Abstract
We evaluated a new microtiter assay for antifungal susceptibility testing based on a colorimetric reaction to monitor fungal substrate utilization. This new method (rapid susceptibility assay [RSA]) provides quantitative endpoint readings in less than 8 h compared with visual determination of MIC by the National Committee for Clinical Laboratory Standards (NCCLS) broth microdilution method, which requires a minimum of 48 h of incubation. In this study, we tested clinical isolates from each of the following species: Candida albicans (20 isolates), C. glabrata (20 isolates), C. krusei (19 isolates), C. tropicalis (19 isolates), and C. parapsilosis (28 isolates). RSA and NCCLS broth dilution methods were used to determine the MICs of amphotericin B, fluconazole, itraconazole, and 5-flucytosine for all 106 isolates. RPMI 1640 medium buffered with morpholinopropanesulfonic acid was used for both methods; however, glucose and inoculum concentrations in the RSA were modified. RSA MICs were determined as the lowest drug concentration that prevented glucose consumption by the organism after 6 h of incubation. MICs obtained from the RSA were compared with those obtained from the NCCLS M-27A method read at 24 and 48 h. MIC pairs were considered in agreement when the difference between the pairs was within 2 twofold dilutions. For the 106 isolates tested, amphotericin B and 5-flucytosine demonstrated the highest agreement in MICs between the two methods (100 and 98%, respectively), whereas fluconazole and itraconazole produced less favorable MIC agreement (63.2 and 61.3%, respectively). The azole MIC differences between the two methods were significantly reduced when lower inocula were used with a prolonged incubation time. This preliminary comparison suggests that this rapid procedure may be a reliable tool for the in vitro determination of MICs of amphotericin B and 5-flucytosine and warrants further evaluation.
Opportunistic fungal infections have dramatically increased in recent years along with the incidence of drug-resistant disease in immunocompromised patient populations (6–8). The increased incidence and severity of mycoses have prompted the pharmaceutical industry to respond with the development of several new antifungal agents. As a result of these combined factors, interest has increased in developing standardized tests to determine antifungal drug susceptibility as well as in optimizing these tests to accurately predict clinical outcome (4).
Methods for in vitro susceptibility testing have been available since the early years of antifungal drug development. However, such procedures lacked standardization and reproducibility among laboratories performing these assays (1–3). Collaborative efforts to develop standardized methods for in vitro susceptibility testing of antifungal agents began in 1982 with the establishment of the National Committee for Clinical Laboratory Standards (NCCLS) Subcommittee for Antifungal Susceptibility Testing. As a result of several multicenter studies, a standardized reference method (M-27A) was published in 1997 (5). This broth dilution method addressed the key variables of inoculum preparation size, medium, temperature and duration of incubation, and MIC endpoint determination. The application of the M-27A method has allowed reproducible results, made interlaboratory data comparison possible, and permitted the development of clinically relevant breakpoints (9). However, the standard method (and its microdilution version) requires 48 h of incubation, and the interpretation of endpoints can sometimes be subjective. Faster and more convenient methods are essential for routine antifungal susceptibility testing for clinical laboratories.
Riesselman et al. (10) described the development of a novel colorimetric method for determination of MICs of antifungal agents against Candida species. This rapid susceptibility assay (RSA) is based on the hypothesis that uptake of an exogenous substrate such as glucose will be suppressed in susceptible fungi in the presence of antifungal drugs. By plotting optical density (OD), which reflects the relative residual glucose concentration, against the increasing concentration of a drug, the susceptibility of a fungal isolate to an antifungal drug can be determined (J. E. Cutler, J. Turner, M. H. Riesselman, K. A. Glase, and K. C. Hazen, Abstr. 97th Gen. Meet. Am. Soc. Microbiol. 1997, abstr. C-247, 1997). Riesselman and colleagues (10; Cutler et al., Abstr. 97th Gen. Meet. Am. Soc. Microbiol. 1997) tested amphotericin B (AMB) and fluconazole (FLU) against six isolates of Candida species in their development of the RSA method. Variables evaluated included glucose concentration, inoculum concentration, and length of incubation. MICs obtained by RSA were compared with those obtained with the NCCLS M-27A method. The results indicated that susceptibility testing of AMB could be accomplished in 6 h for the RSA method but that testing of FLU required up to 19 h.
