Abstract
In vitro engineering of salivary glands relies on the availability of synthetic matrices presenting essential cell instructive signals to guide tissue growth. Here, we describe a biomimetic, hyaluronic acid (HA)-based hydrogel platform containing covalently immobilized bioactive peptides derived from perlecan domain IV (TWSKV), laminin 111 (YIGSR, IKVAV), and fibronectin (RGDSP). The HA network was established by the thiol/acrylate reaction and bioactive peptides were conjugated to the network with high efficiency without significantly altering the mechanical property of the matrix. When encapsulated as single cells in peptide modified HA hydrogels, human salivary gland stem/progenitor cells (hS/PCs) spontaneously organized into multicellular spheroids with close cell-cell contacts. Conjugation of RGDSP and TWSKV signals in HA gels significantly accelerated cell proliferation, with the largest spheroids observed in RGDSP-tagged gels. Peptide conjugation did not significantly alter the expression of acinar (AMY1), ductal (TFCP2L1), and progenitor (KRT14) markers at the mRNA level. Characterization of 3D cultures by immunocytochemistry showed positive staining for keratin-5 (K5), keratin-14 (K14), integrin-β1, and α-amylase under all culture conditions, confirming the maintenance of the secretory progenitor cell population. 2D adhesion studies revealed that integrin-β1 played a key role in facilitating cell-matrix interaction in gels with RGDSP, IKVAV, and TWSKV signals. Overall, conjugation of RGDSP peptide to HA gels improved cell viability, accelerated the formation of epithelial spheroids and promoted the expansion of the progenitor cell population in 3D. This work represents an essential first step towards the development of an engineered salivary gland.
Keywords: salivary gland, progenitor cells, cell-adhesive peptide, hyaluronic acid, hydrogels
Graphical Abstract
1. Introduction
During the treatment of head and neck cancers with radiation therapy, irreversible damage can occur in the nearby radiosensitive salivary glands, resulting in glandular hypofunction for >70% of cancer patients.1 Chronic xerostomia (dry mouth) impedes not only basic human needs such as mastication and phonation, those affected also suffer from complications that develop from compromised oral health.2 Common treatment with systemic sialogogues requires the preservation of basic tissue structures that are responsive to sialogogue stimulation.3 Because radiotherapy leads to tissue fibrosis and acinar cell loss, this approach does not provide a long-term relief.4 Other treatment options, such as artificial saliva substitutes, only provide temporary symptom management with minimal therapeutic efficacy.5 Alternative cell-based therapies and tissue engineering strategies are being actively pursued for the restoration of salivary gland function.6–7 Towards this goal, we have characterized primary salivary human stem/progenitor cells (hS/PCs) isolated from adult human parotid gland tissues prior to radiation therapy.8 These cells can be readily expanded in culture, expressing markers of salivary gland progenitors, keratin-5 (K5) and keratin-14 (K14).9 Thus, hS/PCs represent an accessible cell source with regenerative potential for salivary gland tissue engineering.
The salivary gland epithelium resides in the basement membrane, a specialized extracellular matrix (ECM) that regulates cell differentiation and tissue development.10 Basement membrane components, such as laminin-111 and perlecan (heparan sulfate proteoglycan 2), have been shown to be indispensable for the proper development of salivary glands during organogenesis.11–13 In addition, fibronectin is required for salivary gland branching morphogenesis and is highly expressed by salivary gland epithelium during cleft formation.14 From the tissue engineering perspective, it is desirable to culture cells in synthetic matrices containing instructive signals from the native salivary gland ECM. Unlike the full-length proteins,15–16 short synthetic peptides can be readily synthesized, exhibit decreased immunogenicity and enhanced stability, and can be incorporated in hydrogel matrixes with tunable ligand density without altering the matrix viscoelasticity.17–20 Furthermore, in challenging organoid applications, poly(ethylene glycol)-based hydrogels containing immobilized RGD peptide have performed at comparable levels to native ECM proteins and basement membrane extract.21–22 Separately, Baker and colleagues showed that a fibrin matrix containing laminin-III derived peptides not only enhanced the development of spheroids with a central lumen from Par-C10 cells, a mouse salivary cell line,20 but also accelerated salivary gland tissue regeneration in wound healing mouse models.23–24 Previously, we cultured hS/PCs in a hyaluronic acid (HA)-based hydrogel containing a mixture of basement membrane derived peptides. When stimulated with β-adrenergic and cholinergic agonists, a pro-acinar phenotype was maintained in hS/PCs under the 3D culture conditions.8 To date, a complete understanding of the role of discrete ECM cues on hS/PC fate remains undefined.
In this work, HA-based synthetic matrices with immobilized bioactive peptides were developed, characterized, and used to probe the microenvironment requirements for the expansion of hS/PCs in 3D. hS/PCs were maintained in HA gels containing covalently immobilized bioactive peptides, TWSKV (perlecan domain IV),25 YIGSR (laminin β1), IKVAV (laminin α1),26–29 or RGDSP (fibronectin).30–31 Peptide conjugation was determined to be highly efficient, and matrix mechanics was not affected by peptide incorporation and irrespective of the peptide composition. We found that TWSKV and RGDSP stimulated hS/PC proliferation and growth. We showed that peptide incorporation facilitated the expansion of an amylase-expressing K5/K14 positive progenitor population. Spheroids grown in all bioactive matrices expressed integrin-β1, but hS/PCs exhibited the highest affinity for RGDSP. Antibody blocking completely abolished cell attachment to RGDSP and TWSKV-containing surfaces, suggesting a key role of integrin-β1 interactions in mediating hS/PC growth.
2. Materials and Methods
2.1. Hydrogel Synthesis and Characterization.
2.1.1. Chemical modification of HA.
Thiol and acrylate functionalized HA, as HA-SH and HA-AES, respectively, were prepared according to our previously reported procedures.32–33 In brief, sodium hyaluronate (430 kDa, Sanofi-Genzyme Corporation, Cambridge, MA) was dissolved in deionized water and reacted with 3,3’dithiobispropanoic dihydrazide in the presence of N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride at pH 4.75. The reaction mixture was treated with 1,4-dithiothreitol, purified by dialysis, and lyophilized to yield HA-SH with 60% thiol incorporation as confirmed by 1H-NMR. Separately, sodium hyaluronate (5 kDa, Lifecore Biomedical, Chaska, MN) was converted to the tetrabutyl ammonium salt and dissolved in dimethyl sulfoxide before the addition of mono-2-(acryloyloxy)ethyl succinate (AES). The reaction was allowed to proceed at 50 °C for 24 h. The conjugate was precipitated in ether, purified with ion exchange resin, and dialyzed before lyophilization. 1H-NMR analysis confirmed an acrylate degree of incorporation of 50%.
2.1.2. Peptide synthesis.
Maleimide functionalized peptides, MI-GGGRGDSPG, MI-GGGIKVAVSADR, MI-GGGYIGSR, and MI-GGGTWSKVGGHLRPGIVQSG, were synthesized on a PS-3 peptide synthesizer (Protein Technologies, Tucson, AZ) using Rink Amide-MBHA resin (EMD Millipore, Burlington, MA). Standard Fmoc solid phase peptide synthesis (SPPS) was conducted at 0.25 mmol scale using 1.0 mmol amino acid, 1.0 mmol O-benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) in the presence of 4-methylmorpholine in N,N′-dimethylformamide (DMF) for 1 h. Automated Fmoc deprotection was performed for 20 min using 20% (v/v) piperidine in DMF. After removal of the N-terminal Fmoc, the resin was transferred to a 25 mL fritted reaction vessel, and the N-terminal amine was reacted with 1.0 mmol of 4-maleimidobutyric acid using 1.0 mmol HBTU, and 1.75 mmol of N,N-diisopropylethylamine (DIPEA) for 1 h. RGDSP without the MI group was prepared similarly, and the N-terminus was reacted with 20% (v/v) acetic anhydride in DMF with 1.75 mmol DIPEA. Peptides were cleaved and deprotected in trifluoroacetic acid (TFA)/H2O/triisopropylsilane (TIPS) (95/2.5/2.5% v/v/v) for 3 h and precipitated in cold diethyl ether. Purification was performed on a preparative-scale reverse-phase high-performance liquid chromatography (RP-HPLC) using a Waters Xbridge BEH C18 column and a mobile phase of acetonitrile/water with 0.1% v/v TFA. Peptide M/Z was confirmed by electrospray ionization mass spectrometry (ESI-MS) using a Thermo LCQ LC-MS system with an ion trap mass analyzer (San Jose, CA). Peptide purity was analyzed using a Shimadzu HPLC (Kyoto, Japan) equipped with a Phenomenex C18 column (100 Å, 5 μm, 250 × 4.6 mm) by monitoring absorbance at 220 nm and 280 nm.
2.1.3. Hydrogel preparation.
To prepare peptide modified HA hydrogels, maleimide functionalized peptides were reconstituted in PBS at 1.0, 0.5, 0.25, or 0.125 mM. HA-SH was introduced to the peptide solution at 10 mg/mL and equilibrated at ambient for 1 h to allow dissolution and peptide conjugation. The HA-SH solution was neutralized to pH 7.4 with 1M NaOH, then combined with 10 mg/mL HA-AES at a 20/1 (v/v) ratio to initiate gelation. Hydrogel swelling was determined from gravimetric measurements. Hydrogel precursor solutions were aliquoted into 12-mm cell culture inserts. Each hydrogel was incubated in 800 μL of PBS at 37 °C for 24 h. The swollen gel mass was recorded and compared to the mass of the dry starting components to calculate the swelling ratio. Reported results represent an average of 3 measurements, and the error represents the standard deviation.
2.1.4. Oscillatory rheology.
The viscoelastic properties of the peptide-modified hydrogels were analyzed using an AR-G2 rheometer (TA Instruments, New Castle, DE). Hydrogel discs were prepared by loading 30 μL of hydrogel solution into cylindrical molds with a 4.6 mm diameter. The solution was allowed to gel for 30 min, then equilibrated in PBS for 24 h at 37 °C. Hydrogels were loaded under a 150 mN normal force, and a frequency sweep was performed from 0.01 Hz to 100 Hz at 5% strain. Measurements were conducted using a 20 mm parallel plate geometry at 25 °C and 200 μL of PBS was added around the geometry to ensure the hydrogels remained swollen. Values of storage and loss moduli are reported from measurements within the linear viscoelastic regime at 0.25 Hz.
2.1.5. Peptide incorporation.
Hydrogel precursor solutions were prepared from each peptide at 1 mM and allowed to gel for 30 min in 12-mm cell culture inserts. Hydrogel samples were then incubated in 800 μL PBS at 37 °C. After 1, 3, 7, 10, and 14 days, the supernatant was aspirated and fresh PBS was added. The supernatant was analyzed at 220 nm using a Shimadzu HPLC with a Phenomenex C18 column. Standard curves were developed at peptide concentrations of 0–110 μM. Control gels were prepared using 1 mM RGDSP with MI functionality and peptide release was monitored. Supernatant from a peptide-free HA hydrogel was included in the analysis to characterize the UV active hydrogel degradation products.
2.2. Cell Isolation and Maintenance.
Human parotid glands were collected from consenting patients following protocols approved by institutional review boards at Christiana Care Health Systems and the University of Delaware. Following a reported procedure,8, 34 the tissue was disinfected, minced into a slurry, and suspended into HepatoSTIM™ medium (Corning Inc., Corning, NY) supplemented with 100 IU/mL penicillin-streptomycin, 1% (v/v) Fungizone, and 10 ng/mL epidermal growth factor (EGF, Corning Inc.). After reaching 70–80 % confluence, cells were trypsinized with 0.05% (w/v) trypsin-EDTA for subculture. Trypsin was neutralized using a trypsin soybean inhibitor (Sigma Aldrich, St. Louis, MO). Experiments were conducted with at least three different donors at passages of 4–6.
