Abstract
We have used serological proteome analysis in conjunction with tandem mass spectrometry to identify and sequence a novel protein, Mtb81, which may be useful for the diagnosis of tuberculosis (TB), especially for patients coinfected with human immunodeficiency virus (HIV). Recombinant Mtb81 was tested by an enzyme-linked immunosorbent assay to detect antibodies in 25 of 27 TB patients (92%) seropositive for HIV as well as in 38 of 67 individuals (57%) who were TB positive alone. No reactivity was observed in 11 of 11 individuals (100%) who were HIV seropositive alone. In addition, neither sera from purified protein derivative (PPD)-negative (0 of 29) nor sera from healthy (0 of 45) blood donors tested positive with Mtb81. Only 2 of 57 of PPD-positive individuals tested positive with Mtb81. Sera from individuals with smear-positive TB and seropositive for HIV but who had tested negative for TB in the 38-kDa antigen immunodiagnostic assay were also tested for reactivity against Mtb81, as were sera from individuals with lung cancer and pneumonia. Mtb81 reacted with 26 of 37 HIV+ TB+ sera (70%) in this group, compared to 2 of 37 (5%) that reacted with the 38-kDa antigen. Together, these results demonstrate that Mtb81 may be a promising complementary antigen for the serodiagnosis of TB.
Tuberculosis (TB) is a chronic pulmonary disease caused by infection with Mycobacterium tuberculosis. It is a major disease in developing countries as well as an increasing problem in many developed areas of the world, with about 8 million new cases and 3 million deaths each year (21). A resurgence of TB, largely due to the emergence of drug-resistant strains of M. tuberculosis (28) and the increased risk for TB in human immunodeficiency virus (HIV)-infected persons (9, 14, 29), has magnified the need for rapid, inexpensive, and accurate methods for the diagnosis of TB.
The most common immunologic method used for the diagnosis of M. tuberculosis infection is the purified protein derivative (PPD) (tuberculin) skin test. Although this test is used throughout the world, it is not optimal in terms of either sensitivity or specificity. Individuals vaccinated with bacillus Calmette-Guérin to prevent TB may show a false-positive PPD response. Furthermore, TB is a frequent occurrence in AIDS patients, and the sensitivity of the tuberculin skin test is substantially reduced during HIV infection (4, 13, 16). Direct detection of acid-fast bacilli in sputum can also be accomplished by bacterial staining, culturing, or PCR. Drawbacks to this approach include difficulty in obtaining sputum from children as well as the overall low sensitivity rate, particularly for extrapulmonary TB (12). An alternative approach for diagnosis involves the detection of serum antibodies.
Serodiagnostic tests based on the presence of antibodies against mycobacterial antigens in sera have been described (reviewed in reference 10). Antigens such as 38-kDa PhoS (1), the 30-kDa antigen (antigen 6, alpha antigen, MPB, or 85B) (27), 16-kDa HSP (31), LAM (18), and A60 (5) have been identified, purified, and tested, with various degrees of success. Diagnostic tests based on the 38-kDa antigen, antibodies to which are associated with severe and recurrent disease (3), have achieved sensitivities of as high as 70 to 80% and 95 to 100% specificities (6). However, this antigen has markedly lower sensitivity in smear-negative populations as well as in individuals infected with HIV (19, 32). Several studies have addressed the problems of detecting M. tuberculosis-specific antibodies in TB patients coinfected with HIV. For example, in a field test in Mexico, an enzyme-linked immunosorbent assay (ELISA) based on the 30-kDa mycobacterial antigen had a sensitivity of 70% in patients with culture-positive or smear-positive pulmonary TB and a specificity of 100% in 125 control donors (26). The same test was evaluated with HIV-positive and -negative patients in Uganda (11). Although the sensitivity and specificity in HIV-negative donors were the same as in the Mexico test, the ELISA was positive for only 28% of 128 sera from HIV-positive donors. Accordingly, there is a need for improved diagnostic methods for detecting TB in HIV-positive individuals.
More recently, the reactivity of high-molecular-weight antigens with patient sera has been described. In particular, an 88-kDa antigen was found to elicit a strong antibody response in patients with TB (23). Most importantly, the 88-kDa antigen, which is present in M. tuberculosis culture filtrate proteins (CFP), also has been described as a surrogate marker for TB in HIV-seropositive individuals, but its peptide sequence has been elusive (22). Monoclonal antibody IT-57 has been known to react with an 88-kDa M. tuberculosis antigen in the high-molecular-weight region of CFP, although the identity of the protein antigen has not been previously determined (20). In this report, we used two-dimensional (2D) gel electrophoresis and immunoblot analysis to identify a protein reactive with IT-57 and then nano-liquid chromatography–electrospray ionization tandem mass spectrometry (nano-LC/MS/MS) to identify and sequence a novel protein, Mtb81. Recombinant Mtb81 was expressed in Escherichia coli and evaluated by an ELISA. Based on serological analysis, Mtb81 appears to be a promising antigen for the serodiagnosis of TB, especially for patients coinfected with HIV.
MATERIALS AND METHODS
M. tuberculosis CFP.
CFP from M. tuberculosis strain H37Rv were obtained from Colorado State University.
Antibody reagents.
Murine monoclonal antibody IT-57 (20) was obtained from the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases (Centers for Disease Control and Prevention, Atlanta, Ga.).
Antigens.
The 38-kDa antigen was expressed in E. coli by using a hexahistidine tag similar to that used for Mtb81 (see below). The TB lysate was prepared by alternately homogenizing and sonicating three 100-mg ampoules of dessicated M. tuberculosis H37Ru (Difco, Franklin Lakes, N.J.) three times in 25 ml of 10 mM Tris (pH 8) containing 2% Nonidet P-40. The mixture was spun for 10 min at 13,000 × g. The supernatant was used as the TB lysate.
