Dear Editors:
Veklury (remdesivir) is the first FDA-approved drug for treating COVID-19 (FDA, 2020a). The drug is approved for use in adult and pediatric patients 12 years of age and older and weighing at least 40 kilograms for the treatment of COVID-19 requiring hospitalization. The new approval, granted to Gilead Sciences, Inc., does not comprise the whole population that had been authorized to use Veklury under an Emergency Use Authorization (EUA) originally issued on May 1, 2020. Gilead has entered into voluntary licensing agreements with nine generics manufacturers to expand the supply of remdesivir (Gilead, 2020).
On July 30, 2020, Gilead Sciences Inc. said that it expects sales of remdesivir to help lift its full-year revenue by as much as $2.8 billion in 2020, offsetting the negative impact of the pandemic on some of its other medicines (Walker, 2020). The US government purchased over 500,000 courses of remdesivir (Staines, 2020). On October 8, 2020, Gilead executed a new agreement to supply the European Union with remdesivir as a treatment for COVID-19, which is a deal potentially worth more than $1 billion (Cohen, 2020).
In its approval FDA cites effectiveness from one randomized, double-blind, placebo-controlled clinical trial (ACTT-1), which was conducted by the National Institute of Allergy and Infectious Diseases. ACTT-1 assessed how much time was needed for subjects to recover from COVID-19 within 29 days of treatment (FDA, 2020a). Recovery was defined as either being discharged from the hospital, or being hospitalized but not needing supplemental oxygen and no longer needing ongoing medical care. The median time to recover from COVID-19 was 10 days for the Veklury group compared to 15 days for the placebo group, which was a statistically significant difference. The odds of clinical improvement at Day 15 were also statistically significantly higher in the Veklury group compared to the placebo group.
In spite of the FDA marketing approval, many trials of remdesivir show limited or no effectiveness. A few smaller studies found no impact of treatment on the disease (Cohen, 2020). On October 15, 2020 the fourth and largest controlled study of remdesivir provided what some believe is the most powerful statement on the effectiveness of remdesivir against COVID-19. The World Health Organization’s (WHO’s) Solidarity trial revealed that remdesivir does not reduce mortality or the amount of time COVID-19 patients take to recover.
Adulterated drugs may lack potency, leading to poor efficacy (Figueras, 2003). Adulterated drugs may also be dangerous to use due to impurities that may lead to adverse reactions (Buckley, 2013). Weaknesses in process control can lead to excess impurities and adulteration.