2.3. Development and Characterization of Cellular Construct.
2.3.1. Cell encapsulation.
Reconstituted peptide and HA-AES solutions were sterilized using 0.2-μm syringe filters (Pall Corporation, Port Washington, New York). Dilute HA-SH solution was sterile filtered using 0.2 μm Steriflip® Filter Units (EMD Millipore) prior to lyophilization and maintained under aseptic conditions. hS/PCs were suspended at a concentration of 1×106 cells/mL in the HA-SH solutions with or without conjugated peptide. The cell suspension was combined with HA-AES to initiate gelation. Hydrogels (30 μL each) were prepared in 48-well glass bottom MatTek plates and HeptoSTIM™ media (500 μL) which was exchanged every 72 h. For gene expression analysis, 100 μL of hydrogels were prepared in 12 mm cell culture inserts (EMD Millipore) with 800 μL HeptoSTIM™ media.
2.3.2. Metabolic activity.
The metabolic activity of hydrogel encapsulated hS/PCs was assessed using the PrestoBlue® Cell Viability Reagent (ThermoFisher Scientific, Waltham, MA). At days 1 and 14 of culture, media was replaced with a 10% (v/v) PrestoBlue® medium mixture and incubated for 3 h at 37 °C and 5% CO2. The solution was aspirated and fluorescence was recorded using a PerkinElmer (Waltham, MA) microplate reader using a 550 nm excitation and 590 nm emission wavelength. The fold change is represented as the 14 day measurement relative to the day 1 reading for each condition. Analysis was performed in triplicate.
2.3.3. Viability and proliferation.
The viability of hydrogel encapsulated hS/PCs was analyzed at days 1, 7, and 14 using the LIVE/DEAD™ viability assay (ThermoFisher Scientific, Waltham, MA) supplemented with a membrane permeable nuclear stain. Calcein AM, ethidium homodimer-1 (EthD-1), and Hoeschst 3342 (Thermo Fisher Scientific) were diluted to 2 μm using warm PBS. After aspirating media and washing with 300 μL of warm PBS, the samples were incubated with 300 μL of the dye solution at 37 °C for 15 min. The hS/PC laden hydrogels were assayed using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Oberkochen, Germany). Images were captured as 101.5 μm z-stacks with a z-axis step size of 6.77 μm. Viability was quantified using Perkin Elmer Volocity v6.2 software using hoeschst stained nuclei to determine the total cell count, double stained EthD-1 and hoeschst nuclei as the dead cell count, and viable cells represented as the difference of the two. hS/PC proliferation was determined from the fold change in viable cell number at days 1, 7, and 14 using Perkin Elmer Volocity v6.2 Software. The viable cell number at each time point was normalized against the average day 1 total cell count. Calcein AM positive area (calcien+) and feret diameter were quantified from maximum intensity projections using ImageJ software (National Institutes of Health, Bethesda, MD).
2.3.4. Gene expression.
Hydrogel constructs were snap frozen on dry ice / isopropanol and stored at −80 °C before RNA extraction following our reported methods.32 Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) and reverse transcription was performed using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). Real-time quantitative polymerase chain reaction (qPCR) was carried out using an ABI 7300 real-time PCR system where cDNA templates were combined with primers (400 nM) and Power SYBR green master mix. The relative expression levels of selected genes were monitored with primers listed in SI Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous reference. The fold changes were calculated with the Pfaffl method, using qbase+ software (Biogazelle, Zwijnaarde, Belgium). A total of three biological repeats were analyzed for each gel composition.
2.3.5. Immunofluorescence.
Hydrogel constructs were fixed with 4% (w/v) paraformaldehyde (PFA, Sigma Aldrich) in PBS for 20 min. For K5 and K14 staining, blocking was conducted for 16 h at 4 °C with 3% (w/v) BSA in 0.2% (w/v) saponin PBS (BSA/sap). Next, anti-K5 and anti-K14 antibodies were diluted 1/50 in a (BSA/sap) solution and incubated with the samples at room temperature for 48 h. The primary antibody solution was then aspirated and samples were washed three times by performing a 15 min incubation in 0.2% (w/v) saponin PBS (PBS/sap). Secondary antibodies Alexa Fluor™ 488 goat anti-mouse and Alexa Fluor™ 647 goat anti-rabbit were diluted at 1/200 in a BSA/sap solution and incubated for 48 h at room temperature. The solutions were then aspirated and washed three times with a 15-min PBS/sap incubations. Next, Alexa Fluor™ 568 Phalloidin (Life Technologies, Carlsbad, CA) and 4′,6-diamidino-2-phenylindole (DAPI, Life Technologies, Carlsbad, CA) were diluted to 1/250 and 1/1000 respectively in BSA/sap and incubated for 90 min. Amylase and Integrin-β1 stainings were conducted with a modified procedure where BSA/sap and PBS/sap solutions were prepared at a 0.05% (w/v) saponin concentrations. Extended antibody information can be found in SI Table 2. Fluorescent microscopy was performed using a Zeiss LSM 880 equipped with an Airyscan detector in Fast Airyscan mode. Images were captured as 100 μm z-stacks with a 5 μm z-axis step size.
2.3.6. Attachment assay.
HA-SH (20 mg/mL) was separately modified with 1 mM of each peptide and combined with 100 mg/mL HA-AES at a 1/20 (v/v) ratio to a final volume of 30 μL. The hydrogel components were mixed and allowed to gel for 30 min before equilibrating with HeptoSTIM™ media for 1 h. Then 50,000 hS/PCs were seeded on the hydrogel surfaces and allowed to attach for 72 h. Unattached cells were removed by agitating for 5 min at 250 RPM on a rotational shaker, followed by three PBS washes. Cells were fixed in 4% (w/v) PFA for 15 min, permeabilized for 15 min with 0.2% (w/v) Triton, and blocked with 3% (w/v) BSA. Cells were incubated with K5 and integrin-β1 primary antibodies, diluted to 1/100 dilution in 3% (w/v) BSA, at room temperature for 1 h. Following PBS rinses, Alexa Fluor™ 488 goat anti-mouse, Alexa Fluor™ 647 goat anti-rabbit, and Alexa Fluor™ 568 Phalloidin were diluted in 3% BSA at 1/200, 1/200, 1/250 respectively and incubated on the hydrogel constructs for 30 minutes. After rinsing with PBS, DAPI was diluted prepared at 1/1000 in 3% (w/v) BSA and incubated for 5 min. The hS/PCs on the peptide modified hydrogel surfaces were then visualized using a Zeiss LSM 880 confocal microscope. Utilizing the DAPI labeling, cells were enumerated with ImageJ software, and the mean percentage of cells for each peptide is expressed relative to those on the HA control hydrogel.
2.3.7. Integrin-β1 inhibition.
Hydrogels were prepared following the procedure described in section 2.3.6 and equilibrated in HepatoSTIM™ medium for 1 h. hS/PCs were pre-incubated in medium containing 10 μg/mL anti-Integrin β1 (clone AIIB2, Sigma-Aldrich) or rat IgG antibody (Sigma-Aldrich) for 5 min before seeding onto the hydrogel surfaces. hS/PCs were maintained in medium containing 10 μg/mL AIIB2 or IgG and allowed to adhere for 72 h. Cultures were then placed on a shaker rotated at 250 RPM for 5 min, washed three times with PBS, and fixed in 4% PFA for 15 min. Attached cells were permeablized with 0.2% (w/v) Triton and blocked with 3% (w/v) BSA and labelled for enumeration using Alexa Fluor™ 568 Phalloidin,1/250, and DAPI,1/1000, in 3% (w/v) BSA for 30 min. Images were captured using a Zeiss LSM 880 confocal microscope and ImageJ software was used to quantify cell number based on the DAPI labeling. Inhibition is expressed as the amount of attachment on AIIB2 treated conditions compared to the mean of the same condition treated with IgG antibodies.
2.4. Statistical Analysis.
Statistical significance was analyzed by performing a Student’s t-test, one-way ANOVA, or two-way ANOVA, as appropriate and detailed in figure captions. When a one-way or two-way ANOVA was performed and p < 0.05, pairwise comparisons were made using Tukey HSD post-hoc. Statistical analysis was conducted using JMP Pro 15 (SAS Institute Inc.). Error bars represent standard error of the mean unless otherwise indicated.
3. Results.
3.1. Slow/fast Michael reactions permit efficient peptide conjugation without altering matrix mechanics.
Bioactive peptides were produced following standard SPPS protocols (Figures S1–S10). Maleimide functionality was introduced via the reaction of maleimidobutyric acid with the N-terminal amine, allowing for orthogonal conjugation of the peptide epitopes to the HA hydrogel network via Michael addition. Hydrogels were prepared by dissolving HA-SH in the peptide stock solution, followed by the addition of pH neutralized HA-AES solution. Under these conditions, peptide conjugation occurred through the rapid thiol-maleimide reaction, while the covalent network was established through the slower thiol-acrylate reaction32–33 (Figure 1). Peptide incorporation was quantified by analyzing the supernatant from swollen hydrogels with HPLC and comparing the chromatographs to standard curves constructed using unconjugated peptides (Figure S11–16). First, hydrogels were made with 1 mM maleimide-free, non-reactive peptide Ac-RGDSP. After 24 h equilibration in PBS, 83.2 ± 0.1% of Ac-RGDSP was detected in the supernatant. With an additional 48 h incubation in PBS, >98.0% of the peptide was recovered (Figure 2A). When maleimide functionalized peptides were used, unconjugated peptides were undetectable in the supernatant after 24 h of PBS equilibration for RGDSP, IKVAV, and YIGSR, although 3.8 ± 0.1 % of TWSKV was detected. With an additional 48 h of PBS equilibration, no peptides were detected in the supernatant for any peptide.
Figure 1.