Study population.
Blood was drawn from all patients after informed consent was obtained. Serum samples were obtained from individuals who had pulmonary TB alone prior to treatment (culture and/or acid-fast bacillus smear positive; see Results) or who had documented coinfections with HIV, as evidenced by a positive HIV type 1 and 2 antibody ELISA. These included samples from Uganda and South Africa that were obtained from Milton Tam (Program for Appropriate Technology in Health, Seattle, Wash.). Additional HIV+ TB+ serum samples from South Africa and samples from patients with lung cancer and pneumonia (China) were obtained from Robert Cole (AMRAD-ICT, French's Forest, Australia). This second group of HIV+ TB+ samples was selected on the basis of their negativity for the 38-kDa antigen in the AMRAD-ICT rapid test but smear- and/or culture-positive results (6, 34). To further evaluate the specificity of the Mtb81 antigen, we obtained sera from individuals who were PPD positive (>10-mm reaction zone) (culture, clinically, and radiographically negative for TB) and PPD negative (King County Tuberculosis Clinic, Seattle, Wash.). Samples from healthy blood donors (United States) were obtained from Boston Biomedica (West Bridgewater, Mass.), and sera from individuals who were HIV seropositive alone were obtained from Robert Ackridge (Fred Hutchinson Cancer Research Center, Seattle, Wash.).
Reverse-phase fractionation of M. tuberculosis CFP.
Proteins were separated by reverse-phase chromatography on either a C18 or a diphenyl column.
(i) C18 fractionation.
Approximately 75 mg of CFP was dissolved in water containing 0.1% trifluoroacetic acid (TFA), and the mixture was injected onto a C18 reverse-phase column (22 by 250 mm; The Separations Group, Hesperia, Calif.) by using a preparative liquid chromatograph (Waters, Milford, Mass.). Fractions were eluted with a binary gradient of 0.1% TFA in water (solvent A) and acetonitrile (solvent B) at a flow rate of 10 ml/min. The gradient increased from 0 to 100% solvent B in 60 min, and fractions were collected at 1-min intervals. Fraction 38, which contained reactivity to antibody IT-57, was identified by Western blot analysis as described below.
(ii) Diphenyl fractionation.
Approximately 5 mg of CFP was dissolved in water containing 0.5% TFA, and the mixture was injected onto a Vydac diphenyl reverse-phase column (catalog no. 219TP5415; The Separations Group). Fractions were eluted with a binary gradient of 0.5% TFA in water (buffer A) and acetonitrile (buffer B) on an AKTA Explorer 100 separation system (Amersham Pharmacia Biotech, Uppsala, Sweden). The column was equilibrated with 30% buffer B, and a linear gradient was run from 30 to 65% buffer B at 2 ml/min over the course of 30 min. Fractions were collected at 1.5-ml intervals and analyzed by Western blotting with antibody IT-57. Fractions containing a protein recognized by this antibody eluted at about 50% buffer B and were pooled for further analysis.
SDS-PAGE and 2D PAGE of CFP.
Individual high-pressure liquid chromatography (HPLC) fractions were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on 4 to 20% gradient gels (Bio-Rad, Hercules, Calif.) in accordance with the manufacturer's instructions and transferred to nitrocellulose. HPLC fraction 38 was concentrated to approximately 400 μl, and 40 μl was added to 400 μl of rehydration solution containing 8 M urea, 0.5% (wt/wt) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 15 mM dithiothreitol (DTT), and 0.2% (wt/vol) Parmalyte (pH 3 to 10). The solution was placed in a rehydration cassette, and 18-cm pH 3 to 10 Immobiline DryStrips (Pharmacia Biotech, Uppsala, Sweden) were allowed to hydrate overnight. The hydrated strips were rinsed and focused by using a Multiphor II electrophoresis system with an Immobiline DryStrip kit and an EPS 3500 XL power supply (Pharmacia Biotech) according to the following gradient: 0 to 300 V for 5 min, 300 to 3,500 V for 6 h, and thereafter to 80,000 V · h.
Tube gels for the one-dimensional (1D) control lanes were cast by adding 10 μl of each fraction to 10 μl of Tris-acetate equilibration buffer (ESA, Chelmsford, Mass.) containing 2% (wt/vol) DTT and 2% (wt/vol) agarose. The solution was heated at 100°C for 5 min. Tube gels for molecular weight standards were cast by adding 2 μl of low-molecular-weight silver standards (Bio-Rad) to 8 μl of water and heating the solution as described above.
Each focused Immobiline DryStrip was equilibrated in 10 ml of equilibration buffer containing 6 M urea, 2% (wt/vol) SDS, and 2% (wt/vol) DTT and rocked gently for 15 min. The buffer was decanted, and each DryStrips was equilibrated in 10 ml of Tris-acetate equilibration buffer containing 6 M urea and 2.5% iodoacetamide and rocked gently for 15 min. Strips were placed on top of 10% homogeneous double gels (ESA) alongside the 1-cm tube gels containing the same HPLC fraction as the DryStrip and the standards. The gels were run at 20 mA/gel overnight on an ESA Investigator 2D electrophoresis system containing Tris acetate running buffer in the lower (anode) tank and Tris-Tricine-SDS buffer in the upper (cathode) tank.
Immunoblotting.
Proteins subjected to SDS-PAGE or 2D PAGE analysis were transferred to nitrocellulose membranes (Hybond C Extra; Amersham Corp., Arlington Heights, Ill.) and blocked with 0.5 M NaCl in phosphate-buffered saline (PBS) containing 0.05% Tween (PBST). Blots were washed with PBS and probed with antibody IT-57 at a 1:50 or 1:70 dilution of culture supernatant in 0.5 M NaCl in PBST for the 1D or 2D gels, respectively. After overnight incubation, blots were washed and probed with horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin G (Jackson Immuno Research, West Grove, Pa.). Western blots were developed by use of a Pierce Super Signal enhanced chemiluminescence protocol (Pierce, Rockford, Ill.).
