Abstract
Cardiovascular diseases are the leading cause of death worldwide. Discovering new therapies to treat heart disease requires improved understanding of cardiac physiology at a cellular level. Extracellular vesicles (EVs) are plasma membrane-bound nano- and microparticles secreted by cells and known to play key roles in intercellular communication, often through transfer of biomolecular cargo. Advances in EV research have established techniques for EV isolation from tissue culture media or biofluids, as well as standards for quantitation and biomolecular characterization. EVs released by cardiac cells are known to be involved in regulating cardiac physiology as well as in the progression of myocardial diseases. Due to difficulty accessing the heart in vivo, advanced in vitro cardiac ‘tissues-on-a-chip’ have become a recent focus for studying EVs in the heart. These physiologically relevant models are producing new insight into the role of EVs in cardiac physiology and disease while providing a useful platform for screening novel EV-based therapeutics for cardiac tissue regeneration post-injury. Numerous hurdles have stalled the clinical translation of EV therapeutics for heart patients, but tissue-on-a-chip models are playing an important role in bridging the translational gap, improving mechanistic understanding of EV signalling in cardiac physiology, disease, and repair.
Keywords: cardiomyocyte, myocardium, extracellular vesicle, exosome, heart, tissue engineering, regeneration
Graphical Abstract
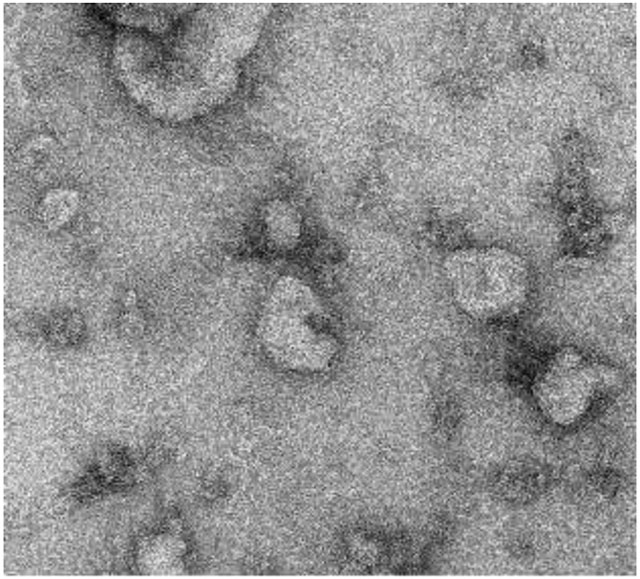
Extracellular vesicles (EVs) are cell-secreted nano and micro-particles having important functions in intercellular communication and in the progression of heart diseases. EVs harvested in the lab show significant promise as a regenerative therapeutic for the injured heart. 3D models of heart ‘tissue-on-a-chip’ represent promising platforms to advance understanding of EVs in the heart towards designing new therapies for heart patients.
1. Introduction
Cardiovascular diseases represent the leading cause of death worldwide, with ischemic heart disease (IHD) representing one of the primary causes of morbidity in heart patients. [1] The coronary arteries supply cells in the heart with oxygenated blood and are particularly susceptible to build-up of fat and cholesterol plaques. [2,3] These plaques can restrict the flow of oxygenated blood to cardiac cells, termed ‘ischemia’, and may rupture to initiate a cascade of events leading to clots that completely block blood flow and cause acute myocardial infarction (MI), or ‘heart attacks’. [4] One of the most critical consequences of IHD involves cardiomyocyte cell death. Adult cardiomyocytes do not divide on a meaningful timescale, meaning that tissue damage sustained during ischemic injury is generally irreversible. [5] Thus, ‘heart failure’ is a common outcome for IHD patients, in which a damaged heart is unable to adequately pump blood through the body. Due to the limited regenerative capacity of the native heart and its tendency for long-term, maladaptive tissue remodeling in response to dysfunction, heart failure is a common final outcome for the majority of other prevalent cardiovascular diseases as well. These include genetic and secondary cardiomyopathies, myocarditis, and hypertension. [6] Heart failure affects over 26 million people globally and represents a major socioeconomic burden, with consequences including shortness of breath in daily activities, weight gain, and ultimately death. [7,8]
Current strategies for inducing tissue repair and regeneration in situ in the injured or dysfunctional heart are limited. This is evidenced by the fact that heart transplant remains the only true ‘cure’ for a failing heart. [6] Thus, a detailed understanding of cardiac physiology and methods for modulating cardiomyocyte cell behaviour must be further developed to realize new therapies for treating cardiac disease.
Recent studies indicate that cell-signalling in the cardiac environment plays an integral role in tissue physiology as well as pathology. [9,10] Extracellular vesicles (EVs) represent one such mode of communication used by cells to coordinate functions and modulate the activities of other cells within tissues and organs. EVs are membrane-bound cell secreted particles containing genetic material and proteins that have been identified as a significant component of numerous tissue systems. It has been suggested that EV release and uptake by cells in the heart is critical to regulating healthy cardiac function, and that dysregulation and dysfunction in EV communication may be both a signal and mechanism of disease propagation. Thus, a better understanding of the roles that EVs play in heart function and disease remains a significant point of interest in the future development of cardiac diagnostics and regenerative therapeutics that harness the power of native and engineered EVs. [11,12]
Since the cardiac environment is difficult to access in vivo to perform detailed mechanistic studies of EVs in the heart, the advancement of in vitro models of heart tissue represents a promising way to improve understanding of EV-associated cardiac physiological processes. In an attempt to recapitulate the function and complex interactions present in native human tissues, the organ-on-a-chip industry has emerged in recent decades at the intersection of microfluidics and tissue engineering. [13] In vitro tissue-on-a-chip models combine cells and biomaterials in arrangements that mimic the structure and function of organs in the body. Such platforms can facilitate controlled, isolated, and accessible investigations of human physiology ex vivo. Creating these models using human cells derived from induced pluripotent stem cells (iPSC) enables a high degree of physiological relevance. [14] The flexibility of these models also opens the door to applications such as disease modeling and the discovery and testing of novel regenerative therapies for restoring function in damaged organs and tissues. [13]
The role and mechanisms of EV signalling in the cardiac environment remain poorly defined and have not been well-classified in a physiologically relevant system, making the adaptation of tissue-on-a-chip models a novel way to better understand cardiac physiology from an EV standpoint. Investigating EV-mediated modulation in healthy and diseased heart tissue represents the first step towards developing new therapies for heart patients that induce tissue repair through a cell-signalling approach by applying specific sources and populations of EVs to damaged hearts. Thus, integrating the fields of tissue-on-a-chip and EV research has the potential to revolutionize the quality of life and outcomes for heart failure patients and reduce the societal burden of cardiovascular disease moving forward.
2. Extracellular Vesicles
2.1. EVs and Intercellular Communication
Intercellular communication is a critical phenomenon that has been observed in a wide variety of organisms, from plants and animals to bacteria, and occurs in virtually all organs and tissues in the human body. Communication at the cellular level has evolutionary origins. An individual bacterium can influence the actions of a group based on environmental cues, and cells in human organs can coordinate normal function or response to disease as a single unit for improved efficiency or survival. [15] It has been recognized for many decades that direct cell-to-cell contact and the secretion and uptake of molecules are two important ways by which cells communicate. [16] These mechanisms explain the ability of cells to signal and influence each other both in a local environment and in distant or remote locations via circulation.
More recently, a third mode of communication involving the release and uptake of ‘extracellular vesicles’ (EVs) has started to gather interest for its key role in regulating and modulating tissue physiology. EVs are cell-secreted particles bound by a lipid bilayer membrane that contain various classes of biomolecular cargo including proteins, lipids, and nucleic acids. [16] The earliest reports of EVs actually date back to the 1940s, however understanding of their universality and mechanisms of action in the human body only started to develop significantly in the past decade. In the earliest description of EVs, particles associated with platelets were found in the blood that were involved in events of the clotting cascade. Over the ensuing decades, similar functional sub-cellular particles were found in virtually all types of bodily fluids cultured cells. [15] The most crucial step towards understanding the functional significance of EVs came in the years 2006 and 2007, when researchers first described the variety of nucleic acids, namely microRNA (miRNA), and proteins present within EVs that facilitated their role as bioactive particles. [17,18] The International Society for Extracellular Vesicles was established in 2011, and since then interest in the role and mechanisms of vesicles in various cells, tissues, and diseases has risen rapidly. [15] Current EV research focuses largely on their potential use as a biomarker for disease based on changes in EVs release from healthy versus pathological tissues, as well as a regenerative therapeutic that induces tissue repair via cell-signalling. [19] Mechanistic understanding and standardization of techniques are some of the major gaps that have delayed clinical implementation of EV diagnostics and therapies, however they represent a promising direction for revitalizing the field regenerative medicine. [20]
2.2. EV Biogenesis, Subgroups, and Mechanisms of Action
EVs are classified in subgroups based on their intracellular origin. The three commonly recognized subgroups of EVs are exosomes, microvesicles (MVs) or microparticles, and apoptotic bodies, as depicted in Figure 1a. [16]
Figure 1.
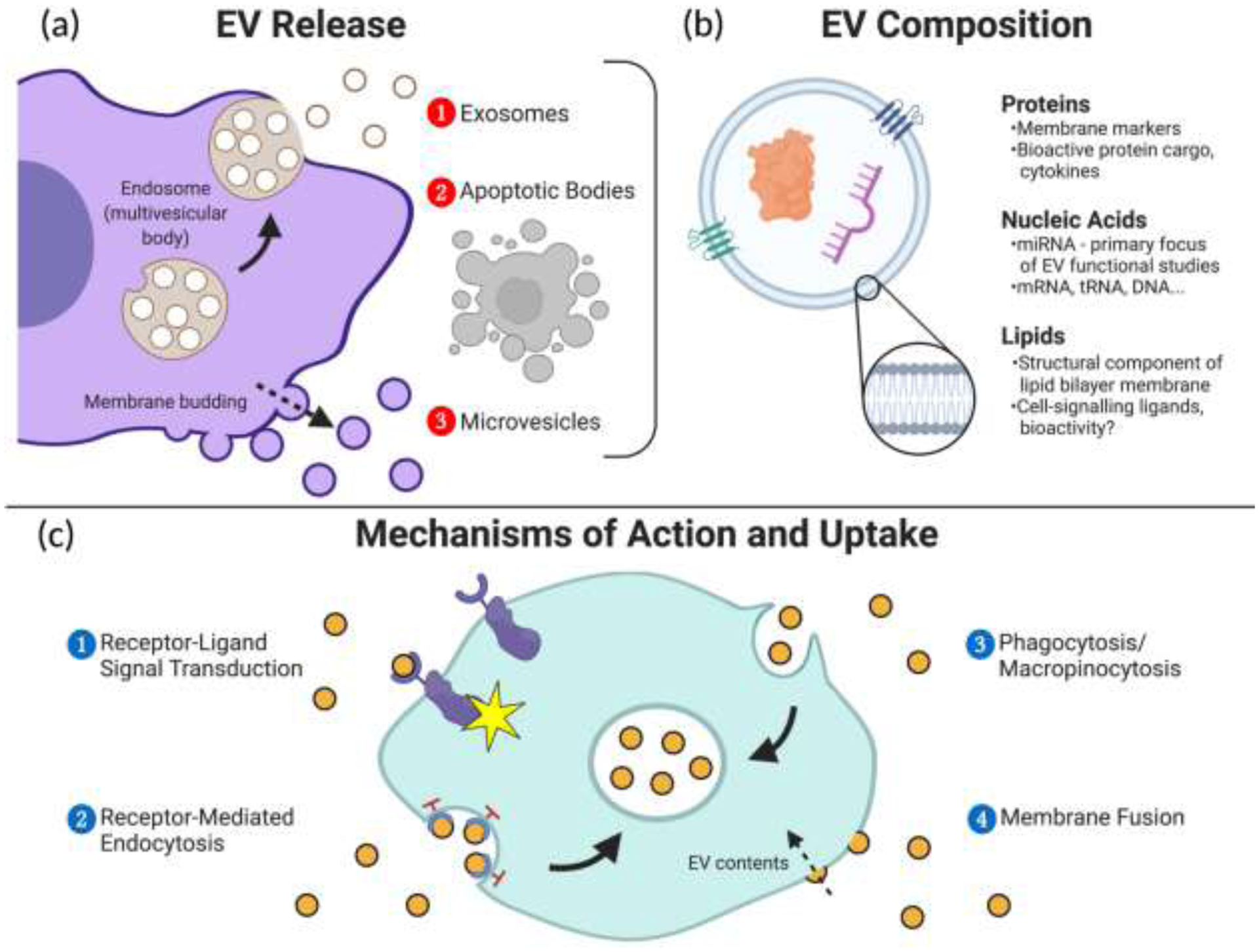
Extracellular vesicles (EVs) play an integral role in intercellular communication. (a) EVs are often classified into subtypes based on their mechanisms of biogenesis from source cells. (b) Various classes of biomolecules are present in the membranes and cargo of secreted EVs and contribute to their functions in cell signalling. (c) Secreted EVs can interact with cells through several mechanisms in order to elicit a response or transfer their cargo into recipient cells.
2.2.1. Exosomes
Exosomes are EVs that mostly range in size from 30–150nm. [21] They originate as intraluminal vesicles (ILVs) within endosomes inside the cell referred to as multivesicular bodies (MVBs). MVBs often fuse with lysosomes for degradation of their contents, however some fuse with the cell membrane to release exosomes via exocytosis. [22]
Several intracellular mechanisms can initiate ILV formation. Protein complexes referred to as the endosomal sorting complex required for transport (ESCRT) have been shown to play a key role in ILV biogenesis and as such, pathways are typically classified as ESCRT-dependent or ESCRT-independent. [15,22] Though the mechanisms of these pathways have been a significant area of investigation, the implications of pathway differences on EV cargo and function remains relatively uncertain. [22]
ESCRT-dependent biogenesis relies on the coordinated action of four protein complexes, ESCRT-0, -I, -II, and -III. [22] Studies indicate that ESCRT-0 has role in initiating endosomal membrane protein sequestration, with ESCRT-I and -II complexes assisting in subsequent membrane budding. Other proteins such as HSC70, TSG101, and ALIX provide support in the trafficking and sorting of biomolecules into membrane buds, as well as the recruitment of ESCRT-III which initiates detachment of individual ILVs within endosomes. [22]
Evidence for ESCRT-independent biogenesis pathways emerged from studies that observed ILV formation after ESCRT knockdown. [23] Some such pathways involve tetraspanin membrane proteins (such as CD63 and CD81), indicating that tetraspanins can initiate sequestration, sorting, and budding of ILVs without the assistance of ESCRT complexes. [22] Lipid generation is another ESCRT-independent mechanism of ILV biogenesis. Enzymes including neutral sphingomyelinase and phosplipase D2 have shown roles in stimulating the production of lipids at the endosomal membrane, a process that can induce budding of lipid bilayer-bound ILVs into the MVB lumen. [22]
During ILV formation, trafficking proteins play a role in directing which molecules in the cytosol are to be sorted into vesicles, as will be addressed in the following sections. Intracellular signals that initiate exosome release have been difficult to discern, though environmental factors such as the presence of serum, neurotransmitters, and gamma irradiation have shown to affect the rate of EV release from several cell types. The Ras-related proteins in brain (RAB) are key effectors in transporting MVBs to the cell membrane for exosome release. [22]
2.2.2. Microvesicles
MVs refer to EVs formed by direct budding and scission of particles from the outer cell membrane. [22] They may range anywhere from 100nm to 1μm in size, though some may be smaller than exosomes. [10,15] Release of MVs may be spontaneous but is usually enhanced by stimuli such as increased intracellular calcium concentrations which can induce enzymatic remodelling of the cell cytoskeleton and bud formation. Similar to ILV biogenesis, members of the ESCRT family and lipid generating enzymes can play a role in initiating budding and scission of MVs directly from the cell membrane, and does the protein ADP-ribosylation factor 6 (ARF6). [22]
2.2.3. Apoptotic Bodies
Apoptotic bodies are particles that originate from the detachment of membrane bulges of cells undergoing apoptosis, and have been a subject of limited focus in regenerative medical applications of EVs thus far. [15] MVs and exosomes released by apoptotic cells during and directly related to the processes of programmed cell death are also often classified as apoptotic bodies. Apoptotic bodies typically range in size from 50nm to 5μm. [24] They often contain fragments of cellular organelles and machinery in their cargo, as well as molecules related to apoptotic pathways. Traditionally regarded as useless by-products of apoptosis destined for macrophage digestion, recent studies have revealed potential roles of apoptotic bodies in local tissue signalling, immune regulation, and even cancer, justifying further mechanistic investigations of these particles. [24]
2.2.4. Mechanisms of Action and Uptake
Once in the extracellular space, EVs can function in their local environment or enter systemic circulation until they reach or interact with target cells. EVs can interact with cells to influence physiology through four primary pathways summarized in Figure 1c, with numerous sub-mechanisms dependent on cell and EV types. Firstly, EVs may bind to specific target cells via receptor-ligand interactions to activate internal cell signalling pathways. [22] Active uptake and internalization of EVs into cells via endocytosis has been suggested to be the most common mode of interaction based on observations of markedly decreased cell-EV interactions in the absence of sufficient energy and a functional cytoskeleton. [25] Receptor-mediated endocytosis is one key pathway for EV uptake, typically involving receptor-ligand binding of EVs on the cell surface followed by membrane deformation and pinching to ultimately transport EVs and their contents into internal endosomes. Clathrin, calveolins, and lipid rafts have all shown functions in mediating this pathway. [25] EVs can also be taken up via phagocytosis or macropinocytosis, where cell membrane protrusions may engulf EVs present in extracellular fluid. Finally, EVs can also attach to cells via membrane fusion, allowing them to open and deposit their cargo directly into the cytosol of a recipient cell. [25] For all mechanisms of uptake, once internalized the bioactive contents in EVs can then exert various functions in regulating cellular physiological processes. As shown in Figure1b, many of these functions are mediated by the biomolecular composition of the EV membrane and internal cargo, which include proteins, lipids, and nucleic acids.