In this work, we further evaluated the RSA method with AMB, 5-flucytosine (5-FC), FLU, and itraconazole (ITRA) against 106 isolates of five Candida species. MICs obtained by the RSA were compared to those obtained by the NCCLS M-27A method.
MATERIALS AND METHODS
Antifungal agents.
The following antifungal drugs were used in the study: AMB (Bristol-Myers Squibb, Princeton, N.J.), FLU (Pfizer Inc., New York, N.Y.), ITRA (Janssen Pharmaceutica, Piscataway, N.J.), and 5-FC (Hoffmann-La Roche Inc., Nutley, N.J.). Stocks of AMB and ITRA were prepared in 100% dimethyl sulfoxide. FLU and 5-FC were prepared in sterile water. Stock solutions containing 0.4-ml aliquots of each drug were prepared and stored at −70°C. All other reagents were purchased from Sigma Chemical Company, St. Louis, Mo., unless otherwise indicated.
Fungal isolates.
A total of 106 Candida isolates were obtained from the culture collection of the Mycotic Diseases Branch, Centers for Disease Control and Prevention. Isolates tested were Candida albicans (20 isolates), C. glabrata (20 isolates), C. krusei (19 isolates), C. tropicalis (19 isolates), and C. parapsilosis (28 isolates). Yeasts were stored in 50% glycerol at −70°C. Isolates were cultured on Sabouraud dextrose agar (BBL Microbiology Systems, Cockeysville, Md.) plates at 35°C and transferred 24 h prior to in vitro susceptibility testing. A strain of C. krusei (ATCC 6258) was included for quality control.
Drug preparations.
The medium used for the NCCLS microbroth dilution method was RPMI 1640 medium with l-glutamine, without sodium bicarbonate, and buffered with 0.165 M morpholinopropanesulfonic acid at pH 7.0. This medium contains 0.2% glucose. Stock solutions were diluted in RPMI 1640 medium to achieve twice the final concentrations. One hundred microliters from each dilution was dispensed into appropriate wells of a U-shaped 96-well plate (Costar Corp., Cambridge, Mass.). The final concentration ranges after inoculation were 0.03 to 16 μg/ml for AMB, 0.125 to 64 μg/ml for FLU and 5-FC, and 0.015 to 8 μg/ml for ITRA.
For the RSA, similar procedures were used for drug preparation except that RPMI 1640 medium containing 0.1% glucose was used. This medium was obtained by combining, in a 1:1 ratio, regular RPMI 1640 (with 0.2% glucose) with glucose-deficient RPMI 1640 medium. One hundred microliters of each drug dilution was pipetted into the wells of flat-bottomed microtiter plates (Costar). All plates were stored at −70°C and thawed at room temperature on the day of assay.
Antifungal susceptibility testing.
All standard antifungal susceptibility testing was performed according to document M-27A as published by the NCCLS (5). Briefly, yeast inocula were standardized to a turbidity equivalent to that of a 0.5 McFarland standard with a spectrophotometer at 530 nm. The suspension was further diluted in RPMI 1640 medium to yield an inoculum concentration of approximately 1 × 103 to 5 × 103 cells/ml. One hundred microliters was inoculated into wells of each row containing diluted drugs. Drug-free purity controls and growth controls were included for each preparation. Plates were incubated at 35°C, and results were determined at 24 and 48 h by visual inspection. The MICs of the azoles and 5-FC were defined as the dilutions at which the turbidity was equal to or less than that of an 80% dilution of the no-drug growth control. The MICs of AMB were defined as the lowest drug dilution with no visible growth, as recommended by the NCCLS (5).