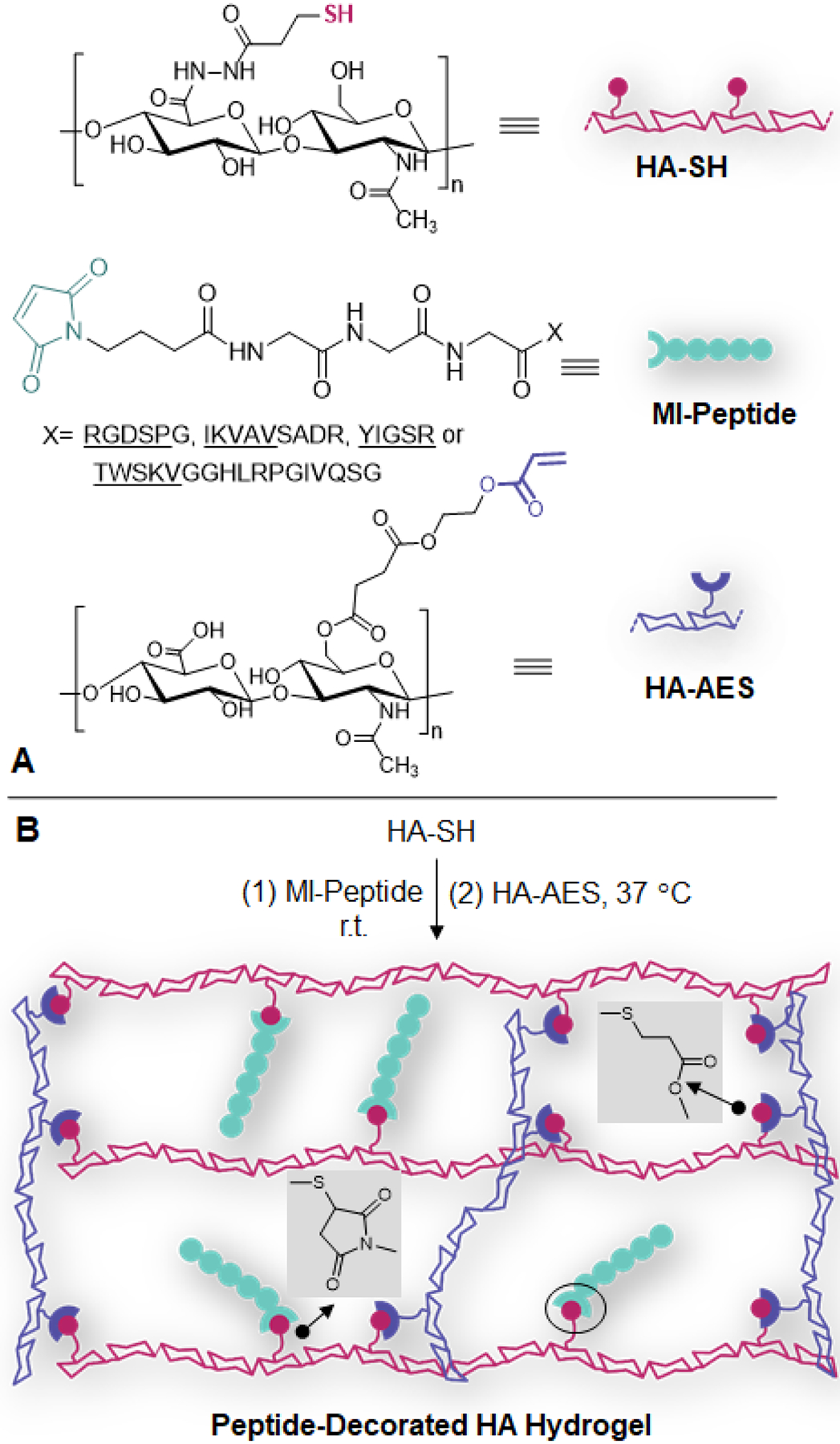
Establishment of peptide-decorated HA hydrogels. (A) Chemical structure of HA-SH, HA-AES and MI-peptide. (B) To create the synthetic matrix, peptide is first conjugated to HA through thiol-maleimide reaction, then HA-AES is added to establish the covalent network via thiol-acrylate reaction.
Figure 2.
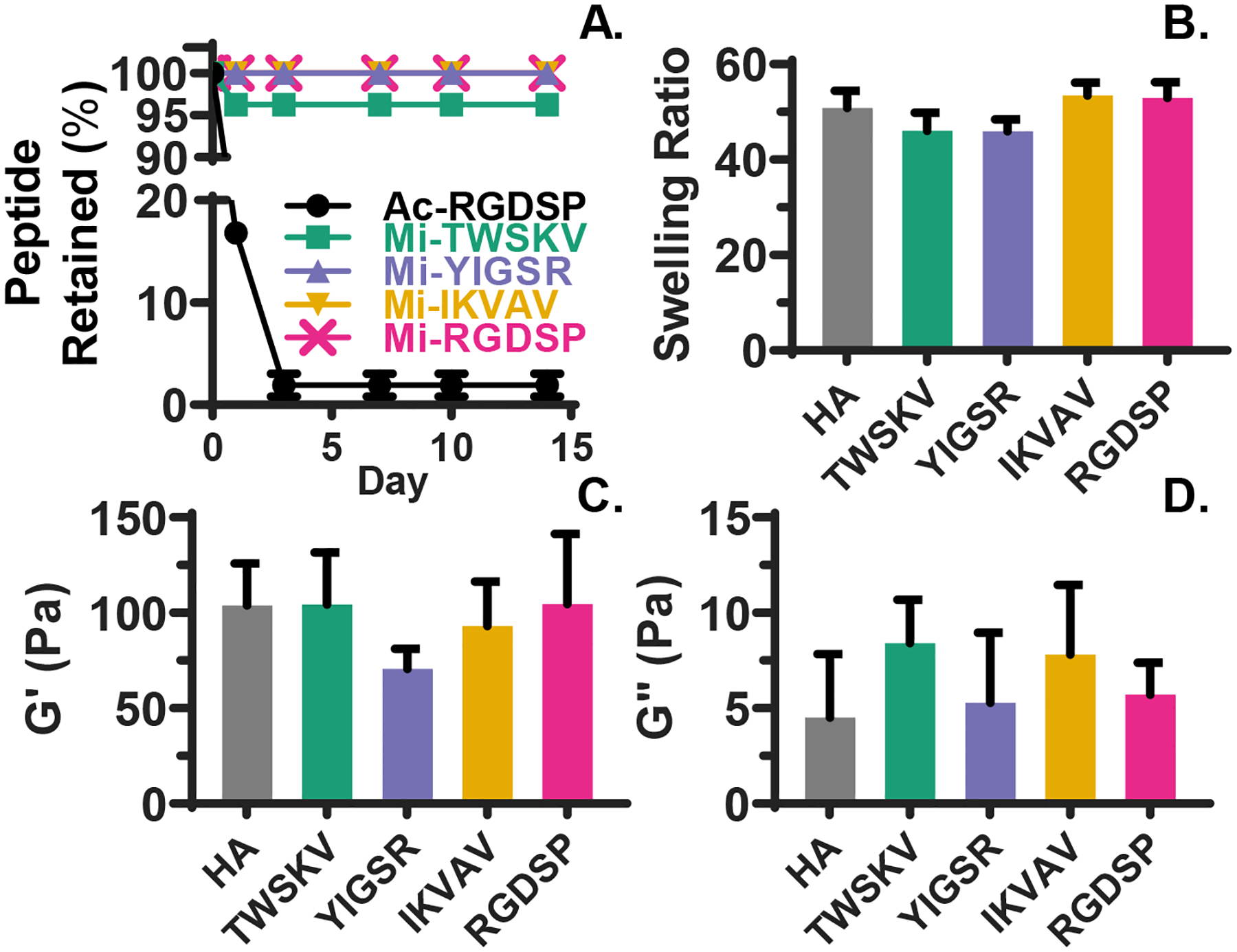
Characterization of peptide conjugation and hydrogel viscoelasticity. (A) HPLC analyses of supernatant equilibrated with the hydrogels show that peptides are quantitatively and stably conjugated to the HA network. (B) Hydrogel swelling is not affected by peptide incorporation. Hydrogels were equilibrated in PBS for 24 h before the wet weight was measured. (C-D) Rheological analyses indicate hydrogel storage and loss moduli are not significantly altered by peptide incorporation. Hydrogels were equilibrated in PBS for 24 h before the test. Error bars represent standard deviation. A one-way ANOVA on data shown in (B-D) did not reveal any statistical significance (p>0.05).
To examine the stability of peptide-HA conjugates throughout the time span of hS/PC culture, the hydrogel PBS solution was sampled after 7, 10, and 14 days of PBS incubation. After 7 days of equilibration, hydrogel degradation products34 were detected at similar levels in each condition (Figures S11–S16). These products were detected again at day 14, though unconjugated peptides were not detected with extended incubations. HA hydrogels without peptide modification exhibited an equilibrium swelling ratio of 50.8 ± 3.7 in PBS. Peptide conjugation did not significantly alter hydrogel swelling (Figure 2B). Particle retention experiments demonstrated that the HA networks had an average pore size of 50 nm (Figure S17). Analysis by oscillatory rheology (Figure 2C–D) indicated that fully swollen HA hydrogels had a storage (G’) modulus of 104 ± 13 Pa. Although incorporating YIGSR peptide led to a moderate, but non-significant decrease in G’, hydrogels containing other peptides exhibited a similar stiffness as the HA hydrogels (p>0.05). The loss modulus (G”) varied from 5 ± 2 Pa for HA hydrogels to 8 ± 2 for TWSKV-decorated gels, yet the differences were non-significant (p>0.05). Overall, the combination of slow and fast chemistries permits the establishment of elastic hydrogels with simultaneous peptide conjugation.
3.2. Bioactive matrices differentially modulate hS/PC growth in 3D.
hS/PCs were encapsulated homogeneously in the hydrogels at a single cell state (Figure S18). Constructs containing bioactive peptides at concentrations of 1.0 mM to 0.125 mM were prepared and the metabolic activity was assessed at day 1 and 14 by PrestoBlue® assay (Figure 3A). Immobilization of RGDSP and TWSKV to the HA network led to a significant (p<0.0001) enhancement in cell metabolism. RGDSP significantly increased the metabolic activity at all concentrations examined and was maximized at a concentration of 0.25 mM with a 4.02 ± 0.09 fold increase in cell metabolism after 14 days of cultures. At a concentration of 1 mM, the TWSKV peptide stimulated a 2.20 ± 0.17 fold enhancement in cell metabolism after 14 days of culture. hS/PCs cultured in hydrogels bearing the laminin-derived peptides, IKVAV and YIGSR, exhibited a similar metabolic activity to those maintained in HA hydrogels. In subsequent experiments, the RGDSP peptide was maintained at 0.25 mM, while other motifs (TWSKV, IKVAV, and YIGSR) were presented at 1 mM.
Figure 3.
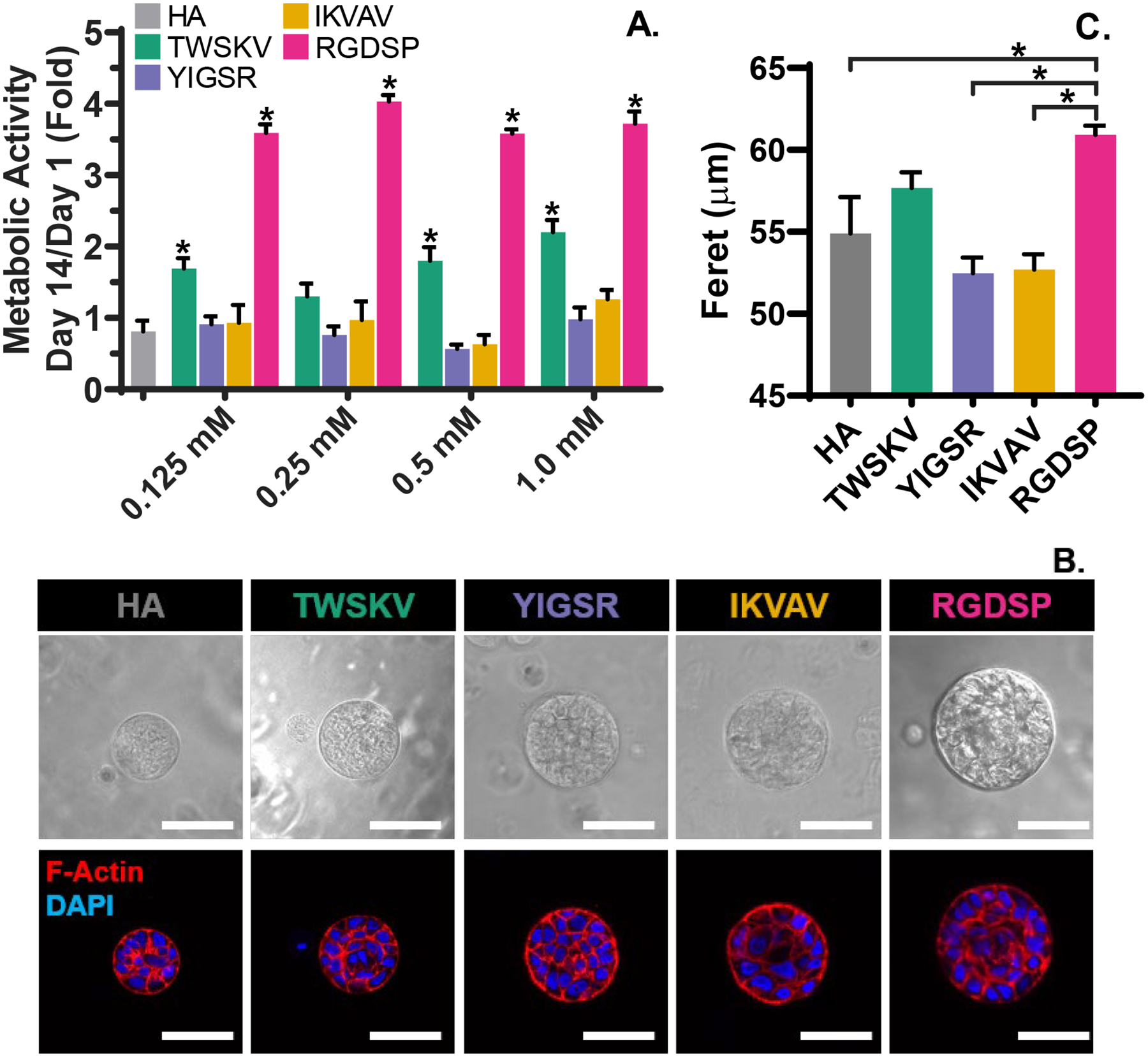
Metabolic screening of bioactive peptide (A) and morphological characterization of 3D grown spheroids (B, C). (A) Conjugation of RGDSP and TWSKV significantly increases the metabolic activity of hSPCs at day 14. *: significantly different compared to the blank HA controls determined by one-ANOVA and post-hoc Dunnett’s Test, p<0.05. (B) Confocal images of hS/PCs after 14 days of culture. Cells dispersed in a single cell state in bioactive hydrogels self-organize into multicellular spheroids reminiscent of the native parotid acini. Top panel: Differential interference contrast (DIC) images, Bottom panel: F-actin (phalloidin) and nuclei (DAPI) labeled hS/PC spheroids. Scale bar = 50 μm. (C) Feret diameter as a function of gel composition. Larger spheroids are develop in RGDSP modified hydrogels. *: significantly different, One-way ANOVA with post-hoc Tukey test, p<0.05.
