Table 1.
Adjusted difference in the mean of each group compared with the control group
| 6 Months | P value | 12 Months | P value | 18 Months | P value | 24 Months | P value | |
| Cognitive-attitudinal outcomes | ||||||||
| Knowledge (DIATEK): F=47.3 P<0.001; ICC PHCP=0.06; ICC subject|PHCP=0.35 | ||||||||
| PTI | – | – | 0.64 (0.17 to 1.11) | 0.007 | – | – | 0.65 (0.2 to 1.11) | 0.005 |
| PFI | – | – | −0.38 (−0.85 to 0.09) | 0.11 | – | – | −0.6 (−1.06 to −0.14) | 0.01 |
| CBI | – | – | 0.63 (0.16 to 1.11) | 0.008 | – | – | 0.34 (−0.12 to 0.8) | 0.15 |
| Empowerment (DES-SF): F=17.3 P<0.001; ICC PHCP=0.08; ICC; subject|PHCP=0.08 | ||||||||
| PTI | – | – | 1.58 (−0.59 to 3.75) | 0.15 | – | – | 3.04 (1.08 to 4.99) | 0.002 |
| PFI | – | – | 3.95 (1.9 to 6) | <0.001 | – | – | 1.84 (−0.11 to 3.79) | 0.07 |
| CBI | – | – | 3.97 (1.9 to 6.04) | <0.001 | – | – | 2.63 (0.68 to 4.58) | 0.008 |
| Behavioural outcomes | ||||||||
| Adherence dietary recommendations (MEDAS): F=25.0 P<0.001; ICC PHCP=0.03; ICC subject|PHCP=0.20 | ||||||||
| PTI | 0.22 (−0.25 to 0.69) | 0.36 | 0.71 (0.17 to 1.24) | 0.01 | 0.93 (0.46 to 1.41) | <0.001 | 0.87 (0.4 to 1.35) | <0.001 |
| PFI | −0.58 (−1.04 to −0.11) | 0.01 | −0.96 (−1.46 to −0.47) | <0.001 | 0.17 (−0.31 to 0.64) | 0.49 | 0.03 (−0.44 to 0.5) | 0.90 |
| CBI | 0.44 (−0.03 to 0.91) | 0.06 | 0.06 (−0.47 to 0.58) | 0.83 | 0.88 (0.4 to 1.35) | <0.001 | 0.7 (0.22 to 1.17) | 0.004 |
| Medication adherence (MGLS): F=14.4 P<0.001; ICC PHCP=0.04; ICC subject|PHCP=0.20 | ||||||||
| PTI | 0.09 (−0.11 to 0.3) | 0.37 | 0.09 (−0.12 to 0.3) | 0.39 | 0.13 (−0.09 to 0.34) | 0.24 | 0.16 (−0.04 to 0.36) | 0.12 |
| PFI | 0.01 (−0.2 to 0.22) | 0.90 | −0.13 (−0.34 to 0.08) | 0.24 | −0.06 (−0.26 to 0.15) | 0.58 | 0.09 (−0.11 to 0.3) | 0.39 |
| CBI | 0.03 (−0.18 to 0.24) | 0.77 | −0.19 (−0.41 to 0.03) | 0.08 | 0 (−0.21 to 0.21) | 0.98 | −0.1 (−0.31 to 0.11) | 0.36 |
| Affective outcomes | ||||||||
| Depression (BDI-II): F=53.6 P<0.001; ICC PHCP=0.05; ICC subject|PHCP=0.34 | ||||||||
| PTI | – | – | −1.91 (−3.99 to 0.17) | 0.07 | – | – | −0.76 (−2.68 to 1.16) | 0.44 |
| PFI | – | – | −2.99 (−4.99 to −1) | 0.003 | – | – | 0.37 (−1.56 to 2.3) | 0.71 |
| CBI | – | – | −3 (−5.13 to −0.87) | 0.006 | – | – | 0.23 (−1.73 to 2.19) | 0.82 |
| Anxiety (STAI-S): F=36.0 P<0.001; ICC PHCP=0.07 ICC; subject|PHCP=0.32 | ||||||||
| PTI | – | – | −2.25 (−5.75 to 1.25) | 0.21 | – | – | −2.18 (−5.54 to 1.18) | 0.20 |
| PFI | – | – | −3.47 (−6.95 to 0.02) | 0.05 | – | – | −0.39 (−3.78 to 2.99) | 0.82 |
| CBI | – | – | −5.4 (−8.99 to −1.81) | 0.003 | – | – | −0.50 (−3.9 to 2.9) | 0.77 |
| Distress (DDS2): F=14.9 P<0.001; ICC PHCP=0.05 ICC; subject|PHCP=0.25 | ||||||||
| PTI | – | – | −0.23 (−0.53 to 0.07) | 0.13 | – | – | −0.34 (−0.62 to −0.07) | 0.01 |
| PFI | – | – | −0.24 (−0.53 to 0.05) | 0.10 | – | – | −0.31 (−0.58 to −0.04) | 0.03 |
| CBI | – | – | −0.36 (−0.65 to −0.07) | 0.01 | – | – | −0.24 (−0.51 to 0.03) | 0.08 |
| Health-related quality of life and symptoms | ||||||||
| Health-related quality of life (ADDQoL-19): F=25.3 P<0.001; ICC PHCP=0.04; ICC subject|PHCP=0.34 | ||||||||
| PTI | 0.09 (−0.24 to 0.42) | 0.60 | 0.40 (0.04 to 0.76) | 0.03 | 0.39 (0.05 to 0.72) | 0.02 | 0.16 (−0.17 to 0.48) | 0.34 |
| PFI | −0.09 (−0.42 to 0.23) | 0.56 | 0.51 (0.16 to 0.86) | 0.005 | −0.02 (−0.35 to 0.31) | 0.89 | −0.06 (−0.38 to 0.26) | 0.71 |
| CBI | 0.03 (−0.3 to 0.35) | 0.88 | 0.84 (0.49 to 1.18) | <0.001 | 0.21 (−0.13 to 0.54) | 0.23 | −0.05 (−0.38 to 0.28) | 0.77 |
| Neuropathic symptom (MNSI): F=59.8 P<0.001; ICC PHCP=0.02 ICC; subject|PHCP=0.32 | ||||||||
| PTI | – | – | −0.35 (−0.8 to 0.09) | 0.12 | – | – | −0.08 (−0.49 to 0.33) | 0.70 |
| PFI | – | – | −0.42 (−0.87 to 0.03) | 0.07 | – | – | 0.35 (−0.07 to 0.78) | 0.11 |
| CBI | – | – | −0.57 (−1.04 to −0.1) | 0.02 | – | – | 0.31 (−0.12 to 0.74) | 0.16 |
The models were adjusted by the baseline value of dependent variables and time elapsed since diagnosis.