For RSA, yeasts were suspended in RPMI 1640 medium without glucose to a turbidity equal to that of a 0.5 McFarland standard. Without further dilution, 100 μl of this suspension was inoculated into wells of each row of previously prepared plates containing drug dilutions. After the plates were incubated for 3 h at 35°C, 25 μl of RPMI 1640 medium containing 0.1% glucose was added carefully to each well to avoid splashing and cross-contamination. At the end of another 3 h of incubation at 35°C, 50 μl of complete color mix was added to each well. The complete color mix was prepared just prior to its use and contained 4-amino antipyrine (360 μg/ml), N-ethyl-N-sulfopropyl-m-toluidine (490 μg/ml), horseradish peroxidase (0.68 U/ml), and glucose oxidase (0.4 U/ml) in 0.6 M sodium phosphate buffer at pH 6.0. Plates were incubated at room temperature for 15 min to allow color development. The OD of each well was determined spectrophotometrically with a microtiter plate reader (SpectraMax 250; Molecular Devices Corp., Sunnyvale, Calif.) at 550 nm with a 710-nm reference filter. The OD values, which reflect the relative glucose concentrations, were plotted against drug concentrations. A line was drawn across the drug-saturation plateau for each curve. The MIC was defined as the last drug dilution on the plateau preceding a 10% or greater drop of the OD. Controls included wells without drug and with yeast growth control (lower OD limit) and wells without drug and without yeast glucose control (upper OD limit).
For FLU and ITRA, inoculum concentrations and incubation times were further investigated. The 0.5-McFarland standard yeast preparation was diluted (1/10, 1/20, 1/100, 1/200, and 1/1,000) in RPMI 1640 medium without glucose before being dispensed into the wells of the drug-containing plate. In addition to the 6 h of incubation time, incubation times of 8 and 19 h were tested for these azole drugs.
MICs obtained by the two methods were compared for each drug and isolate. MICs were considered in agreement when the difference was within 2 dilutions.
RESULTS AND DISCUSSION
MICs obtained by NCCLS and RSA methods are summarized in Table 1. In general, both methods gave similar MIC ranges for all four drugs. For FLU and ITRA, however, the RSA method usually resulted in a higher MIC at which 90%, and occasionally 50%, of the isolates tested were inhibited. The RSA method also gave a broader MIC range for AMB compared to the narrow AMB MIC range given by the NCCLS method, which often hinders detection of resistant isolates. Further work with AMB-resistant isolates is needed to determine if this wider MIC range will allow easier detection of AMB resistance.
TABLE 1.
Distribution of MICs by NCCLS and RSA (6-h) methods
| Yeast (no. of isolates) | Drug | MIC (μg/ml) determined by methoda:
|
|||||
|---|---|---|---|---|---|---|---|
| NCCLS
|
RSA (6 h)
|
||||||
| Range | 50% | 90% | Range | 50% | 90% | ||
| C. albicans (20) | AMB | 0.25–1 | 1 | 1 | 0.125–4 | 0.5 | 2 |
| 5-FC | ≤0.125–16 | ≤0.125 | 1 | ≤0.125–8 | ≤0.125 | 1 | |
| FLU | ≤0.125–>64 | 1 | >64 | ≤0.125–>64 | 1 | >64 | |
| ITRA | ≤0.015–2 | 0.25 | 1 | ≤0.015–8 | 1 | >8 | |
| C. glabrata (20) | AMB | 0.5–2 | 1 | 1 | 0.25–4 | 1 | 2 |
| 5-FC | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | |
| FLU | ≤0.125–>64 | 4 | 64 | 0.5–>64 | >64 | >64 | |
| ITRA | ≤0.015–>8 | 0.5 | 1 | 0.06–>8 | 1 | >8 | |
| C. krusei (19) | AMB | 0.5–1 | 1 | 1 | 0.25–4 | 1 | 4 |
| 5-FC | 4–16 | 16 | 16 | 4–64 | 16 | 64 | |
| FLU | 16–64 | 32 | >64 | 16–64 | 32 | >64 | |
| ITRA | 0.25–1 | 0.5 | 0.5 | 0.06–>8 | 0.5 | 8 | |
| C. parapsilosis (28) | AMB | 0.25–1 | 0.5 | 1 | 0.125–2 | 0.5 | 2 |
| 5-FC | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125–0.5 | ≤0.125 | 0.25 | |
| FLU | ≤0.125–4 | 1 | 4 | ≤0.125–>64 | 1 | >64 | |
| ITRA | ≤0.015–0.25 | 0.125 | 0.125 | ≤0.015–>8 | 0.125 | 8 | |
| C. tropicalis (19) | AMB | 0.5–2 | 1 | 1 | 0.25–4 | 1 | 2 |
| 5-FC | ≤0.125–32 | ≤0.125 | 8 | ≤0.125–16 | ≤0.125 | 2 | |
| FLU | 0.25–64 | 1 | >64 | 0.25–>64 | 0.5 | >64 | |
| ITRA | <0.015–>8 | 0.25 | >8 | 0.03–8 | 0.25 | 8 | |
50% and 90% indicate concentrations required to inhibit 50 and 90% of the strains tested, respectively.
