Abstract
Stem cell therapy for retinal degenerative diseases such as age-related macular degeneration is a promising clinical option for the replacement of photoreceptors and retinal pigment epithelium (RPE). Induced pluripotent stem cell technology has emerged as a viable potential source of cells for transplantation in retinal degenerative disorders. Induced pluripotent stem cells have been used to derive RPE and have been tested for their functional behavior. These cells have the ability to express RPE-specific proteins and morphologically resemble native RPE. Induced pluripotent stem cell-derived RPE are also able to contribute to the visual cycle by their ability to metabolize all-trans retinol, a critical function of RPE in maintaining visual function. Advances in induced pluripotent stem cell technology will contribute to the development of clinical therapies for retinal degenerative diseases as well as provide a tool to understand the pathology of these disorders.
1. INTRODUCTION
There has been well-founded optimism that stem cell therapy may provide a novel avenue for the treatment of disease by replacing dying or malignant cells with healthy ones. To mention every disease treatment being investigated by stem cells is beyond the scope of this chapter. Suffice it to say that the medical applications of stem cells are broad, and there is optimism that stem cell treatment may become available for diseases such as autism, epilepsy, Parkinson, and Alzheimer. Pertinently, stem cell treatments to restore sight from blinding diseases are currently being developed.
In this chapter, we present a review of the current state of stem cells for treatments of blinding diseases, such as age-related macular degeneration (AMD). Additionally, the merit of stem cell-derived models of normal visual function, including retinoid processing, will be discussed.
2. STEM CELL DEFINITIONS AND TYPES
The term “stem cell” refers—not to a single cell type—but to two groups of different cell types: adult stem cells and embryonic stem cells (ESCs). Adult stem cells (or tissue cells) replenish tissues throughout the life of the adult. For example, blood cells make blood; fat cells make fat. Thus, adult stem cells are dedicated to repair and tissue maintenance throughout life. Embryonic (or pluripotent) stem cells are present in early development, before tissue is formed. Stem cells, whether adult or embryonic, are defined by two properties: self-renewal and differentiation. Self-renewal refers to the ability to complete many cycles of cell division while still retaining the undifferentiated state. Differentiation means these cells can become different types of cells.
The greater the number of cell types these stem cells can become, the greater their potency. Stem cells are categorized into five levels by their potency: totipotent, pluripotent, multipotent, oligopotent, bipotent, and unipotent. Totipotent stem cells have the ability to differentiate into embryonic cell types. These cells are produced by the fusion of the ovum and sperm cell. Additionally, the first several divisions of the zygote are totipotent. Pluripotent stem cells descend from totipotent stem cells and can potentially take on many cell fates derived from any of the three germ layers.1 Multipotent stem cells can differentiate into several cell types, but fewer than those of pluripotent stem cells. Oligopotent, bipotent, and unipotent stem cells can develop into a few, two, or a single cell type.2,3 Only embryonic cells are naturally pluripotent.
Despite the value of embryonic cells as a resource for basic and translational research, their use presents some major challenges for research. In order to utilize these cells, embryos must be destroyed. Groups citing concerns pertaining primarily to the moral, ethical, and religious implications of embryo research have, in various countries, led to the restriction of such study. Additionally, as with any transplantation procedure, graft rejection can occur, and has serious health consequences for the patient.
3. INDUCED PLURIPOTENT STEM CELLS
Induced pluripotent stem cells (iPSCs) were initially generated by the Yamanaka group in 2006.4 The group used an adenovirus-based vector to introduce transcriptional regulators SOX2, OCT3/4, Klf4, and c-MYC to reprogram mouse fibroblasts, which became iPSCs after 2–3 weeks. This combination of transgenes has since been used to reprogram somatic cells of a number of species, including the rat,5 dog,6 rhesus monkey,7 and human8. After reprogramming, these iPSCs are capable of differentiating into the three germ layer cell types (mesoderm, ectoderm, and endoderm), similar to that of embryonic cells.4,8–10 Valuably, just like embryonic cells, these induced stem cells are pluripotent and thus can take on many cell fates. Therefore, this significant advancement of the stem cell research field through iPSCs has allowed researchers to generate stem cells without the controversial use of embryos or the serious health consequences of graft rejection.
The applications for such iPSCs are vast, but of particular importance are two application classes: disease modeling and cell-based therapy. iPSCs offer a platform to study the mechanism of disease as the cells are patient specific. By generating iPSCs from patients with disease, models that simulate disease phenotypes can be used to better understand the pathophysiology of these diseases. Such models have been used for understanding diseases such as Down syndrome and Type 1 diabetes (for an in-depth review, see Ref. 11). Importantly, models can also be developed to better understand inherited diseases given their clinical and genetic heterogeneity.12 Additionally, cells derived from a particular patient can be used for patient-specific testing of drug discovery and/or toxicity, providing a new standard for personalized medicine.13 These applications have already had an impact on the understanding and treatment of blinding disorders such as AMD and retinitis pigmentosa, and are discussed below.
