Abstract
The University of California Los Angeles (UCLA) and University of California San Francisco (UCSF) codeveloped 68Ga-PSMA-11 by conducting a bicentric pivotal phase 3 clinical trial for PET imaging for prostate cancer. On December 1, 2020, 2 separate new drug applications (NDAs) submitted by each institution (NDA 212642 for UCLA and NDA 212643 for UCSF) were approved by the Food and Drug Administration as the first drug for PET imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer. This article briefly describes the background, clinical development, regulatory approach, and regulatory process for NDA filing and approval. In the second part of this article, key chemistry, manufacturing, and controls (CMC) information is provided to facilitate abbreviated new drug application (ANDA) submission.
Keywords: oncology: GU, PET/CT, radiochemistry, radiopharmaceuticals, 68Ga-PSMA-11, approval, FDA, new drug application
NOTEWORTHY
The unique NDA approach (2 separate NDAs sharing the same clinical and nonclinical information) paved the way for collaborative PET drug development by academic institutions.
Both NDAs waived market exclusivity, so that the PET imaging providers can submit an ANDA immediately, using either NDA drug product as a reference drug.
Academic institutions are indispensable for regulatory approval of PET drugs, whereas regular drug development and commercialization depends nearly exclusively on industry sponsorship.
PET imaging with 68Ga-PSMA-11 has been adopted in many countries for initial staging and restaging and for localizing biochemical recurrence of prostate cancers. In the United States, it was under investigational use until December 1, 2020, when University of California San Francisco (UCSF, New Drug Application [NDA] 212643) and University of California Los Angeles (UCLA, NDA 212642) received Food and Drug Administration (FDA) approval for both NDAs.
Led by the sponsor-investigators from both institutions (Drs. Thomas Hope–UCSF and Johannes Czernin–UCLA), the NDA process was based on a highly collaborative effort by a multidisciplinary and interinstitutional team (nuclear medicine, radiochemistry, urology, radiation oncology, radiology, and regulatory affairs) supported by the Society of Nuclear Medicine and Molecular Imaging Clinical Trials Network.
This article describes the background and regulatory pathway for these NDAs, the key drug development milestones and regulatory interactions, and the implications to the PET community. It also provides the key Chemistry, Manufacturing and Controls (CMC) information for 68Ga-PSMA-11 to facilitate abbreviated NDA (ANDA) submission.
PART I: 68GA-PSMA-11 DEVELOPMENT AND REGULATORY APPROVAL PROCESS
68Ga-PSMA-11 Background and Regulatory Pathway
Prostate-specific membrane antigen (PSMA)–targeted PET imaging has received increased attention (1) and is clinically used in many countries (2,3). Use of 68Ga-labeled PSMA ligands has been embraced enthusiastically worldwide (4). 68Ga-PSMA-11 was developed by investigators from the German Cancer Research Center and Heidelberg University and was shared with the scientific community free of patent.
In the United States, some academic institutions perform PSMA-targeted PET imaging under effective Investigational New Drug (IND) applications with FDA’s approval to recover the manufacturing cost of the probe from patients (Title 21 of the Code of Federal Regulation section 312.8). However, this approach posed a significant financial toxicity to the patients because 68Ga-PSMA-11 was investigational only and was reimbursed by neither Medicare nor private health insurance companies. Given the unmet clinical need for accurately staging and restaging prostate cancer as well as detecting sites of biochemical recurrence and lack of industry support, we initiated a pivotal phase 3 clinical trial for 68Ga-PSMA-11 to study its efficacy and safety in prostate cancer detection.
At the beginning of 2018, the UCSF and UCLA teams consulted with the FDA Division of Medical Imaging and Radiation Medicine to develop a unique regulatory approach for the NDA submission. UCSF and UCLA would submit 2 separate NDAs around the same time for the same PET drug, waiving market exclusivity. Both NDAs would share the same nonclinical and clinical information, similar labeling information, but with site-specific CMC modules. Both submissions would be 505(b)(2) NDAs, referencing published literature for nonclinical and clinical pharmacology and clinical dosimetry. Clinical information was analyzed jointly by both centers. In addition, a metaanalysis of published literature of relevant clinical trials would serve as confirmatory evidence, corroborating the clinical data derived from the bicentric, prospective pivotal trial conducted by UCSF and UCLA.