To correlate the immunoblots to spots on the silver-stained 2D polyacrylamide gel, AurodyeForte (Amersham) total protein stain was applied to the membranes. Briefly, after immunoblots were developed by enhanced chemiluminescence, the membranes were washed in PBS–0.3% Tween three times for 5 min each time, rinsed in nanopure water, and then incubated in 40 ml of AurodyeForte stain at room temperature with gentle rocking until the desired proteins were visible.
Silver stain protocol.
Polyacrylamide and 2D polyacrylamide gels were silver stained by the method of Blum et al. (2) with slight modifications. After the gels were sufficiently developed, the chemical process was stopped by the addition of 5% acetic acid. Prior to storage at 4°C, the gels were rinsed in nanopure water and placed in 0.1% acetic acid solution.
In situ digests and mass spectrometric analysis.
The silver-stained gel pieces were excised and digested in situ (30). An aliquot of the tryptic peptides was loaded onto a C18 microcapillary column (75 μm [inner diameter] by 12 cm) and eluted with a binary gradient of acetonitrile and 0.1 M acetic acid, with the concentration of acetonitrile increasing from 0 to 80% over 12 min, into a triple-quadrupole mass spectrometer (TSQ7000; Finnigan MAT, San Jose, Calif.) equipped with an electrospray ionization source. Mass spectra were acquired every 1.5 s over a mass range of 300 to 1,400 atomic mass units. Candidate peptide masses were identified by comparing the tryptic digest to a control digest. Collision-activated dissociation (CAD) mass spectra were recorded for the peptide-free acids. The CAD spectra were interpreted de novo (17) or by use of peptide sequence tags (24). The H37Rv genomic sequence used was from the published literature (7).
Mtb81 cDNA cloning, expression, and purification.
Mtb81 cDNA representing the entire open reading frame was generated by PCR from genomic M. tuberculosis DNA using the primers 5′-CTAAGTAGTACTGATCGCGTGTCGGTGGGC-3′ and 5′-CAGTGAGAATTCACTAGCGGGCCGCATCGTCAC-3′. Hexahistidine-tagged Mtb81 was obtained using custom PCR primers with the hexahistidine tag and Mtb81 cDNA as a template. The amplified product was subcloned into the pET28 vector system (Novagen, Madison, Wis.), and the sequence was confirmed. His-tagged Mtb81 protein was expressed and purified by standard methodology and affinity purified from inclusion bodies by detergent extraction and Ni2+-nitrilotriacetic acid affinity chromatography.
ELISAs.
ELISAs were performed with 96-well microtiter plates (Corning Costar, Cambridge, Mass.) coated with Mtb81, 38-kDa antigen (200 ng/well), or TB lysate (100 ng/well). Coating was done overnight at 4°C. Plates were then aspirated and blocked with PBS containing 1% (wt/vol) bovine serum albumin for 2 h at room temperature, followed by a wash with PBS containing 0.1% Tween 20 (PBST2). Serum (diluted 1/25 in PBST2 for Mtb81 and 1/100 for the 38-kDa antigen and the lysate) was added to the wells and incubated for 30 min at room temperature. Following incubation, the wells were washed six times with PBST2 and incubated with protein A-horseradish peroxidase conjugate (Sigma Chemical Co., St. Louis, Mo.) at a 1/20,000 dilution for 30 min. Plates were then washed six times with PBST2 and incubated with tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) for a further 15 min. The reaction was stopped by the addition of 1 N sulfuric acid, and plates were read at 450 nm with an ELISA plate reader (Biotek, Hyland Park, Va.). The cutoff for the assays was the mean of the negative population plus three standard deviations of the mean.
RESULTS
Antibody IT-57 recognizes a single HPLC fraction of CFP.
To identify a diagnostic Mtb antigen, CFP were separated by reverse-phase chromatography on a C18 column. Each fraction was individually tested by immunoblot analysis to identify fractions containing reactivity against IT-57. Fraction 38 reacted with IT-57; all other fractions were negative (Fig. 1). To simplify this mixture of proteins, fraction 38 was further analyzed using 2D Western blot analysis. This methodology combines the resolving power of 2D gel electrophoresis, which can separate proteins to within 1 kDa in the molecular mass dimension and a pI of 0.1 in the isoelectric dimension, with the specificity of immunoblot analysis. Because ultra-high-sensitivity protein sequencing can be achieved from silver-stained gels (33), we analyzed fraction 38 by 2D SDS-PAGE in duplicate. One gel was transferred to nitrocellulose, immunoblotted with antibody IT-57, and developed by chemiluminescence, and the other gel was silver stained for subsequent mass spectrometric analysis. Primary antibody binding conditions were achieved using a high-salt and detergent blocking protocol. Because of the blocking conditions used, we used gold to stain the membrane for a total protein stain to allow an accurate correlation between the immunoblot and the silver-stained 2D gel. Gold stain has a sensitivity similar to that of silver stain. The spot of reactivity on the immunoblot that corresponds to the visible spot on the silver-stained gel is shown in Fig. 2.
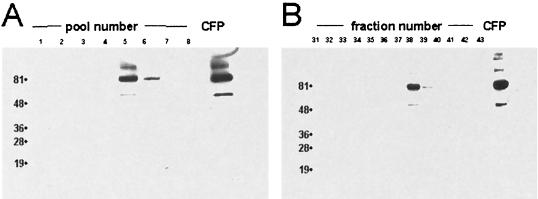
FIG. 1.
Immunoblot analysis of reverse-phase fractionated M. tuberculosis CFP. CFP were separated on a C18 column, and fractions were collected at 1-min intervals. Pooled (A) and individual (B) aliquots were separated by SDS-PAGE and, after being transferred to a membrane, immunoblotted with antibody IT-57. Numbers to the left of lanes are kilodaltons.