4. RETINAL PIGMENT EPITHELIUM, THE VISUAL CYCLE, AND AGE-RELATED MACULAR DEGENERATION
Retinoid metabolism is a principal function of the visual system, and the retinal pigment epithelium (RPE) plays an important role in this process. The uptake and processing of retinoids via the visual cycle is a critical aspect of phototransduction and there is a flow of these molecules between the photoreceptors and RPE.14,15 As depicted in Fig. 1, this process involves the shuttling of 11-cis-retinal to the rod photoreceptor cell from the RPE, where it binds to a protein called opsin to form the visual pigment, rhodopsin.14,16,17 Absorption of a photon catalyzes the isomerization of 11-cis-retinal to all-trans-retinal and results in its release.18,19 This isomerization triggers a cascade of events, leading to the generation of an electrical signal to the optic nerve resulting in vision. All-trans retinal is reduced to all-trans-retinol within the photoreceptors and then transported to the RPE, where it is converted to an all-trans retinyl ester. All-trans retinyl ester is then isomerized by the protein retinal pigment epithelium-specific 65 (RPE65) and hydrolyzed to release 11-cis retinol. 11-cis retinol is then oxidized by 11-cis retinol dehydrogenase into 11-cis-retinal and then transported back to the photoreceptors as mentioned above. This loop is critical for light perception and eventual vision. The Ablonczy lab has demonstrated this process in vitro in fetal RPE cells (Fig. 2).20
Figure 1.
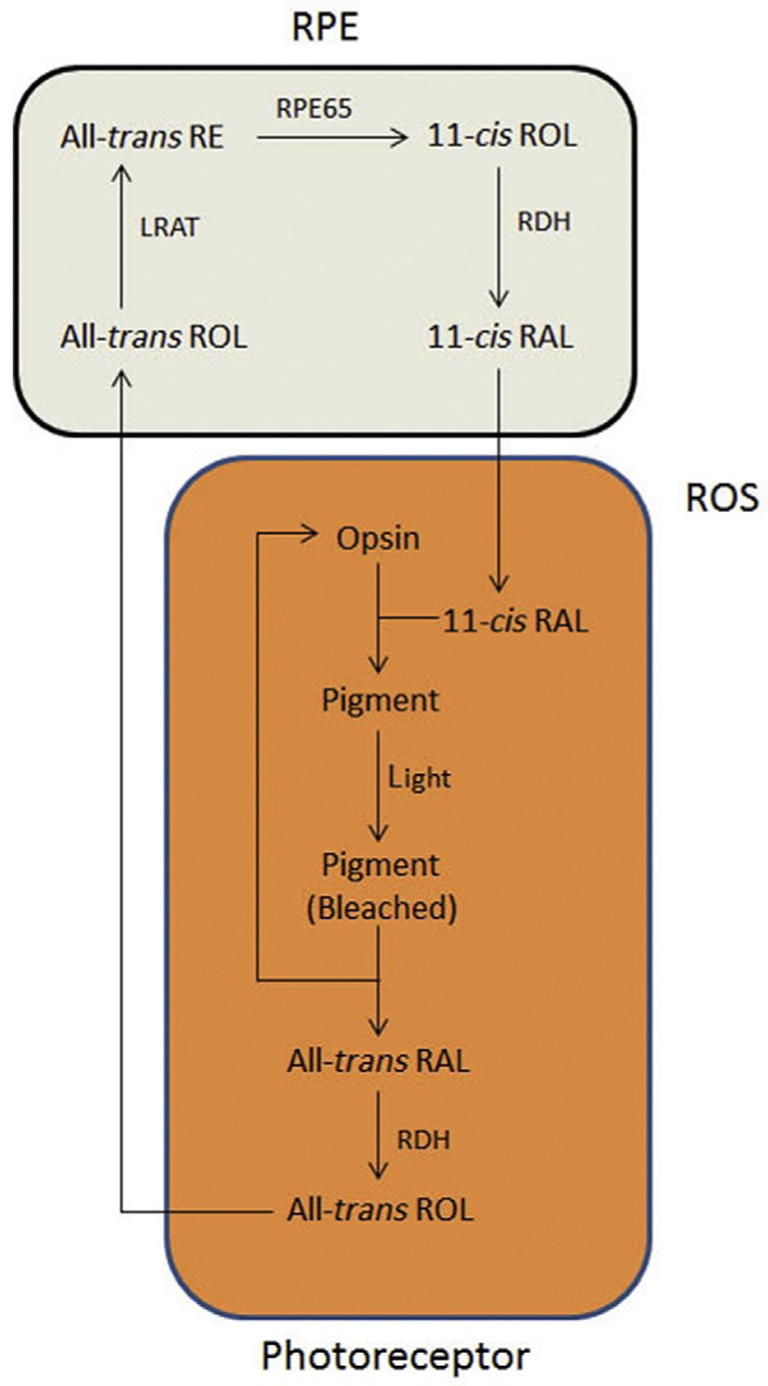
Classic visual cycle. Retinoids are metabolized and shuttled between the RPE (beige; light gray in the print version) and both rod and cone OS (outer segments; depicted generically in orange; dark gray in the print version). RAL, retinal; ROL, retinol; RDH, retinol dehydrogenase; LRAT, lecithin retinol acetyltransferase; RE, retinyl-ester. With the full permission of all authors of the original publication, Fig. 4 of Ref. 14 has been modified for inclusion here.
Figure 2.