Key Drug Development Milestones and Regulatory Interactions with the FDA
In late 2016, pivotal phase 3 clinical trial protocols were submitted to IND application 127621 (UCSF) and 130649 (UCLA). Uniform clinical protocols were designed and followed to collect safety and efficacy data. Even though the 68Ga-PSMA-11 was supplied locally by UCLA and UCSF radiochemistry facilities, they were produced and controlled in a similar way, resulting in highly compatible final products. At the end of 2017, UCSF and UCLA teams assessed the clinical data and determined that they were adequate for NDA filing. A pre-NDA meeting request and briefing package were filed with the FDA in the spring of 2018. In August 2018, a joint pre-NDA meeting was held on the FDA Silver Spring campus. At the meeting, the data assembled in the briefing package were deemed substantive for NDA review. Furthermore, FDA offered positive feedback and constructive recommendations for the NDA submission plan.
After the pre-NDA meeting, the UCSF/UCLA team submitted Prescription Drug User Fee Act (PDUFA) user application fee waiver requests based on Barrier-to-Innovation provision for both NDAs (PDUFA section 736(d)(1)(B)). In late 2018, both fee waiver requests were granted by the FDA. Without a waiver, PDUFA application fees for the NDA with clinical data would be around $2.6 million each for that fiscal year.
In the following months, a multidisciplinary team consisting of regulatory, nonclinical, clinical, statistical, and CMC experts performed clinical data analyses, registration batch and method/process validations. On September 6, 2019, NDA 212643 (UCSF) and NDA 212642 (UCLA) were submitted to the FDA. Both NDAs received FDA’s Day-74 letters, in which FDA determined that both NDAs were sufficiently complete in content and format to permit a substantive review of the application. The initial PDUFA goal date was September 6, 2020. The goal date was later extended to December 6, 2020, due to major amendments during the NDA review.
During the NDA review, multiple iterations of clinical, statistical, CMC, and labeling information requests were issued by the FDA. Both UCSF and UCLA nuclear medicine clinics and PET manufacturing facilities were inspected by the FDA in 2020.
On December 1, 2020, the FDA approved both NDAs for PET imaging of PSMA-positive lesions in men with prostate cancer:
with suspected metastasis who are candidates for initial definitive therapy.
with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
The approved package inserts can be accessed via the FDA website (https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212642s000lbl.pdf; https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212643s000lbl.pdf)
Implications to the PET Community
In this joint effort, the UCSF/UCLA team obtained the approval of the first drug for PET imaging of PSMA-positive lesions in men with prostate cancer. These NDAs are based on academic initiatives for a radiopharmaceutical without patent protection.
Since market exclusivities were waived for both NDAs, the PET imaging providers can submit ANDAs immediately, using either drug product as reference drug. PET drug ANDAs are exempt from Generic Drug User Fee Act (GDUFA) user fees and are subject to a 10-mo GDUFA goal date. A detailed outline of the ANDA contents can be found in the FDA’s “Guidance for Industry: ANDA Submissions—Content and Format” (June 2019, https://www.fda.gov/media/128127/download), and FDA Guidance “PET Drug Applications—Content and Format for NDAs and ANDAs” (August 2011, https://www.fda.gov/media/72271/download).
In October 2020, the FDA published a guidance document titled “Referencing Approved Drug Products in ANDA Submissions” (https://www.fda.gov/media/102360/download), which outlines how to choose reference drug and describes the basis for ANDA submissions.
An ANDA submission will most likely only include Module 1 (administrative and labeling information), Module 2 (Common Technical Document [CTD] summary), and Module 3 (CMC information; key information is provided in Part II of this article). Module 1, section 1.12.11, contains the reference drug information, where section 1.12.12 requires information demonstrating that the generic product is the same as the reference drug. PET providers can support the ANDA by demonstrating that the active ingredients, route of administration, dosage form, strength, and conditions of use are the same as those of the reference drug.