Results observed by using the RSA method followed two major patterns. First, for AMB and 5-FC, plots generated from the RSA followed a simple pattern of a drug-saturation plateau representing drug sensitivity, a steep decline through the dose-dependent range, and finally a baseline OD indicating glucose consumption by the yeast cells (Fig. 1 and 2). With this pattern, the MIC was defined as the lowest drug concentration on the plateau that preceded the sharp decline in OD value. For these two drugs, OD values (reflecting glucose concentrations) were well separated between growth inhibition wells with higher drug concentrations and growth wells with lower drug concentrations. As a consequence, the MIC was easily determined from the curve. Results from this pattern correlated closely with the NCCLS method.
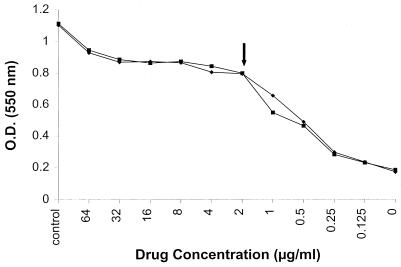
FIG. 1.
RSA susceptibility curve of AMB for an isolate of C. glabrata. Duplicate experiments with the same isolate are shown. The MIC for this organism was 2 μg/ml, as determined by RSA and the NCCLS method. Note the drug-saturation plateau above 2 μg/ml. The control well contained no drug and no yeast inoculum. An inoculum of an 0.5 McFarland standard was added to the wells containing 0 to 16 μg of drug per ml and incubated for 6 h before the complete color mix was added. OD values represent relative glucose concentrations.
FIG. 2.
RSA susceptibility curve of 5-FC for an isolate of C. tropicalis (MIC = 2 μg/ml by NCCLS and RSA). Duplicate experiments with the same isolate are shown. See the legend to Fig. 1 for more information.
The second RSA susceptibility pattern was demonstrated by the use of FLU and ITRA, where MIC endpoints were within an arc which spanned three to five drug concentrations. There was not a sharp decline in the transition from drug-sensitive to dose-dependent region, as observed in the first pattern. In addition, there was less difference in the OD values between the higher-drug concentration wells and lower-drug concentration wells, resulting in shallow curves (Fig. 3A and 4A). This often resulted in a higher MIC reading than that obtained by the NCCLS method.
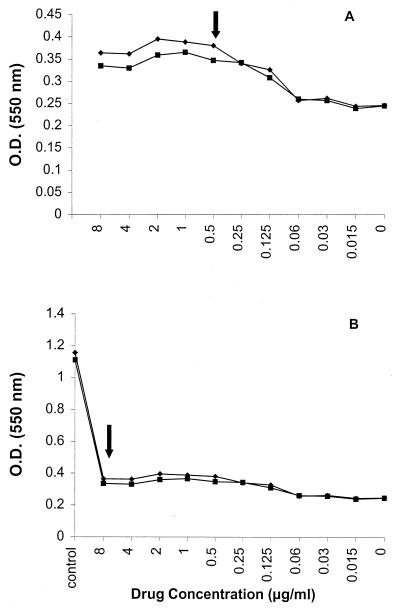
FIG. 3.