There have been recent concerns about the quality of imported drugs (Atlas, 2020)(FDA, 2018). It has been argued that generics, often made in India with ingredients from China, are a danger to US national security. The FDA cannot be assured of conducting unannounced inspections in foreign countries (FDA, 2019). With the onset of the COVID-19 pandemic, unannounced foreign inspections were halted entirely (FDA, 2020b).
The ongoing University of Kentucky (UK) Drug Quality Study (DQS) examined 7 lots of remdesivir using FTNIR (Fourier Transform Near-Infrared Spectrometry), with findings that resulted in the filing of a MedWatch report.
On Monday, November 2, 2020, at approximately 1:30 PM EST, an FDA Form 3500 MedWatch report was filed with FDA detailing our findings. Based on the the fields related to our findings the following information was provided:
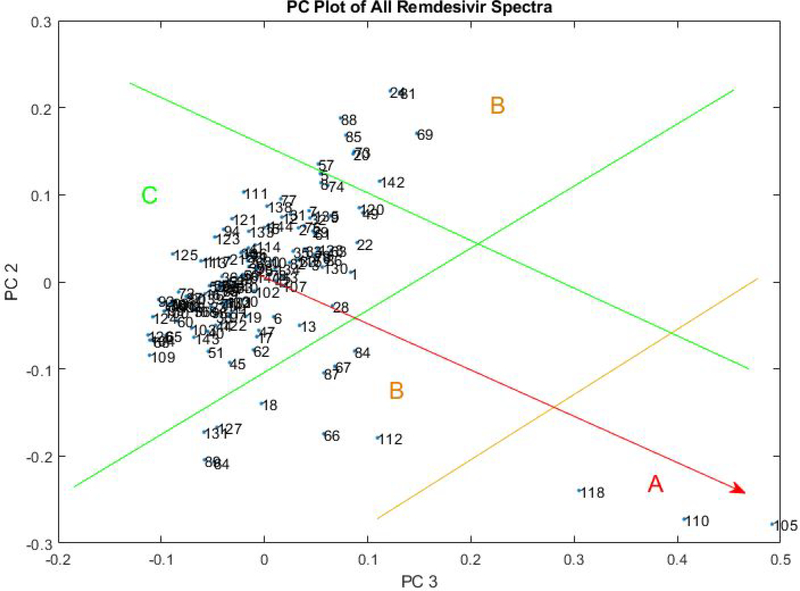
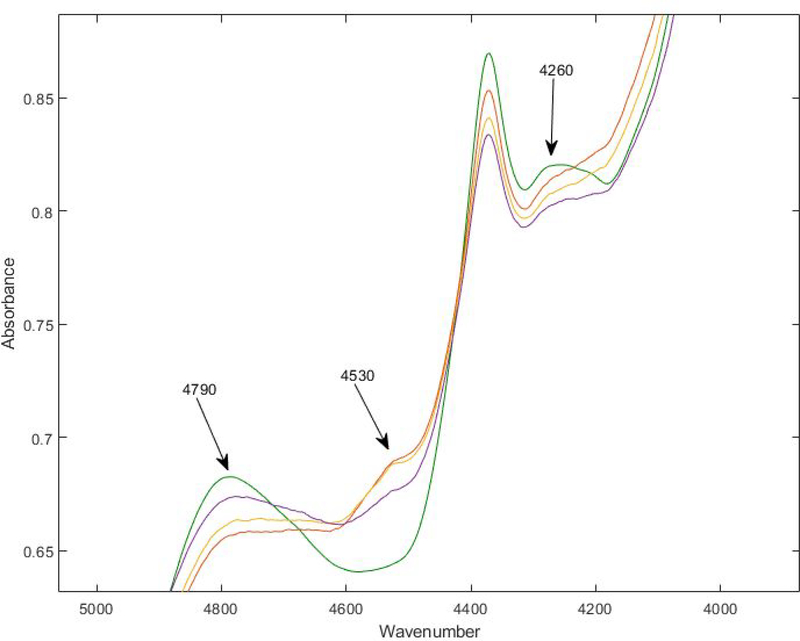
The following seven lots of 100 mg remdesivir lyophilized powder for injection, supplied as a single-dose vial with NDC 61958–2901-2, were scanned with FTNIR as part of the UK DQS workflow: EW2010A1A, EW2012A1B, EW2016A1A, AN4304BA, AN3681BA, 2009103–1A, and 362343–00002A. Upon principal component analysis (see Figure 1) lots 2009103–1A and 362343–00002A both demonstrated significant variation from the other “like” lots. Specifically, the observed variations occurred at wavelengths 4790 cm−1, 4530 cm−1, and 4260 cm−1 (see Figure 2). The expiration dates on lots 2009103–1A and 362343–00002A are 07/31/2023 and 06/30/2023 respectively.
Figure 1.
Principal component plot of 144 FTNIR spectra of remdesivir from 7 different lots. Zone C (bounded by green lines) includes most of the remdesivir vials, which are assumed to be good at this point. Zone B spectra appear to have moisture levels higher or lower than the Zone C spectra. Zone A spectra have three peak changes from Zone C that are shown in Figure 2.
Figure 2.
FTNIR spectrum of the center of Zone C (green) and the three spectra from Zone A. The Zone A spectra (purple, yellow, and orange) show a loss in the peaks at 4790 and 4260 cm−1 relative to Zone C, and an increase in the peak at 4530 cm−1 relative to Zone C.
The report asked a specific question regarding Emergency Use Authorizations: Was this event associated with any of the following COVID-19 treatments under Emergency Use Authorization (EUA)? With this question a selection was made to indicate this was related to remdesivir under EUA.
Upon filing the report an email receipt email was generated to confirm the report was filed. No follow up from the FDA has occurred between the filing and when this manuscript was published.
In Figure 1, the spectral changes in the direction of the red, zone A vector correspond to losses in the peaks at 4260 and 4790 cm−1, and the appearance of a new peak at 4530 cm−1. The chemical cause(s) of these peak changes are currently under investigation (see Figure 2).