In March 2020, the FDA issued eCTD guidance document revision 7, titled “Providing Regulatory Submissions in Electronic Format—Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications” (https://www.fda.gov/media/135373/download). Section III D of this guidance document outlined a possible long-term eCTD waiver pathway for certain PET NDA/ANDA submissions from qualified PET drug facilities. On the basis of our experience, it is feasible to submit long-term waivers and propose alternative submission format. The alternative submission format could be files in PDF format, with content organized by folders and subfolders mirroring the eCTD structure. The final ANDA application can be submitted via the Center for Drug Evaluation and Research Nextgen Portal (http://edm.fda.gov).
PART II: KEY CMC INFORMATION FOR 68GA-PSMA-11
UCSF and UCLA NDAs share the similar body of CMC data, with some minor differences. UCSF uses the Eckert and Ziegler (E&Z) GalliaPharm 68Ge/68Ga Generator or cyclotron-produced 68Ga, whereas UCLA uses only the E&Z GalliaPharm Generator for 68Ga. The key CMC information for both NDAs is provided below.
Drug Substance
3.2.S.1 General Information
General properties are given, with a short description to highlight nomenclature, physical structure characteristics, and external radiation.
3.2.S.2 Manufacture
PSMA-11 precursor is supplied and manufactured by ABX Advanced Biochemical Compounds Biochemische Forschungsreagenzien GmbH.
3.2.S.3 Characterization
The chemical purity and identity of the incoming PSMA-11 precursor are tested on receipt. PSMA-11 precursor is released for clinical use based on Certificates of Analysis (CoA) provided by the supplier and passing results from the in-house acceptance testing.
3.2.S.4 Control of Drug Substance
The specifications for the PSMA-11 precursor are summarized in Table 1. The details of the precursor specification are detailed within the Drug Master File (DMF) 033682 from ABX.
TABLE 1.
PSMA-11 Precursor Specifications
| Criteria | Acceptance |
| Specific filling | |
| Amount of filling | 1 mg ± 10% |
| Identity (HPLC) | Reference retention time ± 0.5 min |
| Impurities (HPLC) | Any unspecified impurity ≤ 2.0% |
| Total impurities ≤ 6.0% | |
| Bioburden/vial | TAMC ≤ 100 cfu |
| TYMC ≤ 10 cfu | |
| BET (LAL) | ≤ 10 IU/vial |
| Bulk precursor batch | |
| Appearance | White to off-white solid |
| Monoisotopic mass (net peptide) | 946.1 ± 1 m.u. |
| 1H-NMR | Conforms to structure |
| 13C-NMR | Conforms to structure |
| Impurities (HPLC) | Any unspecified impurity ≤ 2.0% |
| Total impurities ≤ 3.0% | |
| Residual solvents (GC) | Acetonitrile ≤ 5,000 ppm |
| Tertbutylmethylether ≤ 5,000 ppm | |
| Water (GC) | ≤ 10% |
| Heavy metals (ICP-MS) | Iron ≤ 100 ppm; |
| Copper ≤ 100 ppm; | |
| Zinc ≤ 100 ppm; | |
| Palladium ≤ 100 ppm | |
| Assay (HPLC) | ≥50% |
| Trifluoroacetic acid (GC) | ≤40% |
HPLC = high performance liquid chromatography; TAMC = total anaerobic microbial count; TYMC = total yeast/mold count; BET = bacterial endotoxin test; LAL = limulus amebocyte lysate; NMR = nuclear MR; GC = gas chromatography; ICP-MS = inductively coupled plasma mass spectrometry.
3.2.S.5 Reference Standards or Materials
A batch of precursor is annually qualified as in-house precursor standard to support the precursor acceptance test.
3.2.S.6 Container Closure System
Once accepted, the PSMA-11 precursor is dissolved and aliquoted into single-use 1.5-mL tubes.