(A) Representative RSA susceptibility curve of FLU for a C. glabrata isolate. The NCCLS MIC of FLU for this organism was ≤0.125 μg/ml. The RSA MIC falls in the arc between 0.25 and 1 μg/ml. Note the relatively small difference in OD between the wells with the highest drug concentration and the well without drug. (B) Plot of data for the same isolate as in panel A except that glucose control is included. Note the consumption of glucose even at high concentration of FLU (arrow). In the presence of the high-glucose control OD value, the curve appears flat and erroneously indicates resistance. Duplicate experiments with the same isolate are shown.
FIG. 4.
(A) Representative RSA susceptibility curve of ITRA for a C. glabrata isolate. MICs obtained by RSA and NCCLS methods were both 0.5 μg/ml. (B) Plot of data for the same isolate as in panel A except that glucose control is included. Note the reduction in glucose level even at high concentrations of ITRA (arrow). In the presence of glucose control, the curve appears flat and erroneously indicates resistance. Duplicate experiments with the same isolate are shown.
Comparison of MICs obtained by the RSA and NCCLS M-27A methods resulted in excellent agreement for AMB and 5-FC (Table 2). For all five species, there was total agreement for AMB (106 of 106) and 98% agreement for 5-FC (104 of 106). Similar observations were recorded when RSA data were compared with M-27A MICs read at 24 h (data not shown). Less favorable comparison was observed for the azoles (Table 2). The overall agreement for all isolates between the two methods was 63.2 and 61.3% for FLU and ITRA, respectively. C. krusei demonstrated the highest MIC agreement between the two methods for FLU (100%). This high level of correlation reflects the fact that isolates of C. krusei are intrinsically resistant to FLU, thus contributing to the high MICs for FLU obtained by both methods.
TABLE 2.
Distribution of MIC differences between RSA (6-h) and NCCLS methods
| Yeast (no. of isolates) | Drug | No. of isolates with MICs differing by log, dilution:
|
% Agreementa | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | >2 | |||
| C. albicans (20) | AMB | 2 | 13 | 5 | 100 | |
| 5-FC | 9 | 6 | 3 | 2 | 90 | |
| FLU | 1 | 4 | 4 | 11 | 45 | |
| ITRA | 1 | 3 | 2 | 14 | 30 | |
| C. glabrata (20) | AMB | 8 | 11 | 1 | 100 | |
| 5-FC | 20 | 100 | ||||
| FLU | 2 | 5 | 1 | 12 | 40 | |
| ITRA | 3 | 5 | 5 | 7 | 65 | |
| C. krusei (19) | AMB | 5 | 9 | 5 | 100 | |
| 5-FC | 11 | 5 | 3 | 100 | ||
| FLU | 8 | 9 | 2 | 100 | ||
| ITRA | 3 | 7 | 7 | 2 | 89.5 | |
| C. parapsilosis (28) | AMB | 14 | 14 | 100 | ||
| 5-FC | 25 | 2 | 1 | 100 | ||
| FLU | 6 | 10 | 4 | 8 | 71.4 | |
| ITRA | 7 | 11 | 5 | 5 | 82 | |
| C. tropicalis (19) | AMB | 5 | 13 | 1 | 100 | |
| 5-FC | 14 | 4 | 1 | 100 | ||
| FLU | 5 | 3 | 3 | 8 | 57.9 | |
| ITRA | 3 | 2 | 1 | 13 | 31.6 | |
| All isolates (106) | AMB | 34 | 60 | 12 | 100 | |
| 5-FC | 79 | 17 | 8 | 2 | 98 | |
| FLU | 22 | 31 | 14 | 39 | 63.2 | |
| ITRA | 17 | 28 | 20 | 41 | 61.3 | |
RSA MICs were compared with NCCLS MICs recorded at 48 h. MICs differing within 2 dilutions were considered in agreement.