hS/PCs encapsulated in the synthetic matrices spontaneously formed compact and organized multicellular spheroids, with apical localization of cortical F-actin (Figure 3B). The spheroid forming efficiency for RGDSP and HA cultures was ~15% and ~3.5 %, respectively (Figure S19A). These values are similar to what has been reported for primary murine salivary gland cultures in Matrigel.35 Spheroids developed in RGDSP gels were significantly larger (60.9 ± 0.5 μm) than those grown in YIGSR, IKVAV and HA gels. hS/PCs in the HA hydrogel develop into spheroids with an average Feret diameter of 54.0 ± 0.2 μm (Figure 3C). Spheroids in TWSKV cultures were slightly larger (57.0 ± 0.2 μm). Spheroids developed in YIGSR and IKVAV gels were similar in size to those in the HA hydrogels, having an average diameter of 54.0 ± 0.2 μm. Notably, spheroids with highly organized nuclei around a central lumen were frequently observed in RGDSP cultures. Spheroids grown in HA gels exhibited a narrow size distribution around 40 μm (Figure S19B). Peptide incorporation not only increased the spheroid size but also broaden the size distribution. Compared to YIGSR/IKVAV cultures, TWSKV cultures produce a wider range of spheroid sizes. RGDSP cultures contained spheroids predominantly in the 50–70 μmrange, although spheroids smaller than 40 μm or larger than 80 μm were also present.
The role of adhesive peptides in maintaining cell viability and promoting cell proliferation was investigated by conducting LIVE/DEAD™ assays after 1, 7, and 14 days of culture (Figure 4A, Figure S20). The day 1 live dead images detail the even dispersion of single cells without the presence of pre-aggregated cell clusters. 3D Volocity® image analysis software was used to perform unbiased automated viability quantification. hS/PC viability was at ~75% on day 1 for the HA hydrogel, and significant alterations in viability were not detected with peptide incorporation (Figure 4B). Extending the culture time to day 7 led to a moderate increase in cell viability for RGDSP containing hydrogels, but a significant decrease under the HA hydrogel conditions HA (60.3 ± 2.6 %), YIGSR (59.1 ± 1.2%), and IKVAV (64.9 ± 2.9%). By day 14, the average cell viability was 81.9 ± 3.5% and 59.6 ± 4.0% for RGDSP and HA cultures, respectively. Cells cultured in hydrogels containing the laminin-derived peptides exhibited a similar time-dependent decrease in viability as those maintained in the HA hydrogels (59.6 ± 3.9 %), YIGSR (62.8 ± 1.7%) IKVAV (63.7 ±3.8 %). hS/PCs cultured in TWSKV exhibited a viability of (69.3 ±1.3%) at day 1 that did not significantly change over the 14 day culture period.
Figure 4.
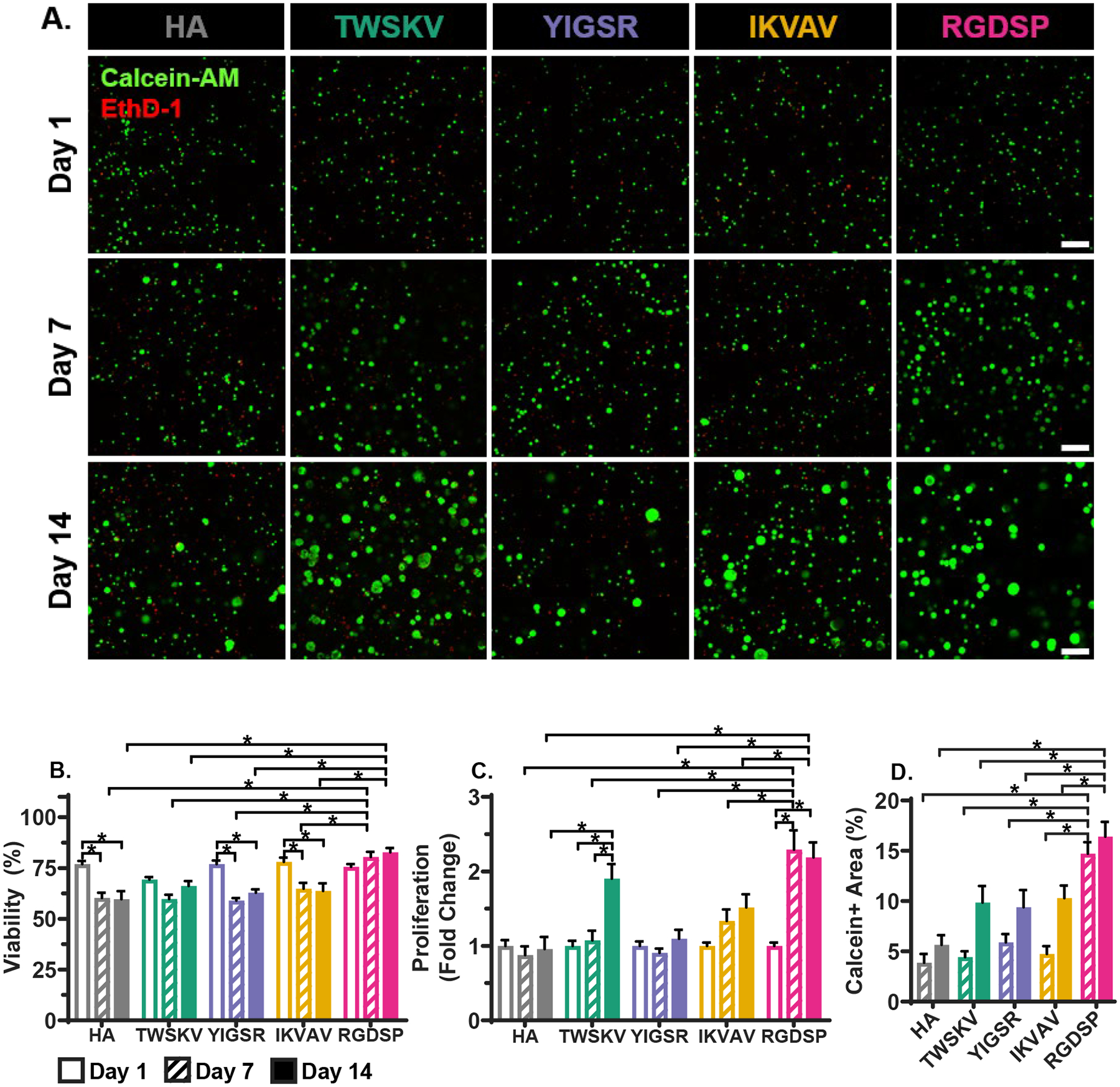
Bioactive peptides increase cell viability and proliferation, (A) Confocal images of hS/PCs cultured in peptide modified hydrogels after 1, 7 and 14 days. Live cells were stained green by calcein and dead cells were stained red by EthD-1. Scale bar = 200 μm. (B) Percent viable cells as a function of culture time and gel composition. RGDSP incorporation significantly improves cell viability. (C) Effects of peptide conjugation on hS/PC proliferation. Conjugation of TWSKV and RGDSP results in an increase in cell proliferation. (D) Calcein+ area, of 3D constructs as a function of gel composition. RGDSP significantly stimulated hS/PC growth. Error bars represent standard error of the mean. *: Significance determined using a two-way one-way ANOVA with post-hoc Tukey test, p<0.05; n = 9.
RGDSP stimulated rapid cell proliferation 91.7 ± 0.2 fold) within the first 7 days, while limited proliferation was observed beyond day 7 of culture (Figure 4C). The stimulatory effect from the TWSKV peptide was delayed until day 7 culture, reaching a 1.5 ± 0.2 fold increase in cell proliferation by day 14. No significant proliferation was observed over 14 days of culture for HA hydrogel cultures, as well as those that were presented with IKVAV and YIGSR peptides. hS/PC growth was further investigated by quantifying the calcein+ area of acquired confocal images based on maximum intensity projected z-volumes (Figure 4A and 4D). Thus, the calcein+ area is an indicator of the viable cell area of the engineered construct. Within the first 7 days of culture, the calcein+ area in RGDSP bearing hydrogels was more than 3 times higher than constructs containing other peptide signals as well as the peptide free controls. While little additional growth was observed from day 7 to day 14 in RGDSP cultures, a non-significant increase in the mean calcein+ area was observed for hS/PC cultured in TWSKV, IKVAV, YIGSR, or HA hydrogel. Collectively, RGDSP and TWSKV peptides maintain high cell viability and stimulate the growth of organized multicellular structures in 3D.
3.3. Bioactive peptides maintain the progenitor status.
hS/PCs were cultured in the peptide modified HA hydrogels for 14 days, and the expression of progenitor (KRT5, KRT14), acinar (AMY1), and ductal (TFCP2LI) markers were analyzed by qPCR (Figure 5). TWSKV and RGDSP modification significantly downregulated the expression of the progenitor marker KRT5, but did not significantly alter the expression of KRT14. On the other hand, expression of the acinar marker AMY1 was not significantly altered with the addition of bioactive peptides. Similarly, the expression of TFCP2L1, a marker for developing ductal cells,36 was not significantly affected by the introduction of peptides in HA matrices.
Figure 5.