3.2.S.7 Stability
One PSMA-11 precursor vial (e.g., 1 mg) is portioned into 5-μg aliquots. These precursor aliquots are stored in a freezer and used within their expiration date established by the stability study.
Drug Product
3.2.P.1 Description and Composition of the Drug Product
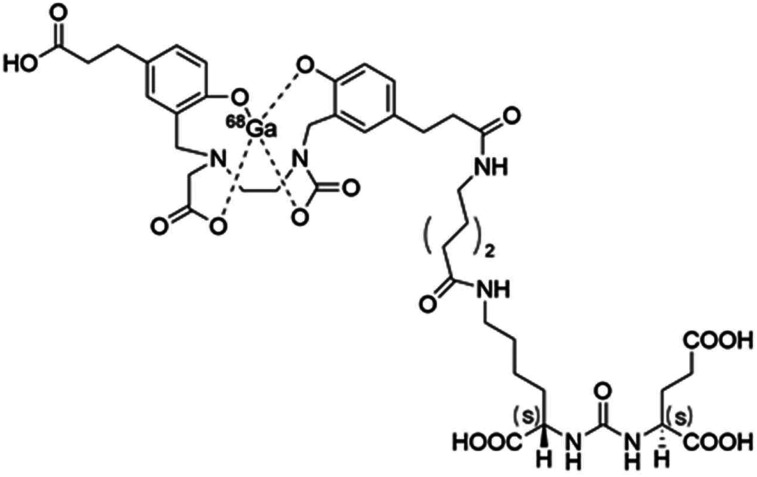
The structure, chemical name, code, and other nonproprietary names are included in Figure 1 and Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
FIGURE 1.
Structural formula of 68Ga-PSMA-11.
3.2.P.2 Pharmaceutical Development
68Ga can be produced by either commercially available generators or by a cyclotron. Each source provides 68Ga trichloride in hydrochloric acid.
3.2.P.3 Manufacture
This section lists the manufacturer, the batch formula (component, amount, function, and quality standard), the manufacturing process, control of critical steps and intermediates, and process validation/evaluation (i.e., media fill simulation).
The suitability of the manufacturing process with the E&Z GalliaPharm generator and cyclotron-produced 68Ga is demonstrated with 3 consecutive successful quality control releasable runs.
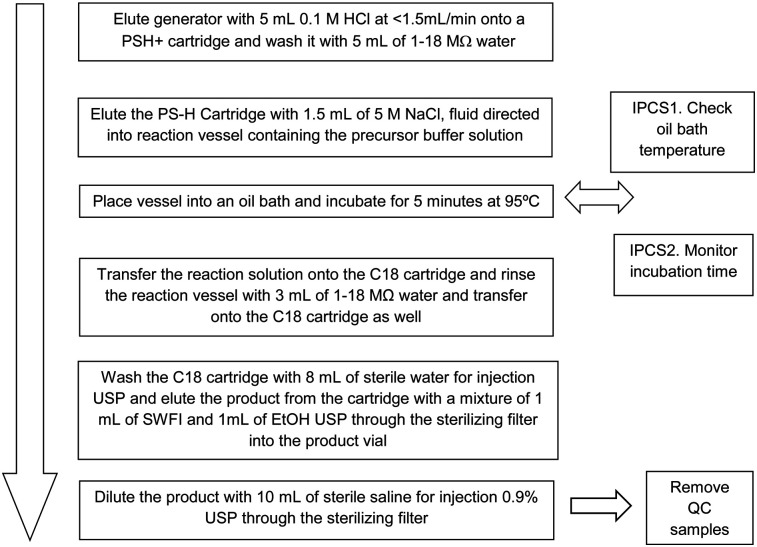
For UCLA, 5 μg of freshly thawed precursor in 1.5 mL 1.5 M HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) are mixed with generator-eluted 68Ga (5 mL). Then, the vessel containing the reaction mixture is incubated at 95°C for 5 min in an oil bath. At the completion of the reaction phase, the vessel is cooled for 5 min. Then the reaction solution is loaded onto a C18 Sep-Pak Light cartridge (Waters) and washed with sterile United States Pharmacopeia (USP) water and the product eluted with 1 mL of Sterile Water for Injection and 1 mL of Ethanol USP. Finally, the product is diluted with 10 mL of sterile saline for injection 0.9% USP through the sterilizing filter. The process is described in Figure 2. The final formulation is provided in Table 2.
