Abstract
Aging is a major risk factor of developing breast cancer. Despite the fact that post-menopausal women have lower levels of estrogen, older women have a higher rate of estrogen receptor alpha (ERα) positive breast cancer. Conversely, young women who have elevated levels of estrogen tend to develop ERα negative disease that is associated with higher rate of metastasis. This perspective proposes a unifying model centered around the importance of mitochondrial biology in cancer and aging to explain these observations. Mitochondria are essential for the survival of cancer cells and therefore pathways that maintain the functionality of the mitochondrial network in cancer cells fulfill a critical role in the survival of cancer cells. The ERα and the mitochondrial sirtuin-3 (SIRT3) have been reported to be key players of the mitochondrial unfolded protein response (UPRmt) 1–5. The UPRmt is a complex retrograde signaling cascade that regulates the communication between the mitochondria and the nucleus to restore mitochondrial fitness in response to oxidative stress 5–7. SIRT3 is a major regulator of aging 8. Its level decreases with age and single nucleotide polymorphisms (SNPs) that preserve its expression at higher levels are observed in centenarians 9,10. We propose a model whereby the ERα axis of the UPRmt acts to compensate for the loss of SIRT3 observed with age, and becomes the dominant axis of the UPRmt to maintain the integrity of the mitochondria during transformation, thus explaining the selective advantage of ERα positive luminal cells in breast cancer arising from older women.
Keywords: Breast cancer, SIRT3, estrogen receptor alpha, mitochondria, mitochondrial unfolded protein response, aged breast, aging
Graphical Abstract:
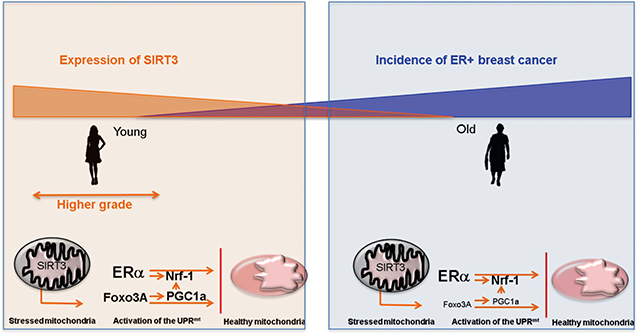
Aging is a major risk factor of developing breast cancer. Despite the fact that post-menopausal women have lower levels of estrogen, older women have a higher rate of estrogen receptor alpha (ERα) positive breast cancer. Conversely, young women who have elevated levels of estrogen tend to develop ERα negative disease that is associated with higher rate of metastasis. This perspective proposes a unifying model centered around the switch between the SIRT3 and estrogen receptor axes of the mitochondrial unfolded protein response with age to explain these observations.
The association between age and the development of breast cancer is clearly indicated by the fact that 80% of breast cancers occur in women that are 50 years old or older 11–15. This observation suggests that post-menopausal involution of the breast and/or cessation of ovarian function impose changes to the breast that create a more favorable environment for transformation. Curiously, while the levels of estrogen decrease drastically after menopause, breast cancers in women aged more than 75 years old tend to be positive for the estrogen receptor alpha (ERα). This observation suggests that the activation of the ERα in these women may be mainly driven by estrogen-independent or by other events that cooperate with low level of estrogen for its activation. In order to gain a better understanding of the mechanism by which the ERα can be activated in presence of low concentration of estrogen, the modes of activation of the transcriptional activity of the ERα must be considered.
Phosphorylation of the ERα by Akt leads to its activation
The ERα is composed of one DNA binding domain and two activation function (AF) domains. The AF2 contains the ligand-binding domain and is activated through binding of estrogen 16,17. The AF1 domain however is activated through phosphorylation by AKT in an estrogen-independent fashion. Several studies have now shown that the ERα binds to several hundred binding sites across the genome 17. A follow up study from this original report described that approximately 300 genes are activated when cells are treated with estrogen in presence of constitutively active AKT but not by estrogen alone 18. Further the phosphorylation of the ERα by AKT was shown to prolong the binding of the ERα to certain promoters 18. These observations raise the possibility that the synergy between AKT and estrogen may be a mechanism to explain not only the activation of the ERα despite low levels of estrogen in post-menopausal women but also that the transcriptional programs that are turned on vary whether the ERα is activated through the AF1 and/or AF2 functions (Fig. 1). As described in this perspective, some of the ERα target genes that are activated upon mitochondrial stress are only transcribed upon phosphorylation of the ERα by AKT.