As mentioned previously, the RSA method resulted in a higher azole MIC than that obtained from the NCCLS method when the two were not in agreement. One explanation for the higher azole MICs is that these fungistatic drugs, even at high concentrations, did not prevent glucose consumption by susceptible organisms even at concentrations higher than their MICs. As a result, there was not a distinct difference between the OD of growth-inhibited wells and that of the drug-free control wells. Thus, susceptibility could not be readily distinguished from resistance. As shown in Fig. 3B and 4B, for isolates that were susceptible to both FLU and ITRA by the NCCLS method, a high concentration of drugs did not prevent glucose consumption as compared to the no-yeast glucose control. It is evident that, for the azole drugs, these testing conditions did not produce a sufficient detection interval in the OD values to allow interpretation of MICs.
Several factors influence the MIC determination of any susceptibility testing method (1, 11). Perhaps more prominent for the RSA are the inoculum density, incubation time, and mechanism of drug action. From our results, it is evident that AMB and 5-FC have a rapid effect on glucose uptake at concentrations above their MIC. However, azoles were less effective in inhibiting glucose uptake under the conditions tested. Therefore, in an attempt to increase the sensitivity of the RSA for azole drugs, the assay was modified by lowering the inoculum size and prolonging the incubation time. Several inoculum dilutions and incubation periods were investigated. With lower yeast concentrations, glucose consumption was not sufficient at 6 and 8 h of incubation to allow MIC determination (data not shown). The optimal conditions were found by diluting the initial inoculum 1,000-fold and increasing incubation time from 6 to 19 h. The RSA MICs of azoles obtained under these conditions were comparable to those obtained from the NCCLS M-27A method (Table 3). As illustrated in Fig. 5 and 6, these modifications have allowed a greater detection interval of OD values between higher-drug concentration (growth-inhibited) and lower-drug concentration (growth) wells, thus permitting accurate readings of MICs of the azole drugs. Our results confirmed the previous experience of others (10) that diluted inocula and prolonged incubation facilitate azole MIC determination and reproducibility for this assay.
TABLE 3.
RSA azole MICs determined with diluted inoculum and prolonged incubation
| Isolate no. | Species | Drug | MIC (μg/ml) determined by:
|
||
|---|---|---|---|---|---|
| 48- NCCLS | RSA
|
||||
| Undiluted, 6 ha | Diluted, 19 hb | ||||
| 97-049 | C. glabrata | FLU | 64 | >64 | 64 |
| ITRA | 2 | >8 | 2 | ||
| 97-050 | C. glabrata | FLU | 8 | >64 | 8 |
| ITRA | 1 | >8 | 0.5 | ||
| 97-055 | C. tropicalis | FLU | 1 | >64 | 0.25 |
| ITRA | 0.125 | 0.125 | 0.125 | ||
| 97-012 | C. albicans | FLU | 4 | >64 | 8 |
| ITRA | 0.5 | 0.25 | 0.5 | ||
| 97-056 | C. parapsilosis | FLU | 0.25 | 4 | 0.5 |
| ITRA | 0.125 | 0.125 | 0.125 | ||
In the RSA, an inoculum with a turbidity of an 0.5 McFarland standard was used initially without dilution, with an incubation time of 6 h. Various inoculum dilutions and incubation times were subsequently tested for the azole compounds.
The original 0.5-McFarland standard inoculum preparation was diluted 1:1,000 in RPMI 1640 medium without glucose before inoculation. Plates were incubated for a total of 19 h before the complete color mixture was added.
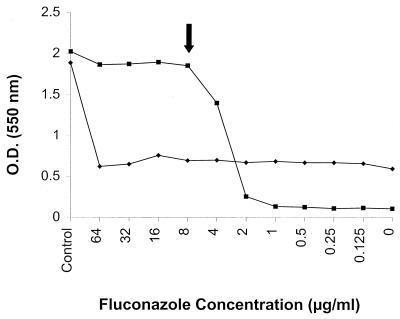
FIG. 5.