FIGURE 2.
68Ga-PSMA-11 production method from UCLA NDA 212642.
TABLE 2.
Batch Formula for UCLA Final Product
| Component | Amount | Function | Quality standard |
| 68Ga-PSMA-11 | 18.5–185 MBq/mL (0.5–5 mCi/mL) | Drug substance | In-house |
| PSMA-11 | 5 μg | Drug substance precursor | GMP |
| HEPES* | 1.5 mL (1.5 M) | Processing aid | PharmaGrade |
| Hydrochloric acid*1 | 5 mL (0.1 M) | Processing aid | GMP |
| Sodium chloride 0.9% injection | 10 mL | Tonicity | USP |
| Ethanol | 1 mL | Eluent | USP |
| Water for injection USP | 1 mL | Eluent | USP |
HEPES and hydrochloric acid are completely removed during processing and are not present in final formulation. GMP = good manufacturing practice; HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.
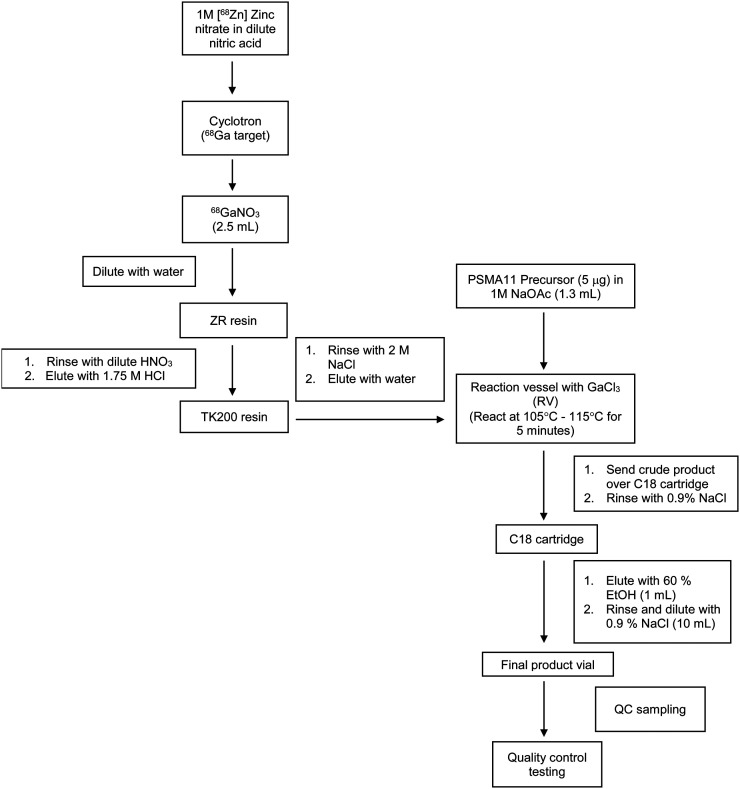
For UCSF, 68Ga is produced using a PETtrace cyclotron (GE Healthcare). The process involves proton irradiation of a 1 M 68Zn-zinc oxide solution in dilute nitric acid. The solution is irradiated for 60 min at a beam current of 35 μA. After irradiation, the 68Ga-nitrate solution is delivered to a FastLab (GE Healthcare) synthesis module, where the solution is purified and prepared as 68GaCl3, which is the same form of 68GaCl3 eluted from the generator. The 68GaCl3 is then reacted with 5 μg of PSMA-11 precursor in 0.5 M NaOAc at 100°C–115°C for 5 min. The crude reaction mixture is loaded onto a C18 Sep-Pak Light cartridge, and the cartridge is rinsed with 0.9% sodium chloride for injection USP. The 68Ga-PSMA-11 is eluted with 1 mL of 60% ethanol through a 0.22-μm sterilizing filter into the final product vial. Finally, the product is diluted with 10 mL of 0.9% sodium chloride for injection USP through the sterilizing filter. The cyclotron-based process is outlined in Figure 3. The final formulation for cyclotron-based production is provided in Table 3.