ADDQoL, Audit of Diabetes-Dependent Quality of life; BDI-II, Beck Depression Inventory II; CBI, combined intervention for patients and professionals; DDS2, Diabetes Distress Scale; DES-SF, Diabetes Empowerment Scale-Short Form; ICC, intraclass correlation coefficient; MEDAS, Mediterranean Diet Adherence Screener; MGLS, Morisky Medication Adherence Scale; MNSI, Michigan Neuropathy Screening Instrument; PFI, intervention only for healthcare professionals at primary care; PHCP, Primary Care Health Practices; PTI, intervention only for patients and family members; STAI-S, State Trait Anxiety Inventory.
Table 2.
Adjusted means for each group and intragroup differences compared with the baseline measurement (cognitive-attitudinal, behavioural and affective outcomes)
| Adjusted means in each group (95% CI) | Difference in intragroup of adjusted means compared with baseline (95% CI) | ||||||||||||
| Baseline | 6 Months | 12 Months | 18 Months | 24 Months | B-6M | P value | B-12M | P value | B-18M | P value | B-24M | P value | |
| Cognitive-attitudinal outcomes | |||||||||||||
| Knowledge (DIATEK) | |||||||||||||
| PTI | 6.4 (6.3 to 6.5) | – | 7.2 (6.9 to 7.5) | – | 7.4 (7.1 to 7.7) | – | – | 0.82 (0.48 to 1.2) | <0.001 | – | – | 1.03 (0.71 to 1.36) | <0.001 |
| PFI | 6.5 (6.3 to 6.7) | – | 6.2 (5.8 to 6.5) | – | 6.1 (5.8 to 6.5) | – | – | −0.31 (−0.63 to 0.02) | 0.07 | – | – | −0.32 (−0.64 to 0.01) | 0.058 |
| CBI | 6.5 (6.4 to 6.6) | – | 7.2 (6.8 to 7.5) | – | 7.1 (6.8 to 7.4) | – | – | 0.7 (0.36 to 1.03) | <0.001 | – | – | 0.6 (0.27 to 0.94) | <0.001 |
| UC | 6.2 (6.1 to 6.3) | – | 6.5 (6.2 to 6.9) | – | 6.7 (6.4 to 7.1) | – | – | 0.3 (−0.04 to 0.63) | 0.08 | – | – | 0.5 (0.18 to 0.82) | 0.002 |
| Empowerment (DES−SF) | |||||||||||||
| PTI | 26.4 (25.8 to 27.0) | – | 29.5 (27.9 to 31.0) | – | 33.5 (32.1 to 34.9) | – | – | 3.08 (1.6 to 4.6) | <0.001 | – | – | 7.1 (5.7 to 8.5) | <0.001 |
| PFI | 26.3 (25.2 to 27.4) | – | 31.9 (30.5 to 33.2) | – | 32.3 (30.9 to 33.7) | – | – | 5.6 (4.2 to 6.9) | <0.001 | – | – | 6.02 (4.7 to 7.4) | <0.001 |
| CBI | 27.6 (27.0 to 28.3) | – | 31.9 (30.4 to 33.3) | – | 33.1 (31.7 to 34.5) | – | – | 4.3 (2.8 to 5.7) | <0.001 | – | – | 5.7 (4.1 to 6.9) | <0.001 |
| UC | 26.1 (25.5 to 26.7) | – | 27.9 (26.4 to 29.4) | – | 30.5 (29.1 to 31.8) | – | – | 1.8 (0.26 to 3.3) | 0.02 | – | – | 4.3 (2.9 to 5.7) | <0.001 |
| Behavioural outcomes | |||||||||||||
| Adherence dietary recommendations (MEDAS) | |||||||||||||
| PTI | 8 (7.8 to 8.1) | 7.6 (7.2 to 7.9) | 9.1 (8.7 to 9.4) | 8.3 (7.9 to 8.6) | 8.7 (8.3 to 9) | −0.43 (−0.77 to −0.09) | 0.01 | 1.1 (0.71 to 1.5) | <0.001 | 0.27 (−0.07 to 0.62) | 0.12 | 0.68 (0.34 to 1.02) | <0.001 |
| PFI | 8.2 (7.9 to 8.5) | 6.8 (6.4 to 7.1) | 7.4 (7.1 to 7.7) | 7.5 (7.1 to 7.8) | 7.8 (7.5 to 8.2) | −1.5 (−1.8 to −1.1) | <0.001 | −0.82 (−1.2 to −0.49) | <0.001 | −0.82 (−1.2 to −0.49) | <0.001 | −0.4 (−0.74 to −0.07) | 0.018 |
| CBI | 8.3 (8.1 to 8.5) | 7.8 (7.4 to 8.1) | 8.4 (8.0 to 8.8) | 8.2 (7.9 to 8.5) | 8.5 (8.1 to 8.8) | −0.51 (−0.84 to −0.17) | 0.003 | 0.13 (−0.24 to 0.51) | 0.49 | −0.08 (−0.43 to 0.26) | 0.63 | 0.2 (−0.14 to 0.54) | 0.26 |
| UC | 8.02 (7.9 to 8.2) | 7.3 (7.0 to 7.7) | 8.4 (8.0 to 8.7) | 7.3 (7.0 to 7.7) | 7.8 (7.5 to 8.1) | −0.69 (−1.0 to −0.36) | <0.001 | 0.34 (−0.02 to 0.7) | 0.07 | −0.7 (−1.0 to −0.37) | <0.001 | −0.24 (−0.57 to 0.1) | 0.16 |
| Medication adherence (MGLS) | |||||||||||||
| PTI | 3.1 (3.1 to 3.2) | 3.5 (3.4 to 3.7) | 3.6 (3.4 to 3.7) | 3.6 (3.5 to 3.8) | 3.6 (3.5 to 3.7) | 0.41 (0.26 to 0.56) | <0.001 | 0.45 (0.29 to 0.6) | <0.001 | 0.5 (0.35 to 0.65) | <0.001 | 0.48 (0.33 to 0.62) | <0.001 |
| PFI | 3.3 (3.2 to 3.3) | 3.5 (3.3 to 3.6) | 3.3 (3.2 to 3.5) | 3.4 (3.3 to 3.6) | 3.5 (3.4 to 3.7) | 0.18 (0.03 to 0.33) | 0.02 | 0.08 (−0.07 to 0.22) | 0.32 | 0.16 (0.02 to 0.31) | 0.026 | 0.25 (0.11 to 0.4) | 0.001 |
| CBI | 3.3 (3.3 to 3.3) | 3.5 (3.3 to 3.6) | 3.3 (3.1 to 3.4) | 3.5 (3.3 to 3.6) | 3.3 (3.2 to 3.5) | 0.17 (0.02 to 0.32) | 0.02 | −0.01 (−0.18 to 0.15) | 0.87 | 0.2 (0.05 to 0.35) | 0.01 | 0.04 (−0.11 to 0.2) | 0.60 |
| UC | 3.2 (3.1 to 3.3) | 3.4 (3.3 to 3.6) | 3.5 (3.3 to 3.6) | 3.5 (3.3 to 3.6) | 3.4 (3.3 to 3.6) | 0.23 (0.08 to 0.38) | 0.002 | 0.27 (0.12 to 0.42) | <0.001 | 0.29 (0.14 to 0.43) | <0.001 | 0.23 (0.08 to 0.37) | 0.002 |