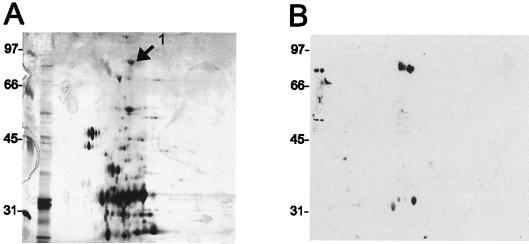
FIG. 2.
2D Western blot analysis of HPLC fraction 38. Fraction 38 was separated by 2D gel electrophoresis in duplicate. One gel was silver stained (A), and the other was transferred to nitrocellulose and immunoblotted with antibody IT-57 (B). Data from the two gels were correlated, and the 81-kDa spot of reactivity in the immunoblot is labeled in the corresponding silver-stained gel (arrow labeled “1”). Numbers to left of panels are kilodaltons.
Identification of Mtb81.
To determine the identity of the protein in spot 1 (Fig. 2), the gel piece was excised and digested in situ with trypsin (30), and the total peptide mixture was extracted, concentrated, and analyzed by nano-LC/MS. By comparing the tryptic digest of spot 1 to that of a control tryptic digest, we identified two peptides, with (M + 2H)2+ ions at m/z of 444 and 531, which were present in spot 1 but absent in the control.
To sequence the candidate peptides, an additional aliquot of CFP was generated by diphenyl fractionation and digested in situ as described in Materials and Methods. CAD mass spectra were recorded for the (M + 2H)2+ ions at m/z 531 and 444, as shown in Fig. 3D and E, respectively. The information in the spectrum for 531 was sufficient to assign seven of nine residues of the sequence as XQAQXDK (where X is Leu or Ile) and the mass of the b2 ion as 245 (17). This information was assembled as a peptide sequence tag (24) and used to search the published M. tuberculosis H37Rv genomic sequence (7). A single match to the sequence DELQAQLDK was obtained from genomic clone 1773. Spectra generated for the (M + 2H)2+ ion at m/z 769 confirmed this result by identifying an additional tryptic peptide corresponding to the sequence NYTAPGGGQFTLPGR, which is also encoded by the same genomic clone. We have designated the novel protein Mtb81 (Table 1). A similar analysis was performed on the CAD mass spectra for the ions at m/z 444. The amino acid sequence TFGFGFR was deduced from the mass spectral data, and a database search with the corresponding peptide sequence tag revealed the source protein to be KatG. Hence, protein spot 1 contained a mixture of at least two proteins, Mtb81 and KatG.
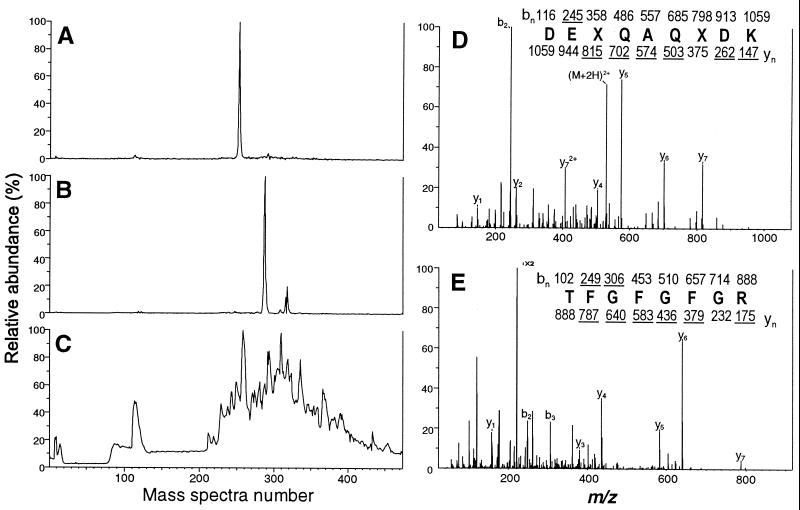
FIG. 3.
Identification of proteins in spot 1 by nano-LC/MS and nano-LC/MS/MS analyses. The silver-stained gel spot 1, shown in Fig. 2, from the 2D Western blot analysis of HPLC fraction 38 was excised, digested in situ with trypsin, and analyzed by nano-LC-MS. Ion current chromatograms for the candidate peptides at m/z 531 and 444 as well as the total ion current chromatogram are shown in panels A, B, and C, respectively. CAD mass spectra of (M + 2H)2+ ions at m/z 531 and 444 are shown in panels D and E, respectively. Ions observed in each spectrum are underlined. Deduced amino acid sequences are shown, where X refers to Leu or Ile, which were not differentiated in this experiment.
TABLE 1.