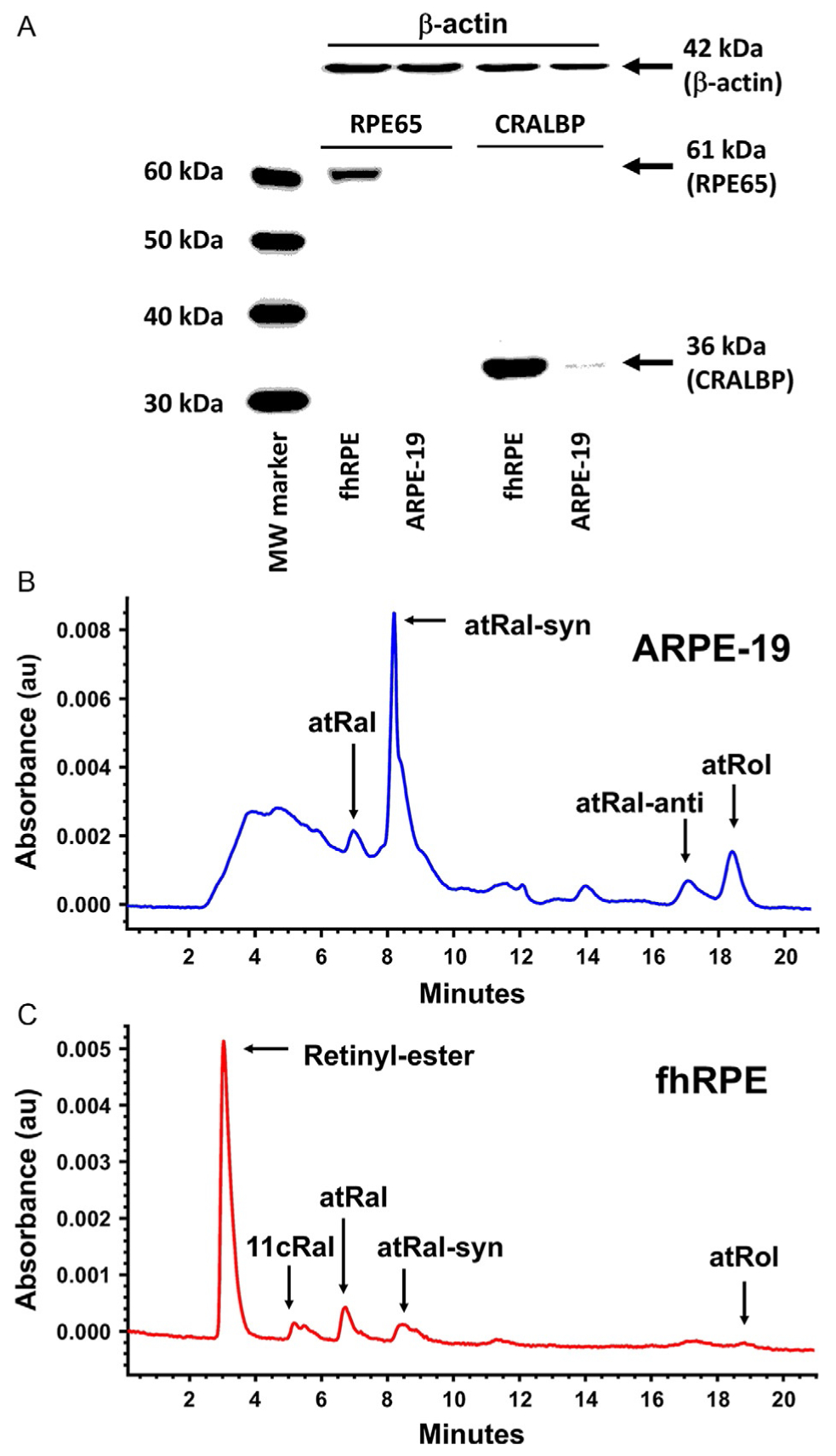
Retinoid metabolism in monolayer RPE cultures. Expression of RPE visual cycle proteins (A) showing immunoblots against RPE65 and CRALBP in both fhRPE and ARPE-19 cells. While fhRPE cells abundantly express both proteins, ARPE-19 cells express only low quantities of CRALBP. β-Actin indicated approximately equal loading of the lanes. Functional retinoid metabolism was missing from ARPE-19 cells (B); but was present in fhRPE cells (C) as shown by the corresponding retinoid profiles (aldehydes indicated both as unconverted and converted to oximes by hydroxylamine) taken by monitoring the HPLC at 360 nm 2 days postadministration of 5 μmol/L all-trans retinal (atRal) in phosphatidylcholine (PC) vesicles. The profile for ARPE-19 cells (B) was dominated by unconverted all-trans retinal, while the profile for fhRPE cells (C) and was dominated by retinyl esters and 11-cis-retinal (11cRal), indicating functional retinoid metabolism. The data are representative of three independent experiments. With the full permission of all authors of the original publication, Fig. 3 of Ref. 20 has been included here.
AMD, a blinding disease that affects 30–50 million elderly people worldwide, is associated with RPE loss.21,22 Approximately 30% of adults over the age of 75 show signs of AMD and around 10% of patients develop the late stage of disease.23,24 AMD primarily exists in two forms: dry (atrophic or nonexudative) and wet (neovascular or exudative). Early in the course of disease, there is cellular dysfunction without cell death. In late-stage disease, AMD is characterized by extensive cell death. In the case of dry AMD, loss of vision develops due to loss of the RPE, photoreceptors, and/or choriocapillaris; this can lead to patches of geographic atrophy (GA) which result clinically in paracentral scotomas.25 Replacement of dying RPE cells with new healthy RPE cells may be a useful treatment for AMD. Further, models of in vitro cell cultures of RPE from patients with AMD/GA will likely be useful for studying RPE-related AMD pathogenesis.
5. INDUCED PLURIPOTENT STEM CELL-DERIVED RETINAL PIGMENT EPITHELIUM
There have been many clinical challenges in the transplantation of intact sheets and suspensions of primary RPE cells in patients with retinal disorders.26–31 Stem cell engineering allows us to overcome these obstacles inherent in this treatment through the use of iPSCs, which can then be differentiated into RPE using well-established differentiation protocols. Stem cells are an ideal replacement source for these lost or damaged cells, since stem cells have a significant ability to proliferate in vitro—prior to transplantation—and in vivo—after subretinal transplantation.