| Affective outcomes | |||||||||||||
| Depression (BDI−II) | |||||||||||||
| PTI | 10.9 (10.4 to 11.5) | – | 8.5 (7.1 to 9.9) | – | 6.1 (4.7 to 7.5) | – | – | −2.4 (−3.7 to −0.96) | 0.001 | – | – | −4.9 (−6.2 to −3.5) | <0.001 |
| PFI | 11.0 (9.9 to 12.1) | – | 7.5 (6.1 to 8.8) | – | 7.2 (5.8 to 8.6) | – | – | −3.6 (−4.9 to −2.2) | <0.001 | – | – | −3.8 (−5.2 to −2.4) | <0.001 |
| CBI | 11.7 (10.9 to 12.4) | – | 7.5 (5.9 to 8.9) | – | 7.1 (5.7 to 8.5) | – | – | −4.2 (−5.7 to −2.7) | <0.001 | – | – | −4.6 (−5.9 to −3.1) | <0.001 |
| UC | 11.4 (10.9 to 11.9) | – | 10.5 (8.9 to 11.9) | – | 6.7 (5.5 to 8.2) | – | – | −0.94 (−2.4 to 0.55) | 0.22 | – | – | −4.5 (−5.9 to −3.2) | <0.001 |
| Anxiety (STAI−S) | |||||||||||||
| PTI | 21.5 (20.7 to 22.2) | – | 18.4 (15.9 to 20.9) | – | 14.5 (12.0 to 16.9) | – | – | −3.0 (−5.5 to −0.55) | 0.017 | – | – | −7 (−9.4 to −4.6) | <0.001 |
| PFI | 20.6 (18.8 to 22.4) | – | 17.2 (14.8 to 19.6) | – | 16.2 (13.8 to 18.7) | – | – | −3.4 (−5.8 to −1) | 0.006 | – | – | −4.4 (−6.8 to −1.9) | <0.001 |
| CBI | 23.2 (22.0 to 24.3) | – | 15.3 (12.8 to 17.8) | – | 16.1 (13.7 to 18.6) | – | – | −7.9 (−10.4 to −5.4) | <0.001 | – | – | −7.0 (−9.5 to −4.6) | <0.001 |
| UC | 21.9 (21.2 to 22.7) | – | 20.7 (18.1 to 23.2) | – | 16.6 (14.3 to 19.0) | – | – | −1.3 (−3.8 to 1.3) | 0.32 | – | – | −5.3 (−7.7 to −2.9) | <0.001 |
| Distress (DDS2) | |||||||||||||
| PTI | 2.8 (2.6 to 2.8) | – | 1.9 (1.7 to 2.2) | – | 1.6 (1.4 to 1.8) | – | – | −0.72 (−0.93 to −0.51) | <0.001 | – | – | −1.1 (−1.2 to −0.86) | <0.001 |
| PFI | 2.5 (2.3 to 2.6) | – | 1.9 (1.8 to 2.1) | – | 1.7 (1.5 to 1.9) | – | – | −0.5 (−0.7 to −0.31) | <0.001 | – | – | −0.79 (−0.98 to −0.6) | <0.001 |
| CBI | 2.7 (2.6 to 2.8) | – | 1.8 (1.6 to 2.0) | – | 1.7 (1.5 to 1.9) | – | – | −0.91 (−1.1 to −0.71) | <0.001 | – | – | −1.01 (−1.2 to −0.82) | <0.001 |
| UC | 2.6 (2.5 to 2.6) | – | 2.1 (1.9 to 2.4) | – | 1.97 (1.8 to 2.2) | – | – | −0.36 (−0.58 to −0.15) | 0.001 | – | – | −0.58 (−0.77 to −0.39) | <0.001 |
The models were adjusted by the baseline value of dependent variables and time elapsed since diagnosis.
B, baseline; BDI-II, Beck Depression Inventory II; CBI, combined intervention for patients and professionals; DDS2, Diabetes Distress Scale; DES-SF, Diabetes Empowerment Scale-Short Form; M, month; MEDAS, Mediterranean Diet Adherence Screener; MGLS, Morisky Medication Adherence Scale; PFI, intervention only for healthcare professionals at primary care; PTI, intervention only for patients and family members; STAI-S, State Trait Anxiety Inventory; UC, usual care or control group.
Table 3.
Proportion of patients who stop smoking at each follow-up compared with the control group
| PTI (n=114) | PFI (n=156) | CBI (n=109) | UC (n=145) | P value global | P value PTI versus UC |
P value PFI versus UC |
P value CBI versus UC |
|
| 3 Months | 12.8 | 8.7 | 15.4 | 10.4 | 0.54 | 0.99 | 0.99 | 0.99 |
| 6 Months | 28.5 | 7.5 | 24.2 | 15.4 | 0.003 | 0.11 | 0.22 | 0.99 |
| 12 Months | 33.1 | 17.4 | 28.4 | 14.3 | 0.014 | 0.018 | 0.99 | 0.11 |
| 18 Months | 36.7 | 19.6 | 37.6 | 18.8 | 0.004 | 0.04 | 0.99 | 0.03 |
| 24 Months | 41.5 | 23.4 | 42.3 | 21.2 | 0.002 | 0.012 | 0.99 | 0.012 |
Only basal smokers were included in the analysis.
CBI, combined intervention for patients and professionals; PFI, intervention only for healthcare professionals at primary care; PTI, intervention only for patients and family members; UC, usual care or control group.
Cognitive-attitudinal outcomes
Table 1 shows that the level of knowledge about diabetes is significantly higher for PTI (p=0.007) and CBI (p=0.008), compared with UC at 12 months; and for PTI (p=0.005) at 24 months.
Patient empowerment was significantly higher for PFI and CBI groups, compared with UC at 12 months (p<0.001 for both comparisons). Analysis of non-imputed data led to a p value of 0.05 for the difference between PTI and UC, favouring the former, at this time point. At 24 months, PTI and CBI also attained significantly higher scores than UC (p=0.002 and p=0.008, respectively); while differences with PFI are marginally significant.