Figure 1:
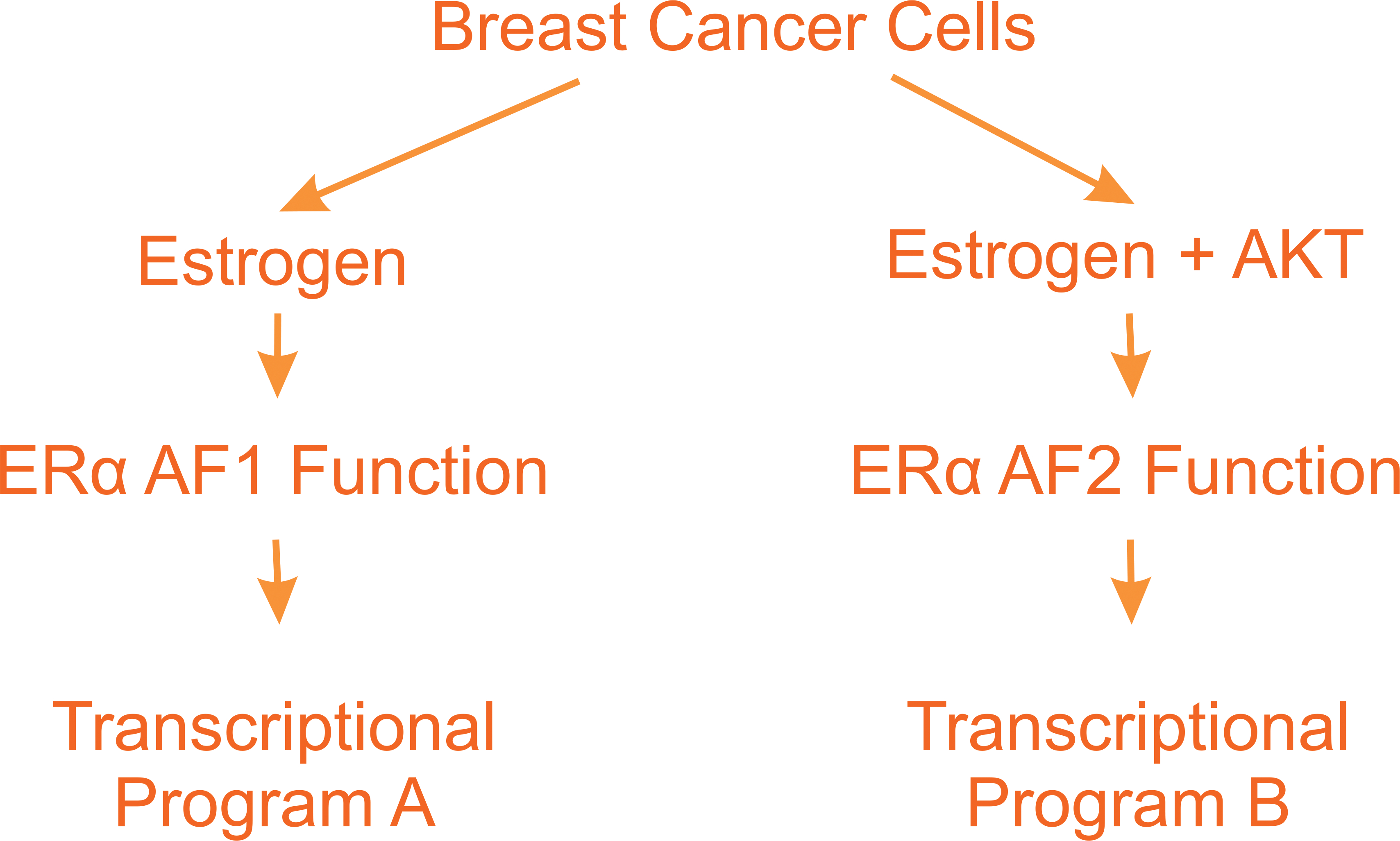
Activation of the ERα can be mediated either by elevated level of estrogen or low levels of estrogen in combination with phosphorylation of the ERα by AKT. These modes of activation result in the activation of distinct transcriptional programs.