FIGURE 3.
68Ga-PSMA-11 production method (cyclotron-based) from UCSF NDA 212643.
TABLE 3.
Batch Formula for UCSF Final Product (Cyclotron-Based Production)
| Component | Amount | Function | Quality standard |
| PSMA-11 | 5 μg | Precursor | GMP grade |
| 68Ga-PSMA-11 | 18.5–185 MBq/mL (0.5–5 mCi/mL) | Drug substance | In-house |
| Sodium acetate, 1 M* | 1.3 mL | Buffering agent | Pharma grade |
| Nitric acid, 0.1 M1 | 4 mL | 68Ga purification | Trace-metal basis |
| Hydrochloric acid, 1.75 M* | 4 mL | 68Ga purification | Ultrapure |
| Sodium chloride, 2 M1 | 4 mL | 68Ga purification | Trace metal basis |
| Ethanol, 60% | 1 mL | Product eluent | USP grade |
| Ultra-high purity water | 50 mL | Solution preparation | Pharma grade |
| Water for injection | 100 mL | Solution preparation | USP grade |
| Sodium chloride, 0.9% | 5–10 mL | Isotonicity | USP grade |
Completely removed during processing and are not present in final formulation. GMP = good manufacturing practice.
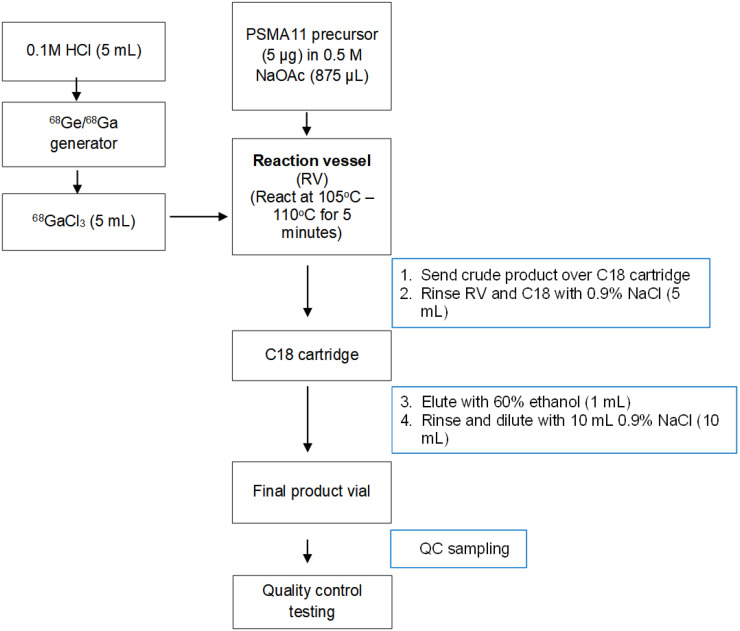
For UCSF generator-produced 68Ga, 5 μg of freshly thawed precursor in 875 μL 0.5 M sodium acetate trihydrate is mixed with generator-eluted 68Ga (5 mL). Then, the vessel containing the reaction mixture is incubated at 105°C for 5 min. At the completion of the reaction phase, the reaction solution is loaded onto a C18 Sep-Pak Light cartridge, washed with 5 mL of 0.9% sodium chloride for injection USP and the product eluted with 1 mL of 60% ethanol USP in water for injection through the sterilizing filter into the product vial. Finally, the product is diluted with 10 mL of 0.9% sodium chloride for injection USP through the sterilizing filter. The generator-based process is described in Figure 4. The final formulation for generator-based production is provided in Table 4.
FIGURE 4.
68Ga-PSMA-11 production method (generator-based) from UCSF NDA 212643.