Behavioral outcomes
Table 1 shows that the PTI group is significantly more adherent to the diet recommendations, compared with UC, after 12 months of follow-up. There is a difference of 0.87 (p<0.001) at 24 months. Adherence improves for CBI from 18 months, compared with UC, with differences of 0.7 (p=0.004) at 24 months. Adherence levels remain moderate for all patient groups throughout follow-up (see table 2).
No differences were found in medication adherence, compared with UC (table 1). However, average levels of medication adherence were significantly improved in all four groups, despite the high baseline levels (>3) (see table 2).
Table 3 shows the reduction in the proportion of smokers who quit smoking during follow-up in PTI (12 months), and CBI (18 months), compared with UC. With non-imputed data the reduction was statistically significant from month 6 for PTI (p=0.023) and month 12 for CBI (p=0.025). The percentage of patients who quit smoking at 24 months was 41.5% for PTI (p=0.012) and 42.3% (p=0.012) for CBI, versus 21.2% for UC group. There were no statistically significant differences between groups in the baseline percentage of smokers (p=0.99).
Affective outcomes
Compared with UC, both PFI and CBI show statistically significant differences at 12 months for depression (p=0.003 and p=0.006, respectively), and anxiety (p=0.05 and p=0.003, respectively) (table 1). These differences disappear at 24 months because all groups of patients improved (table 2).
The diabetes distress score improved significantly compared with the UC group for CBI at 12 months (p=0.01) and for PTI and PFI at 24 months (p=0.01 and p=0.03, respectively). The score remained marginally significant for CBI (table 1). At baseline, all patient groups showed moderate distress, which decreased to a low level from 12 months, except for the UC group, which did so at 24 months (table 2).
Health-related quality of life and symptoms
HRQoL significantly improved for all intervention groups, at 12 months, compared with UC; a difference only maintained for PTI at 18 months (p=0.02) (table 1).
Neuropathic symptom scores were significantly lower for the CBI group at 12 months (p=0.02) compared with the UC group (the analysis of non-imputed data led to a non-significant result, p=0.12). This difference disappeared at 24 months (table 1). Mean baseline scores for all groups were under 4, considerably below the cut-off point of 7 for abnormal classification (table 4).
Table 4.
Adjusted means for each group and intragroup differences compared with the baseline measurement (health-related quality of life and symptoms)
| Adjusted means in each group (95% CI) | Difference in intragroup of adjusted means compared with baseline (95% CI) | ||||||||||||
| Baseline | 6 Months | 12 Months | 18 Months | 24 Months | B-6M | P value | B-12M | P value | B-18M | P value | B-24M | P value | |
| Health-related quality of life and symptoms | |||||||||||||
| Health-related quality of life (ADDQoL-19) | |||||||||||||
| PTI | −1.7 (−1.8 to −1.6) | −1.0 (−1.3 to −0.8) | −1.2 (−1.5 to −0.97) | −0.85 (−1.1 to −0.61) | −0.76 (−0.99 to −0.53) | 0.69 (0.46 to 0.93) | <0.001 | 0.52 (0.27 to 0.76) | <0.001 | 0.89 (0.65 to 1.1) | <0.001 | 0.97 (0.74 to 1.2) | <0.001 |
| PFI | −1.7 (−1.8 to −1.5) | −1.2 (−1.5 to −1) | −1.1 (−1.3 to −0.88) | −1.3 (−1.5 to −1.0) | −0.98 (−1.2 to −0.75) | 0.43 (0.21 to 0.66) | <0.001 | 0.55 (0.32 to 0.78) | <0.001 | 0.4 (0.17 to 0.63) | 0.001 | 0.68 (0.45 to 0.9) | <0.001 |
| CBI | −1.8 (−1.9 to −1.6) | −1.1 (−1.3 to −0.87) | −0.78 (−1.0 to −0.54) | −1.0 (−1.3 to −0.79) | −0.97 (−1.2 to −0.73) | 0.65 (0.42 to 0.88) | <0.001 | 0.98 (0.74 to 1.2) | <0.001 | 0.73 (0.49 to 0.96) | <0.001 | 0.78 (0.54 to 1.0) | <0.001 |
| UC | −2.1 (−2.2 to −1.9) | −1.1 (−1.4 to −0.9) | −1.6 (−1.9 to −1.4) | −1.2 (−1.5 to −1) | −0.92 (−1.2 to −0.69) | 0.92 (0.7 to 1.2) | <0.001 | 0.44 (0.18 to 0.7) | 0.001 | 0.82 (0.59 to 1.1) | <0.001 | 1.1 (0.9 to 1.4) | <0.001 |
| Neuropathic symptom (MNSI) | |||||||||||||
| PTI | 3.1 (3 to 3.2) | – | 2.8 (2.5 to 3.1) | – | 2.4 (2.1 to 2.7) | – | – | −0.29 (−0.61 to 0.02) | 0.07 | – | – | −0.69 (−0.99 to −0.4) | <0.001 |
| PFI | 3.3 (3.0 to 3.6) | – | 2.8 (2.5 to 3.1) | – | 2.9 (2.5 to 3.2) | – | – | −0.55 (−0.86 to −0.23) | 0.001 | – | – | −0.45 (−0.76 to −0.13) | 0.005 |
| CBI | 3.3 (3.1 to 3.4) | – | 2.6 (2.3 to 2.9) | – | 2.8 (2.5 to 3.1) | – | – | −0.67 (−1.0 to −0.31) | <0.001 | – | – | −0.46 (−0.78 to −0.13) | 0.006 |
| UC | 3.3 (3.2 to 3.5) | – | 3.1 (2.9 to 3.5) | – | 2.5 (2.2 to 2.8) | – | – | −0.15 (−0.47 to 0.17) | 0.36 | – | – | −0.82 (−1.1 to −0.54) | <0.001 |
The models were adjusted by the baseline value of dependent variables and time elapsed since diagnosis.
ADDQoL-19, Audit of Diabetes-Dependent Quality of life; B, baseline; CBI, combined intervention for patients and professionals; M, month; MNSI, Michigan Neuropathy Screening Instrument; PFI, intervention only for healthcare professionals at primary care; PTI, intervention only for patients and family members; UC, usual care or control group.
Satisfaction
Table 5 shows the patients’ satisfaction with the intervention received. While average scores were higher than 9/10, in all dimensions, for the group educational sessions, satisfaction with the web platform and SMS obtained scores above 8.
Table 5.