2.3. Composition of EVs
2.3.1. Proteins
A wide range of proteins with many different functions have been identified in EVs. One of the primary current interests in proteins associated with EVs is their existence as markers on the surface of EV membranes. [26] It has been suggested that presence of certain surface markers, notably tetraspanins, can illuminate the cell-source of secreted EVs, the physiological state of the parent cell, and the sub-type or biogenesis pathway that led to EV secretion among other properties. This information may be especially useful in characterizing EVs to understand their mechanistic effects as well as harnessing their potential as biomarkers of healthy and diseased tissue states. [15] There is currently no recognized universal surface marker for EVs, however the enrichment of several key proteins has been successfully used to identify EVs and EV sub-populations and sources, as will be discussed later in characterization techniques. [26] Besides markers, EV proteins have shown function in sorting intracellular contents into EVs during biogenesis and as ligands for specific cell surface receptors. These ligands can induce responses including cellular uptake via phagocytosis and activating signal transduction pathways such as the mitogen-activated protein kinase (MAPK) and natural killer group 2D (NKG2D) pathways to induce transcriptional behavioural changes in cells. [15] Finally, EVs may also contain cytokine proteins within their luminal space. EVs may be useful for protecting cytokines from degradation or trafficking of cytokines out of the cell and to the desired target for molecules lacking a signal peptide. A notable example includes the release of transforming growth factor-β (TGF-β)-containing EVs by the thymus as an immunomodulatory regulator. Understanding of the complete spectrum of proteins associated with EVs, their mechanistic roles, and the specificity of proteins to EV sub-types, cell-types, and pathologies remains limited. [15] Trafficking and sorting of proteins into exosomes in particular has not been extensively defined, though studies have suggested that chaperone proteins such as HSC70 can recognize certain amino acid sequences on intracellular proteins that mark them for binding to the MVB membrane and inclusion within ILVs. [22]
2.3.2. Nucleic Acids
Nucleic acids are one of the components of EVs that have garnered the most interest in terms of regenerative medical applications. Since the discovery of RNA in EVs just over a decade ago, a wide spectrum of nucleic acids have been observed in EVs including various types of both RNA and DNA. [15] RNA species that have been directly associated with EVs include messenger RNA (mRNA) as well as numerous classes of non-coding RNA including microRNA (miRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), long non-coding RNA (lncRNA), small nuclear RNA (snRNA), small nucelolar RNA (snoRNA), PIWI-interacting RNA (piRNA), Y RNA, vault RNA (vtRNA), and circular RNA (circRNA).[27]
EV-associated miRNA has been the focus of a large proportion of current studies due to its enrichment in EVs and potential for modulating cell behaviour upon uptake. miRNA are strands of RNA typically 17–24 nucleotides in length that do not code for proteins like mRNA molecules. [28] They are transcribed from DNA in the nucleus as double stranded loop structures, followed by cleavage, separation, and single-strand binding to Argonaute proteins in the cytosol to form an “RNA-induced silencing complex” (RISC). [15] These complexes can then bind to mRNA molecules with a complementary nucleotide sequence to the miRNA to either initiate degradation of target mRNA or repress its translation into proteins in the cytosol. In this way, miRNAs can modulate the post-transcriptional expression of certain genes. [28] The amount and types of miRNA required to induce appreciable modulation of physiology in specific circumstances, however, is generally unclear. [15]
Transport of miRNAs in EVs is desirable due to protection from degradation via RNAase once released by a cell into the extracellular space. EV transport can also facilitate targeted delivery of miRNA cargo to specific sites. RNA sorting into EVs is known to be an active process, as the RNA profile of EVs often differs significantly from that of their parent cells. [15] miRNAs produced by a cell that are targeted for EV incorporation typically contain a short universal nucleotide sequence referred to as an “EXOmotif” that differs them from miRNA intended for intracellular functions, the most common of which is the GGAG sequence. A small protein called heterogeneous ribonucleoprotein A2B1 (hnRNPA2B1) can then recognize the EXOmotif sequence to bind and transport specific miRNA into vesicles. [15,29] Physiological functions of EV-transferred miRNA include immunomodulation, cell differentiation, and protective responses to damage and disease. [15]
Of the other classes of EV-associated non-coding RNA described earlier, lncRNA has drawn particular interest in cardiac applications, with one study finding a significant role of the EV-transferred lncRNA NEAT1 in the cardiac response to injury, as will be discussed in section 4.2.[27,30] snRNAs and snoRNAs are known to play important roles in the maturation of cellular RNA, while EV-tRNAs may also be involved in regulating gene expression, though non-functional fragments of such species can also likely be found in secreted EVs. The specific functions of these and other listed non-coding RNA species have yet to be examined at same depth as miRNAs in the context of cardiac disease and repair, and the biological implications of diverse EV-associated RNA species remains a subject of ongoing investigation. [27]
mRNA and DNA molecules have also been identified in EV cargo. There is evidence that some mRNA in EVs can be translated into proteins by recipient cells to influence physiology, however limited studies have investigated their roles compared to miRNA. Most crucially, mRNA and DNA content in EVs has been proven to change to reflect the physiological state of parent cells. Hypoxia causes the release EV-mRNA that may modulate the resistance of nearby cells to oxidative stress, while tumour cells release EVs carrying increased levels of oncogenic DNA. Thus, changes in nucleic acid composition may be significant in the application of EVs as a biomarker for detecting disease or assessing the physiological state of tissues. [15]
2.3.3. Lipids
Though lipids are a significant part of EV composition, considerably fewer lipidomic studies have been performed compared to protein and nucleic acid analyses, specifically when it comes to EVs from different cell types and tissues. [15] Active sorting of lipids into EVs mirrors that for proteins and nucleic acids, in that lipids such as sphingomyelin, glycosphingolipids, cholesterol, and phosphatidylserine are noticeably upregulated in EVs compared to parent cell expression. [31] The most recognized role of lipids in EVs is their presence in the bilayer membrane structure. Due to the aforementioned upregulation sphingomyelin and cholesterol, EV membranes are noted to be more robust than cell membranes, imparting excellent post-release stability and resistance to physical, chemical, and enzymatic destruction. EV lipids may also act as ligands to activate cell-signalling pathways, contributing factors in EV biogenesis, biomarkers of disease or as bioactive molecules themselves. [15,31]
3. Techniques in EV Research
As interest in EVs has grown, a multitude of techniques have been used to isolate, characterize, and apply EVs from various sources. Due to its infancy, the techniques in EV research have suffered from poor standardization and high variability which are also exacerbated by the inherent variability and ill-defined nature of EV secretion and composition. [19] The International Society for Extracellular Vesicles (ISEV) has released two reports defining recommendations outlining the minimal information for studies of extracellular vesicles (MISEV), referred to by their respective years of publication as MISEV2014 and MISEV2018. In these reports, the society has created guidelines for EV researchers that aim to set standards as to how different techniques should be used to investigate EVs and how to properly attribute observations to EV properties. [26,35] Another attempt to improve the flow of information in the EV field has involved the creation of databases of EV protein, lipid, and nucleic acid composition based on secretion source to which researchers can contribute, examples of which include ExoCarta and EVpedia. [15,19] The following section summarizes common techniques for EV processing used in literature, MISEV recommendations for each, and their significance within the scope of this proposal.
3.1. Sourcing and Preparation of Samples
As mentioned, large variability in the profiles of isolated EVs is common, even when using identical protocols. [19] Thus, one of the most important pre-processing steps recommended by MISEV is thorough reporting of sample conditions. In isolation of EVs from cell culture media, the primary source in the proposed research, the parameters that should be reported include: cell type, cell density/number at time of sample collection, cell viability, passage number, culture vessel and coating, special culture conditions implemented, culture time, method of media collection, and type of culture media. [26] Culture media is of particular concern, as many media supplements use serum or an alternative which inherently contains EVs that can be co-isolated with samples as contaminants. [32] Thus, it is recommended that either cells are cultured in serum-free media for the period before collection, EV-depleted serum is purchased as an alternative supplement, or culture media is depleted of EVs before it is applied to cells. [26] It is important to note that EV-depletion or removal of serum from media may affect cell growth and thus EV release. A recent study compared several techniques for EV removal from media, including ultracentrifugation and ultrafiltration. It was concluded that media ultrafiltration through a 100kDa membrane produced optimal EV removal from media while inducing significantly less stress in cultured cells compared to other techniques. [36] Regardless of media pre-treatment conditions, all experiments should include a conditioned media control for baseline assessment. For EVs isolated from 3D tissues, bulk transport of EVs is of note, and any methods of tissue disruption used should be reported. Storage of EVs should also be reported, including time, conditions, and number of freeze-thaw cycles. For long-term storage, EVs should be frozen quickly and kept at −80°C. [26]
3.2. EV Isolation
EVs are secreted by cultured cells into media, which also contains cellular debris, protein complexes, extracellular RNA, and other soluble ions and molecules. Thus, techniques for isolating cell-secreted EVs aim to reduce background contribution from contaminants as much as possible while also concentrating EVs in smaller volumes so that effective characterizations can be performed and any observations made from samples can accurately be attributed to secreted EVs rather than other contaminating species. [26] Selection of isolation techniques must be carefully considered, as different techniques have been shown to produce drastically different compositional profiles of isolated EVs. [32]
Many different methods for isolating EVs from culture media have been used in literature, each producing varying degrees of concentration, recovery, and purity as summarized in Figure 2. Preferred techniques may vary depending on sample and application. One of the simplest EV isolation techniques is differential centrifugation (DC). Conditioned media samples are centrifuged for set time periods at sequentially increasing speeds, starting slow to remove large cell debris and finishing with ultracentrifugation (UC) at speeds up to 200 000g. [32] At each step, the supernatant is removed and spun in a new tube, leaving pellets of EVs fractionated by size and density for each step performed. Unfortunately, high centrifugation speeds may damage EVs, DC is low throughput and takes several hours to complete, and cannot separate non-EV particles from EVs of a similar size. Modifications to DC include density gradient centrifugation (DGC), employing additives such as sucrose during UC to facilitate spatial fractionation of particles by density after which they can be selectively removed from their vertical position in the centrifuge tube. Protein removal is very efficient, however the cholesterol carrying low- and high-density lipoproteins (LDL and HDL) can interact with or have similar densities to EVs and are major contaminants from DGC. Additionally, DGC protocols can last upwards of 24h for best effect and produce low EV yields. [32]
Figure 2.
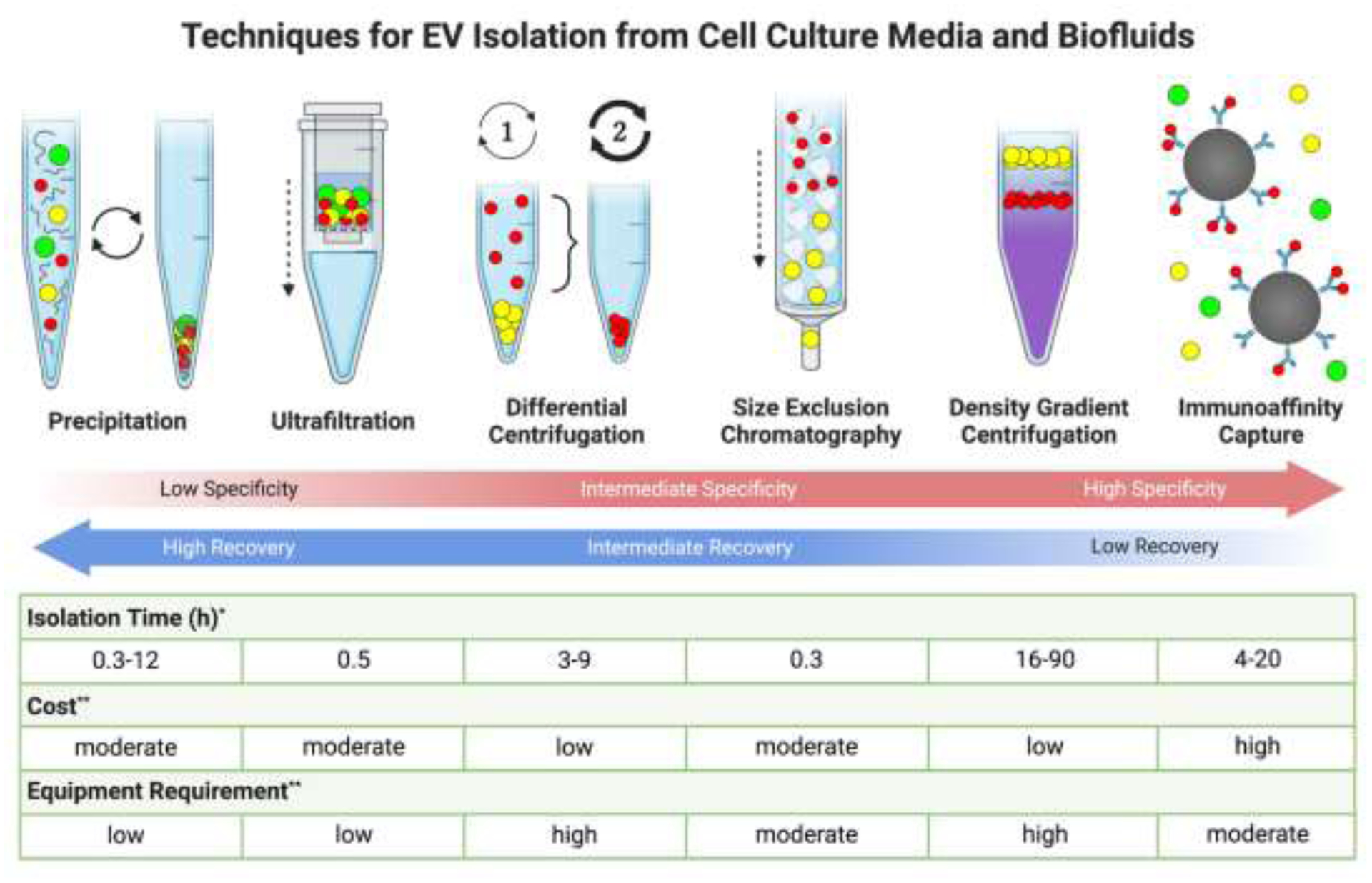
Numerous techniques exist for isolating EVs from cell culture media and biofluids. Different techniques can yield significant differences in the profiles of isolated EVs, with a trade-off existing between recovery of EVs and the specificity of the technique for isolating pure populations EVs from other non-vesicular contaminants. *Ref: [32]; **Ref: [33,34].
Size-exclusion chromatography (SEC) separations run samples through a column containing a matrix of porous beads, such as sepharose. Particles differentially interact with beads based on their size, with larger particles eluting earlier than small particles, facilitating the collection of EVs in size fractions. In application, SEC has produced high purity EV fractions with very low protein and HDL content, superior yields up to 90%, and no damage to EVs. Drawbacks include co-isolation of LDL particles similar in size to EVs, low volume throughput, and no concentration EVs. Thus, samples require combination with other techniques such as GC before and after SEC to concentrate EVs. Elution is highly variable based on column design and sample, and must be extensively characterized. [32]
Ultrafiltration (UF) of EVs is one of the quickest isolation techniques. Similar to EV depletion of culture media, conditioned media can be run through a filter with a size cut off, such as 100kDa, that retains most EVs while allowing smaller species to pass through. UF can concentrate EVs more than any other technique, but also retains some of the highest levels of contaminants, as any particles larger than the size cut off of the filter will remain in isolates. Selection of filter pore size is critical to determining yield and purity, and pre-centrifugation to pellet particles larger than EVs is a necessity. [32]
Immunoaffinity capture isolations employ antibodies that target and bind specific ligands on the surface of a desired population of EVs. These antibodies can be immobilized on a surface, in a column, or on magnetic beads prior to exposure with conditioned media. Washing and decoupling of bound EVs produces a highly specific isolate of only EVs that bind to the antibody’s epitopes, facilitating cell-specific or even subgroup-specific EV isolation. Yields are very low using this method, however, and limited knowledge of the EV ligand presentation in different cell types and conditions means that antibody selection to produce a desired isolation specificity is difficult. Cross-reactive and non-specific binding of proteins by antibodies can also produce contamination. [19,26,32]
Precipitation of EVs represents another common isolation method in literature. Commercially available EV precipitation kits are often used, and contain a high molecular weight polymer in buffer, usually polyethylene glycol (PEG). PEG is hydrophilic, and reduces the solubility of small particles in solution via steric volume exclusion. After addition of precipitation buffer, samples are usually agitated or refrigerated for minutes to hours, after which EVs can be pelleted by short centrifugations at low speeds. EV pellets can then be resuspended in a desired volume of buffer for downstream assays and applications. Due to the mechanism of precipitation employed, all manner of suspended particles will be pelleted during EV isolation, including extracellular protein and RNA. Thus, very high recovery and concentration is produced at the expense of high contamination. [32] Due to its speed, simplicity, and high recovery, precipitation can be favourable for preliminary studies and when low yields are expected. It is important, however, to combine precipitation with other isolation techniques to reduce contamination or to perform rigourous characterizations of EVs to verify that any observations made from assays or functional studies can be attributed to EVs and not to co-isolated molecules. [26,37]
Other techniques have also been utilized in literature for EV isolation. Building upon a chromatography-style approach, column-based membrane affinity systems pass supernatant through a filter that binds EVs via hydrophobic interactions between their phospholipid bilayer and column substrate. Bound EVs can then be eluted using an inverse salt gradient.[38] The application of microfluidic chip-based techniques represent another growing field of interest in the future of EV isolation. Microfluidics possess advantages such as reduced scale, complexity, and cost while facilitating high simultaneous throughput, in situ on-chip isolation, and the possibility of combining isolation and downstream characterizations on a single device. [39] Examples of microfluidic isolation techniques tested to date include static techniques, such as nano-porous membranes and adsorption-based systems, or dynamic techniques, such as electric field gradient focusing and separation via differential flow velocity. [39] Though less established and standardized compared to classical techniques, the flexibility and customizable nature of novel microfluidic systems have the potential to overcome some of the traditional challenges in EV separation moving forward.