Deduced amino acid sequence of Mtb81a
| Posi- tion | Sequence |
|---|---|
| 1 | MTDRVSVGNL RIARVLYDFV NNEALPGTDI DPDSFWAGVD KVVADLTPQN |
| 50 | QALLNARDEL QAQIDKWHRR RVIEPIDMDA YRQFLTEIGY LLPEPDDFTI |
| 100 | TTSGVDAEIT TTAGPQLVVP VLNARFALNA ANARWGSLYD ALYGTDVIPE |
| 150 | TDGAEKGPTY NKVRGDKVIA YARKFLDDSV PLSSGSFGDA TGFTVQDGQL |
| 200 | VVALPDKSTG LANPGQFAGY TGAAESPTSV LLINHGLHIE ILIDPESQVG |
| 250 | TTDRAGVKDV ILESAITTIM DFEDSVAAVD AADKVLGYRN WLGLNKGDLA |
| 300 | AAVDKDGTAF LRVLNRDRNY TAPGGGQFTL PGRSLMFVRN VGHLMTNDAI |
| 350 | VDTDGSEVFE GIMDALFTGL IAIHGLKASD VNGPLINSRT GSIYIVKPKM |
| 400 | HGPAEVAFTC ELFSRVEDVL GLPQNTMKIG IMDEERRTTV NLKACIKAAA |
| 450 | DRVVFINTGF LDRTGDEIHT SMEAGPMVRK GTMKSQPWIL AYEDHNVDAG |
| 500 | LAAGFSGRAQ VGKGMWTMTE LMADMVETKI AQPRAGASTA WVPSPTAATL |
| 550 | HALHYHQVDV AAVQQGLAGK RRATIEQLLT IPLAKELAWA PDEIREEVDN |
| 600 | NCQSILGYVV RWVDQGVGCS KVPDIHDVAL MEDRATLRIS SQLLANWLRH |
| 650 | GVITSADVRA SLERMAPLVD RQNAGDVAYR PMAPNFDDSI AFLAAQELIL |
| 700 | SGAQQPNGYT EPILHRRRRE FKARAAEKPA PSDRAGDDAA R |
The protein sequence was determined from the M. tuberculosis H37Rv genomic sequence. Amino acid sequences obtained by tandem mass spectrometry are shown in bold.
Recently, using expression cloning, researchers identified a clone coding for the protein reactive with antibody IT-57 and determined this protein to be KatG (K. M. Samanich, J. T. Belisle, M. G. Sonnenberg, S. Zolla-Pazner, and S. Laal, Abstr. from the Meeting TB: Molecular Mechanisms and Immunologic Aspects, Keystone, Colo., 1999). However, their subsequent studies using KatG-negative BCG and a KatG deletion knockout mutant of M. tuberculosis demonstrated that sera from TB patients still reacted with an 88-kDa protein in both KatG-negative strains. Interestingly, the strains no longer contained reactivities to IT-57 (J. T. Belisle, personal communication). In our studies, we identified two proteins, KatG and Mtb81. However, because KatG and Mtb81 have identical molecular masses and isoelectric points, we hypothesized that the high-molecular-mass protein described by others as showing patient serum reactivity could copurify with the IT-57-reactive KatG protein. Hence, to test this hypothesis, we decided to pursue the identification of Mtb81. The Mtb81 gene was cloned from the M. tuberculosis H37Rv genomic library and expressed in E. coli as described in Materials and Methods. The recombinant Mtb81 protein produced was found not to be recognized by antibody IT-57, as determined by Western blot analysis (data not shown). This observation either may be due to limitations of the E. coli expression system used or may occur because Mtb81 is not recognized by IT-57 but may represent an antigen recognized by patient sera.
Serological evaluation.
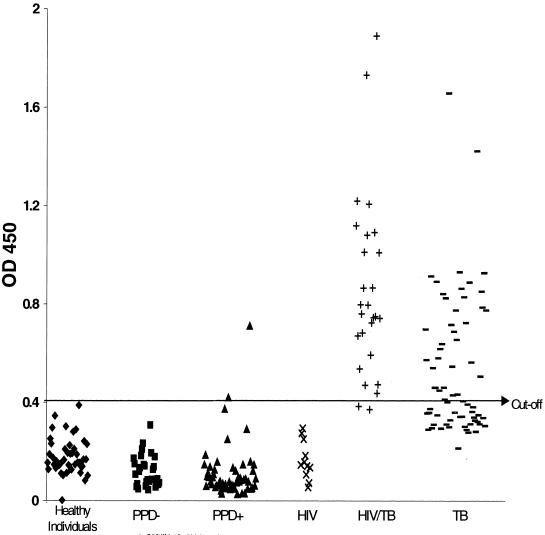
To evaluate Mtb81 as a diagnostic antigen, the purified recombinant protein was tested in an ELISA with sera from individuals from Uganda and South Africa with pulmonary TB and coinfected with HIV, as determined by an HIV type 1 and 2 antibody ELISA. Also tested were sera from the same regions but from individuals with only pulmonary TB. The results showed Mtb81 antibody reactivity in sera from 25 of 27 HIV-positive, TB-positive patients (92%) tested, compared to 38 of 67 (56%) for patients with TB alone (Fig. 4 and Table 2). The reactivity did not appear to be a function of HIV seropositivity, since it was not detected in any U.S. HIV-positive, TB-negative individuals tested (0 of 11). In contrast, the 38-kDa antigen showed reactivity with sera from 18 of 27 patients (67%) in this same group (HIV and TB positive), with ELISA signals barely above the cutoff, and 27 of 67 patients (40.3%) with TB alone, with higher ELISA signals (Table 2 and Fig. 4). To further evaluate the specificity of Mtb81, we also tested sera from individuals who had class II TB, who were PPD positive (>10 mm), but who had no evidence of active disease (culture, clinically, and radiographically negative) and sera from individuals who were PPD negative and had no history of exposure to TB (class 0) or who had a medical history of TB exposure but were PPD negative (class I) (Table 2 and Fig. 4). Sera from healthy blood donors were also tested. Sera from 2 of 57 PPD-positive individuals were positive in the Mtb81 ELISA, whereas sera from 0 of 29 PPD-negative individuals were reactive. No Mtb81 ELISA-positive results were seen for the 45 healthy individuals, indicating that Mtb81 may have a high positive predictive value for TB. These results demonstrate the presence of an antibody response against Mtb81 in sera from patients with active TB both in the presence and in the absence of HIV infection; this response was not detectable in sera from PPD-negative individuals and was detected in sera from only 2 of 57 individuals with PPD-positive responses.
FIG. 4.