The use of iPSC-derived RPE as a research tool and potential therapeutic for cell transplantation therapy holds great potential for the treatment of retinal degeneration. For example, patients with advanced GA in AMD will require repopulation of the atrophic area with new cells, namely, new RPE.32 In principle there are several potential sources for donor RPE, including adult RPE, fetal RPE, immortalized RPE (i.e., ARPE-19), and ESCs. These sources are limited and can often vary in terms of quality and expansion capacity.26,33–35 As noted above, there are also ethical implications as well as the problem of a limited reservoir of donor cells.36 There have been many important studies demonstrating the use of ESCs for the treatment of GA including a Phase 1 clinical trial in humans started in 2011 by Advanced Cell Technology, Inc., for the treatment of retinal degenerative disorders.26,37 This is the first FDA-approved trial for the treatment of macular degeneration using RPE derived from human ESCs that have been injected into the subretinal space of these patients. One caveat is that these patients are on an immunosuppressive regimen for the duration of their treatment.
The use of iPSC technology offers a source of individualized or patient-specific cells that can then be differentiated into iPSC-derived RPE. Many research groups have efficiently differentiated iPS cells into RPE using established protocols.38–41 These cells morphologically and functionally behave like native RPE. Figure 3 demonstrates the differentiation process of establishing iPSC-derived cell lines. Cultures are established within 45–60 days and tested for their ability to produce RPE-specific markers such as tight junction protein zona occluden-1 (ZO-1) (Fig. 4). Functional testing, such as integrity of the monolayer is analyzed (Fig. 5). When treating a disorder such as GA, this approach offers the advantage of potentially circumventing the problem of immune rejection after transplantation in the area of disease as well as being a reservoir for large numbers of cells for replacement. The feasibility of this approach has been demonstrated in animal models of retinal degeneration.39,42
Figure 3.
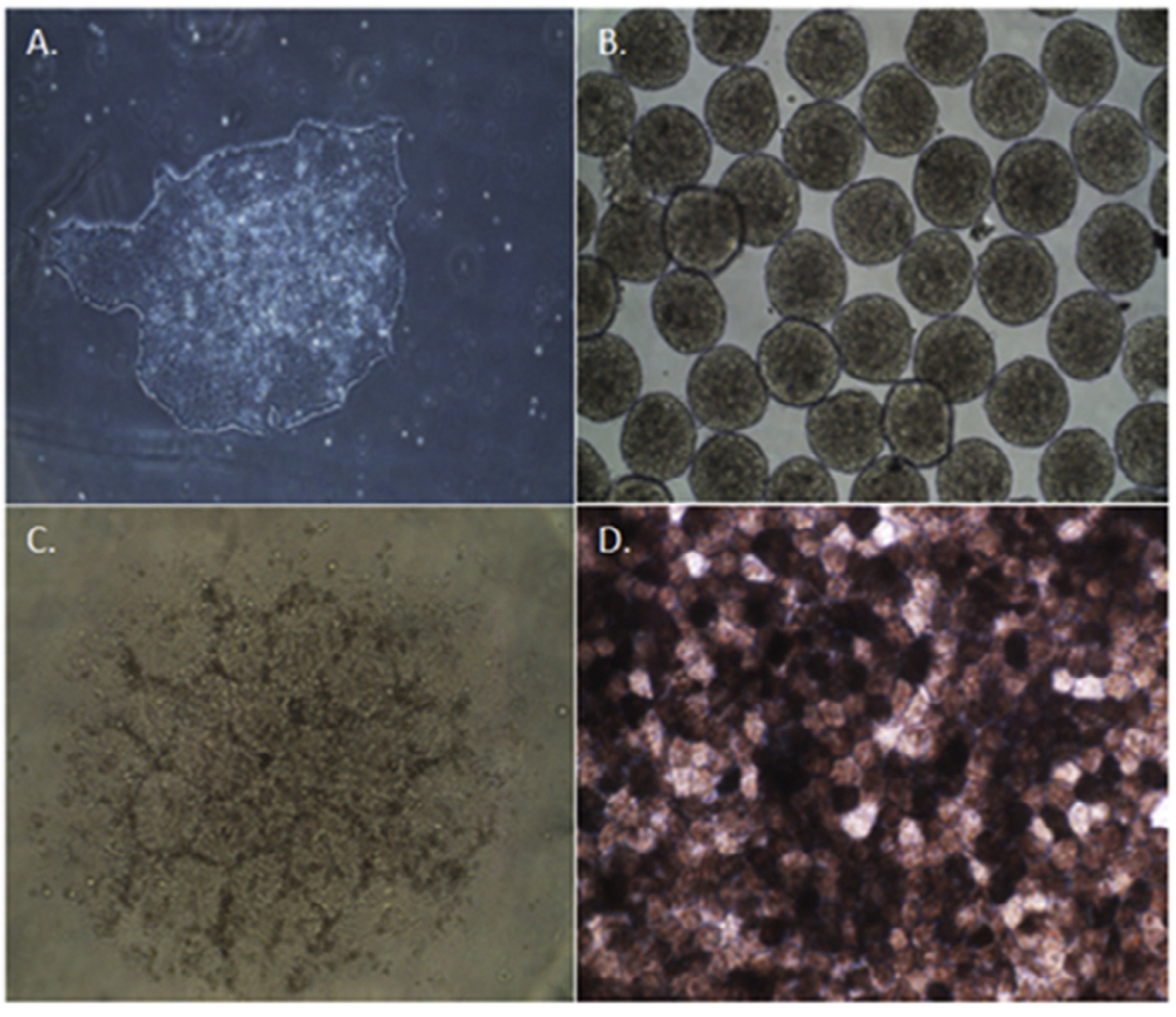
Differentiation of induced pluripotent stem cells toward an RPE fate. Undifferentiated iPSC colony at day 0 (A). Embryonic bodies form by day 7 (B), and eventual formation of neural aggregates (C) by day 14. Pigmented monolayer of iPSC-derived RPE form by day 45 postdifferentiation (D).