For all methods described above, it is generally recognized that combining two or more methods is useful in producing higher quality isolates for improved downstream analyses, and that extensive reporting of parameters is critical for standardization and reduced variability. Decisions in optimizing specificity versus recovery are generally dependent on biological source, experimental goals, assays to be performed, and desired application of isolated EVs. [26]
3.3. Quantification and Single Vesicle Analysis
Quantification and single vesicle analysis of EV samples are used to assess properties such as size, morphology, and concentration as summarized in Figure 3a. MISEV2018 recommends that researchers use at least two different techniques to characterize single vesicles: usually one form of microscopic imaging and one form of statistical or populational analysis. [26] Since many EVs are smaller than the diffraction limit of visible light, electron microscopy (EM) is the most commonly used imaging technology for EVs, namely scanning EM (SEM) and transmission EM (TEM). TEM has been the most widely applied in literature, offering fine resolution down to 1nm and several different imaging modalities for visualizing EVs. [32] In most TEM experiments, suspended EVs are usually placed on an EM grid, dried, and treated with negative stain such as uranyl acetate to enhance contrast around bilayer membranes. Samples may or may not be fixed with glutaraldehyde or alternatives to preserve morphology. Drying causes spherical EVs to become “cup-shaped” when viewed in TEM. [40] Thus, cryoTEM may be preferred in which hydrated, unstained EVs are rapidly frozen and can be imaged without inducing morphological changes. Both widefield and close-up images should be taken, for example at 300 and 30,000x magnification. Size distribution can be estimated from EM images, but concentration cannot be determined. [26,32] Atomic force microscopy and super-resolution microscopy have also been used to image EV samples. [26]
Figure 3.
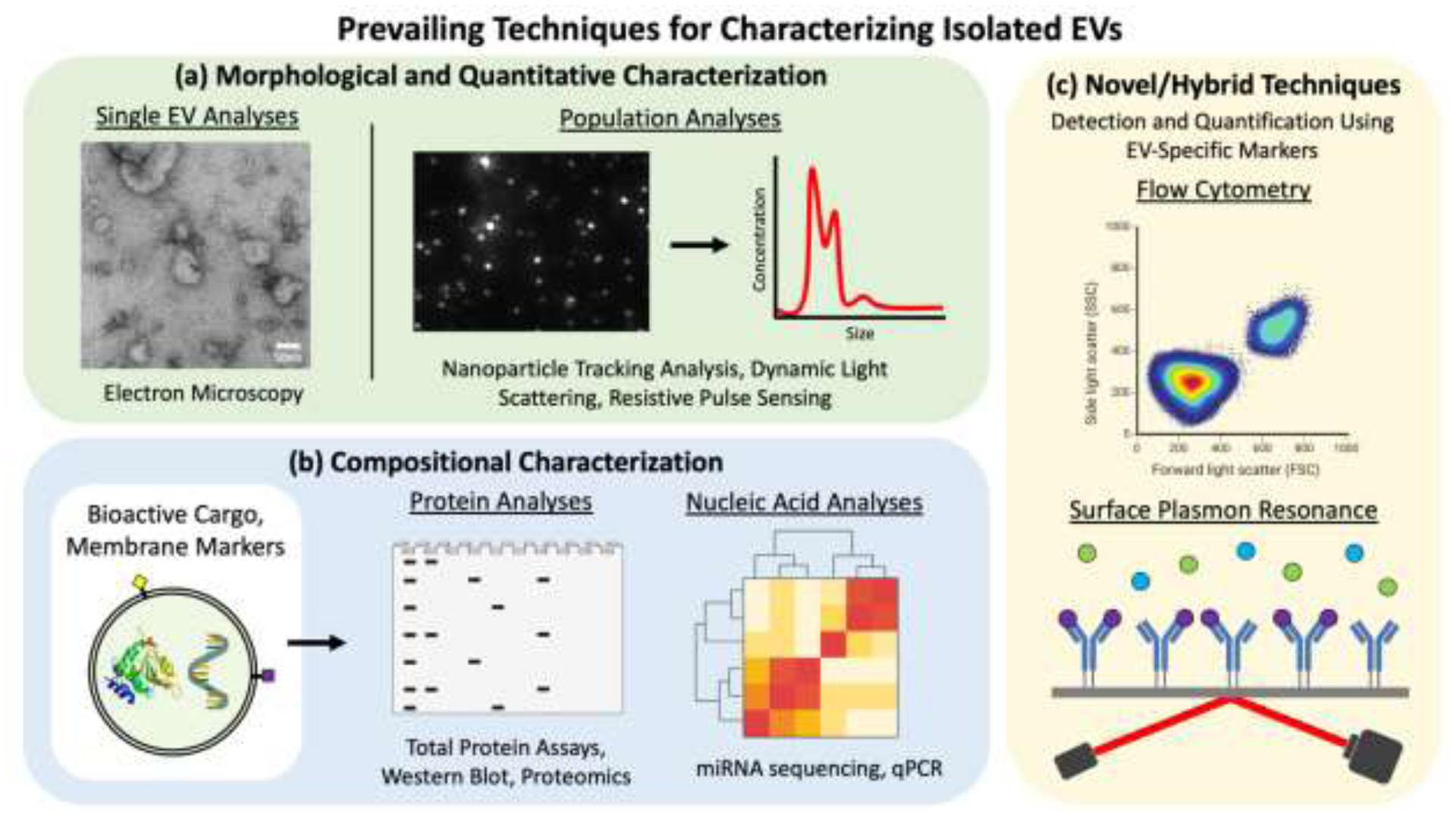
Summary of common techniques for characterization of isolated EVs. Standard characterizations used to assess EV samples include: (a) single and population-based EV analyses to assess morphology and size distribution; (b) biomolecular compositional characterizations; and (c) novel/hybrid techniques that utilize EV-specific markers for population analyses.
Statistical and population techniques can analyze large numbers of EVs to more accurately quantify their properties, and are useful in conjunction with the more qualitative techniques of EM imaging. [26] Nanoparticle tracking analysis (NTA) has been particularly useful for EV research. A laser is directed onto samples that flow through a channel, and the scattering of laser light by particles in suspension is recorded by a video camera. The Brownian motion of particles is analyzed to calculate particle size distribution, and concentration of particles in solution can also be estimated since the volume of sample in the flow cell is known. For increased statistical relevance, higher sample throughput and longer video capture times can be used. Statistical accuracy of NTA can be hindered for samples that are dilute or have high polydispersity. [32] Another limitation is the inability to distinguish between EVs and other non-EV particles in suspension.
Dynamic light scattering (DLS) is a similar technique that has been used for EV quantification, but is usually performed on static samples, reducing EV sample size. Resistive pulse sensing (RPS) offers potential improvement in quantification accuracy over NTA, especially with regard to polydisperse samples. In RPS, particles passing through pores in a membrane induce electrical signatures that are used to calculate size. Unfortunately, RPS still cannot distinguish non-EV particles, pores can easily be clogged by proteins and aggregates, and pore size selection can induce a measurement bias. [26,32]
The European Society of Cardiology recently released a position paper stating that standardizing and improving flow cytometry (FC) characterization of EVs represents a key step towards clinical application of EVs. [19] Ongoing studies are attempting to overcome a number of challenges to adapt FC techniques for EV analyses. [41,42] Just as with conventional FC, EVs are often labelled with fluorophores and pass through a laser one at a time, scattering light and emitting fluorescence. [32] Because of limited application of FC in EV detection, extensive validation and standardization of techniques is required to ensure reproducibility and accuracy. [26] Due to the small size of EVs, novel methods must be developed for measuring size, such as correlation of fluorescent signal to vesicle surface area. [42] However, improved FC techniques for assessing EVs offer the possibility of higher resolution than current standards while facilitating specific measurement of particular cell-type and subgroups of EVs via antibody targeted fluorescent labelling. Thus, FC is considered to be a promising technique in the future of standardized EV quantification. [19,32]
3.4. EV Molecular Composition
A plethora of techniques are have been used in literature to assess the biomolecular content of EVs, with several of the most common ones depicted in Figure 3b. Characterization of EV molecular composition is of particular importance to understanding and assessing their bioactivity in vitro and in vivo. Global characterization of the total amount of proteins and lipids in EV isolates can provide high level insights. Total protein concentration can be measured via detergent lysis of EV samples followed by colorimetric assays such as the bicinchoninic acid (BCA) assay. Total lipid content can also be measured via colorimetric assays, including the sulpho-phospho-vanillin assay. Comparing ratios of total protein and total lipid amounts to the number of particles measured during single EV analyses can be used as rough gauges for the isolate purity and degree of EV enrichment. [26]
In terms of defining EV protein composition, there are two distinct aims. One is to confirm that isolated particles are vesicular in nature via detection of commonly enriched EV proteins in samples. This is usually performed by EV lysis followed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), western blotting, and antibody detection of proteins involved in EV biogenesis processes. [26,43] Enzyme-linked immunosorbent assays (ELISA) can also be used to this effect, as well as surface plasmon resonance (SPR) which has yet to be widely utilized for EVs but promises the potential for vastly improved sensitivity in membrane marker detection (Figure 3c). [32,44] MISEV2018 acknowledges that given isolation and compositional variability, it is not possible to define specific protein markers for certain EV populations; however, there are several recommended categories of commonly enriched EV proteins that together can strongly suggest the presence of EVs. It is recommended that at least one protein from each of the following categories be probed in western blots or equivalent: EV membrane proteins (ie. tetraspanins – CD9, CD63, CD81), cytosolic EV proteins (ie. ESCRT proteins such as TSG101, HSC70, ALIX), and non-EV or contaminating proteins to indicate purity (ie. lipoproteins from HDL, LDL such as APOA1). [26,45,46] Additionally, probing for non-endosomal intracellular proteins, such as GM130 of the golgi apparatus, may be useful in evaluating the presence of non-exosomal vesicles in exosome specific studies. For western blots, EV samples should be loaded in gels beside samples of corresponding cell lysate to assay enrichment of EV proteins in isolates. [26] Flow cytometry for enhanced quantitation of EV markers will likely start to replace western blotting as techniques improve (Figure 3c). [19] Aside from marker detection, EV protein characterization is essential for understanding the role that EV proteins play in modulating the behaviour of recipient cells. Proteomics via mass spectrometry is the most common method used to assess global protein profile of isolated EVs. Protein content can be compared between test samples to reveal changes in expression and can be used to understand biogenesis and functional pathways. Proper controls are essential in protein analyses due to inevitable contamination of isolates with co-isolated non-EV proteins found in culture media and secreted by cells. [32]
Like proteomics, nucleic acid analyses are commonly used to quantify expressional changes in EV populations or to understand EV sources and predict functions. RNA can be purified from EVs via lysis, solvent extraction, and precipitation. Next-generation sequencing (NGS) instruments are used to globally define RNA content. Select sequences of interest should then be validated by a second technique such as quantitative polymerase chain reaction (qPCR) or digital PCR. Though uncommon in literature, DNA analysis of EVs can also be performed by NGS. Since EV samples likely contain co-isolated extracellular RNA or RNA bound to lipoproteins, it is recommended that they be treated with RNAase and DNAase prior to lysis to remove contributions from non-EV nucleic acids. [32] Lipidomics has not been performed to the same extent as proteomics and nucleomics, however liquid and gas chromatography followed by mass spectrometry have been used in some studies to assess global lipid content of EV isolates. [31]
3.5. Considerations and Challenges for Functional EV Studies
Isolated vesicles can be applied to cell cultures or other biological systems to assess their functionality; there are a number of considerations that must be made in regard to controls and experimental design in order to ensure that interpretations do not reach beyond objective observations. Due to the described infancy of EV isolation techniques, it is generally difficult to ascribe observed effects to any one subtype of EVs, such as exosomes. If EV isolates can successfully be fractionated by size, then each size fraction should be functionally tested for activity independently along with proper controls of identically processed conditioned and fresh media to assess the effects of other soluble molecules and background contributions on cells. Until isolation and characterization techniques advance further, only general hypotheses surrounding the activity of EV subtypes are considered viable. When EVs are applied to a culture system, dose-response curves should be generated to measure and optimize activity at different concentrations. Normalizing effect to number of EVs applied, amount of a specific bioactive molecule, or number of recipient cells enhances comparability. Degradation treatments with detergents, nucleases, and proteases have been used in some studies to rule out contributions of non-EV co-isolates and improve rigour. [26] Fluorescent labelling of EVs to visualize uptake have been performed to assess EV bioactivity, but are viewed by researchers with caution as non-EV associated dye aggregates can also enter cells. [26,47] To attribute observations to specific EV proteins or RNAs, researchers have often employed comparison to knock-down EV samples from the same source, though thorough characterization of modified EVs is important to ensure knock-down does not produce significant unintended alterations to EV isolates. Regardless of application, MISEV2018 recommends that claims surrounding EV activity should avoid over-interpretation, controls must be properly applied, characterization should be as thorough as possible, and reporting results to online databases is advisable. [26]
4. Extracellular Vesicles in the Healthy and Diseased Heart
Since their discovery, EVs have been shown to play a role in the physiology of nearly all cells and tissue systems in humans and can also indicate or be involved in a wide range of diseases and pathologies, as summarized in Figure 4. Examples include immunomodulatory functions of monocyte derived EVs, the induction of coagulation by platelet secreted vesicles, and mediation of metabolic activity in the liver via EVs released by hepatocytes. [15] It follows that EV release and uptake by various cell types in the heart plays a key role in regulating cardiac function. Section 4.1 summarizes current knowledge on the roles of cardiac EVs in regulating physiology, while section 4.2 examines how EVs are involved in the initiation and progression of myocardial disease. Current research on EVs in the heart aims to improve mechanistic understanding of the roles that EVs play in mediating both healthy physiology and disease in order to establish targets for novel EV-based diagnostics and therapies, which will be examined in section 5.
Figure 4.
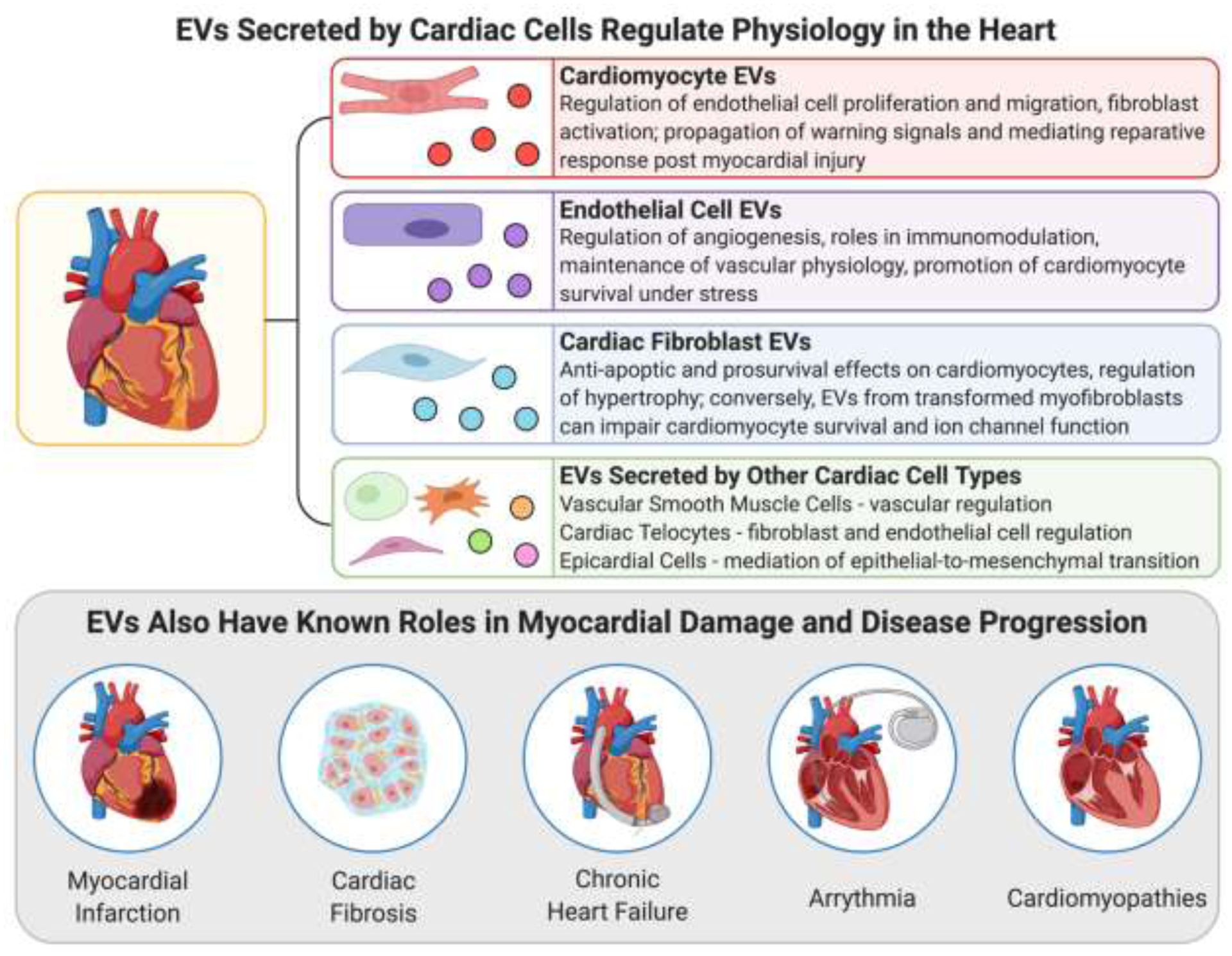
EVs secreted by cardiac cells are known to play important roles in regulating cardiac physiology as well as in the initiation and progression of myocardial diseases.