Patient satisfaction with the intervention received (only those who made use of each intervention component)
| n | Mean (95% CI) | |
| Conventional group educational programme | ||
| Usability | ||
| Environment generated | 592 | 9.53 (9.46 to 9.60) |
| Exchange of experiences with participants and educator | 588 | 9.59 (9.53 to 9.66) |
| Educator’s work | 587 | 9.79 (9.74 to 9.83) |
| Quality of materials | 587 | 9.56 (9.49 to 9.64) |
| Personal satisfaction | ||
| The sessions helped me get to know my diabetes better | 591 | 9.67 (9.61 to 9.73) |
| I found the sessions useful | 593 | 9.60 (9.52 to 9.67) |
| The sessions motivated me to look after my health better | 590 | 9.62 (9.55 to 9.69) |
| General | ||
| General satisfaction | 589 | 9.70 (9.65 to 9.76) |
| I would recommend the sessions | 588 | 9.77 (9.72 to 9.82) |
| Website platform | ||
| Usability | ||
| Access to the content | 253 | 8.30 (8.02 to 8.58) |
| Usability of the web | 251 | 8.59 (8.33 to 8.85) |
| Patient outcomes follow-up charts | 215 | 8.37 (8.03 to 8.72) |
| Quality of materials | 229 | 8.81 (8.53 to 9.08) |
| Access to videos of the sessions | 216 | 8.76 (8.47 to 9.05) |
| General | ||
| General satisfaction | 237 | 8.56 (8.30 to 8.82) |
| I would recommend using the website | 239 | 8.81 (8.56 to 9.05) |
| Semi-automated mobile phone messages | ||
| Usability | ||
| Reading SMS | 585 | 9.51 (9.41 to 9.61) |
| Usefulness of reminders | 576 | 9.33 (9.22 to 9.45) |
| Personal satisfaction | ||
| They adapt to my needs | 579 | 9.04 (8.90 to 9.18) |
| They motivate me to look after myself | 576 | 9.15 (9.02 to 9.28) |
| I would like to continue receiving them | 552 | 8.80 (8.59 to 9.00) |
| General | ||
| General satisfaction | 572 | 9.23 (9.09 to 9.37) |
Table 6 shows a summary of the results at 12 and 24 months. For all PROMs, ICC values were close to zero at the PHCP levelthus reflected a very small effect associated with PHCP for interventions and control groups (similar results among PHCP in every arm). The ICC at the patient level was broad, accounting for considerable variations among individuals.
Table 6.
Significant differences compared with usual care for the three intervention groups
| PTI | PFI | CBI | ||||
| 12 months | 24 months | 12 months | 24 months | 12 months | 24 months | |
| Cognitive/attitudinal | ||||||
| Knowledge (DIATEK) | ** | ** | ↓** | ** | ||
| Empowerment (DES-SF) | ** | *** | *** | ** | ||
| Behavioural | ||||||
| Diet (MEDAS) | ** | *** | ↓*** | ** | ||
| Adherence (MGLS) | ||||||
| Smoking | * | * | * | * | ||
| Affective | ||||||
| Depression (BDI-II) | ** | ** | ||||
| Anxiety (STAI-S) | * | ** | ||||
| Diabetes Distress (DDS2) | ** | * | ** | |||
| HRQOL | ||||||
| HRQoL (ADDQoL-19) | * | ** | *** | |||
| Neuropathy (MNSI) | * | |||||
↓Represent worsening compare to usual care.
*P≤0.05. **P≤0.01. ***P≤0.001.
ADDQoL, Audit of Diabetes-Dependent Quality of life; BDI-II, Beck Depression Inventory II; CBI, combined intervention for patients and professionals; DDS2, Diabetes Distress Scale; DES-SF, Diabetes Empowerment Scale-Short Form; MEDAS, Mediterranean Diet Adherence Screener; MGLS, Morisky Medication Adherence Scale; MNSI, Michigan Neuropathy Screening Instrument; PFI, intervention only for healthcare professionals at primary care; PTI, intervention only for patients and family members; STAI-S, State Trait Anxiety Inventory.
Discussion
This article assesses the effect of interventions implemented by the INDICA study to improve T2DM outcomes on several health measures self-perceived by patients in the cognitive-attitudinal (knowledge, empowerment), behavioural (ie, adherence to the dietary recommendations, medication and tobacco use), affective (anxiety, depression, distress) and health-related quality of life dimensions. The INDICA study is a pragmatic cluster-randomised study with 2 years follow-up that assesses the effectiveness of multicomponent interventions for knowledge transfer and behaviour modification of patients, families and healthcare professionals (physicians and nurses) at the primary care level.
At 1 year follow-up, the combined intervention lead to obtaining significant results in all outcomes except diet and medication adherence. Relative improvements compared with UC ranged between 9.6% (knowledge) and 52.2% (HRQoL), with intermediate values for anxiety (26.1%) and depression (28.7%). Significant improvements in HRQoL were also obtained for the PTI and PFI groups, although of less intensity (24.8% and 31.7%, respectively). However, they showed different results in the remaining variables: the PTI group improved in terms of knowledge and behavioural outcomes (ie, diet and smoking), while the PFI improved in regard to empowerment and depression, but obtained a significantly worse result than the UC group for diet adherence.
After 2 years of follow-up, there were no significant differences in HRQoL, anxiety or depression, mainly due to the improvement experienced by the UC group in these variables. The PTI group obtained the best overall results, with significant improvements in the cognitive (ie, knowledge, empowerment), affective (ie, diabetes distress) and behavioural (ie, diet and tobacco) variables. The same significant results were obtained for the combined intervention, except for knowledge and distress. Finally, the PFI group outperformed UC only for distress, and showed a significantly worse result in regard to knowledge. There were no statistically significant differences in medication adherence during all the follow-up, although a ceiling effect could have occurred, since all groups showed high scores at baseline.
Therefore, the best results were observed in both groups including patients (PTI and CBI), similar to the findings observed on clinical outcomes.13 This is not surprising, given the straightforward and continuous application of these patient interventions, and the high reported satisfaction levels with all the intervention components (educational sessions, web resources and SMS). Previous studies that combined education and training with support phone calls, assessing interventions aimed at empowering diabetes patients to improve self-care and outcomes, showed inconsistent results between clinical variables and PROMs.8 9 The use of one-way messages such as those used in INDICA appears to significantly and consistently improve HbA1c levels, although with a small-to-moderate effect-size (−0.38%, 95% CI −0.53 to −0.23).10 In addition, continuous advances in smart mobile technology provide new possibilities for diabetes self-management, despite the fact that evidence on the effectiveness of these new functionalities remains scarce and uncertain.11 35
Reduction in the number of smokers in interventions applied directly to patients (PTI and CBI) in regard to UC that remain significant at 24 months with percentages of approximately 42% which is 2.5 times the result obtained by the most extended pharmacological intervention (replacement nicotine therapy). This is according to a meta-analysis published recently36 which puts this reduction at 16.9% of the intervened group compared with 10.4% of the control group in studies with follow-up varying from 6 to 24 months.