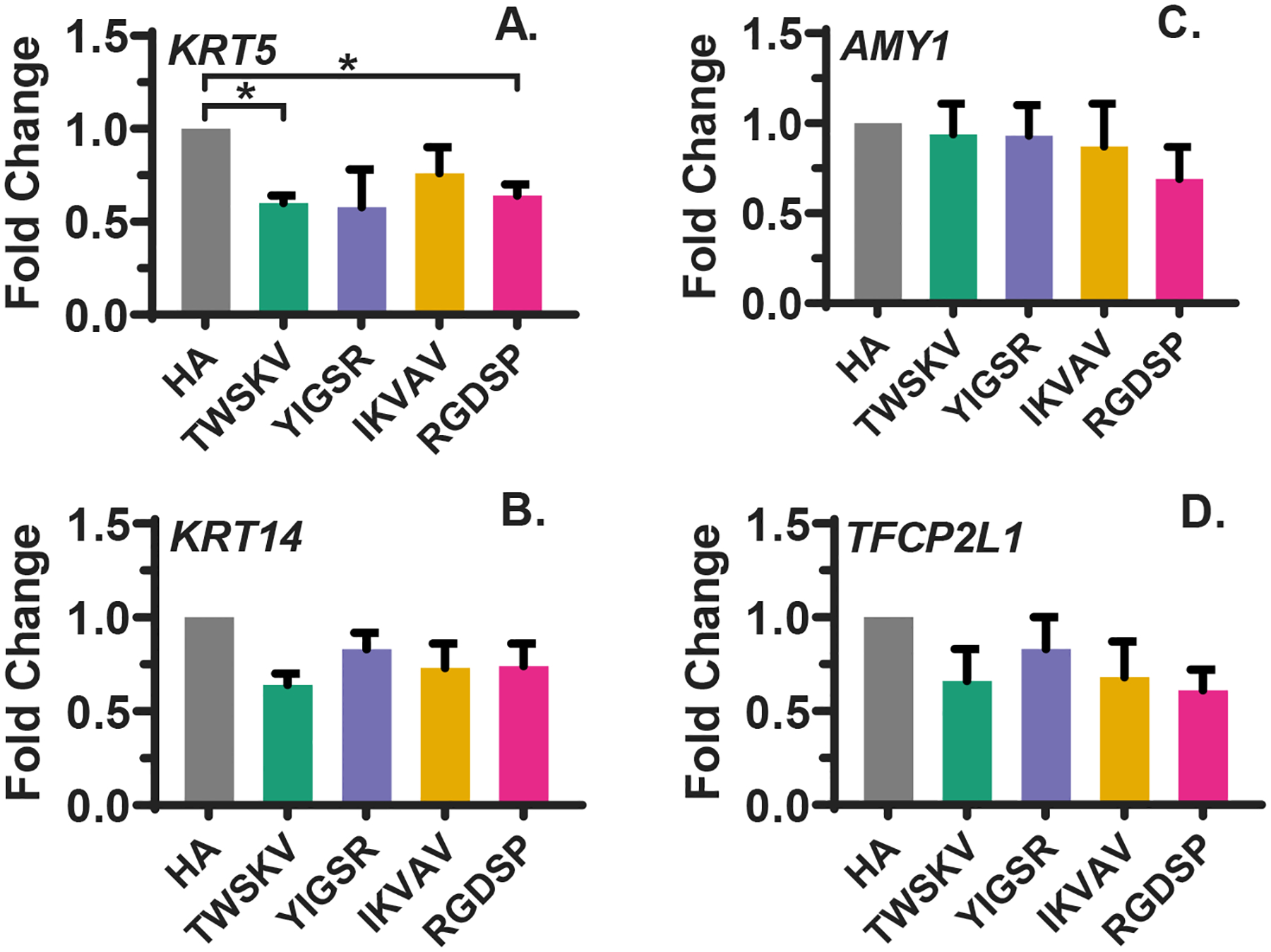
qPCR analysis of the expression of progenitor (A, B) and differentiation (C, D) markers. RGDSP and TWSKV significantly downregulated the expression of progenitor marker KRT5 (A). mRNA levels of progenitor marker KRT14 (B), acinar marker AMY1 (C) and ductal marker TFCP2L1 (D) are not significantly regulated by conjugated peptides. Error bars represent standard error of the mean in all cases. *: Significance determined using a one-way ANOVA with post-hoc Tukey test, p<0.05.
Characterization of progenitor marker expression with immunofluorescence indicated that with the development of the multicellular spheroids, all peptide modified hydrogels supported hS/PC’s maintenance of K5 and K14 expression (Figure 6). As keratin intermediate filaments that can form heterodimers, these proteins were colocalized in 3D cultured hS/PCs to maintain the cytoskeleton structures. Amylase expression was confirmed in the hS/PC spheroids cultured in all peptide modified gels as well as the blank HA gels (Figure 7). Amylase was uniformly expressed throughout the hS/PC population and was intracellularly localized without a specific preference to apical or basal membranes. Previously, we showed that hS/PC spheroids developed in HA gels after 28 days of culture stained weakly/diffusely for laminin and collagen-IV, and these proteins were largely intracellular.34 Similarly, fibronectin was found to be intracellular and weakly expressed after 14 days of culture in hydrogels with or without the peptide signal (Figure S21). hS/PCs spheroids were shown to express integrin-β1 under all 3D culture conditions. Integrin-β1 was localized to basal and basolateral surfaces of the spheroids and enriched on the basal surfaces of the large RGDSP cultured spheroids. (Figure 7). Collectively, hS/PCs cultured in 3D biomimetic hydrogels form multicellular spheroids expressing K5/K14, amylase, and integrin-β1.
Figure 6.
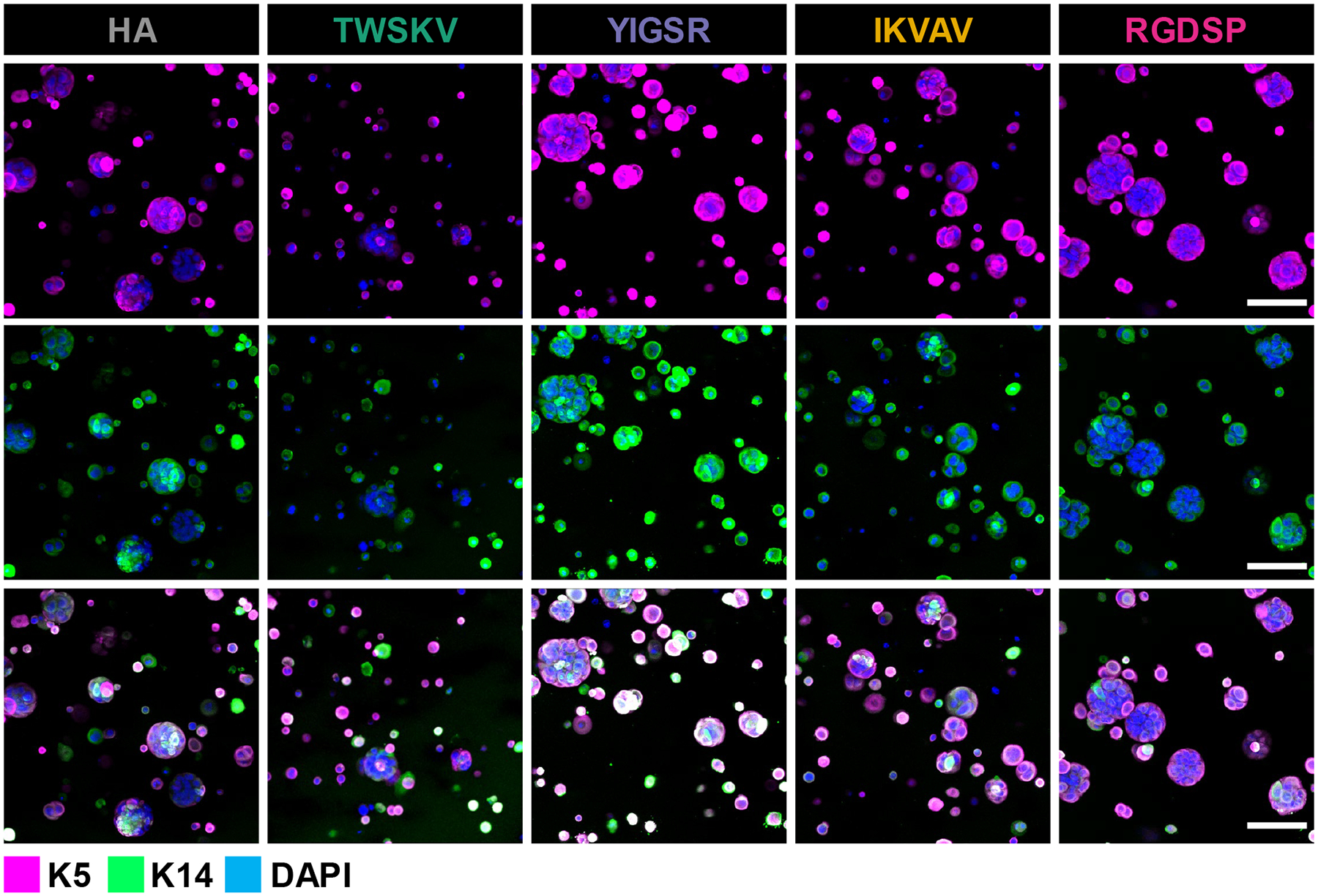
Peptide modified hydrogels promote expansion of the hS/PC population in 3D and cells maintain expression of K5/K14, characteristic of progenitor identity. Confocal microscopy was performed to evaluate K5 (magenta), K14 (green) expression after 14 days of culture in peptide modified hydrogels. Scale bar =100 μm.
Figure 7.
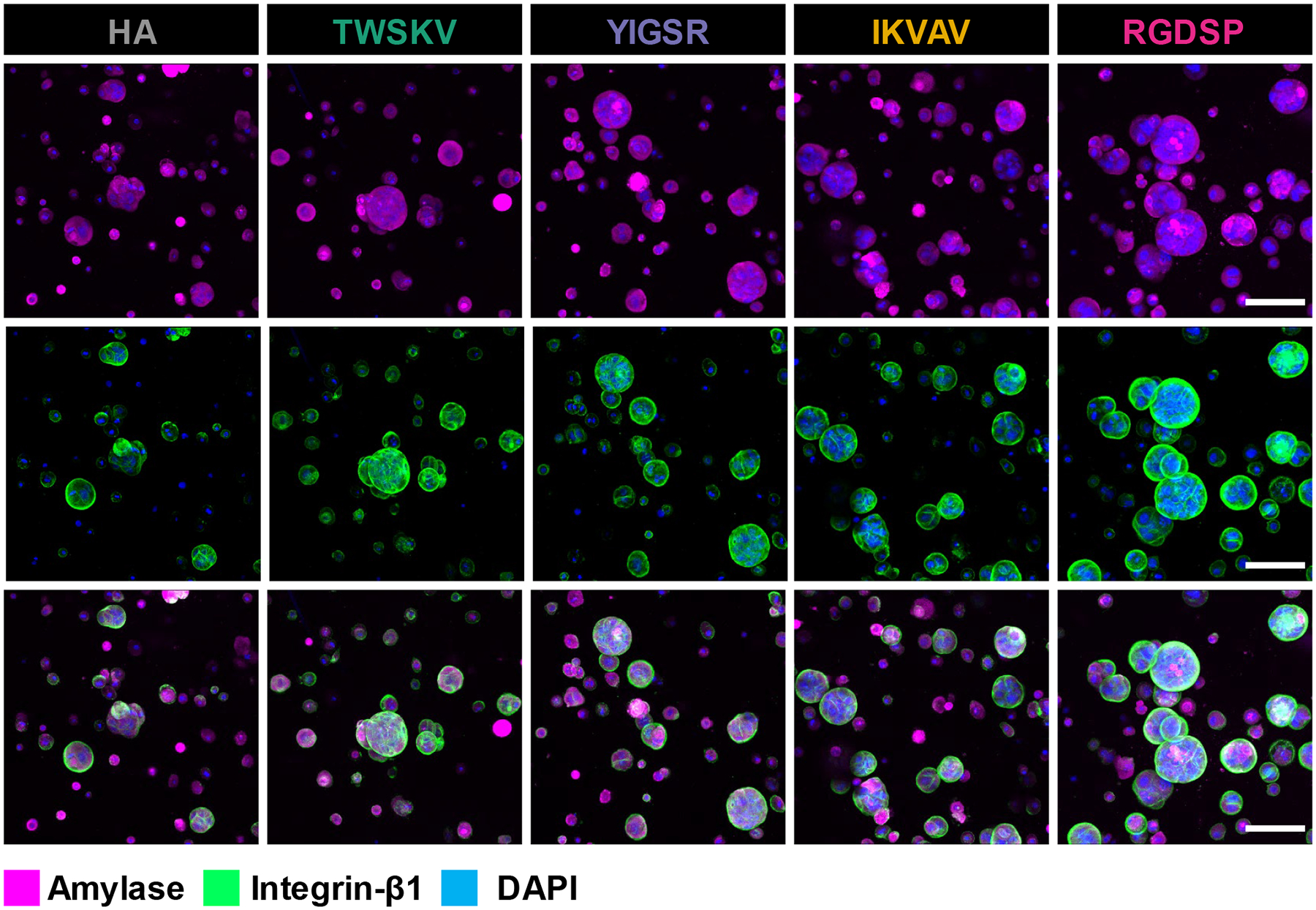
hS/PC spheroids grown in peptide modified hydrogels express integrin-β1 and amylase. Cellular expression of amylase (magenta) and integrin- β1 (green) were visualized by confocal microscopy. Scale bar =100 μm.