Figure 4.
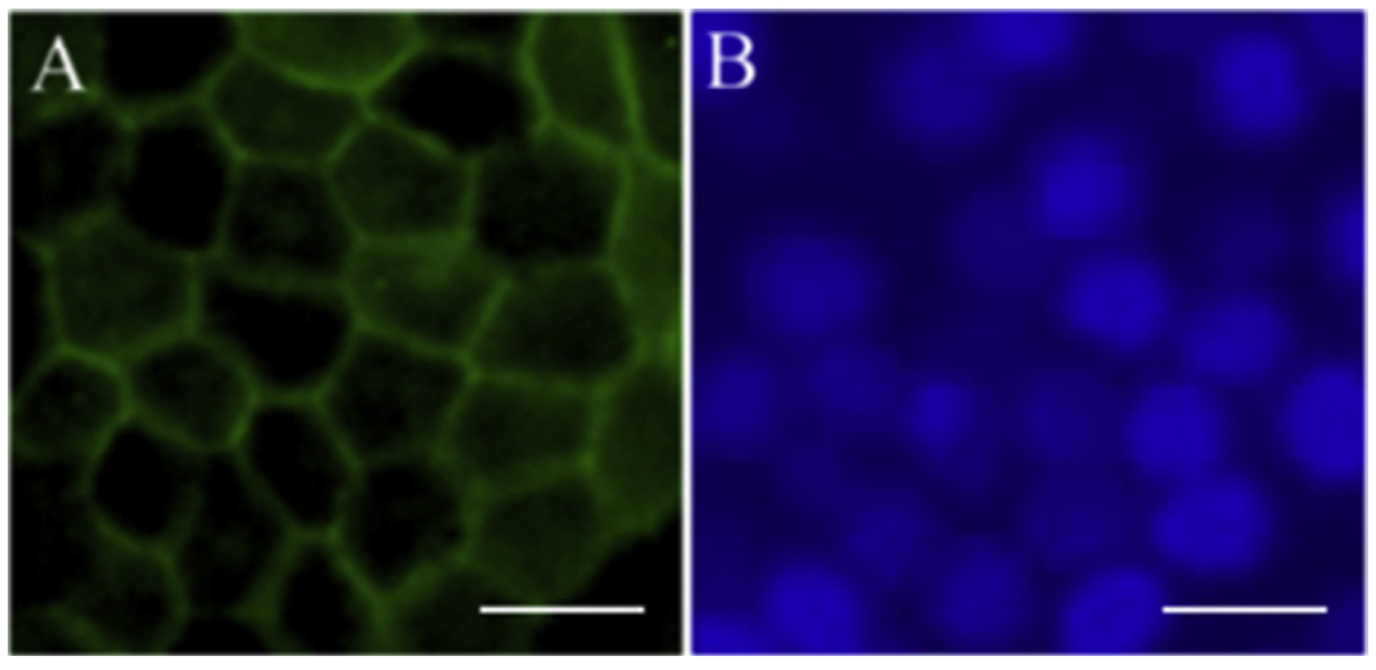
Epithelial expression of RPE cell markers. Immunofluorescent staining of RPE marker ZO-1 (A) in pigmented IPS-derived RPE cells. (B) DAPI image of same cells. Scale bars=50 μm.
Figure 5.
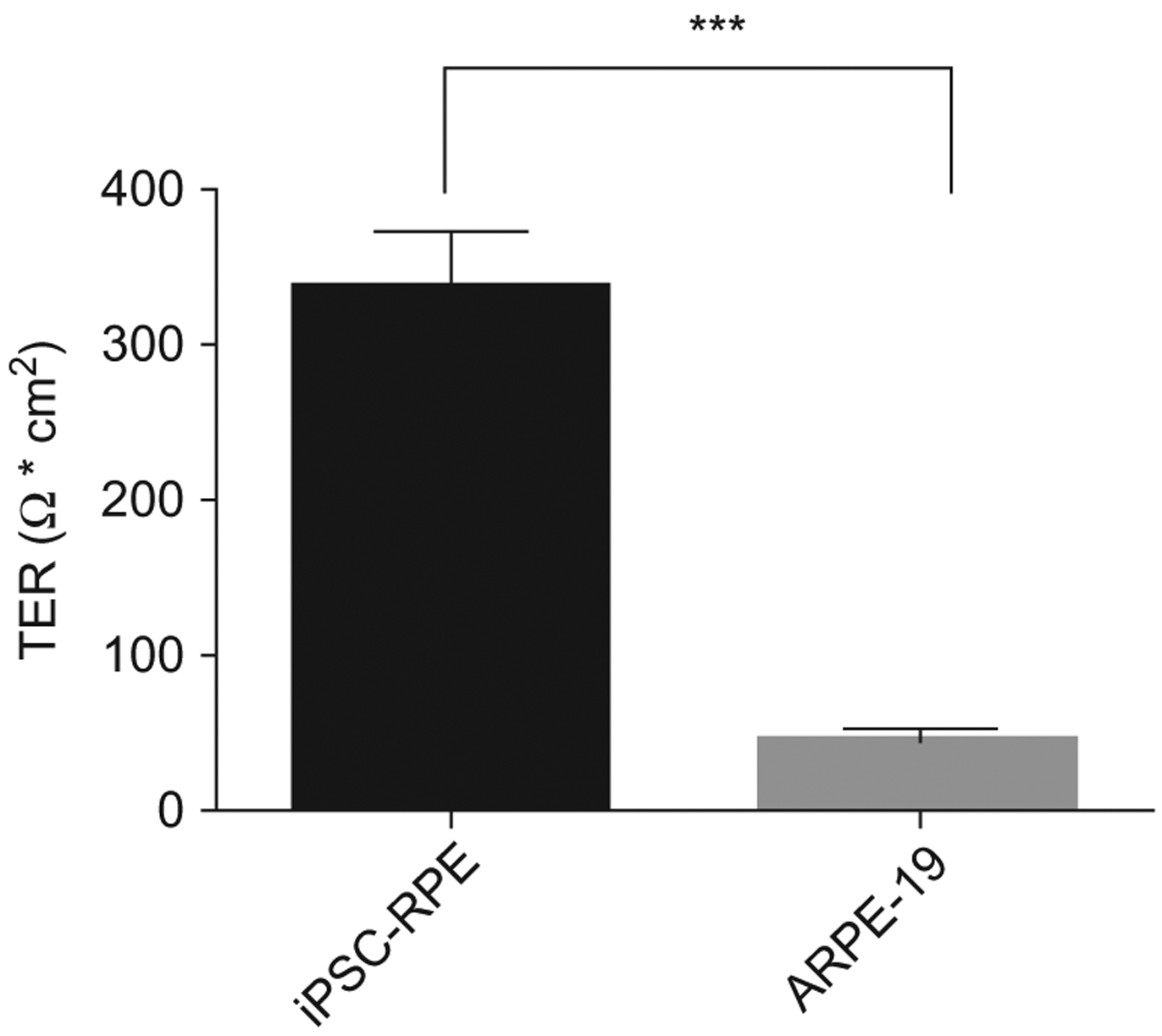
Transepithelial resistance (TER) of iPSC-derived RPE. Monolayer permeability was assessed by transepithelial resistance measurements in polarized iPSC-RPE cells. Transepithelial resistance was tested at an average of 33 ± 36 Ω* cm2 for iPSC-RPE compared with ARPE-19 cells which tested at an average resistance of 46 ± 73 Ω* cm2.
IPSC-derived RPE can also provide a valuable research tool and platform to understand disease. Through the generation of iPS cells from patients with specific diseases such as AMD and Best disease, genetic studies and models to express particular disease phenotypes can be developed which can then be used to understand the pathophysiology of disease and determine the efficacy of therapeutic interventions.13,43,44 These models can help to understand human inherited diseases,12 and can serve as a tool for drug discovery and pharmacological toxicity testing of therapeutic agents, what is often referred to as personalized medicine.13,45,46
6. RETINOID PROCESSING IN INDUCED PLURIPOTENT STEM CELL-DERIVED RETINAL PIGMENT EPITHELIUM
When considering the use of iPSCs derived as a therapeutic option for individuals with retinal degenerations, it is vital that these cells are able to perform and restore visual cycle regeneration.47,48 In vitro assays have been developed to verify visual cycle function in iPSC-derived RPE cultures. While many research groups have efficiently developed iPSC-RPE cells, only a limited number have demonstrated the production of retinoids in these cultures.