The intervention effect on professionals raises questions. At 1 year of follow-up, the PFI and CBI groups obtained improvements in psychological variables not affected by the intervention targeted exclusively at patients (PTI) (ie, empowerment, anxiety, depression). These findings could be interpreted as the lasting result of better shared decision-making/patient-centred care by professionals trained in this care model. However, the PTI group was the only group to show significant improvements in behavioural variables (diet adherence and tobacco consumption); while PFI obtained significantly worse results for diet adherence from the sixth month, and CBI did not show significant benefits for these two outcomes until 18 months. These negative findings from groups containing professionals are repeated after 2 years in the case of knowledge, a variable in which the CBI group did not obtain significant differences. This interpretation should be considered cautiously given the analysis limitations, since the differences between intervention groups have not been statistically contrasted. As a recent Cochrane review37 reported, current evidence on the effect of interventions to promote shared decision-making by healthcare professionals shows benefits when decision-making is assessed by external observers but not by patient’s assessment; furthermore, no significant effects were observed in most patient-reported outcomes.37 Given the paucity and limited quality of available studies, more focused research is needed to draw solid conclusions about the effect of interventions aimed at professionals, and the mechanisms by which these interventions translate into psychological, behavioural and health changes of patients.
The assessment of clinical outcome measures in the INDICA study13 for the total sample recruited regardless of Hb1Ac levels (only 50.6% of all participants had baseline HbA1c concentrations>7%, with a mean of 7.3%), showed an early and significant but temporary reduction in HbA1c for the PTI group, compared with UC, from 3 to 6 months. Even so, more than 30% of the intervened patients (PTI and CBI) attained statistically and clinically relevant reductions in HbA1c (>0.4%); significantly higher than UC at 12 and 18 months.
In the group of patients with baseline HbA1c greater than 7% (uncontrolled patients), the magnitude of the intervention effect on clinical outcomes was greater, especially in the PTI group compared with the UC group, with significant differences up to 18 months, and a significant area under the curve at 24 months for PTI compared with UC.13 These results are supported by other studies that report greater intervention effects in patients with higher HbA1c levels.38 39 Longer term reductions in blood pressure were also found in the two groups in which professionals were intervened, with smaller effects in the remaining clinical measures (lipid profile, body mass index, serum creatinine and glomerular filtration rate). Some of these results are more related to changes in medication than lifestyles. From a cost-effectiveness perspective, small differences were observed between groups after 2 years follow-up. The PTI was more effective and less costly than CBI and PFI, in patients with HbA1c>7%.14 This prompted the conclusion that interventions focused on patients with the highest needs would limit the impact on the healthcare sector budget.
This study has several limitations. The high number of instruments and measurement times increase the risk of type 1 error, which explains the decision not to compare intervention groups with each other. Moreover, the use of PROMs makes it necessary to know the minimum clinically significant differences of every instrument used. This difference, however, has not been investigated for most of them, and there is currently no consensus on the appropriate method (distribution or anchor-based) and/or statistics (eg, absolute vs relative reduction).40 Furthermore, the use of PROMs implies by definition an unblind assessment of results, which is added to the impossibility of blinding the participants regarding the intervention. Finally, the INDICA study was not designed to test the efficacy of every single component of the interventions assessed (eg, text messages vs patient education vs web content). Despite these limitations, the INDICA study presents some distinctive characteristics from other published studies that assess the impact of interventions promoting empowerment, self-management and behaviour modification to patients and professionals: (1) a robust design (pragmatic cluster-randomised controlled trial with a factorial design for intervention arms) with a long follow-up (2 years); (2) incorporation of the different actors involved in disease management (patients and family caregivers and primary care professionals; (3) greater external validity by including patients regardless of their baseline HbA1c levels; (4) incorporation of ICT-based components to the intervention that favours applicability and access, in a cost-effective manner, to a growing number of patients; and (5) inclusion of a large sample size with 2334 patients and 211 healthcare professionals.
In conclusion, all the interventions assessed improved patients HRQoL at 1 year of follow-up, with differences according to the intervention in the remaining PROMs examined. The intervention targeted exclusively at patients (PTI) significantly improved knowledge, empowerment, distress, dietary recommendation adherence and tobacco cessation, up to 2 years of follow-up. Although the clinical relevance of these effects is uncertain, except in the case of smoking cessation, these results are promising since they reflect improvements in all personal domains assessed (cognitive, attitudinal, affective, behavioural), which highlight the importance of behavioural factors to attain good health outcomes. The intervention on professionals improved affective variables at 1 year of follow-up, but showed virtually no effects at 2 years together with a negative effect on diet adherence and no effect on tobacco consumption, which emphasises the need for more focused evaluative research on this type of intervention. For both target groups (patients and professionals), the use of ICT can be a major help to improve care access and continuity; as well as effectiveness and cost-effectiveness in T2DM self-management.
Supplementary Material
Acknowledgments
We thank Prof Clare Bradley and Health Psychology Research Limited (owners and source of the ADDQoL-19 questionnaire) for allowing the use of their questionnaire in the INDICA Study. We also thank Jason Willis-Lee for copyediting services during preparation of the final manuscript, and Thayli León Plasencia for her help in recruiting patients.