3.5. Bioactive peptides regulate hS/PC function via integrin-β1.
The expression of integrin-β1 in 3D implicated its role in mediating the peptide hS/PC interactions and we further characterized this by examining peptide-hS/PC affinity, integrin-β1 localization, and integrin-β1 inhibition. Determining the extent to which hS/PCs interact with the matrix when mechanically confined in a 3D network is non-trivial. 2D cultures are commonly performed to assess cell adhesion and to project cell-matrix interactions in 3D.37–38 Thus, the hS/PC affinity for each peptide was assessed by seeding hS/PCs on a stiffer (~1,800 Pa)33 gel to promote cell adhesion. Each hydrogel was prepared with 1 mM of peptide, and analysis was made by comparing the number of cells on the blank HA gel (Figure 8A). A similar number of cells was observed on TWSKV and YIGSR modified hydrogels when compared to the HA hydrogel. The IKVAV and RGDSP modified hydrogels supported the attachment of more than 3.5 times more cells than the HA hydrogel (Figure 8C). Cells attached to HA hydrogels were organized in small colonies with cells touching each other along the entire cell boundary. Cells attached to TWSKV and YIGSR modified hydrogels were also organized into clusters, but exhibited distinct filopodia (arrowheads, Figure 8A) along the colony edges. Cells on IKVAV and RGDSP decorated hydrogels developed as a closely packed monolayer, with flattened cell bodies and a cobblestone morphology that was not observed on HA gels. Immunostaining performed on adhered cells indicated integrin-β1 was more distinctly localized on the peptide modified surfaces than on the HA hydrogel where it was seen to be largely diffuse. On the peptide modified hydrogels, integrin-β1 expression was concentrated along cell-cell contacts and cell membrane protrusions (arrowheads, Figure 8A), a morphology not exhibited on the HA hydrogels.
Figure 8.
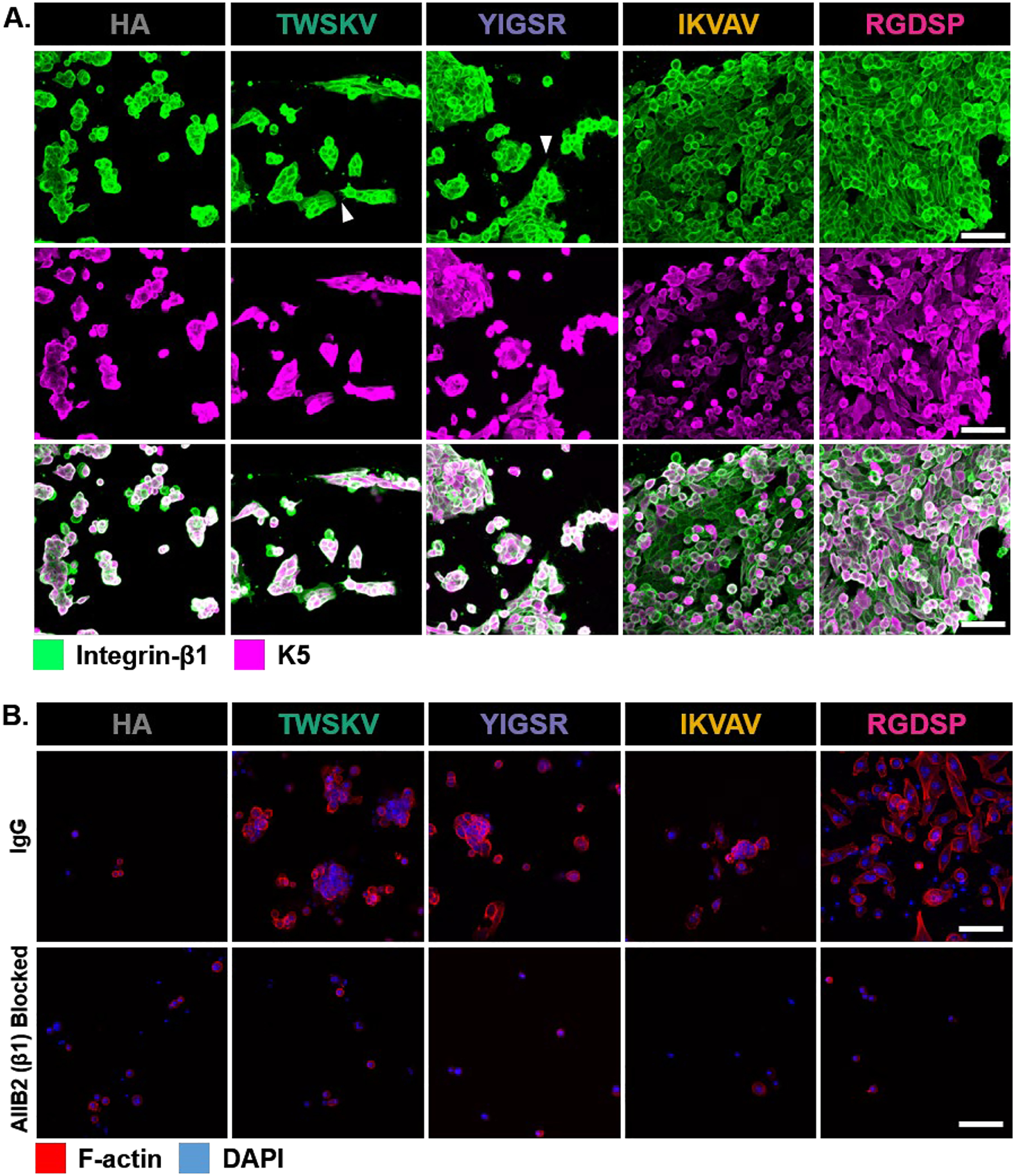
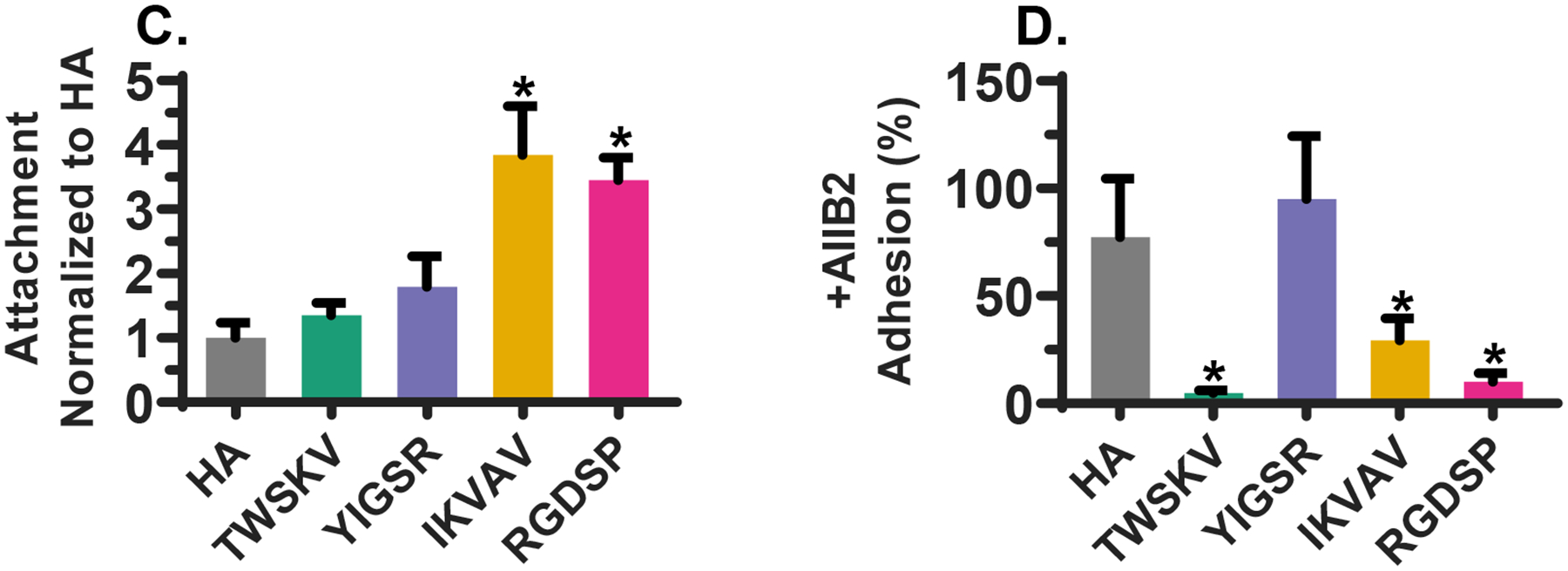
Characterization of integrin-β1-dependent cell adhesion. (A) Confocal images of hS/PCs after 72 h of culture on peptide decorated hydrogels. Integrin-β1 was stained green and K5 was stained magenta. Arrow heads point to elongated cell processes, Scale bar =100 μm. (B) Confocal images of AIIB2-treated hS/PCs cultured on peptide modified hydrogels for 72 h. Nuclei and F-actin was stained blue and red, respectively. Scale bar =100 μm. (C) Cell attachment as a function of peptide identity. Bioactive peptides increased hS/PC adherence to HA hydrogels. *: Significantly different from HA hydrogel. (D) Integrin- β1 inhibition with AIIB2 reduced the number of adhered cells of TWSKV, YIGSR and RGDSP modified hydrogels relative to IgG treated controls. Integrin-β inhibited hS/PC adhesion on peptide modified hydrogels. *: Significantly different from IgG blocked controls, p<0.05, n = 6.
To further characterize peptide/integrin-β1 interactions, hS/PCs were treated with AIIB2, an antibody that recognizes integrin-β1, before being plated on peptide modified hydrogels (Figure 8B). When compared to the IgG-treated controls, cell attachment to HA hydrogels and YIGSR modified hydrogels was not significantly affected by AIIB2 inhibition, suggesting minimal involvement of integrin-β1 in cell attachment. YIGSR is reported to utilize other cell surface receptors, such as the 67-kDa laminin receptor/RPSA protein, for cell adhesion.27, 39 However, treatment with anti-RPSA did not disrupt YIGSR adhesion relative to controls treated with IgG (Figure S22). AIIB2 treatment significantly reduced cell attachment to TWSKV, IKVAV, and RGDSP-containing hydrogels, with only 7 ± 1%, 31 ± 10%, and 12 ± 4% cells anchored on the respective hydrogel surfaces (Figure 8D). Collectively, these results indicate that hS/PCs cultured in TWSKV, IKVAV, and RGDSP-containing hydrogels interact with the peptide epitopes via β1-integrin, with IKVAV and RGDSP providing the highest degree of attachment.