There are different systems that can induce the production and analysis of retinoids. Maeda et al. have demonstrated the ability of iPSC-RPE cells to express visual cycle enzymes both in vivo and in vitro.48 These cells are able to produce visual cycle proteins such as RPE65, lecithin retinol acetyltransferase (LRAT), and cellular retinaldehyde-binding protein (CRALBP).48 It was shown that the cellular environment and culture conditions may play a major role in the maintenance of visual cycle proteins in these cultures. Muniz et al. have also conducted studies on visual cycle production by iPSC-RPE cultures. In these studies, iPSC-RPE cultures were able to uptake all-trans retinol, synthesize retinyl esters, and release 11-cis-retinaldehyde into the culture media after analysis by high-performance liquid chromatography (HPLC).47 Similar experiments have been conducted by Ablonczy et al. in human fetal cultures to assess retinoid metabolism.20 Briefly, iPSC-RPE cells are cultured to confluence, forming cobblestone morphology and allowed to produce pigment. Cultures are treated with all-trans retinal in phosphatidylcholine (PC) vesicles. Media is then collected, cells lysed, and HPLC analysis follows. Separation of retinoids is performed and quantified by comparison of retention times and absorption properties with pure retinoid isomeric standards.20,49 As shown in the HPLC profile in Fig. 6, iPSC-RPE monolayers treated with all-trans retinol form detectable amounts of 11-cis-retinal. These results suggest the ability of these cultures to process retinoids may be similar to that of native RPE. Given the ability of these cells, it is clear that iPSC-RPE could be a potential therapeutic tool for transplantation.
Figure 6.