Distribution of seroreactivity against Mtb81 among sera from healthy donors (n = 45) (⧫), PPD-negative individuals with no medical history of exposure to TB (class 0; n = 11) and PPD-negative individuals with a medical history of exposure to TB (class I; n = 18) (■), PPD-positive individuals who were culture negative and had no clinical or radiographic evidence of TB (class II; n = 57) (▴), HIV-seropositive individuals with no evidence of TB (n = 11) (×), HIV- and TB-positive individuals (n = 27) (+), and TB-positive, HIV-negative individuals (n = 67) (−). OD 450, optical density at 450 nm.
TABLE 2.
ELISA reactivity of Mtb81 and other TB antigens with HIV-positive and -negative TB sera
| Status | Origina | No. of samples | Resultb of the following test:
|
No. of samples found positive for the following antigen or lysate by ELISA:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| HIV | Smear | Culture | Lysate | Mtb81 | 38 kDa | 38 kDa + Mtb81 | |||
| HIV+ TB+ | Uganda | 17 | + | + | + | 7 | 16 | 12 | 16 |
| Uganda | 1 | + | − | + | 0 | 1 | 1 | 1 | |
| Uganda | 1c | + | − | − | 1 | 1 | 0 | 1 | |
| South Africa | 7 | + | + | + | 5 | 6 | 4 | 7 | |
| South Africa | 1 | + | + | − | 1 | 1 | 1 | 1 | |
| Total | 27 | 14 | 25 | 18 | 26 | ||||
| TB | Uganda | 15 | − | + | + | 10 | 14 | 12 | 14 |
| Uganda | 1 | ? | − | + | 0 | 0 | 0 | 0 | |
| South Africa | 37 | − | + | + | 33 | 17 | 12 | 23 | |
| South Africa | 14 | − | + | − | 14 | 7 | 3 | 9 | |
| Total | 67 | 57 | 38 | 27 | 46 | ||||
| HIV | U.S. | 11 | + | ND | ND | 0 | 0 | 0 | 0 |
| Class II TB (PPD positive) | Africa | 3 | − | − | − | 1 | 0 | 0 | 0 |
| Europe or Asiad | 6 | − | − | − | 0 | 1 | 0 | 1 | |
| S.E. Asia | 7 | − | − | − | 1 | 0 | 2 | 2 | |
| U.S. or Latin America | 41 | − | − | − | 4 | 1 | 3 | 3 | |
| Total | 57 | 6 | 2 | 5 | 6 | ||||
| Class 0 or I TB (PPD negative) | Africa | 1 | − | − | − | 0 | 0 | 0 | 0 |
| Europe or Asiad | 3 | − | − | − | 2 | 0 | 0 | 0 | |
| S.E. Asia | 3 | − | − | − | 0 | 0 | 0 | 0 | |
| U.S. or Latin America | 22 | − | − | − | 0 | 0 | 1 | 1 | |
| Total | 29 | 2 | 0 | 1 | 1 | ||||
S.E., Southeast; U.S., United States.
+, positive; −, negative; ?, test results not available; ND, not done.
This individual was found positive for TB by X-ray.
Primarily from Russia and its satellite regions, where TB is endemic.
To evaluate whether the Mtb81 antigen could identify TB patients seronegative for the 38-kDa antigen, we obtained sera from individuals who were smear positive but who were negative when tested with a rapid immunochromatographic assay using the 38-kDa antigen (AMRAD-ICT) (6, 34). We examined a total of 37 HIV- and TB-positive serum samples, along with control sera and sera from individuals with lung cancer and pneumonia. Mtb81 detected 26 of 37 sera (70%) from the HIV- and TB-positive group; 2 of 37 sera (5%) reacted with the 38-kDa antigen (Table 3). Only 19 of 37 of these sera (51%) reacted with the TB lysate. The 38-kDa antigen and the TB lysate reacted with 1 of 45 and 2 of 45 healthy donor sera, for specificities in this group of 98 and 95%, respectively (Table 3). Mtb81, 38-kDa, and TB lysate antigens were also tested with sera from patients with lung cancer (13) and pneumonia (18) and healthy controls from an area in which TB is endemic (9) to further evaluate specificity (Table 3). Both Mtb81 and 38-kDa antigens showed high specificity (98%), whereas the specificity obtained for the TB lysate antigen (74%) indicated the problems associated with using a whole-cell lysate.
TABLE 3.
ELISA reactivity of Mtb81 with sera from individuals infected with both HIV and TB, with TB, and with other lung disorders and shown to be 38-kDa antigen seronegative
| Status | Origin | No. of samples | Resulta of the following test:
|
No. of samples found positive for the following antigen or lysate by ELISA:
|
||||
|---|---|---|---|---|---|---|---|---|
| HIV | Smear | Culture | 38 kDa | Lysate | Mtb81 | |||
| HIV+ TB+ | South Africa | 13 | + | + | + | 0 | 8 | 11 |
| South Africa | 19 | + | + | − | 1 | 6 | 10 | |
| South Africa | 2 | + | − | + | 0 | 2 | 2 | |
| South Africa | 2 | + | + | ? | 0 | 2 | 2 | |
| South Africa | 1 | + | + | ? | 1 | 1 | 1 | |
| Total | 37 | 2 | 19 | 26 | ||||
| TB | South Africa | 4 | − | + | + | 0 | 4 | 0 |
| South Africa | 3 | − | + | − | 0 | 3 | 0 | |
| South Africa | 4 | ? | + | + | 1 | 2 | 1 | |
| South Africa | 5 | ? | + | − | 0 | 3 | 3 | |
| China | 6 | − | + | + | 0 | 1 | 1 | |
| China | 11 | − | + | − | 0 | 4 | 1 | |
| China | 15 | − | − | + | 0 | 7 | 2 | |
| Total | 48 | 1 | 23 | 8 | ||||
| Lung cancer | China | 13 | − | 0 | 2 | 0 | ||
| Pneumonia | China | 18 | − | 1 | 5 | 1 | ||
| Healthy | China or Caucasia | 9 | − | 0 | 2 | 0 | ||
| Controls | United States | 45 | − | 1 | 2 | 0 | ||
| Total | 85 | 2 | 11 | 1 | ||||
See Table 2, footnote b.