Footnotes
Collaborators: The INDICA team included the following members (alphabetical order): Abraham Pérez de la Rosa (Canary Islands Health Research Institute Foundation, FIISC), Alicia Pareja Ríos (University Hospital of Canary Islands), Andrés Sifre Perello (Molina Orosa Hospital), Ángela Trinidad Gutiérrez Pérez (Primary Care of Gran Canaria), Antonio Cabrera de León (Ntra. Sra. de la Candelaria University Hospital), Antonio García Quintana (Dr. Negrín University Hospital), Armando Carrillo Domínguez (Insular University Hospital), Bernardo Eusebio Herrera Domínguez (General de La Palma Hospital), Carlos Sedeño Pérez (Primary Care of Tenerife), Carlos Ramírez Álamo (Primary Care of Gran Canaria), Cecilia Lobos Soto (Insular University Hospital), Cristina Padrón Pérez (Canary Islands Health Research Institute Foundation, FIISC), Dácil Alvarado Martel (Dr. Negrín University Hospital), Daniel Hernández Obregón (Dr. Negrín University Hospital), Dulce N. Hernández Correa (Primary Care of Gran Canaria), Elsa Espinosa Pozuelo (Diabetes Patient' association of Tenerife), Elsa Florido Mayor (Canary Islands Health Research Institute Foundation, FIISC), Engracia Pinilla Domínguez (Ntra. Sra. de la Candelaria University Hospital), Fátima Herrera García (University Hospital of Canary Islands), Félix Bonilla Aguiar (Dr. José Molina Hospital), Francisco Cabrera López (Insular University Hospital), Gloria Guerra de la Torre (Primary Care of Gran Canaria), Gregorio Muelas Martín (Dr. Negrín University Hospital), Héctor de la Rosa Merino (Canary Islands Health Research Institute Foundation, FIISC), Ignacio García Puente (Dr. Negrín University Hospital), Ignacio Llorente Gómez de Segura (Ntra. Sra. de la Candelaria University Hospital), Isabel García Calcerrada (Ntra. Sra. de la Candelaria University Hospital), Jacqueline Álvarez Pérez (Canary Islands Health Research Institute Foundation, FIISC), Jorge Federico Aldunate Page (Insular University Hospital), Jose Antonio García Dopico (University Hospital of Canary Islands), Juan Andrés Báez Hernández (Primary Care of La Palma), Juan José Pérez Valencia (Primary Care of Tenerife), Julia Charlotte Wiebe (Dr. Negrín University Hospital), Lilisbeth Perestelo Pérez (Evaluation Unit, SESCS, Canary Islands Health Service, SCS), Leopoldo Martín Martín (Hospital General de La Palma), Luis Morcillo Herrera (University Hospital of Canary Islands), Marcos Estupiñán Ramírez (Canary Islands Health Service, SCS), María Inmaculada González Pérez (Ntra. Sra. de la Candelaria University Hospital), María Isabel Visuerte Morales (University Hospital of Canary Islands), María Pino Afonso Medina (Dr. Negrín University Hospital), Marta Riaño Ruiz (Insular University Hospital), Marta Tejera Santana (Dr. Negrín University Hospital), Mauro Boronat (Insular University Hospital), Mercedes Lorenzo Medina (Dr. Negrín University Hospital), Miguel Juan Mora García (Primary Care of Gran Canaria), Nayra Pérez Delgado (Ntra. Sra. de la Candelaria University Hospital), Pablo Pedrianez Martín (Dr. Negrín University Hospital), Pedro de Pablos- Velasco (Dr. Negrín University Hospital), Pilar Peláez Alba (La Laguna University), Rafael Valcárcel (Primary Care of Tenerife), Remedios Castro Sánchez (Primary Care of Gran Canaria), Rodrigo Abreu González (Ntra. Sra. de la Candelaria University Hospital), Rosa Borges Trujillo (Dr. Negrín University Hospital), Víctor Lorenzo Sellarés (University Hospital of Canary Islands).
Contributors: YR-F is the guarantor. YR-F, LG-P, LR-R, AMW, MR-R and PGS-A contributed to the study design. SK-G, GM-M, CG-M, CD-A and MR-R developed the contents and gave the educational sessions to patients. Also, SK-G, GM-M, CG-M, CD-A and MR-R recruited participants and collected data. YR-F, MAG-B and HG-P contributed to the statistical analyses. YR-F, AR-S, LG-P, AMW and PGS-A were part of the writing committee of the manuscript. All authors reviewed, commented on, and approved the final manuscript.
Funding: This work was supported by the Spanish Ministry of Economy, Industry and Competitiveness (Instituto de Salud Carlos III), grant number: ADE10/00032 and PI16/00769 co-funded by the European Regional Development Fund (ERDF) “A way to make Europe”.
Disclaimer: The funders did not participate in the study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
INDICA Team:
Abraham Pérez de la Rosa, Alicia Pareja Ríos, Andrés Sifre Perello, Ángela Trinidad Gutiérrez Pérez, Antonio Cabrera de León, Antonio García Quintana, Armando Carrillo Domínguez, Bernardo Eusebio Herrera Domínguez, Carlos Sedeño Pérez, Carlos Ramírez Álamo, Cecilia Lobos Soto, Cristina Padrón Pérez, Dácil Alvarado Martel, Daniel Hernández Obregón, Dulce N Hernández Correa, Elsa Espinosa Pozuelo, Elsa Florido Mayor, Engracia Pinilla Domínguez, Fátima Herrera García, Félix Bonilla Aguiar, Francisco Cabrera López, Gloria Guerra de la Torre, Gregorio Muelas Martín, Héctor dela Rosa Merino, Ignacio García Puente, Ignacio Llorente Gómez de Segura, Isabel García Calcerrada, Jacqueline Álvarez Pérez, Jorge Federico Aldunate Page, Jose Antonio García Dopico, Juan Andrés Báez Hernández, Juan José Pérez Valencia, Julia Charlotte Wiebe, Lilisbeth Perestelo Pérez, Leopoldo Martín Martín, Luis Morcillo Herrera, Marcos Estupiñán Ramírez, María Inmaculada González Pérez, María Isabel Visuerte Morales, María Pino Afonso Medina, Marta Riaño Ruiz, Marta Tejera Santana, Mauro Boronat, Mercedes Lorenzo Medina, Miguel Juan Mora García, Nayra Pérez Delgado, Pablo Pedrianez Martín, Pedro de Pablos-Velasco, Pilar Peláez Alba, Rafael Valcárcel, Remedios Castro Sánchez, Rodrigo Abreu González, Rosa Borges Trujillo, and Víctor Lorenzo Sellarés
Data availability statement
Data are available upon reasonable request. The datasets generated during and/or analysed during the current study, including deidentified participant data are available from the corresponding author on reasonable request in the next 10 years. The study protocol is available at https://implementationscience.biomedcentral.com/articles/10.1186/s13012-015-0233-1.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All participants provided written informed consent. The scientific and ethics committees of both the University Hospital of Canarias (ID: 2012_44) and the University Hospital Nuestra Señora de la Candelaria (ID: EPA-07/10) approved the study protocol. The study was performed in accordance with Good Clinical Practice standards, prevailing local regulatory requirements, and Declaration of Helsinki recommendations.