4. Discussion.
We have developed a modular HA based 3D culture platform that permits orthogonal incorporation of bioactive adhesive ligands. Using this platform, we investigated the roles of ECM signals in mediating hS/PC growth and maintaining progenitor status and secretory functions. The relatively slow thiol/acrylate reaction enabled adequate mixing of hydrogel components and consistent manufacturing of cell-laden gel constructs with hS/PCs homogeneously distributed in 3D. The large excess (×18) of thiol groups relative to the acrylate groups ensured complete consumption of the reactive acrylates. The excess thiols were not detrimental to cells and did not compromise spheroid forming ability. The percentage of unreacted thiols decreased linearly with time, and within seven days, were entirely converted to disulfides.34 Though the acrylate functional group is optimal for the establishment of the hydrogel network, faster and more efficient chemistry is required for quantitative in situ peptide conjugation. The maleimide group is the most efficient Michael type acceptor and can be easily incorporated into standard SPPS protocols.40 The maleimide-thiol reaction is not optimal for the development of hydrogel networks due to its reactivity kinetics. Networks formed from maleimide-thiol reactions are prone to rapid gelation that can occur before the monomer components can be fully mixed. These resultant networks exhibit heterogenic microdomains and give rise to inconsistent cellular responses, confounding the interpretation of experimental results.41–42 The combined usage of slow and fast reactions ensured the establishment of consistent hydrogel networks with a high degree of peptide incorporation, permitting cell-matrix interactions to be reliably investigated.21, 43
Our results show that the bioconjugation reaction is not dependent on the peptide sequence and more than 95% of peptides remained stably conjugated for 14 days as the thiol-maleimide adduct is not susceptible to reverse Michael type reaction.44–45 Peptide conjugation did not perturb the network structure, as evidenced by swelling ratio and rheological measurements. While the incorporation of non-elastic chains can affect network properties, these peptide sequences are small and are not included at such a degree (≤1 mM) so that they did not contribute significantly to the network mechanics.
The hydrogel matrix is relatively soft, with stiffness comparable to that of murine embryonic salivary glands.46 Acini-like multicellular structures were developed from single cells under all 3D culture conditions and the largest structures were found in the RGDSP hydrogels after 14 days of culture. Spheroid size distribution was also broadened in RGDSP cultures. This is not unexpected because hS/PCs encapsulated in the gels were in different cell cycle stages, therefore responding differently to the growth stimulating signals. Although all peptide-decorated HA gels, and peptide-free hydrogels, maintained high cell viability at day 1, only TWSKV and RGDSP containing networks promote robust cell proliferation. Cell viability was compromised over time for constructs derived from peptide-free HA gels and those with IKVAV and YIGSR; however moderate cell proliferation occurred under these conditions to sustain a stable cell population and to enable the development of multicellular spheroids, albeit smaller in size, by day 14. Membrane compromised dead cells detected under 3D culture conditions were largely hS/PC at the single cell state, in agreement with our previous publication.34 Significant cell proliferation was observed in RGDSP constructs starting day 7, but was delayed until day 14 for TWSKV gels. hS/PCs were uniformly dispersed throughout the hydrogel matrices, which are nanoporous and not conducive to cellular migration, therefore hS/PCs spheroids proliferate from single cell clones. Furthermore, hS/PC can be expected to behave as epithelial cells that proliferate more readily when cell-cell contacts are established. Taken together, this suggests that RGDSP is a more potent pro-proliferative motif than TWSKV, even at a significantly lower concentration (0.25 mM RGDSP, 1.0 mM TWSKV).
At the mRNA level, RGDSP and TWSKV conjugation induced a modest downregulation of KRT5, while KRT14 expression remains unaltered. This is similar to what was previously reported where KRT5 but not KRT14 was downregulated in peptide modified hydrogels.8 The salivary gland contains diverse stem cell populations, and a K14+ population can contribute to multipotent epithelial lineages that retain the regenerative capacity after radiation-induced damage.47 Introduction of peptide signals to the HA network did not give rise to changes in the expression of differentiation markers AMY1 and TFCP2L1 either. Our immunostaining results show that at the protein level, K5 and K14 expression was maintained under all 3D culture conditions. As the spheroids grew larger, there was more pronounced basal localization of the K5/K14 observed in the RGDSP cultures. In agreement with the qPCR results, peptide modified hydrogels provide an ECM microenvironment that supports the development of amylase expressing spheroids. As progenitor markers, K5/K14 are coexpressed and serve as cytoskeletal intermediate filaments. Our results indicate that the bioactive peptides promote the expansion of amylase expressing progenitor cells in 3D. On average, spheroids in RGDSP modified hydrogels were larger, yet we did not detect any loss of progenitor status at the protein level. There is no direct correlation between spheroid size and cell differentiation either.
Because cellular secretion of ECM proteins is minimal, hS/PCs engage with the synthetic matrix via immobilized cell adhesive peptides. It has been shown that integrin-β1 is important for salivary gland development and the assembly of multicellular salivary gland spheroids.48–50 While receptors such as CD44, RHAMM, and ICAM can bind the HA matrix, the bioactive peptides are reported to target multiple isoforms of integrin-β1.51 The RGDSP peptide predominantly ligates α5β1 and αvβ3 integrin subunits.52–53 The laminin derived peptides, YIGSR and IKVAV, interact to varying extents with α3β1, α4β1 and α6β126,28,30 YIGSR also has an affinity for a 67-kDa laminin receptor, to which much of its biological activity has been attributed.27, 29 Derived from perlecan domain IV, TWSKV is reported to primarily target a non-integrin receptor, with limited integrin-β1 affinity.25, 31 While all 3D cultures were stained positive for integrin-β1, more pronounced staining was found on the larger spheroids localized to the basal domains. Our comparative adhesion studies show that conjugation of RGDSP and IKVAV to the HA network significantly enhanced cell attachment. Cells plated on hydrogels with YIGSR and TWSKV, as well as the HA gels, form small colonies sparsely distributed on the surface. Integrin-β1 staining was largely diffuse in colonies grown on HA gels indicating limited cell-ECM interaction. On TWSKV and YIGSR hydrogels, integrin-β1 was highly localized along the cell-ECM interface and distinctly along spreading cells. These observations indicate that cell clusters anchored on the HA surface were largely maintained by cell-cell interactions and those on the TWSKV and YIGSR surfaces are more extensively engaged with the synthetic ECM.54–55 The strong binding with IKVAV and RGDSP motifs led to the rapid development of closely packed monolayers with flattened and spread-out cells.
Antibody blocking experiments indicate that TWSKV, IKVAV, and RGDSP rely on integrin-β1 to promote hS/PC attachment. AIIB2 or RPSA treatment did not significantly inhibited cell adhesion to YIGSR hydrogels, but the presence of integrin-β1 rich processes at the cell periphery suggested the involvement of integrin-β1 in the adhesion. YIGSR may utilize RPSA for initial adhesion that is later stabilized by integrin-β1. Alternatively, in the absence of available integrin binding sites, YIGSR can access other cell surface receptors. The integrin-β1 affinity correlates with the effects we observed on proliferation and spheroid growth. RGDSP and TWSKV dependent cell adhesion was largely inhibited by AIIB2 and these peptides stimulated the highest levels of hS/PC proliferation. IKVAV exhibited a lower degree of integrin-β1-mediated adhesion and, while non-significant, promoted a positive trending relationship with proliferation.
In summary, the HA-based hydrogels developed here with optimized stiffness and conjugated peptide ligands robustly stimulate the development of compact, acini-like spheroids seen in native salivary gland tissues. Specifically, we have shown that the RGDSP peptide stimulates the rapid expansion and organized growth of amylase-expressing salivary gland progenitor cell population in 3D in an integrin-β1 dependent manner. This is critically important from a tissue regeneration perspective, as a pool of amylase expressing progenitors with self-renewal capacity is required to reconstitute the developing organ. To date, there have been few reports of amylase expressing salivary gland cells in 3D. Future studies will focus on the identification of appropriate soluble factors to promote acinar and ductal differentiation. Incorporation of potent regulatory signals in the synthetic microenvironment will lead to the development of engineered salivary glands with secretory functions.
5. Conclusion.
Using HA-based hydrogels containing covalently tagged bioactive peptide motifs, we investigated the roles of ECM signals in mediating hS/PC growth and spheroid development. The tunability of our hydrogel platform enabled rapid screening of multiple peptides at varying concentrations for optimization of human salivary gland cell cultures. The modular hydrogel design allowed the equal presentation of peptide motifs without compromising the biomechanical properties of the hydrogel network. The RGDSP and TWSKV peptides improved hS/PC viability and proliferation while maintaining K5/K14 expressing spheroids. While analysis at the mRNA level did not reveal enrichment of differentiation markers, the protein level analysis confirmed the maintenance of amylase expression by hS/PCs cultured in the peptide modified hydrogels. 2D adhesion study showed peptides investigated here exhibited varying affinities for integrin-β1, and those with the highest affinity (RGDSP, and to a lesser extent, TWSKV) for integrin-β1 were most effective in maintaining high cell viability, promoting rapid establishment of acini-like structures. Work is in progress to identify growth factors and ECM cues that stimulate acinar or ductal differentiation of adult salivary gland cells.
Supplementary Material
Acknowledgement.
This work was supported in part by National Institutes of Health (NIDCD, R01DC014461; NIDCR R01 DE029655), National Science Foundation (NSF, DMR 1809612) and Delaware Bioscience Center for Advanced Technology (DE-CAT 12A00448). EWF and AR acknowledge the University of Delaware for the Graduate Fellowship and Dissertation Fellowship. Instrumentation support was made possible by NIH (S10 RR027273, P20 GM103446) and NSF (CHE-0840401, CHE-1229234, IIA-1301765, DMR-2011824) grants. We thank Sanofi/Genzyme for generously providing HA.
Footnotes
Supporting Information:
Primer/antibody information, characterization of peptides by ESI-MS and HPLC, characterization of hydrogel pore size by particle retention, confocal images of Hoechst stained hS/PCs in 3D, spheroid forming efficiency and size distribution under different 3D culture conditions, confocal images of cellular constructs stained by Hoechst and ethidium homodimer-1 and confocal images of cellular constructs stained for fibronectin and DAPI. This material is available free of charge via the Internet at http://pubs.acs.org.
References.