TABLE 4.
Batch Formula for UCSF Final Product (Generator-Based Production)
| Component | Amount | Function | Quality standard |
| PSMA-11 | 5 μg | Precursor | GMP grade |
| 68Ga-PSMA-11 | 18.5–185 MBq/mL (0.5–5 mCi/mL) | Drug substance | In-house |
| Sodium acetate, 0.5 M* | 0.1 mL | Buffering agent | Pharma grade |
| Hydrochloric acid, 0.1 M* | 5 mL | Generator eluent | GMP grade |
| Ethanol, 60% | 1 mL | Product eluent | USP grade |
| Ultra-high purity water | 10 mL | Solution preparation | Pharma grade |
| Water for injection | 4 mL | Solution preparation | USP grade |
| Sodium chloride, 0.9% | 5–10 mL | Isotonicity | USP grade |
Completely removed during processing and are not present in final formulation. GMP = good manufacturing practice.
3.2.P.4 Control of Excipients
Compendial excipients (Supplemental Table 2) are tested and released according to the referenced Pharmacopeia. CoAs for each excipient are usually provided.
3.2.P.5 Control of Drug Product
Every batch of the 68Ga-PSMA-11 drug product manufactured for human use conforms to the specified prerelease acceptance criteria as described in Table 5. Brief descriptions of quality control testing methods are provided below.
Appearance
TABLE 5.
Final Product Specifications
| Test | Test method | Acceptance criteria |
| Prerelease | ||
| Radiochemical purity | Thin-layer chromatography | ≥90% |
| Appearance | Visual observation | Clear solution, free of particulates |
| pH | pH paper | 4.0–7.0 |
| Endotoxin content | PTS Endosafe, USP < 85 > | <17.5 EU/mL |
| Radiochemical identity | Thin layer chromatography* | UCLA: Rf value = 0.75–0.95 |
| UCLA: Rf value = 0.3–0.6 | ||
| Radionuclidic identity | Half-life determination | 64.4–71.2 min |
| Filter integrity | Bubble point pressure reading, USP < 823 > / < 797 > | >50 psi |
| Postrelease | ||
| Sterility | Direct inoculation. USP < 71 > / < 823 > | Sterile |
UCLA and UCSF use different mobile phases.
The appearance of the 68Ga-PSMA-11 is determined by observing the contents of the vial for color, clarity, and particulate matter.
pH
The pH of the final drug product is determined by a colorimetric test. A 1-μL aliquot is spotted on pH indicator paper and then compared with the color reference chart.
Radiochemical Purity
The amount of free 68Ga in the radiolabeled 68Ga-PSMA-11 preparations is evaluated using instant thin-layer chromatography (iTLC). The stationary phase is an iTLC-silicic acid paper strip. The mobile phase for UCLA is a 50:50 (v/v) solution of 1 M ammonium acetate and methanol and for UCSF 1 M ammonium acetate. Briefly, an approximately 37- to 185-kBq (1–5 μCi) sample of the radiolabeled 68Ga-PSMA-11 (used without dilution) is spotted on an iTLC-silicic acid strip and developed in the mobile phase. The test is considered valid if the retardation factor of gallium(III) and gallium in colloidal form is <0.3 and the retardation factor of 68Ga-PSMA-11 is 0.75–0.95 (UCLA) or 0.3–0.6 (UCSF).
Radionuclidic Identity
The half-life method is used to determine the identity of the radionuclide. The half-life test result for 68Ga should be between 64.4 and 71.2 min.
68Ga Radionuclidic Purity
A test is performed annually to determine the 68Ga radionuclidic purity and identity using the γ-spectrum obtained with a multiple-channel analyzer. The radionuclidic purity of 68Ga is confirmed by absence of peaks on the γ-spectrum that are attributable to radionuclidic impurities and the detection of peaks at 511 keV on the γ-spectrum. Potential contaminants for UCLA drug product are summarized in Supplemental Table 3. Potential contaminants for UCSF drug product are summarized in Supplemental Tables 4 and 5 (generator-based and cyclotron-based, respectively).