4.1. EVs Secreted by Cardiac Cells Regulate Physiology in the Heart
The cardiac environment is difficult to access and observe in vivo, meaning that knowledge surrounding the role and mechanisms of EV signalling in regulating normal heart function has mainly been generated indirectly through animal models, analyzing human biofluids, or from in vitro cell cultures. Since it is not possible to study EV release and uptake directly in native human heart tissue, biofluids such as pericardial fluid and blood have represented the best way to collect and study human cardiac EVs, since secreted vesicles from cardiac cell types will often enter these fluid compartments. [19] In vitro cell cultures offer the added benefit of direct visualization of EV release and uptake from specific cardiac cells in a highly controllable environment. Even when using co-cultures, most studies to date have been performed in 2D monolayers on plastic substrates which poorly replicate structure and phenotype of native tissues. [48,49] Thus, the composition and functions of EVs released by and interacting with cells in the heart have not been extensively classified in a physiologically relevant environment in which in vitro observations can be closely correlated to in vivo processes. Due to these challenges in characterizing the role of EVs in baseline cardiac physiology, many studies use chemical or physical stimuli or stressors followed by measurement of significant changes in EV profile. Populations of isolated EVs can also be applied to the media of different types of cardiac cell cultures that are then monitored for functional and phenotypic changes. These types of information can then be used to narrow down which EV components are likely critical to maintaining normal physiology, and how upregulation or downregulation of certain factors enriched in cardiac EVs may be involved in tissue dysregulation or damage. [49] The following section provides a brief summary of current knowledge surrounding the composition and postulated functions in the heart of EVs secreted by cardiac cells, focusing on the most common cardiac cell types. In the interest of space, discussion of EVs from very rare cell populations (e.g. c-kit+ cells) is omitted. The roles of EVs secreted by non-cardiac cells in regulating normal heart function are even more difficult to define. Many cell types throughout the body release EVs into circulation that can potentially act on the heart and selective uptake of specific EV populations cannot be easily investigated in vivo. Though it may be more difficult to determine their distinct roles in the native heart, non-cardiac cell EVs have been tested in a significant number of studies as novel therapeutics for cardiac repair post-injury as will be discussed later in section 5.3.4.
4.1.1. EVs Secreted by Cardiomyocytes
As the major cell-type responsible for the contractile function of the heart, characterization of cardiomyocyte (CM) EVs has been the subject of many cell culture studies utilizing primary animal cardiomyocytes or those differentiated from iPSC or embryonic stem cells (ESC). CM EVs have been shown to be enriched in heat shock proteins including HSP20, 60, and 70, which regulate heart function as well as survival and response to stress. Several proteins such as interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) that are involved in cardiac remodeling and fibroblast activity and may be implicated in cardiac fibrosis post-infarction have been characterized in CM EVs. Glucose transporters such as GLUT4 and GLUT1 along with the enzyme lactate dehydrogenase in CM-secreted EVs likely modulate metabolism in endothelial cells. [50] A number of recent studies have characterized and postulated functions for a wide-variety of miRNA species present in CM EVs. Some of these include miR-217 and miR-155 involved the development of hypertrophy during heart failure; miR-29b and miR-208a, components that can both inhibit and promote fibroblast activity, respectively; and miR-939 and miR-320, which can both promote and inhibit angiogenesis and endothelial sprouting, respectively. [49–51] Exposure of cultured CM to hypoxia for 2h caused cells to release EVs at twice the normal rate, however reports on pro-reparative versus pro-inflammatory dominant functions of hypoxic CM EVs have differed depending on time and conditions of hypoxic stress. [11]
Similar conclusions have been drawn from studies applying exogenously derived CM EVs to in vitro and in vivo models of cardiac injury. CM EVs have been found to act on endogenous CMs to reduce apoptosis, prevent hypertrophy, and reduce abnormalities in potassium channels through the action of biomolecular cargo such as HSP20, miR-1, miR-133a, and miR-499. [12,52–58] They have also shown therapeutic efficacy in acting on cardiac fibroblasts to reduce cardiac fibrosis via HSP20 and miR-133a. [52,53,58] Acting on endothelial cells (EC) in the heart, CM EVs may induce angiogenesis by promoting the proliferation and migration of EC into tubule formations via HSP20, miR-143, and miR-222. [52,59,60]
Overall, these observations reveal that a multitude of proteins and nucleic acids can be enriched in CM EVs, often having antagonistic effects that balance and regulate the activity of various cell types in the cardiac environment. CM EV cargo has been shown to vary greatly between studies depending on environmental cues and physiological state of the secreting cells, contributing to their widely varied mechanisms, functions, and profiles in different systems. [19]
4.1.2. EVs Secreted by Cardiac Fibroblasts
Cardiac fibroblasts (CF) are also critical members of the cardiac environment, forming about one third of the heart’s volume. [49] miR-21-3p localized in CF EVs was found to be upregulated via stimulation from angiotensin II, an event which occurs in vivo during the maladaptive renin-angiotensin response to heart damage. [49,61] In a study from Bang et al., miR-21-3p-containing EVs interacted with CM in vitro to initiate mechanisms of cardiac hypertrophy. [62] Both CF and CM in mice exhibiting heart failure secreted EVs containing enriched levels of miR-27a, miR-28-3p, and miR-34a which inhibited antioxidant and cardioprotective signalling pathways. [63] Conversely, other studies have indicated that CF EVs can also play a role in cardiac repair or cardioprotection from ischemia-reperfusion injury (IRI), though they remain more limited in number and scope. Wang et al. found that CF EVs were able to improve the survival of CM under stress via delivery of miR-21 and miR-210; however, it was noted that these bioactive miRNA were not as significantly enriched in CF EVs compared to those isolated from iPSC. [64] Another study found that CF EV transfer of miR-423-3p to CM during and post-infarction improved CM survival and led to reduced infarct size. [49,65]
4.1.3. EVs Secreted by Endothelial Cells
As the cells mediating contact between blood flow and cardiovascular tissues, endothelial cells (EC) have also been found to secrete EVs with cardiac functionality. A study by Balkom et al. showed the importance of EC EVs in angiogenic sprouting; it was discovered that EC EVs contained miR-214 that repressed cell-cycle arrest in nearby recipient ECs to facilitate cellular migration and angiogenesis. [66] EC EVs have been implicated in immunomodulation, such as through miR-10a inhibition of pro-inflammatory genes in monocytes or, in the case of cardiac damage and disease, through stimulated release of EVs containing HSP70 to activate and induce adhesion of monocytes to ECs. [67,68] EC EVs have also exhibited possible functions in protecting against atherosclerotic blockage of coronary arteries via the miR-143/145 cluster as well as the progression of peripartum cardiomyopathy in those who are pregnant via miR-146 upregulation. [49,69] ECs exposed to hypoxia showed upregulation of miR-126 and miR-210 in EVs which promoted increased angiogenesis in ECs and improved survival of recipient cardiac progenitor cells. [11,70] A recent study from Yadid et al. noted the critical importance of EC EV protein cargo in their ability to improve CM survival and contractility during and after ischemic stress. [71] Protein intermediates in the adenosine monophosphate-activated protein kinase (AMPK) signalling pathway were enriched in EC EVs and likely contributed to increased spare respiratory capacity in ischemic CM, helping them adapt to metabolic stress and recover contractile function afterwards. [71]
4.1.4. EVs Secreted by Other Cardiac Cell Types
Telocytes are supporting cells found in many tissues. Their role and origins in the heart are still widely debated, but cultured cardiac telocyte-like cells contained several angiogenic miRNA and precursors that can modulate ECs and may be transferred in EVs, namely let-7e, miR-10a, miR-21, mi-R27b, miR-100, miR-126-3p, miR-130a, miR-143, miR-155, and miR-503. [49,72,73] Other observations indicate that telocyte EVs may regulate CFs and modulate cardiac fibrosis, though mechanisms that have not yet been defined. [73] Vascular smooth muscle cells (VSMCs) also release EVs that affect heart function, but have primarily been the focus of studies surrounding mechanisms of atherosclerosis and vascular pathology outside of the myocardium. [49] Epicardial cells play a pivotal role in cardiac biology and have represented a point of interest in novel strategies for inducing cardiac tissue repair. [74] Few studies have thoroughly characterized the profile and role of epicardial cell EVs in the heart, but there are indications that epicardial-derived EVs contain clusterin which may have anti-apoptotic properties and could mediate epithelial-to-mesenchymal transition and cellular migration into heart tissue during cardiac remodeling post-MI. [11] Another study by Villa del Campo et al. found that epicardial EVs enhanced cell cycle re-entry as a mechanism of functional recovery in cryoinjured in vitro cardiac tissues, mediated by the action of miR-30a, miR-100, miR-27a, and miR-30e.[75]
4.2. EVs Play Important Roles in Myocardial Diseases and Pathologies
Besides regulating normal heart function, EV signalling between cardiac cells is also known to play a role in numerous myocardial disease processes. Some biomolecular cargo isolated from EVs present in pathological heart tissue has been implicated in protective tissue responses to minimize damage or promote healing postinjury, while other EV components have been found to stimulate maladaptive pathways that can actually further the progression of disease and tissue dysregulation. [76] The following section will examine current knowledge surrounding the compositional profiles and roles of EVs in human myocardial disease. A better understanding of the role and mechanisms of EVs in the mediation of cardiac disease is a critical step towards finding new therapeutic targets for cardiac repair.
4.2.1. EVs in Myocardial Infarction
During acute MI, ischemic conditions due to thrombotic blockage of coronary arteries leads to local necrosis of oxygen deprived tissue. The necrotic area continues to expand until perfusion of blood is restored. Post-infarction, CMs are known to release increased quantities of EVs, possibly as a type of “warning signal” to surrounding cells that damage has occurred. These EVs are enriched in miR-1, miR-133a, miR-208, and miR-499, which are associated with genetic regulation of cardiac features such as sarcomeres and ion channels, and have been suggested as potential novel biomarkers for MI. These miRNAs may also confer cardioprotection on surrounding cells to limit damage via anti-apoptotic, anti-fibrotic, and anti-oxidant properties. [11] As another example of a cardioprotective role of EVs, Kenneweg et al. found that post-hypoxia, EVs secreted by CMs in vitro were enriched in the lncRNA NEAT1. This effect was also observed in vivo post-MI and NEAT1 was found to play a role in improving heart function and recovery after ischemic injury, possibly through cardioprotective activation of fibroblasts. [30]
Besides cardioprotection, EVs also play known roles in the progression of injury post-MI. A study by Yang et al. found that EVs isolated from the serum of MI patients as well as CM grown in hypoxia were enriched in miR-30a which directly impaired the natural autophagic injury response in recipient CM and instead increased CM apoptosis, suggesting a role for cardiac EVs in enhancing disease progression post-MI. [77] Other studies have found that EVs secreted by other cell types in the heart can also play a role in injury progression post-MI. Notably, CF EVs have been found to interact with CM in the infarcted heart to initiate maladaptive hypertrophy via the transfer of miR-21-3p. [52,61,62] EVs derived from macrophages are also thought to act on CF soon after ischemia, inhibiting their proliferation and promoting inflammation via miR-155. This may potentially impede the native short-term protective response of fibroblasts that is meant the reduce risk of rupture in the weakened infarct region. [78]
Beyond local signalling, systemically circulating EVs secreted by cells in the infarcted heart have been implicated in the targeted regulation of a number of different organs and systems throughout the body as part of disease response. [52] Post-MI, CM EVs enriched in miR-1, miR-208, and miR-499 can preferentially target bone marrow progenitor cells, decreasing CXCR4 expression and promoting their mobilization into circulation to initiate tissue repair. [79] Gao et al. found that EVs produced by the infarcted heart can activate pro-angiogenic signalling in adipose-derived mesenchymal stem cells (MSC) via delivery of miR-1956. [80] Other studies have indicated that cardiac EVs are enriched in the spleen post-MI, can mobilize splenic monocytes through the action of miR-126, and can induce pro-inflammatory activation of circulating monocytes. [81,82]
4.2.2. EVs in Cardiac Fibrosis and Chronic Heart Failure
After acute cardiac damage and CM death, immune cells invade necrotic tissue and eventually fibroblasts are activated and remodel the infarcted zone with collagenous scar. [11] This process is initially adaptive to protect the mechanical integrity of the heart, but long term activation of fibroblasts leads to myocardial fibrosis, ventricular wall thinning, reduced heart function, and heart failure. [83] Over time, EVs released by CM in the damaged heart start to shift from a cardioprotective to pro-fibrotic phenotype, acting on CF to enhance their viability, promote fibroblast to myofibroblast transformation, and increasing collagen expression through the action of cargo including miR-217, miR-208a, HSP90, and IL-6. [11,84–86] Numerous studies have also shown that many cell types tend to upregulate expression of TGF-β and TGF-β transcripts in EV cargo in response to hypoxia and inflammation, a factor that is well-known for its role in promoting fibrosis. [11,52,87–89] In turn, overactive CFs may release EVs enriched in miR-21 that promotes maladaptive hypertrophy of CM. [49]
Long term progression of disease and tissue dysfunction towards chronic heart failure is another major concern and source of morbidity for MI patients, with EVs known to play several key roles. EVs secreted by CM and CF are dysregulated in the chronically injured heart and, through the action of miR-27a, miR-28-3p, and miR-34a, have been found to cause long-term translational inhibition of proteins with important antioxidant functions in CM. [63] Dysregulated CM EVs have also been shown to promote chronic cardiac hypertrophy and remodeling in a number of studies, with key molecular mechanisms including miR-27b, miR-155, miR-217, tumor necrosis factor-α (TNF-α), and mir-208a. [52,84,90–93] Other plasma EVs are also believed to modulate immune cells to support chronic inflammation in the heart after injury, though specific sources and mechanisms have not yet been identified. [94] Together, these mechanisms suggest that significant changes in EV signalling and cargo occur after cardiac injury that contribute to extending the progression of tissue damage and dysfunction well beyond the end of acute injury. Restoring physiological EV signalling to the injured heart may thus prove to be a useful target for future therapies that aim to prevent heart failure, induce cardiac repair, and improve quality of life for heart patients.