RSA curves of FLU for a C. albicans isolate (97-012) when inoculated with an undiluted 0.5-McFarland standard yeast suspension and incubated for 6 h (⧫) or with a 1/1,000-diluted inoculum and incubated for 19 h (■). The MICs for this organism are reported in Table 3.
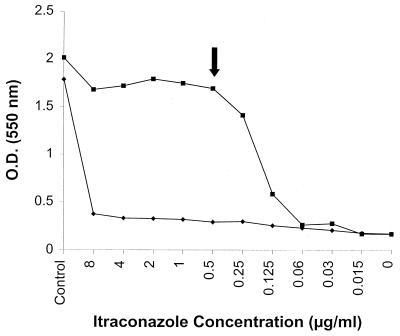
FIG. 6.
RSA curves of ITRA for a C. glabrata isolate (97-050). An undiluted 0.5-McFarland standard yeast suspension was used for inoculation and incubated for 6 h (⧫). The inoculum was diluted 1/1,000 and incubated for 19 h (■). The latter resulted in a MIC comparable to that obtained by the NCCLS method. MICs for this isolate are listed in Table 3.
In conclusion, the RSA method appears to be a potential alternative for the determination of MICs of AMB and 5-FC for Candida isolates. MICs obtained from RSA are in close agreement with results obtained from the published reference method. In addition, MICs can be determined in 1 day, compared with the 48-h incubation time for the accepted method. Furthermore, the broader MIC range of AMB from the RSA method may offer an advantage for detecting AMB-resistant isolates. Experience with the RSA suggests that this method can be readily applied to other fast-acting drugs and fast-growing organisms. This method may also be applicable to azole or other slow-acting drugs with modifications such as overnight incubation. However, these modifications may offer little advantage over the existing NCCLS M-27A method since yeast growth is already prominent after 19 h of incubation. Further evaluation of the RSA method with other antifungal drugs and fungal species is warranted.
REFERENCES
- 1.Espinel-Ingroff A, Pfaller M A. Antifungal agents and susceptibility testing. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 1405–1414. [Google Scholar]
- 2.Fromtling R A, Galgiani J N, Pfaller M A, Espinel-Ingroff A, Bartizal K F, Bartlett M S, Body B A, Frey C, Hall G, Roberts G D, Nolte F B, Odds F C, Rinaldi M G, Sugar A M, Villareal K. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993;37:39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galgiani J N, Reiser J, Brass C, Espinel-Ingroff A, Gordon M A, Kerkering T M. Comparison of relative susceptibility of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrob Agents Chemother. 1987;31:1343–1347. doi: 10.1128/aac.31.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graybill J R, Montalbo E, Kirkpatrick W R, Luther M F, Revankar S G, Patterson T F. Fluconazole versus Candida albicans: a complex relationship. Antimicrob Agents Chemother. 1998;42:2938–2942. doi: 10.1128/aac.42.11.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M-27A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 6.Perfect J R, Schell W A. The new fungal opportunists are coming. Clin Infect Dis. 1996;22:S112–S118. doi: 10.1093/clinids/22.supplement_2.s112. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller M A, Jones R N, Messer S A, Edmond M B, Wenzel R P. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn Microbiol Infect Dis. 1998;30:121–129. doi: 10.1016/s0732-8893(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 8.Powles R L, Mehta J. Systemic fungal infections: major problems in cancer patients. Indian J Cancer. 1996;31:180–184. [PubMed] [Google Scholar]
- 9.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 10.Riesselman M H, Hazen K C, Cutler J E. Determination of antifungal MICs by a rapid susceptibility assay. J Clin Microbiol. 2000;38:333–340. doi: 10.1128/jcm.38.1.333-340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan D J, Espinel-Ingroff A, Moore L S, Webb C D. Antifungal susceptibility testing of yeasts: a brief overview. Clin Infect Dis. 1993;17(Suppl. 2):S494–S500. doi: 10.1093/clinids/17.supplement_2.s494. [DOI] [PubMed] [Google Scholar]