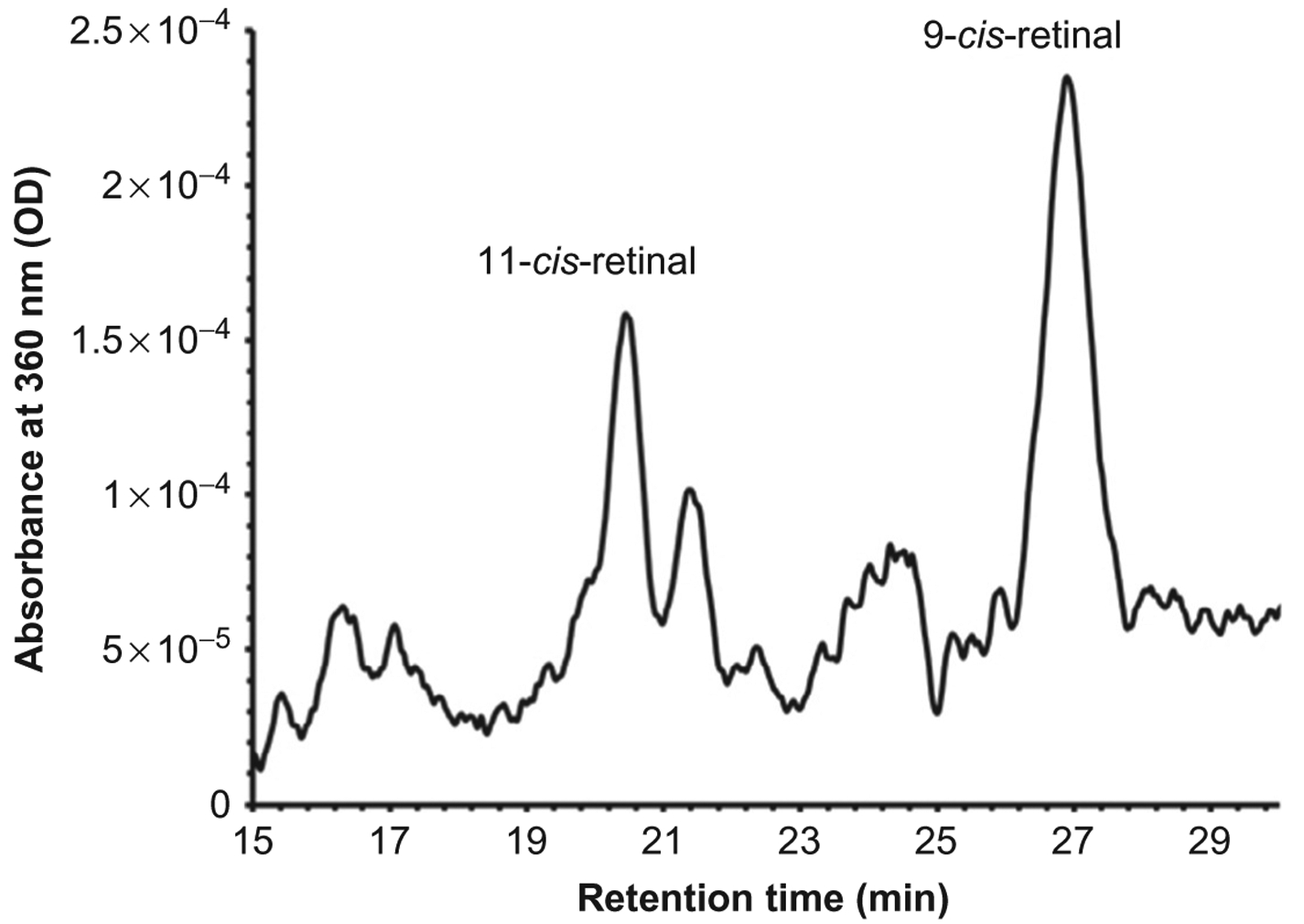
Retinoid metabolism in iPSC-RPE monolayers. iPSC-RPE were cultured in 6-well plates until a confluent and pigment monolayer was observed. Retinoid profiles were taken by monitoring the HPLC at 360 nm, 2 days postadministration of 5 μmol/L all-trans retinol (atRol) in 1% bovine serum albumin. iPSC-RPE cultures express 11-cis-retinal indicating functional retinoid metabolism. No hydroxylamine was used in these experiments, therefore oximes are not detected. Note that significant quantities of the more stable 9-cis isomer are also formed with the administration of all-trans retinol, and that the solvent system/column used in this experiment was different from that in Fig. 2 to better resolve the retinal isomers.
7. FUTURE DIRECTIONS
iPSCs are a promising technology as a research tool and potential reservoir of cells for the treatment of many diseases, but challenges do remain. For example, IPSCs derived from the affected patient contain the predisposing mutation that initially caused the disease. This can provide a challenge when assessing use of these particular cells given their dysfunction, but it also provides a unique opportunity for disease models. Use of technology such as clustered regularly interspaced short palindromic repeats (CRISPR) has also begun to change the way disease mutations are approached. This technology has the ability to correct mutations directly within the cell allowing for precise and targeted gene therapy.50 However, challenges remain in efficient RPE generation. Optimizing protocols to provide homogenous populations in sufficient numbers suitable for transplantation will have to be addressed. Better understanding of iPSC technology and refining the methodology of iPSC-derived RPE generation will create new inroads in regenerative medicine and in treatment of retinal degenerations.
ACKNOWLEDGMENTS
Supported in part by NIH grant EY19065 (Z. A.) and an unrestricted grant to MUSC-SEI from Research to Prevent Blindness, New York, NY; and Foundation Fighting Blindness, Columbia, MD. The authors would also like to thank Dr. Rosalie Crouch for critically reading this chapter.