4.2.3. EVs in Other Cardiovascular Pathologies
Distinct changes in cardiac EV signalling are not unique to MI, fibrosis, and heart failure, and have been observed in numerous other cardiovascular pathologies. Cardiac arrythmias have been correlated with CM-, CF-, and platelet-EV dysregulation by several studies which have suggested mechanisms for EV-associated miRNA in the promotion of calcium channelopathies. [95–97] A number of mechanisms have also been suggested for the role of cardiac EVs in cardiomyopathies, including peripartum cardiomyopathy, diabetic cardiomyopathy, and septic cardiomyopathy, with the deleterious effects of pathologic EVs on cardiac endothelium likely playing a part in tissue dysfunction for all three diseases. [98–104] CM EV dysregulation is also thought to contribute to adverse myocardial remodeling in patients with dilated cardiomyopathy, though definitive molecular mechanisms have yet to be confirmed. [105–107] Contributions from pathologic EVs have been implicated in a multitude of other cardiovascular diseases beyond the myocardium as well, including in the initiation of coronary artery disease, suggesting that further investigations of EVs in the heart will help continue to improve our mechanistic understanding of cardiovascular diseases and to discover new treatments. [10,68]
5. Applying tissue engineering and tissue-on-a-chip models to improve mechanistic understanding of EVs in cardiac disease and repair
5.1. Current Challenges and Future Opportunities in EV Research and Clinical Translation
Due to their recognized roles in regulating cardiac physiology and pathology, EVs have been touted as a promising vehicle for improving understanding of myocardial disease processes and for designing novel, targeted therapies for restoring cardiac function in patients. However, a number of distinct challenges have yet to be addressed before experimental EV diagnostics and therapies can be effectively translated and implemented clinically. Publication of the MISEV2018 guidelines has served as a useful start towards standardizing practices in EV isolation, characterization, and functional testing. [26] Unfortunately, the relatively recent emergence of interest in cardiac EVs has meant that techniques for producing therapeutic populations of EVs still suffer from significant variability, while scaling production to clinically relevant quality and quantities remain challenging. Major gaps in knowledge surrounding cardiac EVs persist, such as a lack of universally accepted surface markers for EV subgroups and fragmented understanding of the mechanisms by which EVs modulate cardiac physiology, disease, and repair. Beyond the preliminary recommendations from ISEV, highly efficient and reliable practices for therapeutic EV collection and testing must be defined and adopted across the board before the clinical potential of cardiac EVs can be fully realized.[19,26,52]
Due to these limitations, researchers and international bodies, including the European Society for Cardiology (ESC), have described a need for the development and application of ‘advanced cell models…with multiple cell types in a 2D or 3D structure’ towards the investigation of cardiac EV signalling and therapeutics. [19,52,108] In particular, engineered cardiac tissue-on-a-chip platforms combining various types of cardiac cells and biomaterials in biomimetic constructs have shown significant promise for creating physiologically relevant in vitro models of the heart. [13,14] As will be summarized in the following sections, the advantages of novel tissue-on-a-chip platforms over in vivo studies in humans and animal models have opened the door to a wide range of new insights and applications for EVs in cardiac tissue engineering and regenerative medicine including in-depth studies of EV signalling in cardiac physiology, screening for new EV biomarkers of cardiac disease, discovering new targets and therapeutics for cardiac repair using EVs, and testing pharmacokinetics and delivery strategies for EV therapeutics. Such applications promise to hasten the clinical translation of EV-based regenerative cardiac therapies in the coming years.
5.2. Extracellular vesicles in cardiac tissue engineering
5.2.1. Advantages of Cardiac Tissue-on-A-Chip Models in EV Research
Cardiac tissue engineering, defined by the application of engineering principles towards understanding physiological processes in the heart and developing substitutes or strategies to restore heart function, has steadily grown as a leading field in revolutionizing knowledge and care for heart patients. [14] As part of this revolution, organ-on-a-chip engineering has combined microfabrication techniques and microfluidics with biomaterial scaffolds and cells to create 3D tissue constructs that can closely replicate the structure, phenotype, and function of tissues in the human body. The advent of induced pluripotent stem cells (iPSC) and directed differentiation protocols has provided a virtually infinite source of personalized cell types for building such constructs. [13] Thanks to these advances in cardiac tissue-on-a-chip engineering, it is increasingly possible to create samples of physiologically relevant and mature adult tissue samples of heart tissue in vitro.
Difficulty in accessing the cardiac environment in humans and animal models means that gaining detailed mechanistic insight into the function of cardiac EVs in vivo can be challenging. The variability and interactions in the in vivo environment are key aspects that influence tissue phenotype, but they also contribute to the difficultly in controlling or isolating behaviours and responses when studying tissue physiology. Due to these challenges, robust in vitro models of mature and physiologically relevant human heart tissues-on-a-chip provide a promising platform for controlled and accessible study of cardiac physiology, especially as it relates to their ability to accurately recapitulate native cardiac EV signalling processes. [13,14,109] Such models also impart the ability to replicate the myocardial disease phenotypes discussed in section 4, facilitating mechanistic investigations of the role of EVs in myocardial disease initiation and progression. Techniques that have been used thus far include hypoxic culture and media adjustment to simulate ischemia-reperfusion injury (IRI) in engineered tissues, as well as the use of patient-specific iPSC to create in vitro platforms that recapitulate genetic cardiac diseases such as hypertrophic cardiomyopathy. [110,111] Current progress in studying the role of EVs in healthy and diseased hearts using in vitro tissue models will be examined further in section 5.2.2.
Beyond ascertaining the role of EVs in regulating heart function and disease, tissue-on-a-chip models have also proven advantageous for screening potential EV biomarkers and testing the efficacy novel cardiac EV therapeutics for restoring heart function. 2D cell cultures and animal models remain the gold standards for preclinical investigations. Unfortunately, cells grown in simple 2D monolayers have shown significant phenotypic differences to those in complex 3D environments in vivo, including major differences in the profile of secreted EVs. [14,112] Distinct physiological differences between humans and animals also exist, and together these factors can create misleading preclinical results related to the safety and efficacy of investigational diagnostics and therapeutics, ultimately slowing clinical translation or increasing the chance of missing high-risk side effects. [13,109] Cardiac tissue-on-a-chip models have the ability to overcome these deficits, combining matured human cells in complex 3D environments that more closely resemble those in vivo, making them a useful platform for enhanced preclinical screening of EV diagnostics and therapeutics in the heart. A number of novel tissue platforms designed in recent years have also incorporated built-in readouts that can be used to monitor functional effects and assess mechanisms of applied therapeutics on engineered tissues. These include measurement of changes in tissue contractile force, electrophysiology, and genetic expression. [111,113,114] Specialized functional readouts enabled by tissue-on-a-chip platforms may be more clinically relevant than data obtained from 2D cultures. For example, a number of cardiac platforms designed to date can assess changes in tissue contractility which can potentially be extrapolated to predict impacts on cardiac ejection fraction in vivo, a parameter of particular interest for predicting a heart patient’s clinical outcome. [110] Further discussion on current and future possibilities for EV diagnostics and regenerative therapeutics for the heart is detailed in section 5.3.
Besides screening therapeutic efficacy, cardiac tissue-on-a-chip platforms also open the door to facilitating enhanced preclinical in vitro studies of therapeutic logistics. Dosing regimes, pharmacokinetic profiles, and methods of delivery are all important considerations that need to be investigated and defined to bring novel cardiac EV therapies to the clinic. The flexibility and relevance of tissue-on-a-chip models make it possible to perform such investigations in vitro to study differences in biodistribution and functional effects of EVs administered to tissues. These applications represent another potential avenue by which tissue engineering may accelerate the implementation of cardiac EVs in the clinic, though current studies remain limited. [52]
5.2.2. In Vitro Tissue Engineered Models of Cardiac EV Signalling: Current Progress
Though it is evident that cardiac tissue-on-a-chip models possess significant potential to generate new mechanistic insight into the role of EVs in cardiac physiology and disease, limited studies of EV signalling have been performed in tissue engineered models with most in vitro work to date performed in simpler 2D systems. [52] The studies outlined below represent early examples of cardiac tissue-on-a-chip platforms applied to three of the previously outlined areas of interest in cardiac EV research: studying EVs in cardiac physiology, disease, and rengeneration.
In 2017, Mayourian and colleagues studied to role of mesenchymal stem cell (MSC) EVs in enhancing the maturity and functionality of in vitro cardiac tissues. [115] They utilized a 3D engineered cardiac platform developed by the Costa lab, consisting of human embryonic stem cell (hESC)-derived CM seeded in a collagen-Matrigel matrix suspended between two PDMS posts. [116,117] As part of their study, the investigators treated separate groups of engineered tissues with MSC conditioned media, isolated MSC EVs, and EV-depleted MSC conditioned media and studied the functional effects of treatments on cardiac tissue function. Tissues treated with MSC conditioned media or MSC EVs showed a significant increase in contractility, measured by developed force (DF), compared to pre-treatment and control conditions. Tissues treated with EV-depleted MSC conditioned media did not exhibit any significant difference in DF, suggesting that MSC EVs have the potential to regulate cardiac contractile function and may play an important role in the cardiac environment in vivo. [115]
In a follow-up study in 2018, Mayourian and colleagues used the same engineered cardiac platform to delve into the mechanisms of MSC mediation of cardiac contractility. [118] Modelling including partial least squares regression and ingenuity pathway analysis were used to match highly expressed miRNA with target effects observed in engineered tissues treated with MSC EVs. Combining modelling and experimental results revealed that miR-21-5p was adbundant, significantly increased in treated tissues, and known to regulate cardiac contractility via modulation of the PI3K/Akt signalling pathway. [118] It was found that independent delivery of miR-21-5p to tissues increased the expression of calcium handling genes alongside contractility. Conversely, miR-21-5p knockdown in MSC EVs reduced their ability to enhance tissue contractility. [118] Through both of their studies, Mayourian and colleagues illustrated that engineered cardiac tissues can be useful for studying the role and mechanisms of EV signalling in regulating cardiac function in vitro. [115,118]
Another avenue for analyzing cardiac EVs in vitro pertains to investigating their role in cardiac disease processes. In 2020, Mastikhina et al. adapted an in vitro model of cardiac fibrosis, seeding iPS-derived CM and CF together in a fibrin gel suspended between PDMS rods. [119] CF treated with TGF-β1 prior to seeding were used in some of the platforms to initiate myofibroblast transformation and create a fibrotic phenotype in engineered tissues. Tissue-secreted EVs were isolated and miRNA sequencing was used to compare the difference between whole-tissue versus EV miRNA expression for both control and fibrotic tissues. It was observed that, as expected, miRNA expression differed significantly between control and fibrotic tissues on both the whole-tissue and EV levels. Interestingly, tissue miRNA expression also differed significantly from EV miRNA expression in several instances. Though distinct conclusions related to EV signalling in cardiac fibrosis were not made in this preliminary study, the results suggested that EV cargo is significantly altered in the fibrotic heart and that EVs may serve specific functions related to local signalling and disease progression. [119]
Also in 2020, Yadid et al. investigated the application of engineered heart tissues in screening EC EVs as a cardioprotective therapeutic for IRI. [71] The researchers applied a cantilever model of heart tissue designed by the Parker lab, consisting of a 3D printed device with an embedded strain sensor for detecting beam deflections caused by the contraction of seeded iPS-CM. [113,120] As shown in Figure 5a, EVs were isolated via differential ultracentrifugation from 2D cultures of human umbilical vein ECs (HUVECs) grown in either normoxia (‘Norm EEVs’) or hypoxia (‘Hyp EEVs’) and added to engineered tissue cultures in 2 doses, 3 hours before and then again at the onset of 3 hours of simulated ischemic injury. Ischemia was simulated via culture in hypoxic conditions using an altered media composition for 3 hours prior to reperfusion in normoxia and regular culture media for 1.5 hours. As shown in Figure 5b, tissues preconditioned with a treatment of either normoxic or hypoxic EC EVs maintained a significantly higher twitch stress during ischemic assault compared to untreated tissues. Recovery towards baseline contractility after reperfusion was also significantly improved for EV-treated tissues. [71] Investigations of therapeutic EV delivery in the heart-on-a-chip model were limited primarily to functional assessments, as further mechanistic studies were performed mostly in 2D CM cultures. Overall, Yadid and colleagues illustrated that engineered cardiac tissue-on-a-chip models can be a useful in vitro platform for screening novel EV therapeutics for the heart. The engineered tissue platform used in this study provided data on clinically relevant therapeutic targets, including contractility, that can be difficult to assess in 2D models and give greater insight into the in vivo potential of a novel treatment. [71]
Figure 5.
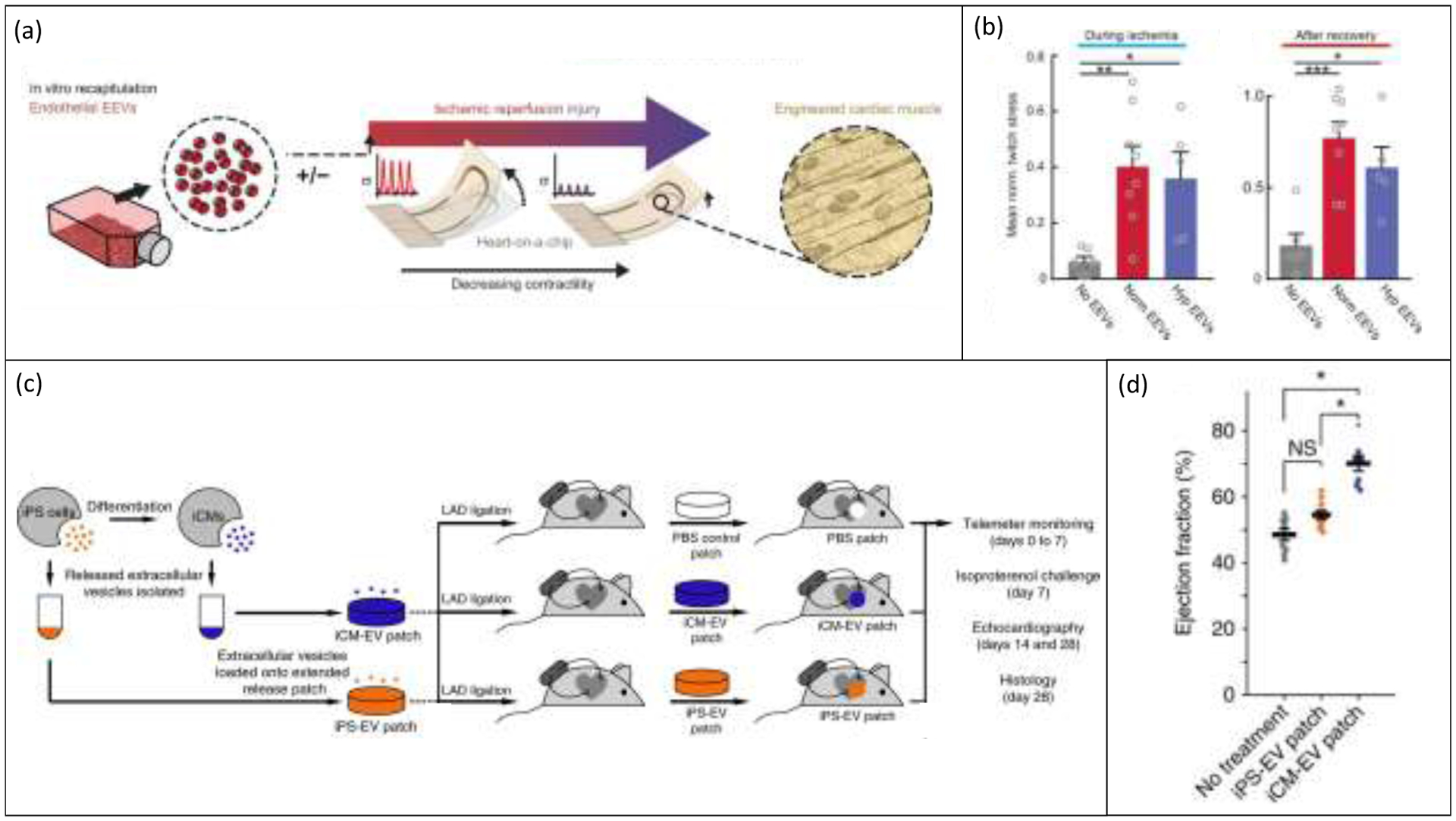
Current progress in the application of tissue engineered models to the investigation of extracellular vesicles in the heart. (a) Endothelial cell-derived EVs (EEVs) have been applied to an engineered cantilever heart-on-a-chip model before simulated ischemia-reperfusion injury to study the effect of EEVs as a prophylactic cardiac therapeutic. Reproduced with permission from [71]. (b) EVs isolated from endothelial cells grown in both hypoxia and normoxia and applied to engineered cardiac tissues before simulated ischemic injury significantly increased the twitch stress of cardiac tissues both during and after ischemia compared to untreated control tissues. Reproduced with permission from [71]. (c) Liu et al. encapsulated iPS and CM EVs in a collagen gelfoam mesh as a method of providing slow-release delivery of therapeutic EVs to the infarcted heart in rats. Reproduced with permission from [12]. (d) CM EV loaded “patches” significantly increased ejection fraction of rat hearts 24 hours after injury and patch application. Reproduced with permission from [12].
In the most recent study published in 2021, Villa del Campo et al. applied a model of cryoinjured engineered heart tissue as part of their study on the effects and mechanisms of epicardial EVs on cardiac functional recovery post-injury. [75] Using a circular engineered human myocardium (EHM) model suspended around flexible pillars that was pioneered by the Zimmerman lab, cardiac injury was induced via treatment with liquid nitrogen. EVs isolated from primary human epicardium-derived cell (EPDC) cultures via ultracentrifugation were applied to EHM tissue cultures post-injury. [75] It was observed that EV-treated tissues expressed an increase in CM cell cycle re-entry and proliferation 3 days after treatment, which manifested as a complete recovery of contractile force to pre-injury levels after 7 days. Untreated tissues still showed some increase in CM proliferation 3 days after injury, but contractile force never recovered and continued to decline through 7 days. [75] These data supported the findings of previous experiments performed in vivo and in 2D cultures. Additionally, viral transfection of EHM to overexpress miR-30a or miR-100 both reproduced the same 7-day post-injury recovery of force that was observed for EPDC EV treatment, supporting the suggestion that these miRNA species had an important mechanistic role in the therapeutic efficacy of epicardial EVs. Overall, engineered tissues proved to be a useful tool for validating in vivo observations of therapeutic EV signalling and for studying its mechanisms in an accessible environment possessing built-in assessment of contractile function. [75]
6. Harnessing the power of EVs in Cardiac Diagnostics and Regenerative Therapeutics
6.1. EVs as a Biomarker of Myocardial Disease
Traditionally, a troponin T assay is standard for the diagnosis of MI. Troponin T is a CM protein released into the bloodstream upon MI-induced CM death. These assays are highly sensitive and specific, with the ability to begin detecting troponin levels 4–8 hours after infarction that peak around 18 hours after onset. During MI, however, significant irreversible damage to the heart via necrosis tends to be concentrated in hours 4–12 after the onset of ischemia. 18 hours after onset, immune cell infiltration and inflammation can already begin. [11] Thus, earlier detection of MI events with alternative biomarkers could enable earlier intervention to limit damage sustained to tissues. Additionally, developing new biomarkers for comprehensive characterization of cardiac disease phenotype will be useful for implementing novel regenerative therapies that can target or reverse specific mechanisms of injury.