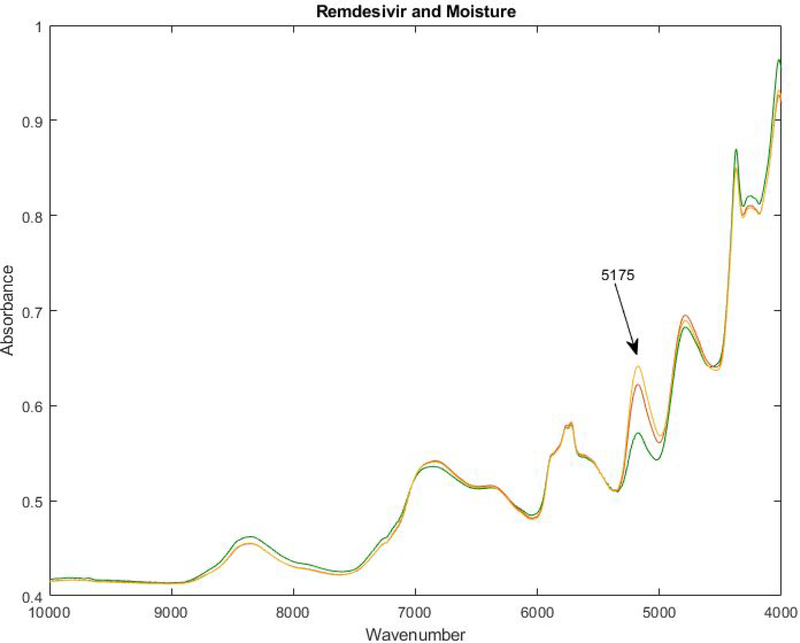
If the two gold B’s are connected in Figure 1, changes along that hyperline correspond to changes in the water peak at 5175 cm−1 (see Figure 3). The orange and yellow B spectra in Figure 3 contain more moisture than the green zone C spectrum.
Figure 3.
FTNIR spectrum of the center of Zone C (green) and the two spectra from Zone B. The difference between the Zone B and C spectra appears to be due to moisture content. (Water has a combination band at 5175 cm−1).
Conclusions
Quality control is important in drug manufacturing. Good drugs lead to good patient outcomes. There is currently no published USP monograph for remdesivir or USP standard available for purchase and comparison with delivered lots. These FTNIR results do not prove an excess level of impurities or adulteration. However, they suggest that the manufacturing process of 2 of the 7 lots may have been operating outside of a state of process control. Additional investigation is needed. Monitoring of remdesivir lots should be stepped up and additional chemical testing performed to ensure that the drug shipped and received matches the approved product.
Acknowledgements
The project described was supported in part by NSF ACI-1053575 allocation number BIO170011 and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Atlas Scott W. and McMaster HR (2020). Relying on Foreign Drugs Is Dangerous. The Wall Street Journal, April 28, 2020. https://www.wsj.com/articles/relying-on-foreign-drugs-is-dangerous-11588093635, retrieved Nov. 4, 2020.
- Buckley GJ, & Gostin LO(Eds.). (2013). Countering the problem of falsified and substandard drugs. National Academies Press.https://www.ncbi.nlm.nih.gov/books/NBK202526/, retrieved Nov. 4, 2020. [PubMed] [Google Scholar]
- Cohen Jon, and Kupferschmidt Kai. (2020) The ‘very, very bad look’ of remdesivir, the first FDA-approved COVID-19 drug. Science,.https://www.sciencemag.org/news/2020/10/very-very-bad-look-remdesivir-first-fda-approved-covid-19-drug, retrieved Nov. 4, 2020.
- FDA, (2018). Imported Drugs Raise Safety Concerns. https://www.fda.gov/drugs/drug-information-consumers/imported-drugs-raise-safety-concerns, retrieved Nov. 4, 2020.
- FDA, (2019). FDA News Release. Securing the U.S. Drug Supply Chain: Oversight of FDA’s Foreign Inspection Program. https://www.fda.gov/news-events/congressional-testimony/securing-us-drug-supply-chain-oversight-fdas-foreign-inspection-program-12102019, retrieved Nov. 5, 2020.
- FDA, (2020a). FDA News Release. FDA Approves First Treatment for COVID-19. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19, retrieved Nov 4, 2020.
- FDA, (2020b). FDA News Release. Coronavirus (COVID-19) Update: FDA prepares for resumption of domestic inspections with new risk assessment system. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-prepares-resumption-domestic-inspections-new-risk-assessment-system, retrieved Nov. 5. 2020.
- Figueras A, & Laporte JR, (2003). Failures of the therapeutic chain as a cause of drug ineffectiveness: promotion, misinformation, and economics work better than needs. BMJ. 2003 Apr 26; 326(7395): 895–896. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1125813/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead, 2020. Gilead Sciences Update on Veklury® (Remdesivir) Manufacturing Network. https://www.gilead.com/news-and-press/company-statements/gilead-sciences-update-on-veklury-remdesivir-manufacturing-network, retrieved Nov. 4, 2020.
- Staines Richard. (2020) US government buys most of world’s supply of COVID-19 drug remdesivir. PharmaPhorum, July 1, 2020, https://pharmaphorum.com/news/us-government-buys-most-of-worlds-supply-of-covid-19-drug-remdesivir/, retrieved Nov. 4, 2020. [Google Scholar]
- Walker Joseph and Armental Maria.(2020) Gilead Raises 2020 Profit Outlook on Remdesivir Demand. The Wall Street Journal, July 30, 2020.https://www.wsj.com/articles/gilead-swings-to-quarterly-loss-on-higher-costs-11596143000, retrieved Nov. 4, 2020.