ABBREVIATIONS
- AMD
age-related macular degeneration
- CRALBP
cellular retinaldehyde-binding protein
- ESC
embryonic stem cell
- GA
geographic atrophy
- HPLC
high-performance liquid chromatography
- iPSC
induced pluripotent stem cell
- iPSC-RPE
induced pluripotent stem cell-derived retinal pigment epithelium
- LRAT
lecithin retinol acetyltransferase
- PC
phosphatidylcholine
- RPE
retinal pigment epithelium
- RPE65
retinal pigment epithelium-specific 65
- TER
transepithelial resistance
- ZO-1
zona occluden-1
REFERENCES
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 2.Cumano A, Paige CJ, Iscove NN, Brady G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature. 1992;356:612–615. [DOI] [PubMed] [Google Scholar]
- 3.Lantz CS, Huff TF. Differential responsiveness of purified mouse c-kit+ mast cells and their progenitors to IL-3 and stem cell factor. J Immunol. 1995;155:4024–4029. [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 5.Liao J, Cui C, Chen S, et al. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2009;4:11–15. [DOI] [PubMed] [Google Scholar]
- 6.Shimada H, Nakada A, Hashimoto Y, Shigeno K, Shionoya Y, Nakamura T. Generation of canine induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol Reprod Dev. 2010;77:2. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Zhu F, Yong J, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3:587–590. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 9.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka S A fresh look at iPS cells. Cell. 2009;137:13–17. [DOI] [PubMed] [Google Scholar]
- 11.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin ZB, Okamoto S, Mandai M, Takahashi M. Induced pluripotent stem cells for retinal degenerative diseases: a new perspective on the challenges. J Genet. 2009;88:417–424. [DOI] [PubMed] [Google Scholar]
- 13.Song M, Paul S, Lim H, Dayem AA, Cho SG. Induced pluripotent stem cell research: a revolutionary approach to face the challenges in drug screening. Arch Pharm Res. 2012;35:245–260. [DOI] [PubMed] [Google Scholar]
- 14.Tang PH, Kono M, Koutalos Y, Ablonczy Z, Crouch RK. New insights into retinoid metabolism and cycling within the retina. Prog Retin Eye Res. 2013;32:48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiser PD, Golczak M, Maeda A, Palczewski K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim Biophys Acta. 1821;2012:137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baylor DA, Burns ME. Control of rhodopsin activity in vision. Eye. 1998;12(Pt. 3b): 521–525. [DOI] [PubMed] [Google Scholar]
- 17.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001;26:318–324. [DOI] [PubMed] [Google Scholar]
- 18.Lamb TD, Collin SP, Pugh EN Jr. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci. 2007;8:960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. [DOI] [PubMed] [Google Scholar]
- 20.Ablonczy Z, Dahrouj M, Tang PH, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011;52:8614–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defoe DM, Ahmad A, Chen W, Hughes BA. Membrane polarity of the Na(+)-K+ pump in primary cultures of Xenopus retinal pigment epithelium. Exp Eye Res. 1994;59:587–596. [DOI] [PubMed] [Google Scholar]
- 22.Tuo J, Grob S, Zhang K, Chan CC. Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul Immunol Inflamm. 2012;20:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad PS, Schwartz SD, Hubschman JP. Age-related macular degeneration: current and novel therapies. Maturitas. 2010;66:46–50. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. [DOI] [PubMed] [Google Scholar]
- 25.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. [DOI] [PubMed] [Google Scholar]
- 27.Binder S, Krebs I, Hilgers RD, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci. 2004;45:4151–4160. [DOI] [PubMed] [Google Scholar]
- 28.Algvere PV, Berglin L, Gouras P, Sheng Y. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1994;232:707–716. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan HJ, Tezel TH, Berger AS, Del Priore LV. Retinal transplantation. Chem Immunol. 1999;73:207–219. [DOI] [PubMed] [Google Scholar]
- 30.MacLaren RE, Bird AC, Sathia PJ, Aylward GW. Long-term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age-related macular degeneration. Ophthalmology. 2005;112:2081–2087. [DOI] [PubMed] [Google Scholar]
- 31.Lappas A, Weinberger AW, Foerster AM, Kube T, Rezai KA, Kirchhof B. Iris pigment epithelial cell translocation in exudative age-related macular degeneration. A pilot study in patients. Graefes Arch Clin Exp Ophthalmol. 2000;238:631–641. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Enzmann V, Ildstad ST. Stem cell-based therapeutic applications in retinal degenerative diseases. Stem Cell Rev. 2011;7:434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MK, Lui GM. Propagation of fetal human RPE cells: preservation of original culture morphology after serial passage. J Cell Physiol. 1990;143:196–203. [DOI] [PubMed] [Google Scholar]
- 34.Gamm DM, Wright LS, Capowski EE, et al. Regulation of prenatal human retinal neurosphere growth and cell fate potential by retinal pigment epithelium and Mash1. Stem Cells. 2008;26:3182–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan CK, Heilweil G, Lanza R, Schwartz SD. Embryonic stem cells as a treatment for macular degeneration. Expert Opin Biol Ther. 2013;13:1125–1133. [DOI] [PubMed] [Google Scholar]
- 38.Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. [DOI] [PubMed] [Google Scholar]
- 39.Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, Phillips MJ, Kuai D, et al. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Invest Ophthalmol Vis Sci. 2013;54:6767–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–131. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Tsai YT, Hsu CW, et al. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol Med. 2012;18:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh R, Shen W, Kuai D, et al. iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013;22:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Li Y, Chan L, et al. Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Hum Mol Genet. 2014;23:3445–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrer M, Corneo B, Davis J, et al. A multiplex high-throughput gene expression assay to simultaneously detect disease and functional markers in induced pluripotent stem cell-derived retinal pigment epithelium. Stem Cells Transl Med. 2014;3:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther. 2011;89:655–661. [DOI] [PubMed] [Google Scholar]
- 47.Muniz A, Greene WA, Plamper ML, et al. Retinoid uptake, processing, and secretion in human iPS-RPE support the visual cycle. Invest Ophthalmol Vis Sci. 2014;55:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda T, Lee MJ, Palczewska G, et al. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J Biol Chem. 2013;288:34484–34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groenendijk GW, De Grip WJ, Daemen FJ. Quantitative determination of retinals with complete retention of their geometric configuration. Biochim Biophys Acta. 1980;617: 430–438. [DOI] [PubMed] [Google Scholar]
- 50.Li HL, Nakano T, Hotta A. Genetic correction using engineered nucleases for gene therapy applications. Dev Growth Differ. 2014;56:63–77. [DOI] [PubMed] [Google Scholar]