CM-secreted EVs have been identified as novel biomarkers for the improved detection of MI. Soon after cardiac damage begins, CM EVs containing high concentrations of cardiac-specific markers such as miR-1, miR-133, miR-208, and miR-499 are secreted into the blood stream. Specific studies have shown that concentrations of miR-208 species in blood samples can increase up to 3000 times post-MI compared to baseline levels, and successful detection less than 4 hours after injury is also possible via miRNA analyses. [11] Another study found that these cardiac miRNAs are also upregulated in urine after MI whereas troponins are not. [121] Overall, vesicular miRNAs assays for detecting MI have exhibited improved sensitivity, specificity, and flexibility than the current standard, making their clinical application a novel way to limit cardiac damage by improving the speed and efficiency of diagnosis. [11] Besides MI, EC-derived EVs have also been identified as a possible biomarker for coronary artery disease. Since MV release from ECs increases during plaque progression, the presence, number, and concentration of EC EVs containing miR-199a, miR-126, or CD144 and CD34 markers may be useful for characterizing the extent of plaques or the likelihood of rupture. [10] Post-infarction, miR-34a, miR-192, and miR-194 containing-EVs have been suggested as predictive signals of heart failure. Before their clinical implementation of EVs as biomarkers of heart disease, larger datasets from diverse phenotypes are required and more studies are needed to characterize the effect of co-morbidities on EVs released during cardiac pathology. [19] As described in section 3.4, novel methods such as flow cytometry and SPR have shown significant promise in enhanced detection of cell- and disease-specific EV markers present in biofluids at low quantities, such as a study from Im et al. [122] that successfully detected the presence of ovarian cancer-related EVs in biofluids via SPR. [26,41,42,44,122] Further optimization of such detection methods in cardiac applications alongside improved understanding of cardiac disease-related EV marker expression is expected to expand the potential of EV-based diagnostics for cardiovascular diseases moving forward. [19,26]
6.2. A Promising Application for EVs in Regenerative Cardiac Therapies
Besides their application as biomarkers, EVs have shown significant promise as key components of engineered regenerative therapeutics. With the advent of stem cell engineering, cell therapies have been widely touted as the future of medicine. Conventional treatments for heart disease focus on either limiting the progression of damage or heart transplant, but stem cell therapies offer the potential to actually replace cardiomyocytes lost during cardiac injury to induce functional repair and recovery. The injection of stem cells, stem cell-derived CM, and other cells into damaged tissues to improve heart function post-infarction has been widely studied in pre-clinical studies, however clinical implementation remains distant. Allogeneic cell transplantation presents immune concerns, cells generally exhibit very poor survival and engraftment post-transplant, and tumorigenicity or cardiac arrhythmias can be dangerous unintended consequences of such therapies. [20] Many researchers have noted that paracrine effects represent the likely mechanism of most of the observed benefits of cell therapies due to poor survival and engraftment of implanted cells. Thus, the application of cell-free EV preparations to damaged heart tissue offers the potential to induce functional cardiac repair through a cell-signalling approach while reducing drawbacks associated with cell therapy. [11,48]
Both cells and biologics such as EVs, offer significant potential for cardiac regeneration and remuscularization. Although both cells and EVs could elicit regenerative effects, the best choice will likely depend on the type of heart disease, the extent of the injury and the time frame available for intervention. Whereas EVs will likely be beneficial in a variety of disease settings, large transmural infarcts in ventricles with significant akinetic regions, where over a billion cardiomyocytes are lost, may require more direct replacement of the lost cells through cell injection. The following sections will briefly overview several key considerations for cardiac EV therapeutics in development, including EV sourcing, preparation, and delivery to the heart.
6.2.1. Sourcing and Preparing EVs as Cardiac Therapeutics
EVs from many different cell types and sources have been proposed and tested for their ability to modulate signalling in pathological heart tissues towards a pro-reparative phenotype. There is no single universally recognized population of EVs that has been identified or implemented clinically to date, however numerous EV-associated molecules and components have been identified for their role in modulating different properties of cardiac function and potentially inducing recovery. Generally, therapeutic EVs are isolated from 2D in vitro cell cultures where conditions can be carefully controlled to produce consistent populations of EVs. [11,19]
Native EVs secreted by unmodified cardiac cell cultures have been an important starting point in therapeutic EV research since the roles and impacts of such EVs in cardiac physiology have already been a point of significant study in literature as discussed in section 4.1. However, engineered EV populations and EVs sourced from other non-cardiac cell types have also shown utility in cardiac repair. The following sections present a brief overview of each class of therapeutic EVs.
6.2.1.1. Native Cardiac EV Therapies
Since EVs are critical to regulating normal physiology and their release and cargo have been observed to be dysregulated in pathological tissues, a large number of novel EV-based cardiac therapies under investigation have applied native EVs isolated from unmodified, healthy cardiac cell cultures in an attempt to artificially restore normal EV signalling profiles in damaged tissues.
EVs isolated from the major cardiac cell types, namely CM, CF, and EC, have been a major focus for current therapeutic research, as EVs from these cells have a significant presence and defined functions in the heart itself and are thus often thought to be the most critical source for restoring healthy physiology to the damaged heart. [19,20,49] Section 4.1 outlined current knowledge of the natural pro-reparative and cardioprotective effects of EVs secreted by the major cardiac cell types, yielding mechanistic insights that also apply to the potential functional benefits of these EVs when harvested and applied as therapeutics to the damaged heart.
6.2.1.2. Engineered Cardiac EV Therapies
Though the benefits of many types of native EVs in cardiac repair have been established, a recent focus in literature has been on improving EV therapeutic capacity and cardiac functional recovery via engineered enhancement of cell-secreted EVs. EVs can be engineered before secretion by altering source cells or modified post-isolation. Primary reasons for engineering EVs include tracking their localization, modulating bioactivity, modifying target specificity, and altering uptake and intracellular trafficking processes.[123]
For tracking purposes, EVs can be labelled with reporter molecules after isolation or cell lines can be genetically modified to cause them to secrete labelled EVs on their own. EV labelling and tracking is useful for in vitro and in vivo studies of physiological EV signalling as well as understanding the targets and mechanisms by which therapeutic EVs act on injured tissues post-application. Fluorophores are common and simple labels for such purposes, though more advanced techniques exist that have utilized reporters for SPECT-CT and PET-MRI tracking of EVs. [123]
Modulating the bioactivity of EVs represents one of the most common objectives of EV engineering in literature. Such techniques aim to adjust the molecular composition of EV cargo through environmental conditioning or genetic engineering to change the biological response in EV-treated tissues. [123] Pre-isolation, cell cultures can be ‘preconditioned’ using various environmental stimulating factors, including exposure to hypoxia or shear stress. [69,70,124] The goal of preconditioning is to use specific stimuli to alter the release profile or cargo of cell-secreted EVs in an attempt to amplify their therapeutic efficacy. [60] In one study, culture of rat CM in hypoxia (95% N2, 5% CO2) and an acidic ischemia-mimicking buffer solution (NaCl, KCl, KH2PO4, MgSO4, CaCl2, NaHCO3, calcium lactate, 2-deoxy-d-glucose, Na-HEPES) significantly upregulated EV expression of miR-143 and miR-222 which led to improved angiogenic proliferation of ECs in vitro and greater post-MI survival of mice in vivo compared to CM EVs secreted in control conditions. [60] In another study, Hergenreider et al. showed that shear stress exposure during HUVEC culture increased the expression of the miR-143/145 cluster in isolated HUVEC EVs. These EVs in turn acted on vascular smooth muscle cells and were found to have an inhibitory effect on atherosclerotic lesion formation in cardiac vessels of mice. [69] Besides chemical and physical preconditioning, genetic engineering can also be used to alter and enhance EV cargo to increase therapeutic efficacy. Ong et al. transfected EC cultures with hypoxia inducible factor 1 (HIF1). ECs overexpressing HIF1 released EVs enriched in miR-126 and miR-210. These EVs were found to improve the survival of cardiac progenitors during hypoxic stress. [70] It is also possible to load biomolecules directly into populations of pre-isolated EVs to enhance their therapeutic capacity. Youn et al. transfected CPC EVs with miR-322 via electroporation and found that these engineered EVs improved post-infarction recovery in mice compared to EVs that were not enriched with miR-322.[123,125]
Similar modifications to EVs can be used to alter their biodistribution, cellular targeting, and mechanisms of uptake and intracellular processing. Such strategies usually involve membrane modifications to EVs with peptides or lipids, which can once again be performed via genetic engineering of cell lines or through physical or chemical integration post-secretion and isolation. [123] As one example in the sphere of cardiac research, specific peptide sequences have been genetically inserted within the membranes of therapeutic EVs to target cell surface receptors highly expressed in the ischemic heart, showing positive signs of enhanced homing, uptake, and retention of engineered EVs in the injured heart.[123,126]
It has generally been recognized that large quantities of EVs are required for efficacy in therapeutic applications. Limited secretion of EVs by cells cultured at the laboratory scale means that obtaining clinically relevant yields of EVs is expensive and time consuming. [127] An emerging branch in vesicle research has sought to engineer ‘vesicle-like’ nanoparticles from cultured cells to improve scale-up of vesicle production while maintaining the therapeutic potential observed for traditional EVs. Several groups have developed techniques that break cultured cells into small, membrane bound nanoparticles that have been deemed ‘nanovesicles’ (NVs). NVs are usually produced from cells cultured in 2D that are subjected to sonication and/or forced extrusion through membranes with decreasing pore size. [127–129] The resulting particles can maintain their lipid bilayer membrane and contain contents derived from their parent cells, including therapeutically relevant proteins and nucleic acids, that can be transferred to target cells in a similar fashion to native EVs. NVs have been successfully produced in quantities over ten times larger than from EVs isolated from a similar number of cells, and have shown therapeutic activity in vitro. [127–129] Direct comparison of efficacy and mechanisms of action between NVs and EVs remains limited, however. A technique for directly increasing the rate of native EV secretion was developed by Yang et al., who delivered key transcriptional factors via electroporation to cells cultured on a biochip to improve EV yield 50-fold. [130] Sourcing and preparing clinically relevant yields of EVs remains an ongoing challenge to their therapeutic translation.
6.2.1.3. Non-Cardiac Cell EV Therapies
Besides therapies utilizing EVs secreted by the primary cardiac cell types, EVs isolated from cultures of other cell types have also shown promising results in pre-clinical studies of cardiac repair and have been tested in both native and engineered forms. Mesenchymal stem cell (MSC) EVs, including those from adipose derived stem cells (ADSC), have been one of the most notable cell sources studied to date as MSCs continue to gain traction in regenerative medicine due to their versatility and efficacy in a multitude of pro-reparative, anti-inflammatory, and anti-microbial applications. [19,131] Numerous studies have identified concentration of pro-angiogenic proteins and miRNAs in MSC EVs. Researchers have indicated that factors including miR-19a, miR-21, miR-22, miR-126 and miR-93-5p can act on CM to mediate anti-apoptotic effects, preserve mitochondrial membrane potential during ischemia, improve contractility and calcium handling, and reduce the production of inflammatory factors. [118,124,132–135] Other mechanistic studies have noted numerous miRNAs and proteins in MSC EVs that mediate cardiac functions including the promotion of angiogenesis in ECs, anti-inflammatory/pro-reparative polarization of macrophages, and inhibition of fibroblast to myofibroblast transformation. [124,133,134,136–142] Engineered cultures have also been employed to enhance the therapeutic cargo and efficacy of MSC EVs for cardiac applications, including GATA-4 overexpression to increase miR-19a content and ischemic preconditioning to increase miR-22 content. [124,132]
Other classes of stem cell EVs have also been studied for their potential in cardiac therapies. Some researchers have theorized that the potency of stem cells and their physiological roles in developmental and reparative signalling in vivo may impart useful bioactivity in their secreted EVs for applications in cardiac repair. [143] One study applied ESC EVs to mouse hearts post-infarction, observing improved survival, reduced fibrosis, and increased angiogenesis. Enrichment of miR-294 in ESC EVs improved survival and proliferation of cardiac progenitor cells, which may be one mechanism of the cardiac improvements. [144] iPSC EVs were compared to CM EVs in a recent study of cardiac repair. iPSC EVs expressed fewer cardiac-specific miRNA than CM EVs. In vivo, iPSC EVs produced a moderate but non-significant reduction in infarct size, while CM EVs noticeably reduced infarct size and improved post-infarct ejection fraction. [12] A different study found that iPSC EVs protected CMs from oxidative stress in vivo and transferred cardioprotective miR-21 and miR-210 to CMs. [64] Generally, studies have reported that iPSC and ESC EV applied to the heart may be useful in improving cell survival, promotion of self-renewal and cell cycle re-entry, imparting improved resistance to stress, and stimulating angiogenesis. [48,64,144,145] CD34+ hematopoietic stem cells (HSCs) have not shown significant cardioprotective properties for heart muscle in vivo, but due to their specific targeting of ECs, HSC EVs have proven useful in engineered therapies for stimulating angiogenesis post-ischemia via miR-126-3p signalling. [9,11]
As opposed to cell culture-derived EVs, vesicles isolated from human biofluids also have potential therapeutic capacity. EVs isolated from human plasma improved CM survival in a rat MI model, partly mediated by HSP70 present in EVs. [11] Pericardial fluid (PF) EVs were found to increase survival and proliferation of ECs in vitro and in vivo post ischemia due in part to the presence of let-7b-5p, which was present in significantly higher quantities in PF compared to plasma. [146] EVs in biofluids originate from many different cell types, making reproducibility, sample control, and matching of specific bioactive EV components to parent cells more difficult. Overall, EVs from many different sources have shown promise in cardiac therapy. Once again, the lack of standardized methods and inherent variabilities in EV research mean that it is difficult to compare efficacy between studies to pinpoint the best sources and components of EVs for cardiac repair. Further mechanistic investigations in relevant disease models are also needed to improve understanding of repair processes. [147]
6.3. Applying Therapeutic EVs to the Diseased Myocardium
Further to sourcing and isolation of EVs, the next focus for translating EV therapeutics lies in the methods in which they are administered to patients to achieve clinically desired outcomes and maximize efficacy. Table 1 highlights the modalities of EV delivery in recent studies of regenerative therapeutics that will be discussed in the following sections.
Table 1.