References
- 1. Renders CM, Valk GD, Griffin SJ, et al. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care 2001;24:1821–33. 10.2337/diacare.24.10.1821 [DOI] [PubMed] [Google Scholar]
- 2. Al Sayah F, Majumdar SR, Williams B, et al. Health literacy and health outcomes in diabetes: a systematic review. J Gen Intern Med 2013;28:444–52. 10.1007/s11606-012-2241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pouwer F, Nefs G, Nouwen A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin North Am 2013;42:529–44. 10.1016/j.ecl.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 4. Peyrot M, Rubin RR, Lauritzen T, et al. Patient and provider perceptions of care for diabetes: results of the cross-national dawn study. Diabetologia 2006;49:279–88. 10.1007/s00125-005-0048-8 [DOI] [PubMed] [Google Scholar]
- 5. Skovlund SE, Lichtenberg TH, Hessler D, et al. Can the routine use of patient-reported outcome measures improve the delivery of Person-Centered diabetes care? A review of recent developments and a case study. Curr Diab Rep 2019;19:84. 10.1007/s11892-019-1190-x [DOI] [PubMed] [Google Scholar]
- 6. Borg S, Eeg-Olofsson K, Palaszewski B, et al. Patient-reported outcome and experience measures for diabetes: development of scale models, differences between patient groups and relationships with cardiovascular and diabetes complication risk factors, in a combined registry and survey study in Sweden. BMJ Open 2019;9:e025033. 10.1136/bmjopen-2018-025033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y-C, Li I-C. Effectiveness of interventions using empowerment concept for patients with chronic disease: a systematic review. JBI Libr Syst Rev 2009;7:1179–233. 10.11124/jbisrir-2009-208 [DOI] [PubMed] [Google Scholar]
- 8. Aquino JA, Baldoni NR, Flôr CR, et al. Effectiveness of individual strategies for the empowerment of patients with diabetes mellitus: a systematic review with meta-analysis. Prim Care Diabetes 2018;12:97–110. 10.1016/j.pcd.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 9. Baldoni NR, Aquino JA, Sanches-Giraud C, et al. Collective empowerment strategies for patients with diabetes mellitus: a systematic review and meta-analysis. Prim Care Diabetes 2017;11:201–11. 10.1016/j.pcd.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 10. Haider R, Sudini L, Chow CK, et al. Mobile phone text messaging in improving glycaemic control for patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2019;150:27–37. 10.1016/j.diabres.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 11. Hou C, Xu Q, Diao S, et al. Mobile phone applications and self-management of diabetes: a systematic review with meta-analysis, meta-regression of 21 randomized trials and GRADE. Diabetes Obes Metab 2018;20:2009–13. 10.1111/dom.13307 [DOI] [PubMed] [Google Scholar]
- 12. Ramallo-Fariña Y, García-Pérez L, Castilla-Rodríguez I, et al. Effectiveness and cost-effectiveness of knowledge transfer and behavior modification interventions in type 2 diabetes mellitus patients--the INDICA study: a cluster randomized controlled trial. Implement Sci 2015;10:47. 10.1186/s13012-015-0233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramallo-Fariña Y, García-Bello MA, García-Pérez L, et al. Effectiveness of Internet-based multicomponent interventions for patients and health care professionals to improve clinical outcomes in type 2 diabetes evaluated through the indica study: Multiarm cluster randomized controlled trial. JMIR Mhealth Uhealth 2020;8:e18922. 10.2196/18922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. García-Pérez L, Ramallo-Fariña Y, Vallejo-Torres L. Cost-effectiveness of multicomponent interventions in type 2 diabetes mellitus in a cluster randomized controlled trial: the INDICA study. Primary Care Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Rekeneire N, Resnick HE, Schwartz AV, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the health, aging, and body composition study. Diabetes Care 2003;26:3257–63. 10.2337/diacare.26.12.3257 [DOI] [PubMed] [Google Scholar]
- 16. Selvin E, Coresh J, Golden SH, et al. Glycemic control and coronary heart disease risk in persons with and without diabetes: the Atherosclerosis risk in Communities study. Arch Intern Med 2005;165:1910–6. 10.1001/archinte.165.16.1910 [DOI] [PubMed] [Google Scholar]
- 17. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 18. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet 1998;352:837–53. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 19. Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 2005;28:2668–72. 10.2337/diacare.28.11.2668 [DOI] [PubMed] [Google Scholar]
- 20. Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997;20:735–44. 10.2337/diacare.20.5.735 [DOI] [PubMed] [Google Scholar]
- 21. Goldney RD, Phillips PJ, Fisher LJ, et al. Diabetes, depression, and quality of life: a population study. Diabetes Care 2004;27:1066–70. 10.2337/diacare.27.5.1066 [DOI] [PubMed] [Google Scholar]
- 22. Michie S, Johnston M, Francis J, et al. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660–80. 10.1111/j.1464-0597.2008.00341.x [DOI] [Google Scholar]
- 23. Anderson RM, Fitzgerald JT, Gruppen LD, et al. The diabetes Empowerment Scale-Short form (DES-SF). Diabetes Care 2003;26:1641–2. 10.2337/diacare.26.5.1641-a [DOI] [PubMed] [Google Scholar]
- 24. Martínez-González MA, García-Arellano A, Toledo E, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 26. Spielberger CD, Gorsuch RL, Lushene R. Manual del Cuestionario de Ansiedad Estado-Rasgo (STAI). Madrid: TEA Ediciones, 1982. [Google Scholar]
- 27. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 28. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–52. 10.1370/afm.842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradley C, Todd C, Gorton T, et al. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res 1999;8:79–91. 10.1023/A:1026485130100 [DOI] [PubMed] [Google Scholar]
- 30. Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–9. 10.2337/diacare.17.11.1281 [DOI] [PubMed] [Google Scholar]
- 31. Finucane MM, Samet JH, Horton NJ. Translational methods in biostatistics: linear mixed effect regression models of alcohol consumption and HIV disease progression over time. Epidemiol Perspect Innov 2007;4:8. 10.1186/1742-5573-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 33. Enders CK. Applied missing data analysis. New York, NY: The Guilford Press, 2010. ISBN: 9781606236390. [Google Scholar]
- 34. StataCorp . Stata statistical software: release 15. College Station, TX: StataCorp LLC, 2017. [Google Scholar]
- 35. Wu Y, Yao X, Vespasiani G, et al. Correction: mobile App-Based interventions to support diabetes self-management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR Mhealth Uhealth 2018;6:e20. 10.2196/mhealth.8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 2018;5:CD000146. 10.1002/14651858.CD000146.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2018;7:CD006732. 10.1002/14651858.CD006732.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behavioural interventions for type 2 diabetes: an evidence-based analysis. Ont Health Technol Assess Ser 2009;9:1–45. [PMC free article] [PubMed] [Google Scholar]
- 39. Peters RM, Lui M, Patel K, et al. Improving glycemic control with a standardized Text-Message and Phone-Based intervention: a community implementation. JMIR Diabetes 2017;2:e15. 10.2196/diabetes.7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masson SC, Tejani AM. Minimum clinically important differences identified for commonly used depression rating scales. J Clin Epidemiol 2013;66:805–7. 10.1016/j.jclinepi.2013.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The datasets generated during and/or analysed during the current study, including deidentified participant data are available from the corresponding author on reasonable request in the next 10 years. The study protocol is available at https://implementationscience.biomedcentral.com/articles/10.1186/s13012-015-0233-1.