- 1.Acauan MD; Figueiredo MA; Cherubini K; Gomes AP; Salum FG, Radiotherapy-induced salivary dysfunction: Structural changes, pathogenetic mechanisms and therapies. Arch. Oral. Biol 2015, 60 (12), 1802–1810. DOI: 10.1016/j.archoralbio.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Grundmann O; Mitchell GC; Limesand KH, Sensitivity of salivary glands to radiation: from animal models to therapies. J. Dent. Res 2009, 88 (10), 894–903. DOI: 10.1177/0022034509343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burlage FR; Faber H; Kampinga HH; Langendijk JA; Vissink A; Coppes RP, Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother. Oncol 2009, 90 (2), 253–256. DOI: 10.1016/j.radonc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Jasmer KJ; Gilman KE; Muñoz Forti K; Weisman GA; Limesand KH, Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J. Clin. Med 2020, 9 (12), 4095. DOI: 10.3390/jcm9124095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villa A; Connell CL; Abati S, Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag 2015, 11, 45–51. DOI: 10.2147/TCRM.S76282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombaert I; Movahednia MM; Adine C; Ferreira JN, Concise Review: Salivary Gland Regeneration: Therapeutic Approaches from Stem Cells to Tissue Organoids. Stem cells 2017, 35 (1), 97–105. DOI: 10.1002/stem.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozdemir T; Fowler EW; Hao Y; Ravikrishnan A; Harrington DA; Witt RL; Farach-Carson MC; Pradhan-Bhatt S; Jia X, Biomaterials-based strategies for salivary gland tissue regeneration. Biomat. Sci 2016, 4 (4), 592–604. DOI: 10.1039/c5bm00358j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan PP; Patel VN; Liu S; Harrington DA; Hoffman MP; Jia X; Witt RL; Farach-Carson MC; Pradhan-Bhatt S, Primary Salivary Human Stem/Progenitor Cells Undergo Microenvironment-Driven Acinar-Like Differentiation in Hyaluronate Hydrogel Culture. Stem Cells Transl. Med 2017, 6 (1), 110–120. DOI: 10.5966/sctm.2016-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knox SM; Lombaert IM; Reed X; Vitale-Cross L; Gutkind JS; Hoffman MP, Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010, 329 (5999), 1645–1647. DOI: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequeira SJ; Larsen M; DeVine T, Extracellular matrix and growth factors in salivary gland development. Front. Oral Biol 2010, 14, 48–77. DOI: 10.1159/000313707. [DOI] [PubMed] [Google Scholar]
- 11.Kadoya Y, Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J. Cell Biol 1995, 129 (2), 521–534. DOI: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebustini IT; Hoffman MP, ECM and FGF-dependent assay of embryonic SMG epithelial morphogenesis: investigating growth factor/matrix regulation of gene expression during submandibular gland development. Methods Mol. Biol 2009, 522, 319–330. DOI: 10.1007/978-1-59745-413-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel VN; Knox SM; Likar KM; Lathrop CA; Hossain R; Eftekhari S; Whitelock JM; Elkin M; Vlodavsky I; Hoffman MP, Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 2007, 134 (23), 4177–4186. DOI: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 14.Sakai T; Larsen M; Yamada KM, Fibronectin requirement in branching morphogenesis. Nature 2003, 423 (6942), 876–881. DOI: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 15.Hosseini ZF; Nelson DA; Moskwa N; Sfakis LM; Castracane J; Larsen M, FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci 2018, 131 (4), jcs208728. DOI: 10.1242/jcs.208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vining KH; Lombaert IMA; Patel VN; Kibbey SE; Pradhan-Bhatt S; Witt RL; Hoffman MP, Neurturin-containing laminin matrices support innervated branching epithelium from adult epithelial salispheres. Biomaterials 2019, 216, 119245. DOI: 10.1016/j.biomaterials.2019.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersel U; Dahmen C; Kessler H, RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24 (24), 4385–4415. DOI: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 18.Zustiak SP; Durbal R; Leach JB, Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 2010, 6 (9), 3404–3414. DOI: 10.1016/j.actbio.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mott JD; Werb Z, Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol 2004, 16 (5), 558–564. DOI: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam K; Jones JP; Lei P; Andreadis ST; Baker OJ, Laminin-111 Peptides Conjugated to Fibrin Hydrogels Promote Formation of Lumen Containing Parotid Gland Cell Clusters. Biomacromolecules 2016, 17 (6), 2293–2301. DOI: 10.1021/acs.biomac.6b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorrentino G; Rezakhani S; Yildiz E; Nuciforo S; Heim MH; Lutolf MP; Schoonjans K, Mechano-modulatory synthetic niches for liver organoid derivation. Nature Commun. 2020, 11 (1), 3416. DOI: 10.1038/s41467-020-17161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spence J; Cruz-Acuna R; Quiros M; Farkas A; Dedhia P; Huang S; Siuda D; Garcia-Hernandez V; Miller A; Spence J; Nusrat A; Garcia A, PEG-4MAL Hydrogels for In Vitro Culture of Human Organoids and In Vivo Delivery to Sites of Injury. Protocol Exchange 2017. DOI: 10.1038/protex.2017.098. [DOI] [Google Scholar]
- 23.Nam K; Maruyama CL; Wang CS; Trump BG; Lei P; Andreadis ST; Baker OJ, Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PloS one 2017, 12 (11), e0187069. DOI: 10.1371/journal.pone.0187069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam K; Wang CS; Maruyama CLM; Lei P; Andreadis ST; Baker OJ, L1 Peptide-Conjugated Fibrin Hydrogels Promote Salivary Gland Regeneration. J. Dent. Res 2017, 96 (7), 798–806. DOI: 10.1177/0022034517695496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farach-Carson MC; Brown AJ; Lynam M; Safran JB; Carson DD, A novel peptide sequence in perlecan domain IV supports cell adhesion, spreading and FAK activation. Matrix Biol. 2008, 27 (2), 150–160. DOI: 10.1016/j.matbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caniggia I; Liu J; Han R; Wang J; Tanswell AK; Laurie G; Post M, Identification of receptors binding fibronectin and laminin on fetal rat lung cells. Am. J. Physiol 1996, 270 (3 Pt 1), L459–L468. DOI: 10.1152/ajplung.1996.270.3.L459. [DOI] [PubMed] [Google Scholar]
- 27.Givant-Horwitz V; Davidson B; Reich R, Laminin-induced signaling in tumor cells. Cancer Lett. 2005, 223 (1), 1–10. DOI: 10.1016/j.canlet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Morais Freitas V; Nogueira da Gama de Souza, L.; Cyreno Oliveira, E.; Furuse, C.; Cavalcanti de Araujo, V.; Gastaldoni Jaeger, R., Malignancy-related 67kDa laminin receptor in adenoid cystic carcinoma. Effect on migration and beta-catenin expression. Oral Oncol. 2007, 43 (10), 987–998. DOI: 10.1016/j.oraloncology.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Kikkawa Y; Hozumi K; Katagiri F; Nomizu M; Kleinman HK; Koblinski JE, Laminin-111-derived peptides and cancer. Cell Adh. Migr 2013, 7 (1), 150–256. DOI: 10.4161/cam.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frith JE; Mills RJ; Hudson JE; Cooper-White JJ, Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012, 21 (13), 2442–2456. DOI: 10.1089/scd.2011.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S; Nih LR; Bachman H; Fei P; Li Y; Nam E; Dimatteo R; Carmichael ST; Barker TH; Segura T, Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater 2017, 16 (9), 953–961. DOI: 10.1038/nmat4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zerdoum AB; Fowler EW; Jia X, Induction of Fibrogenic Phenotype in Human Mesenchymal Stem Cells by Connective Tissue Growth Factor in a Hydrogel Model of Soft Connective Tissue. ACS Biomater. Sci. Eng 2019, 5 (9), 4531–4541. DOI: 10.1021/acsbiomaterials.9b00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravikrishnan A; Fowler EW; Stuffer AJ; Jia X, Hydrogel-Supported, Engineered Model of Vocal Fold Epithelium. ACS Biomater. Sci. Eng 2021. DOI: 10.1021/acsbiomaterials.0c01741. DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozdemir T; Fowler EW; Liu S; Harrington DA; Witt RL; Farach-Carson MC; Pradhan-Bhatt S; Jia X, Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D. ACS Biomater. Sci. Eng 2016, 2 (12), 2217–2230. doi: 10.1021/acsbiomaterials.6b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maimets M; Rocchi C; Bron R; Pringle S; Kuipers J; Giepmans BN; Vries RG; Clevers H; de Haan G; van Os R; Coppes RP, Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Reports 2016, 6 (1), 150–162. DOI: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi Y; Yonemura S; Takada S, Grainyhead-related transcription factor is required for duct maturation in the salivary gland and the kidney of the mouse. Development 2006, 133 (23), 4737–4748. DOI: 10.1242/dev.02658. [DOI] [PubMed] [Google Scholar]
- 37.Galarza S; Crosby AJ; Pak C; Peyton SR, Control of Astrocyte Quiescence and Activation in a Synthetic Brain Hydrogel. Adv. Healthc. Mater 2020, 9 (4), e1901419. DOI: 10.1002/adhm.201901419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark AY; Martin KE; Garcia JR; Johnson CT; Theriault HS; Han WM; Zhou DW; Botchwey EA; Garcia AJ, Integrin-specific hydrogels modulate transplanted human bone marrow-derived mesenchymal stem cell survival, engraftment, and reparative activities. Nat. Commun 2020, 11 (1), 114. DOI: 10.1038/s41467-019-14000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song T; Choi CH; Cho YJ; Sung CO; Song SY; Kim TJ; Bae DS; Lee JW; Kim BG, Expression of 67-kDa laminin receptor was associated with tumor progression and poor prognosis in epithelial ovarian cancer. Gynecol. Oncol 2012, 125 (2), 427–432. DOI: 10.1016/j.ygyno.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Nair DP; Podgórski M; Chatani S; Gong T; Xi W; Fenoli CR; Bowman CN, The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater 2013, 26 (1), 724–744. DOI: 10.1021/cm402180t. [DOI] [Google Scholar]
- 41.Darling NJ; Hung YS; Sharma S; Segura T, Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly(ethylene glycol) hydrogels. Biomaterials 2016, 101, 199–206. DOI: 10.1016/j.biomaterials.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 42.Jansen LE; Negron-Pineiro LJ; Galarza S; Peyton SR, Control of thiol-maleimide reaction kinetics in PEG hydrogel networks. Acta Biomater. 2018, 70, 120–128. DOI: 10.1016/j.actbio.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz-Acuna R; Quiros M; Farkas AE; Dedhia PH; Huang S; Siuda D; Garcia-Hernandez V; Miller AJ; Spence JR; Nusrat A; Garcia AJ, Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol 2017, 19 (11), 1326–1335. DOI: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldwin AD; Kiick KL, Reversible maleimide-thiol adducts yield glutathione-sensitive poly(ethylene glycol)-heparin hydrogels. Polym. Chem 2013, 4 (1), 133–143. DOI: 10.1039/C2PY20576A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyon RP; Setter JR; Bovee TD; Doronina SO; Hunter JH; Anderson ME; Balasubramanian CL; Duniho SM; Leiske CI; Li F; Senter PD, Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotech 2014, 32 (10), 1059–1062. DOI: 10.1038/nbt.2968. [DOI] [PubMed] [Google Scholar]
- 46.Mosier AP; Peters SB; Larsen M; Cady NC, Microfluidic platform for the elastic characterization of mouse submandibular glands by atomic force microscopy. Biosensors 2014, 4 (1), 18–27. DOI: 10.3390/bios4010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May AJ; Cruz-Pacheco N; Emmerson E; Gaylord EA; Seidel K; Nathan S; Muench MO; Klein OD; Knox SM, Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 2018, 145 (21). dev166363. DOI: 10.1242/dev.166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen M; Wei C; Yamada KM, Cell and fibronectin dynamics during branching morphogenesis. J. Cell Sci 2006, 119 (Pt 16), 3376–3384. DOI: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- 49.Daley WP; Gervais EM; Centanni SW; Gulfo KM; Nelson DA; Larsen M, ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 2012, 139 (2), 411–422. DOI: 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu D; Witt RL; Harrington DA; Farach-Carson MC, Dynamic Assembly of Human Salivary Stem/Progenitor Microstructures Requires Coordinated alpha1beta1 Integrin-Mediated Motility. Front. Cell Dev. Biol 2019, 7, 224. DOI: 10.3389/fcell.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X; Jha AK; Harrington DA; Farach-Carson MC; Jia X, Hyaluronic Acid-Based Hydrogels: from a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8 (12), 3280–3294. DOI: 10.1039/C2SM06463D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellis SL, Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32 (18), 4205–4210. DOI: 10.1016/j.biomaterials.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapp TG; Rechenmacher F; Neubauer S; Maltsev OV; Cavalcanti-Adam EA; Zarka R; Reuning U; Notni J; Wester HJ; Mas-Moruno C; Spatz J; Geiger B; Kessler H, A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep 2017, 7, 39805. DOI: 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mertz AF; Che Y; Banerjee S; Goldstein JM; Rosowski KA; Revilla SF; Niessen CM; Marchetti MC; Dufresne ER; Horsley V Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc Natl Acad Sci U S A. 2013, 110(3), 842–847. doi: 10.1073/pnas.1217279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalili AA; Ahmad MR A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int J Mol Sci. 2015, 16(8):18149–18184. doi: 10.3390/ijms160818149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