DISCUSSION
This study combines 2D gel electrophoresis and immunoblot analysis, together with tandem mass spectrometric sequencing, to identify a serological marker for TB. Advances in mass spectrometry have made it uniquely suited for the sequencing of proteins. The sensitivity is 10,000 times higher than that of N-terminal Edman analysis, with sensitivity of sequencing by tandem mass spectrometric data at the low-femtomole to low-attomole level (8, 25). This is an essential criterion for protein identification in situations in which very limited quantities of material are available. In addition to high sensitivity, tandem mass spectrometric techniques are unaffected by a blocked N terminus, can directly identify posttranslational modifications, and can be used to identify individual components in a complex mixture without the need for extensive biochemical purification. Therefore, this approach is well suited to antigen identification.
Knowledge of serological markers for TB patients coinfected with HIV would facilitate the development of diagnostic tests for TB. A study designed to characterize the target of the antibody response revealed that an 88-kDa antigen from CFP reacted with 70% of sera from TB patients (23). This same 88-kDa antigen was able to detect antibody in 74% of sera from HIV-positive patients who later developed TB (22). The authors suggested that the 88-kDa antigen, which was also recognized by antibody IT-57, can serve as a surrogate marker for identifying HIV-infected persons with subclinical TB. Because Mtb81 described in this report does not react with IT-57, is not clear whether these antigens are indeed the same. However, since IT-57 has been shown to react with the protein KatG and KatG knockout strains of M. tuberculosis lose IT-57 reactivity yet still show reactivity with patient sera (J. T. Belisle, personal communication), it is possible that IT-57 reactivity does not correlate with patient serum reactivity. It is interesting to note that in this study, even after reverse-phase fractionation and 2D gel electrophoresis, Mtb81 and KatG copurified. One method to determine if Mtb81 and the 88-kDa antigen described by others are the same is to preincubate patient sera with Mtb81 prior to analysis with the purified 88-kDa antigen fraction to ascertain whether Mtb81 can block reactivity. Sequence analysis suggests that Mtb81 is a putative malate synthase based on its 61.3% identity in a 724-amino-acid overlap with an E. coli enzyme (7). Further work is necessary to identify the exact nature and function of this protein.
Mtb81 appears to be highly specific for the diagnosis of TB. Sera from non-TB patients with no medical history of exposure to TB and who were PPD negative tested negative in the Mtb81 ELISA. This was also the case for previously exposed individuals who were PPD negative. Sera from 2 of the 57 PPD-positive individuals (culture negative and with no clinical or radiologic evidence of TB) reacted with Mtb81. Sera from both of these individuals, who had a history of TB infection, also reacted with TB lysate and the 38-kDa antigen. The sensitivity of the Mtb81 ELISA does not appear to be significantly impaired in HIV-seropositive individuals. Although in the studies described, Mtb81 demonstrated a higher sensitivity in HIV-seropositive individuals (92%) than in HIV-seronegative individuals with TB (56%), further studies are necessary to confirm this finding with HIV and TB smear-negative individuals and to assess the utility of Mtb81 for extrapulmonary TB. The fact that Mtb81 was able to detect specific antibody in approximately 70% of sera from individuals with HIV-TB coinfections that were previously shown to be seronegative for the 38-kDa antigen indicates the utility of Mtb81 as a complementary antigen in the design of a cocktail of antigens for the serodiagnosis of TB. Some evidence for this notion is shown in Table 2, where a higher sensitivity was seen when Mtb81 and the 38-kDa antigen were combined. These results indicate the need to perform expanded studies with Mtb81 in combination with other novel antigens previously described (15), including the 38-kDa antigen, particularly for HIV-TB coinfections in different geographical regions.
In summary, Mtb81, identified by serological proteome analysis, is a promising antigen for the serodiagnosis of TB. This methodology should be useful in the identification of other pertinent diagnostic markers relevant for other diseases. In addition, the data suggest that the combination of Mtb81 and the 38-kDa antigen or, potentially, other novel M. tuberculosis antigens (15) would lead to optimal sensitivity for the serodiagnosis of TB and potentially improve clinical sensitivity for TB-positive individuals coinfected with HIV.
ACKNOWLEDGMENTS
We thank Robert Akridge (FHCRC, Seattle, Wash.) for providing the serum samples from U.S. HIV patients and Charles Nolan (King County TB Clinic, Seattle, Wash.) for providing PPD-positive and -negative samples.
This research was supported by the Small Business Innovative Research grant AI-39879-02 and the National Institutes of Health (NIAID contract N01-AI-75320 for Tuberculosis Research Materials and Vaccine Testing).