Endotoxin Test, USP 85
The 68Ga-PSMA-11 final drug product is tested for endotoxin content using the PTS Endosafe system (Charles River Laboratories) according to the manufacturer’s instructions.
Filter Integrity Test, Bubble Point Pressure reading, USP 823/797
The 0.22-μm sterilizing filter is tested to ensure its integrity during filtration of the final drug product. The procedure involves connecting the filter to a gas line with a pressure gauge and submerging into water. The pressure inside the gas line is slowly increased until gas starts to flow through the filter and into the water, producing visible bubbles. The pressure at which the bubbles become visible is recorded. The test is valid if bubbles are visible at or above the filter integrity pressure specified by the filter manufacturer.
Sterility
Sterility testing is performed within 30 h of the drug product’s end-of-synthesis time using the direct inoculation of media method (USP < 823 > for sterility testing of radiopharmaceuticals). Briefly, 1 tube containing Tryptic Soy Broth (TSB) media and 1 tube containing the Fluid Thioglycollate growth media (FTM) are each inoculated with 0.1–0.2 mL of the final drug product. Negative control samples for each type of media are created by inoculating one additional of each type of media tube with 0.1–0.2 mL of sterile water or saline for injection. FTM and TSB samples are incubated for 14 d at 30°C–35°C and 20°C–25°C, respectively, and are observed daily for signs of bacterial growth.
3.2.P.6 Reference Standards or Materials
Supplemental Table 6 shows the references standards used in the control of 68Ga-PSMA-11.
3.2.P.7 Container Closure System
Commercial preassembled, sterile, and ready-to-use vials are used. Reference to the DMF for the container/closure system and a representative CoA are provided in the application. 68Ga-PSMA-11 is packaged in a presterilized, pyrogen-free primary container closure consisting of a preassembled 20-mL (UCSF) or 30-mL (UCLA) USP Type I glass, a gray butyl preassembled rubber stopper, and an aluminum crimp seal.
3.2.P.8 Stability
An expiration time of 3 h (UCLA) or 4 h (UCSF) after the end of synthesis is used for 68Ga-PSMA-11 (based on stability studies). Briefly, after removal of the quality control samples, the final-product vials containing the 68Ga-PSMA-11 were positioned at room temperature in an inverted position and stored for 3 h (UCLA) or 4 h (UCSF). After each hour of storage, an additional quality control sample was drawn to reassess appearance, pH, radiochemical identity, and purity by radio-TLC and radio-HPLC (high-performance liquid chromatography). The quality control tests met the acceptance criteria after a 3-h (UCLA) or 4-h (UCSF) storage period of the final product vial in an inverted position.
CONCLUSION
A joint academic effort has led to the FDA NDA approval for the first drug for PET imaging of PSMA-positive lesions in men with prostate cancer. Although regular drug development and commercialization depends near exclusively on industry investment, academic institutions are indispensable for the regulatory approval of PET drugs. In recent years, FDA granted NDA approvals for Mayo Clinic’s 11C-choline, University of Iowa’s 68Ga-DOTATOC, and most recently, for Feinstein’s Institute of Medical Research 18F-fluorodopa.
UCSF and UCLA teams closely collaborated in their pivotal phase 3 trials, pre-NDA meeting, and NDA filing/amendment. The success of this academic partnership is evidenced by the final NDA approval. The unique parallel regulatory approach paved the way for a collaborative PET drug development by academic institutions. We gained substantial knowledge from our interactions with the FDA’s NDA review team. The FDA’s rigorous but open-minded review process ensured that the efficacy and safety claims were supported by the totality of evidence. Sharing our strategy and experience for and with the FDA regulatory approval process should assist the imaging community to develop strategies for ANDA submissions.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Schäfer M, Bauder-Wust U, Leotta K, et al. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. [DOI] [PubMed] [Google Scholar]
- 3.Eder M, Eisenhut M, Babich J, Haberkorn U. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging. 2013;40:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillier SM, Maresca KP, Femia FJ, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]