Recent studies highlighting delivery modalities of cardiac regenerative EV therapies
| Mode of EV Delivery | Study Description | Disease Model | EV Source | Key Findings | Ref. |
|---|---|---|---|---|---|
| Intravenous | Intravenous delivery of engineered EVs for targeted treatment of injured myocardium | Mouse model of MI | MSC EVs, cells transfected to express ischemic myocardium-targeting peptide on EV membrane | Enhanced homing of modified EVs to injured myocardium. Engineered EVs reduced inflammation, increased M2 macrophage polarization, increased angiogenesis, reduced infarct size, and preserved cardiac function compared to native EV infusions and controls. | [126] |
| Intracoronary, Intramyocardial | Comparison of intracoronary infusion versus intramyocardial injection of EVs to treat MI | Porcine model of MI | Cardiosphere-derived cell (CDC) EVs | Higher uptake and retention of EVs after intramyocardial injection compared to intracoronary infusion. Intramyocardial delivery exhibited decreased infarct size, decreased apoptosis, and increased functional rescue compared to intracoronary delivery and controls. | [148] |
| Intramyocardial | Intramyocardial injection of preconditioned EVs to treat MI | Mouse model of MI | CM EVs, post-hypoxic and ischemic preconditioning | Preconditioning upregulated angiogenic miR-143 and miR-222 in EVs. EVs promoted angiogenesis, improved survival, and increased ejection fraction post-MI. | [60] |
| Cardiac Patch | Surgical implantation of collagen-gelfoam cardiac patch for slow-release of encapsulated iPSC or CM EVs to treat MI | Rat model of MI | iPSC EVs versus CM EVs | Sustained local release of encapsulated EVs observed over 7 days. CM EV patches reduced hypertrophy, arrythmias, apoptosis, and infarct size, while increasing ejection fraction 4 weeks after implantation compared to iPSC EV patches and controls. | [12] |
| Stent Coating | Designing EV-eluting stent (EES) coatings for the prevention of re-stenosis and late stent thrombosis post-stenting | Rat models of renal ischemia-reperfusion injury and hindlimb ischemia | MSC EVs | EVs were successfully conjugated to stent coatings, exhibiting extended local release and uptake. EES reduced smooth muscle cell migration and re-stenosis compared to bare metal stents. EES exhibited enhanced re-endothelialization compared to conventional drug-eluting stents. | [149] |
| Inhalation, Oral, Intraperitoneal, Intravenous | Development of inhaled cardiac-targeting nanoparticles for delivery of regenerative biomolecules to the heart | Mouse model of diabetic cardiomyopathy, healthy rats and pigs | Synthetic calcium phosphate (CaP) nanoparticles, loaded with peptide or miRNA | Inhalation resulted in the most targeted and efficient delivery of CaP to the heart compared to oral, intraperitoneal, and intravenous methods. Inhaled peptide-loaded CaP led to complete cardiac functional recovery in mice with diabetic cardiomyopathy and did not cause significant toxicity or cardiac side effects in rats or pigs. | [150,151] |
6.3.1. Systemic and Intracoronary Infusion
Systemic delivery techniques, such as intravenous infusions, represent a simple, non-invasive technique for EV delivery but come with several challenges that can significantly reduce therapeutic efficacy. Studies have shown that EVs in circulation tend to have a short half-life on the order of just over an hour, after which time they are preferentially sequestered in organs such as the liver, lung, and spleen where they can be quickly phagocytized and cleared by macrophages. [152] These mechanisms of clearance mean that few EVs may actually reach and interact with target cells in the myocardium, possibly rendering EV therapeutics ineffective at clinically viable doses even if efficacy can be shown at the preclinical stage. EVs can also be infused into circulation directly within the coronary arteries to attempt to enhance local delivery and uptake in the heart, termed ‘intracoronary delivery’. Gallet et al. tested intracoronary infusion of cardiosphere-derived cell (CDC) EVs in a porcine model of acute MI. [148] Though some uptake and retention of CDC EVs was observed in injured myocardium, there was no appreciable increase in function or reduction in infarct size in treated tissues compared to controls. Alternatively, intramyocardial injection of CDC EVs proved to increase EV uptake compared to intracoronary infusion, also resulting in significantly improved functional cardiac recovery and suggesting that localized EV injections may provide clinical benefits over infusions in some contexts. [148]
6.3.2. Intramyocardial Injection
Intramyocardial injections can be used to deliver EVs directly to the desired site of action in the injured heart. Local injections in the heart can be more invasive than systemic delivery, with some techniques utilizing catheters and others performed during surgical intervention. Intramyocardial injections have proven to be a more effective method for cardiac-specific delivery, but injected EVs can still escape the heart through leakage from the needle hole as well as venous drainage. [152,153] Thus, representative studies have found that significant myocardial retention and uptake of EVs is still limited to window of a few hours post-injection. [12]
6.3.3. Patch- and Device-Based EV Therapies
Beyond the EV engineering approaches for enhancing homing and cellular targeting that were discussed in the previous section, a number of physical techniques have been investigated for improving myocardial retention and cardiac specificity of therapeutically delivered EVs. One of the leading methods has been the employment of degradable hydrogel scaffolds for EV encapsulation and slow release in the heart. Shear-thinning hydrogels can be mixed with EV suspensions for intramyocardial injection, improving retention of therapeutic EVs in the desired location of effect. Hydrogels and other scaffolds with encapsulated EVs can also be prepared ex vivo and surgically implanted onto the heart for sustained release directly to the site of cardiac injury. [152] In both cases, improved retention and sustained release of EVs for up to 3 weeks has been observed in vivo. [12,152] In one such in vivo study, Liu et al encapsulated iPS or CM EVs in collagen gelfoam mesh patches that were applied to rat hearts immediately after left anterior descending artery ligation, as shown in Figure 5c. Their results displayed in Figure 5d demonstrated that CM EV patches significantly increased ejection fraction of injured hearts after 24 hours compared to control and iPS EV groups. [12]
Several techniques have been tested to enhance the benefits of local EV retention and extended release exhibited by patch-based therapies over injections while reducing their disadvantages, such as invasive surgical requirements and poor myocardial engraftment related to epicardial delivery methods. A microneedle (MN) cardiac patch developed by Tang et al. in 2018 is one such example and could provide a useful platform for incorporating and delivering therapeutic EVs to the injured heart with greater efficacy. [154] The MN patch contains polymeric protrusions that penetrate tissue when placed epicardially, providing a conduit through which encapsulated EVs could better reach areas of injury deeper within the myocardium. [154] Beyond invasive surgical implantation procedures, there is the potential to deploy EV-loaded scaffolds onto the damaged myocardium using less invasive delivery modalities. The future integration of encapsulated regenerative EVs into existing systems such as the “shape-memory scaffold” for cardiac repair developed by Montgomery et al. could combine the benefits of minimally invasive delivery typically exhibited by injected EVs with localized targeting and improved retention typically observed for implantable scaffold-based therapeutics. [155]
Another approach that has been used to achieve local sustained delivery of EVs is their incorporation in existing implantable medical devices. Hu et al. chemically linked MSC EVs isolated via ultracentrifugation to the surface coating of cardiovascular stents, devices used for percutaneous coronary intervention procedures that open blocked coronary arteries in the heart. [149] It is well known that the deployment of bare metal stents can lead to recurring blockage of stented arteries since the stenting procedure causes vascular injury and induces maladaptive proliferation of smooth muscle cells into the vascular lumen. [149] Drug-eluting stents incorporating anti-proliferative compounds on the stent surface can prevent re-stenosis of stented arteries, but have also been linked to events of “late stent thrombosis” since these drugs prevent proper vascular healing and leave the stent surface exposed and prone to blood clot formation over the long-term. [156] Hu et al. showed that MSC EV-eluting stents promoted endothelial cell proliferation while inhibiting smooth muscle cell migration, and that EV-eluting stents implanted into rats significantly reduced re-stenosis while promoting re-endothelialization of stented arteries when compared to their bare and drug-eluting counterparts. [156] This study exemplifies the future potential for both enhancing existing medical devices by incorporating EVs and for using such devices as a mode for local therapeutic EV delivery.
6.3.4. Inhalation
Inhaled therapeutics represent a less invasive delivery modality than infusions, injections, or patches. They possess the potential to not only reduce cost and efficiency of therapies but also improve patient compliance and comfort, especially for cases of chronic administration. [150] Though clinically viable inhaled EV treatments for cardiac disease have not been extensively studied to date, a recent study by Dinh et al. tested the effects of an inhalation-based EV therapy on induced pulmonary fibrosis in rats.[157] Both lung-spheroid cell (LSC) and MSC EVs delivered via a nebulizer to rats with pulmonary fibrosis significantly improved respiratory function with no observations of organ toxicity. [157] In a separate study, Miragoli et al. found that calcium phosphate (CaP) nanoparticles delivered to mice via inhalation crossed the pulmonary barrier and selectively concentrated in the heart compared to an oral delivery route. [150] CaP nanoparticles loaded with peptide and delivered via inhalation rescued cardiac function in a murine model of diabetic cardiomyopathy while exhibiting safety and feasibility in pigs. [150] Previously, miRNA loading of CaP nanoparticles was also achieved, suggesting that inhalation of synthetic or native EVs may represent a potentially effective and minimally invasive mode of delivery for regenerative cardiac EV therapies in the future. [151]
6.3.5. Other Considerations for Therapeutic EV Delivery
The aforementioned considerations and progress in the delivery of EVs to the heart illustrate the importance of optimizing current methods to achieve desired therapeutic efficacy and viability in a clinical setting that match promising preclinical results. Beyond delivery methods, parameters such as dosing, frequency, and timing of application are other considerations that require optimization for specific therapeutics and remain a point of ongoing investigation towards realizing clinical translation. [152] Strategies for improving the scalability, storage, and transportation of EV therapeutics will also be key to this end. Innovations such as EV lyophilization have already been tested and indicate potential to retain bioactivity while improving the practicality and shelf-life of EV therapeutics, areas that will be of particular interest in the push towards clinical translation. [158,159]
7. Conclusions and Future Perspectives
Though the future potential of EVs is significant in applications as biomarkers of cardiac disease or as engineered therapies for the damaged heart, there are many challenges and uncertainties that must be overcome before promising preclinical results can be viably translated to real clinical outcomes. The lack of standardization in investigational and analytical techniques in the EV field remains one of the greatest hurdles to clinical translation. The relative infancy of the field and variable nature of EV secretion have contributed to reproducibility challenges in EV studies and preparations in literature. [76] Standardization will begin with detailed reporting of culture conditions and experimental parameters along MISEV2018 guidelines but must also include thorough investigation and establishment of how different isolation techniques affect EV samples and which techniques are most effective and efficient in a clinical setting. For reliable and safe application as both biomarkers and therapeutics, EV preparations must be produced reproducibly on a large scale. Predictably controlling the secretion and composition of EVs at such a scale has been difficult to achieve to date, with low yields and intensive purification protocols hampering translational viability. [19,26]
Characterization standards and a more comprehensive library of EV compositions, properties, and functions for various cell types, tissue systems, and diseases must also be established. For biomarker applications, large patient datasets are required to define clinically reliable markers and expression levels for detecting a variety of heart diseases. For regenerative EV therapies, a strong understanding of bioactive components and mechanisms is critical to ensuring that therapeutic EVs produce the desired responses and efficacy without unintended side-effects. With improved characterization and mechanistic understanding of EVs in cardiac physiology, pathology, and repair, it will be easier to select EV populations and sources that produce the most effective therapeutic response based on patient specific disease targets. As an added benefit, improved understanding of EVs will also open the door to targeted engineering of biomolecular expression in EV populations to further enhance clinical viability and performance. Secondary considerations that require further investigation before implementation include pharmacokinetic profiles, optimal routes of delivery, and dosing regimens.
Cardiac tissue-on-a-chip models represent a promising advance towards hastening the clinical translation of EV therapeutics and biomarkers for heart disease. In recent years, novel in vitro platforms have successfully combined patient-specific cells and biomaterial scaffolds in structural arrangements that can recreate mature, vascularized cardiac tissue. Their ever-improving physiological relevance and the added benefits of accessibility and built-in functional readouts have made them an excellent candidate for addressing the shortfalls in knowledge surrounding mechanisms of EVs in cardiac physiology and repair, as evidenced by several recent studies of EVs in cardiac tissues-on-a-chip. Future studies investigating EVs in cardiac physiology, disease, and repair in advanced in vitro models represent a promising path forward for therapeutic EVs, where targets and mechanisms have been very difficult to discern and refine in vivo. These possibilities represent the potential to address the current barriers in EV research and move one step closer to achieving reproducible efficacy in clinical trials towards attaining regulatory approval.
Due to their critical roles in regulating cardiac physiology and in a variety of pathologies, EVs represent a promising route to a better understanding of heart function and to discovering targets for novel cardiac therapies. The application of EVs in a clinical setting has the potential to revolutionize the way heart disease is understood and treated. By combining advances in cardiac tissue-on-a-chip engineering with EV analyses, clinical realization of safe and effective EV therapies can be achieved sooner, addressing the shortfalls that have stalled the implementation of stem cell therapies and providing an effective alternative to transplant in order to improve outcomes and quality of life for heart patients.
Acknowledgements
Figures 1-4 created with Biorender.com.
Our work is funded by the Canadian Institutes of Health Research (CIHR) Foundation Grant FDN-167274, Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN 326982-10), NSERC-CIHR Collaborative Health Research Grant (CHRP 493737-16), and National Institutes of Health Grant 2R01 HL076485. M.R. was supported by Killam Fellowship and Canada Research Chair. K.T.W. was supported by the NSERC CREATE Training Program for Organ-on-a-Chip Engineering and Entrepreneurship (TOeP) scholarship.
Biographies

Karl Wagner is a Ph.D. student in Chemical Engineering with a Collaborative Specialization in Biomedical Engineering at the University of Toronto. He previously obtained a B.Sc. in Materials Engineering and an M.Eng. in Materials Engineering from the University of Alberta. He is currently conducting research with the Laboratory for Functional Tissue Engineering, working under the supervision of Prof. Milica Radisic. Karl’s research is focused on developing in vitro tissue-on-a-chip models of human cardiac injury to study the role of extracellular vesicles in cardiac physiology and their potential applications as a regenerative therapeutic.

Milica Radisic is Canada Research Chair in Functional Cardiovascular Tissue Engineering at the University of Toronto and a Senior Scientist at the Toronto General Research Institute. She is also the Director of the NSERC CREATE Training Program in Organ-on-a-Chip Engineering & Entrepreneurship and Director of Ontario-Quebec Center for Organ-on-a-Chip Engineering. She obtained B.Eng. from McMaster University and Ph.D. from the Massachusetts Institute of Technology. She is a Fellow of the Royal Society of Canada. Her research focuses on organ-on-a-chip engineering and development of new biomaterials that promote healing. She developed new methods to mature iPSC-derived cardiac tissues using electrical stimulation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Karl T. Wagner, Department of Chemical Engineering and Applied Chemistry, University of Toronto, 27 King’s College Circle, Toronto, Ontario, Canada M5S 1A1 Institute of Biomaterials and Biomedical Engineering, University of Toronto, 27 King’s College Circle, Toronto, Ontario, Canada M5S 1A1.
Milica Radisic, Department of Chemical Engineering and Applied Chemistry, University of Toronto, 27 King’s College Circle, Toronto, Ontario, Canada M5S 1A1; Institute of Biomaterials and Biomedical Engineering, University of Toronto, 27 King’s College Circle, Toronto, Ontario, Canada M5S 1A1; Toronto General Research Institute, University Health Network, 200 Elizabeth St, Toronto, Ontario, Canada M5G 2C4.