REFERENCES
- 1.Andersen A B, Hansen E B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989;57:2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 3.Bothamley G H, Rudd R, Festenstein F, Ivanyi J. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax. 1992;47:270–275. doi: 10.1136/thx.47.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caiaffa W T, Graham N M, Galai N, Rizzo R T, Nelson K E, Vlahov D. Instability of delayed-type hypersensitivity skin test anergy in human immunodeficiency virus infection. Arch Intern Med. 1995;155:2111–2117. [PubMed] [Google Scholar]
- 5.Cocito C, Vanlinden F. Preparation and properties of antigen 60 from Mycobacterium bovis BCG. Clin Exp Immunol. 1986;66:262–272. [PMC free article] [PubMed] [Google Scholar]
- 6.Cole R A, Lu H M, Shi Y Z, Wang J, De-Hua T, Zhou A T. Clinical evaluation of a rapid immunochromatographic assay based on the 38 kDa antigen of Mycobacterium tuberculosis in patients with pulmonary tuberculosis in China. Tuberc Lung Dis. 1996;77:363–368. doi: 10.1016/s0962-8479(96)90103-3. [DOI] [PubMed] [Google Scholar]
- 7.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Cox A L, Skipper J, Chen Y, Henderson R A, Darrow T L, Shabanowitz J, Engelhard V H, Hunt D F, Slingluff C L J. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 9.Daley C L, Small P M, Schecter G F, Schoolnik G K, McAdam R A, Jacobs W R J, Hopewell P C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 10.Daniel T M. Immunodiagnosis of tuberculosis. In: Rom W N, Garay S, editors. Tuberculosis. Boston, Mass: Little, Brown & Co.; 1996. pp. 223–231. [Google Scholar]
- 11.Daniel T M, Sippola A A, Okwera A, Kabengera S, Hatanga E, Aisu T, Nyole S, Byekwaso F, Vjecha M, Ferguson L E. Reduced sensitivity of tuberculosis serodiagnosis in patients with AIDS in Uganda. Tuberc Lung Dis. 1994;75:33–37. doi: 10.1016/0962-8479(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 12.Foulds J, O'Brien R. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int J Tuberc Lung Dis. 1998;2:778–783. [PubMed] [Google Scholar]
- 13.Graham N M, Nelson K E, Solomon L, Bonds M, Rizzo R T, Scavotto J, Astemborski J, Vlahov D. Prevalence of tuberculin positivity and skin test anergy in HIV-1-seropositive and -seronegative intravenous drug users. JAMA. 1992;267:369–373. [PubMed] [Google Scholar]
- 14.Havlir D V, Barnes P F. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340:367–373. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 15.Houghton R L, Reynolds L D, Day C, Dillon D, McNeill P D, Hendrickson R C, Skeiky Y A W, Lodes M, Badaro R, Reed S G. The use of multi-epitope recombinant Mycobacterium tuberculosis antigens in the serodiagnosis of active TB. J Infect Dis. 1999;1:1–2. [Google Scholar]
- 16.Huebner R E, Schein M F, Hall C A, Barnes S A. Delayed-type hypersensitivity anergy in human immunodeficiency virus-infected persons screened for infection with Mycobacterium tuberculosis. Clin Infect Dis. 1994;19:26–32. doi: 10.1093/clinids/19.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Hunt D F, Yates J R, Shabanowitz J, Winston S, Hauer C R. Protein sequencing by tandem mass spectrometry. Proc Natl Acad Sci USA. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter S W, Gaylord H, Brennan P J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 19.Jackett P S, Bothamley G H, Batra H V, Mistry A, Young D B, Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988;26:2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanolkar-Young S, Kolk A H, Andersen A B, Bennedsen J, Brennan P J, Rivoire B, Kuijper S, McAdam K P, Abe C, Batra H V. Results of the Third Immunology of Leprosy/Immunology of Tuberculosis Antimycobacterial Monoclonal Antibody Workshop. Infect Immun. 1992;60:3925–3927. doi: 10.1128/iai.60.9.3925-3927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 22.Laal S, Samanich K M, Sonnenberg M G, Belisle J T, O'Leary J, Simberkoff M S, Zolla-Pazner S. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J Infect Dis. 1997;176:133–143. doi: 10.1086/514015. [DOI] [PubMed] [Google Scholar]
- 23.Laal S, Samanich K M, Sonnenberg M G, Zolla-Pazner S, Phadtare J M, Belisle J T. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigens. Clin Diagn Lab Immunol. 1997;4:49–56. doi: 10.1128/cdli.4.1.49-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann M, Wilm M. Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- 25.Pieper R, Christian R E, Gonzales M I, Nishimura M I, Gupta G, Settlage R E, Shabanowitz J, Rosenberg S A, Hunt D F, Topalian S L. Biochemical identification of a mutated human melanoma antigen recognized by CD4(+) T cells. J Exp Med. 1999;189:757–766. doi: 10.1084/jem.189.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sada E, Ferguson L E, Daniel T M. An ELISA for the serodiagnosis of tuberculosis using a 30,000-Da native antigen of Mycobacterium tuberculosis. J Infect Dis. 1990;162:928–931. doi: 10.1093/infdis/162.4.928. [DOI] [PubMed] [Google Scholar]
- 27.Salata R A, Sanson A J, Malhotra I J, Wiker H G, Harboe M, Phillips N B, Daniel T M. Purification and characterization of the 30,000 dalton native antigen of Mycobacterium tuberculosis and characterization of six monoclonal antibodies reactive with a major epitope of this antigen. J Lab Clin Med. 1991;118:589–598. [PubMed] [Google Scholar]
- 28.Schaaf H S, Botha P, Beyers N, Gie R P, Vermeulen H A, Groenewald P, Coetzee G J, Donald P R. The 5-year outcome of multidrug resistant tuberculosis patients in the Cape Province of South Africa. Trop Med Int Health. 1996;1:718–722. doi: 10.1111/j.1365-3156.1996.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 29.Selwyn P A, Hartel D, Lewis V A, Schoenbaum E E, Vermund S H, Klein R S, Walker A T, Friedland G H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 30.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins on silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 31.Verbon A, Hartskeerl R A, Moreno C, Kolk A H. Characterization of B cell epitopes on the 16K antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 1992;89:395–401. doi: 10.1111/j.1365-2249.1992.tb06969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkens E G L. The serodiagnosis of tuberculosis. In: Davies P D O, editor. Clinical tuberculosis. London, England: Chapman & Hall, Ltd.; 1994. pp. 367–379. [Google Scholar]
- 33.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379:466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou A T, Ma W L, Zhang P Y, Cole R A. Detection of pulmonary and extrapulmonary tuberculosis patients with the 38-kilodalton antigen from Mycobacterium tuberculosis in a rapid membrane-based assay. Clin Diagn Lab Immunol. 1996;3:337–341. doi: 10.1128/cdli.3.3.337-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]