References
- [1].Finegold JA, Asaria P, Francis DP, Int. J. Cardiol 2013, 168, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chatzizisis YS, Giannoglou GD, Parcharidis GE, Louridas GE, Int. J. Cardiol 2007, 116, 7. [DOI] [PubMed] [Google Scholar]
- [3].Kyavar M, Alemzadeh-Ansari MJ, Sanati H, in Pract. Cardiol, Elsevier, St. Louis, 2018, pp. 593–630. [Google Scholar]
- [4].Palasubramaniam J, Wang X, Peter K, Arterioscler. Thromb. Vasc. Biol 2019, 39, E176. [DOI] [PubMed] [Google Scholar]
- [5].Zhang J, Zhu W, Radisic M, Vunjak-Novakovic G, Circ. Res 2018, 123, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fedak PWM, Verma S, Weisel RD, Li R-K, Cardiovasc. Pathol 2005, 14, 49. [DOI] [PubMed] [Google Scholar]
- [7].Savarese G, Lund LH, Card. Fail. Rev 2017, 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Naderi N, in Pract. Cardiol, Elsevier Inc., St. Louis, 2018, pp. 193–227. [Google Scholar]
- [9].Davidson SM, Yellon DM, Mol. Aspects Med 2018, 60, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boulanger CM, Loyer X, Rautou PE, Amabile N, Nat. Rev. Cardiol 2017, 14, 259. [DOI] [PubMed] [Google Scholar]
- [11].Chistiakov DA, Orekhov AN, Bobryshevy YV, Int. J. Mol. Sci 2016, 17, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, et al. , Nat. Biomed. Eng 2018, 2, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang B, Radisic M, Lab Chip 2017, 17, 2395. [DOI] [PubMed] [Google Scholar]
- [14].Ahadian S, Civitarese R, Bannerman D, Mohammadi MH, Lu R, Wang E, Davenport-Huyer L, Lai B, Zhang B, Zhao Y, et al. , Adv. Healthc. Mater 2018, 7, 1. [DOI] [PubMed] [Google Scholar]
- [15].Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. , J. Extracell. Vesicles 2015, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Raposo G, Stoorvogel W, J. Cell Biol 2013, 200, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, Nat. Cell Biol 2007, 9, 654. [DOI] [PubMed] [Google Scholar]
- [18].Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ, Leukemia 2006, 20, 847. [DOI] [PubMed] [Google Scholar]
- [19].Sluijter JPG, Davidson SM, Boulanger CM, Buzás EI, De Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, et al. , Cardiovasc. Res 2018, 114, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Adamiak M, Sahoo S, Mol. Ther 2018, 26, 1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Doyle LM, Wang MZ, Cells 2019, 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Colombo M, Raposo G, Théry C, Annu. Rev. Cell Dev. Biol 2014, 30, 255. [DOI] [PubMed] [Google Scholar]
- [23].Stuffers S, Sem Wegner C, Stenmark H, Brech A, Traffic 2009, 10, 925. [DOI] [PubMed] [Google Scholar]
- [24].Kakarla R, Hur J, Kim YJ, Kim J, Chwae YJ, Exp. Mol. Med 2020, 52, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mulcahy LA, Pink RC, Carter DRF, Extracell J. Vesicles 2014, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. , J. Extracell. Vesicles 2018, 7, 1. [Google Scholar]
- [27].O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO, Nat. Rev. Mol. Cell Biol 2020, 21, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S, Genomics, Proteomics Bioinforma 2015, 13, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F, Nat. Commun 2013, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kenneweg F, Bang C, Xiao K, Boulanger CM, Loyer X, Mazlan S, Schroen B, Hermans-Beijnsberger S, Foinquinos A, Hirt MN, et al. , Mol. Ther. - Nucleic Acids 2019, 18, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kreimer S, Belov AM, Ghiran I, Murthy SK, Frank DA, Ivanov AR, J. Proteome Res 2015, 14, 2367. [DOI] [PubMed] [Google Scholar]
- [32].Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, et al. , Circ. Res 2017, 120, 1632. [DOI] [PubMed] [Google Scholar]
- [33].Li P, Kaslan M, Lee SH, Yao J, Gao Z, Theranostics 2017, 7, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, et al. , Theranostics 2020, 10, 3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lotvall J, Hil AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. , J. Extracell. Vesicles 2014, 1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kornilov R, Puhka M, Mannerström B, Hiidenmaa H, Peltoniemi H, Siljander P, Seppänen-Kaijansinkko R, Kaur S, J. Extracell. Vesicles 2018, 7, DOI 10.1080/20013078.2017.1422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, et al. , J. Extracell. Vesicles 2013, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bruce TF, Slonecki TJ, Wang L, Huang S, Powell RR, Marcus RK, Electrophoresis 2019, 40, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gholizadeh S, Draz M, Zarghooni M, Nezhad AS, Ghavami S, Shafiee H, Akbari M, Biosens Bioelectron 2017, 91, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rikkert LG, Nieuwland R, Terstappen LWMM, Coumans FAW, Extracell J. Vesicles 2019, 8, DOI 10.1080/20013078.2018.1555419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Clayborne C, Braig Z, Rosner A, Demberg T, Watson DC, Karpova TS, et al. , Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nolan JP, Duggan E, in Flow Cytom. Protoc (Eds: Hawley TS, Hawley Robert G.), Springer, New York, 2018, pp. 79–92. [Google Scholar]
- [43].Kowal EJK, Ter-Ovanesyan D, Regev A, Church GM, 2017, DOI 10.1007/978-1-4939-7253-1. [DOI]
- [44].Im H, Yang Katherine, Lee H, Castro CM, in Extracell. Vesicles Methods Protoc (Eds: Kuo WP, Jia S), Humana Press, New York, 2017, pp. 133–141. [Google Scholar]
- [45].Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T, Extracell J. Vesicles 2013, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C, Proc. Natl. Acad. Sci. U. S. A 2016, 113, E968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, et al. , Nat. Biomed. Eng 2018, 2, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yuan Y, Du W, Liu J, Ma W, Zhang L, Du Z, Cai B, Front. Pharmacol 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xu MY, Ye ZS, Song XT, Huang RC, Stem Cell Res. Ther 2019, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu H, Wang Z, Front. Physiol 2019, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li H, Liao Y, Gao L, Zhuang T, Huang Z, Zhu H, Ge J, Theranostics 2018, 8, 2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wagner KT, Nash TR, Liu B, Vunjak-Novakovic G, Radisic M, Trends Biotechnol 2020, DOI 10.1016/j.tibtech.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, et al. , Diabetes 2016, 65, 3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al. , Nat. Med 2007, 13, 613. [DOI] [PubMed] [Google Scholar]
- [55].He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW, J. Biomed. Sci 2011, 18, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li S, Xiao FY, Shan PR, Su L, Chen DL, Ding JY, Wang ZQ, J. Hum. Genet 2015, 60, 709. [DOI] [PubMed] [Google Scholar]
- [57].Wang J, Jia Z, Zhang C, Sun M, Wang W, Chen P, Ma K, Zhang Y, Li X, Zhou C, RNA Biol. 2014, 11, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW, Circ. Res 2010, 106, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan GC, PLoS One 2012, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V, Silva AP, Fernandes R, Zuzarte M, Enguita FJ, et al. , Cardiovasc. Res 2017, 113, 1338. [DOI] [PubMed] [Google Scholar]
- [61].Atlas SA, Manag J. Care Pharm. 2007, 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bang, B. S, D. S, G. SK, F. FA, H. A, J. A, R. J, Z. K, Z. A, et al. , J. Clin. Invest 2014, 124, 2136.24743145 [Google Scholar]
- [63].Tian C, Gao L, Zimmerman MC, Zucker IH, Am. J. Physiol. - Hear. Circ. Physiol 2018, 314, H928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, et al. , Int. J. Cardiol 2015, 192, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Luo H, Li X, Li T, Zhao L, He J, Zha L, Qi Q, Yu Z, Cardiovasc. Res 2019, 115, 1189. [DOI] [PubMed] [Google Scholar]
- [66].Balkom B. W. M. va., Jong O. G. d., Smits M, Brummelman J, Ouden K. den, Bree P. M. d., Eijndhoven M. A. J. va., Pegtel DM, Stoorvogel W, Würdinger T, et al. , Blood 2013, 121, 3997. [DOI] [PubMed] [Google Scholar]
- [67].Njock MS, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E, Roufaiel M, Cybulsky MI, Schober A, Fish JE, Blood 2015, 125, 3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gao XF, Wang ZM, Wang F, Gu Y, Zhang JJ, Chen SL, Int. J. Biol. Sci 2019, 15, 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, et al. , Nat. Cell Biol 2012, 14, 249. [DOI] [PubMed] [Google Scholar]
- [70].Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, Gold JD, Wu JC, Circulation 2014, 130, S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yadid M, Lind JU, Ardoña HAM, Sheehy SP, Dickinson LE, Eweje F, Bastings MMC, Pope B, O’Connor BB, Straubhaar JR, et al. , Sci. Transl. Med 2020, 12, DOI 10.1126/scitranslmed.aax8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Manole CG, Cismaşiu V, Gherghiceanu M, Popescu LM, J. Cell. Mol. Med 2011, 15, 2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yang J, Li Y, Xue F, Liu W, Zhang S, Am. J. Transl. Res 2017, 9, 5375. [PMC free article] [PubMed] [Google Scholar]
- [74].Feric NT, Radisic M, Stem Cells Transl. Med 2016, 5, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].del Campo CV, Liaw NY, Gunadasa-Rohling M, Matthaei M, Braga L, Kennedy T, Salinas G, Voigt N, Giacca M, Zimmermann W-H, et al. , Cardiovasc. Res 2021, 44, DOI 10.1093/cvr/cvab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Osteikoetxea X, Németh A, Sódar BW, Vukman KV, Buzás EI, J. Physiol 2016, 594, 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X, J. Mol. Med 2016, 94, 711. [DOI] [PubMed] [Google Scholar]
- [78].Wang C, Zhang C, Liu L, Xi A, Chen B, Li Y, Du J, Mol. Ther 2017, 25, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cheng M, Yang J, Zhao X, Zhang E, Zeng Q, Yu Y, Yang L, Wu B, Yi G, Mao X, et al. , Nat. Commun 2019, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gao L, Mei S, Zhang S, Qin Q, Li H, Liao Y, Fan H, Liu Z, Zhu H, Theranostics 2020, 10, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Akbar N, Digby JE, Cahill TJ, Tavare AN, Corbin AL, Saluja S, Dawkins S, Edgar L, Rawlings N, Ziberna K, et al. , JCI insight 2017, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Loyer X, Zlatanova I, Devue C, Yin M, Howangyin KY, Klaihmon P, Guerin CL, Khelouf M, Vilar J, Zannis K, et al. , Circ. Res 2018, 123, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pattar SS, Hassanabad AF, Fedak PW, Front. Cell Dev. Biol 2019, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nie X, Fan J, Li H, Yin Z, Zhao Y, Dai B, Dong N, Chen C, Wang DW, Mol. Ther. - Nucleic Acids 2018, 12, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yang J, Yu X, Xue F, Li Y, Liu W, Zhang S, Am. J. Transl. Res 2018, 10, 4350. [PMC free article] [PubMed] [Google Scholar]
- [86].Datta R, Bansal T, Rana S, Datta K, Datta Chaudhuri R, Chawla-Sarkar M, Sarkar S, Mol. Cell. Biol 2017, 37, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gennebäck N, Hellman U, Malm L, Larsson G, Ronquist G, Waldenström A, Mörner S, Extracell J. Vesicles 2013, 2, DOI 10.3402/jev.v2i0.20167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH, LeBleu VS, Kalluri R, J. Am. Soc. Nephrol 2013, 24, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Webber J, Steadman R, Mason MD, Tabi Z, Clayton A, Cancer Res. 2010, 70, 9621. [DOI] [PubMed] [Google Scholar]
- [90].Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, Guo S, Wang Y, Fan K, Zhan D, et al. , Cell Res. 2012, 22, 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Seok HY, Chen J, Kataoka M, Huang ZP, Ding J, Yan J, Hu X, Wang DZ, Circ. Res 2014, 114, 1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, et al. , J. Mol. Cell. Cardiol 2012, 53, 848. [DOI] [PubMed] [Google Scholar]
- [93].Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, Van Rooij E, Circulation 2011, 124, 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ye W, Tang X, Yang Z, Liu C, Zhang X, Jin J, Lyu J, Mol. Immunol 2017, 87, 114. [DOI] [PubMed] [Google Scholar]
- [95].Cañón S, Caballero R, Herraiz-Martínez A, Pérez-Hernández M, López B, Atienza F, Jalife J, Hove-Madsen L, Delpón E, Bernad A, J. Mol. Cell. Cardiol 2016, 99, 162. [DOI] [PubMed] [Google Scholar]
- [96].van den Berg NWE, Kawasaki M, Berger WR, Neefs J, Meulendijks E, Tijsen AJ, de Groot JR, Cardiovasc. Drugs Ther 2017, 31, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ibrahim A, Marbán E, Annu. Rev. Physiol 2016, 78, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQN, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, et al. , J. Clin. Invest 2013, 123, 2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC, J. Mol. Cell. Cardiol 2014, 74, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gambim MH, de Oliveira do Carmo A, Marti L, Veríssimo-Filho S, Lopes LR, Janiszewski M, Crit. Care 2007, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Azevedo LCP, Janiszewski M, Pontieri V, Pedro M. de A., Bassi E, Tucci PJF, Laurindo FRM, Crit. Care 2007, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L, PLoS One 2012, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang X, Huang W, Yang Y, Wang Y, Peng T, Chang J, Caldwell CC, Zingarelli B, Fan GC, Biochim. Biophys. Acta - Mol. Basis Dis 2014, 1842, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ailawadi S, Wang X, Gu H, Fan GC, Biochim. Biophys. Acta - Mol. Basis Dis 2015, 1852, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jiang X, Sucharov J, Stauffer BL, Miyamoto SD, Sucharov CC, Am. J. Physiol. - Hear. Circ. Physiol 2017, 312, H818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, Reinhardt F, Liao R, Krieger M, Jaenisch R, et al. , Circ. Res 2009, 105, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dziewięcka E, Totoń-Żurańska J, Wołkow P, Kołton-Wróż M, Pitera E, Wiśniowska-Śmiałek S, Khachatryan L, Karabinowska A, Szymonowicz M, Podolec P, et al. , Adv. Clin. Exp. Med 2020, 29, 285. [DOI] [PubMed] [Google Scholar]
- [108].Wiklander OPB, Brennan M, Lötvall J, Breakefield XO, Andaloussi SEL, Sci. Transl. Med 2019, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ronaldson-Bouchard K, Vunjak-Novakovic G, Cell Stem Cell 2018, 22, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chen T, Vunjak-Novakovic G, Regen. Eng. Transl. Med 2018, 4, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, et al. , Cell 2019, 176, 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Thippabhotla S, Zhong C, He M, Sci. Rep 2019, 9, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, et al. , Nat. Mater 2017, 16, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, Nartiss Y, Keller G, Gepstein L, Nat. Commun 2020, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Mayourian J, Cashman TJ, Ceholski DK, Johnson BV, Sachs D, Kaji DA, Sahoo S, Hare JM, Hajjar RJ, Sobie EA, et al. , Circ. Res 2017, 121, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cashman TJ, Josowitz R, Gelb BD, Li RA, Dubois NC, Costa KD, J. Vis. Exp 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, et al. , FASEB J. 2014, 28, 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mayourian J, Ceholski DK, Gorski PA, Mathiyalagan P, Murphy JF, Salazar SI, Stillitano F, Hare JM, Sahoo S, Hajjar RJ, et al. , Circ. Res 2018, 122, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mastikhina O, Moon BU, Williams K, Hatkar R, Gustafson D, Mourad O, Sun X, Koo M, Lam AYL, Sun Y, et al. , Biomaterials 2020, 233, 119741. [DOI] [PubMed] [Google Scholar]
- [120].Lind JU, Yadid M, Perkins I, O’Connor BB, Eweje F, Chantre CO, Hemphill MA, Yuan H, Campbell PH, Vlassak JJ, et al. , Lab Chip 2017, 17, 3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cheng Y, Wang X, Yang J, Duan X, Yao Y, Shi X, Chen Z, Fan Z, Liu X, Qin S, et al. , 2015, 53, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Im H, Shao H, Il Park Y, Peterson VM, Castro CM, Weissleder R, Lee H, Nat. Biotechnol 2014, 32, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L, Nat. Rev. Cardiol 2020, 17, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Feng Y, Huang W, Wani M, Yu X, Ashraf M, PLoS One 2014, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Youn SW, Li Y, Kim YM, Sudhahar V, Abdelsaid K, Kim HW, Liu Y, Fulton DJR, Ashraf M, Tang Y, et al. , Antioxidants 2019, 8, DOI 10.3390/antiox8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang X, Chen YY, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen YY, Li J, Ma T, et al. , J. Am. Heart Assoc 2018, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lee H, Cha H, Park JH, Int. J. Mol. Sci 2020, 21, DOI 10.3390/ijms21010343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jeong D, Jo W, Yoon J, Kim J, Gianchandani S, Gho YS, Park J, Biomaterials 2014, 35, 9302. [DOI] [PubMed] [Google Scholar]
- [129].Ilahibaks NF, Lei Z, Mol EA, Deshantri AK, Jiang L, Vader P, Sluijter JPG, n.d., 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, et al. , Nat. Biomed. Eng 2020, 4, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Murphy MB, Moncivais K, Caplan AI, Exp. Mol. Med 2013, 45, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M, Int. J. Cardiol 2015, 182, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, et al. , Stem Cells Transl. Med 2017, 6, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M, Cell. Physiol. Biochem 2018, 44, 2105. [DOI] [PubMed] [Google Scholar]
- [135].Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M, et al. , Mol. Ther. - Nucleic Acids 2018, 11, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Vrijsen KR, Maring JA, Chamuleau SAJ, Verhage V, Mol EA, Deddens JC, Metz CHG, Lodder K, van Eeuwijk ECM, van Dommelen SM, et al. , Adv. Healthc. Mater 2016, 5, 2555. [DOI] [PubMed] [Google Scholar]
- [137].Liang X, Zhang L, Wang S, Han Q, Zhao RC, J. Cell Sci 2016, 129, 2182. [DOI] [PubMed] [Google Scholar]
- [138].Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M, Oncotarget 2017, 8, 45200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Han C, Zhou J, Liang C, Liu B, Pan X, Zhang Y, Wang Y, Yan B, Xie W, Liu F, et al. , Biomater. Sci 2019, 7, 2920. [DOI] [PubMed] [Google Scholar]
- [140].Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, et al. , Sci. Rep 2015, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, Xiao P, Meng Q, Geng YJ, Yu XY, et al. , Biomed Res. Int 2017, 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].de Couto G, Exp. Mol. Med 2019, 51, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Witman N, Sahara M, Stem Cells Int. 2018, 2018, DOI 10.1155/2018/8283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, et al. , Circ. Res 2015, 117, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, et al. , Circ. Res 2018, 122, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Beltrami C, Besnier M, Shantikumar S, Shearn AIU, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, et al. , Mol. Ther 2017, 25, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wendt S, Goetzenich A, Goettsch C, Stoppe C, Bleilevens C, Kraemer S, Benstoem C, Sci. Rep 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Gallet R, Dawkins J, Valle J, Simsolo E, De Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, et al. , Eur. Heart J 2017, 38, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hu S, Li Z, Shen D, Zhu D, Huang K, Su T, Dinh PU, Cores J, Cheng K, Nat. Biomed. Eng 2021, DOI 10.1038/s41551-021-00705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, Carullo P, Ramirez-Rodríguez GB, Patrício T, Degli Esposti L, et al. , Sci. Transl. Med 2018, 10, 1. [DOI] [PubMed] [Google Scholar]
- [151].Di Mauro V, Iafisco M, Salvarani N, Vacchiano M, Carullo P, Ramírez-Rodríguez GB, Patrício T, Tampieri A, Miragoli M, Catalucci D, Nanomedicine 2016, 11, 891. [DOI] [PubMed] [Google Scholar]
- [152].Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, Chen M, Theranostics 2021, 11, 2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sahoo S, Kariya T, Ishikawa K, Nat. Rev. Cardiol 2021, DOI 10.1038/s41569-020-00499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L, Hensley MT, Caranasos TG, Zhang J, Gu Z, et al. , Sci. Adv 2018, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, et al. , Nat. Mater 2017, 16, DOI 10.1038/nmat4956. [DOI] [PubMed] [Google Scholar]
- [156].Strohbach A, Busch R, Int. J. Polym. Sci 2015, 2015, 1. [Google Scholar]
- [157].Dinh PUC, Paudel D, Brochu H, Popowski KD, Gracieux MC, Cores J, Huang K, Hensley MT, Harrell E, Vandergriff AC, et al. , Nat. Commun 2020, 11, DOI 10.1038/s41467-020-14344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bari E, Perteghella S, Di Silvestre D, Sorlini M, Catenacci L, Sorrenti M, Marrubini G, Rossi R, Tripodo G, Mauri P, et al. , Cells 2018, 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].El Baradie KBY, Nouh M, O’Brien F, Liu Y, Fulzele S, Eroglu A, Hamrick MW, Front. Cell Dev. Biol 2020, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]