However, mutations in PI3K are frequent in breast cancer but they tend to affect ERα negative breast cancer 19,20. Therefore, this observation argues that activation of AKT in ERα positive cancer cells is mediated through another mechanism.
One possibility for such alternative mechanism arises from the observation that AKT can be activated by reactive oxygen species (ROS). PI3K/AKT pathway is negatively regulated by the phosphatase PTEN. While mutations in PTEN are less frequent, PTEN can be inactivated by ROS. ROS have been shown to oxidize the active site cysteine on PTEN (Cys124) resulting in a disulfide formation to another intra-protein cysteine (Cys71) 21–23. This results in inactivation of PTEN and constitutive activation of AKT. The activation of AKT by ROS is therefore a potential mechanism that would contribute to the activation of the ERα in presence of low level of estrogen in post-menopausal women.
The main source of cellular ROS is the mitochondria and therefore pathways that link increased mitochondrial ROS and the activation of the ERα are potentially key regulators of the increased incidence of ERα positive breast cancer in older women. Mitochondrial ROS is regulated by the sirtuin SIRT3, a deacetylase that localizes to the matrix of the mitochondria 24,25. SIRT3 deacetylates several key regulators of metabolism and DNA repair 24–26. However, one of the best characterized substrate of SIRT3 is the dismutase SOD2, which is implicated in the conversion of superoxide (O2) into hydrogen peroxide (H2O2) 27,28.
SIRT3 was identified as a longevity gene in several experimental models 8–10. These findings are supported by the observations that SNPs leading to increased expression of SIRT3 were identified in centenarians and life-style interventions known to affect aging and longevity such as calorie restriction, exercise, and high fat diet all affect SIRT3 levels with calorie restriction and exercise increasing SIRT3 and high fat diet reducing SIRT3 29–31. Therefore, reduction in SIRT3 in aging directly contributes to an elevation in mitochondrial ROS.
This observation raises the possibility that the reduction of SIRT3 during aging and the resulting increase in ROS levels, leads to activation of AKT, which in turn promotes the activation of the ERα despite low level of estrogen in older women.
Additionally, the functions of SIRT3 and the ERα are intimately interconnected through their common role in the maintenance of the mitochondrial fitness. This interconnection arises from their implication in the mitochondrial unfolded protein response (UPRmt).
The ERα and SIRT3 play important roles in the mitochondrial UPR.
The first unfolded protein response (UPR) to have been identified refers to the signaling cascade that is activated upon accumulation of misfolded proteins in the lumen of the endoplasmic reticulum. This cascade activates three parallel axes, the ATF6, PERK and IRE axes which collectively leads to the activation of a large nuclear transcriptional program that culminates in the reduction of stress in the lumen of the endoplasmic reticulum 32,33.
In the last few years, it has become abundantly clear that a similar signaling cascade orchestrates the communication between the mitochondria and the nucleus upon stress in the mitochondria 3,4. However, the players of the mitochondrial UPR (UPRmt) are distinct from those of the endoplasmic reticulum UPR (UPRER).
Our current understanding of the UPRmt is that it also involves three axes (Fig. 2). The original axis was identified by the Hoogenraad group in mammalian cells and implicates the transcription factor CHOP, which results in the transcription of mitochondrial chaperones and proteases 34–38. CHOP regulates the transcription of ATF5, which was recently found to be the mammalian homolog of ATFS-1 in C. elegans 39,40. Our group identified two additional axes; the ERα axis and the SIRT3 axis.
Figure 2:
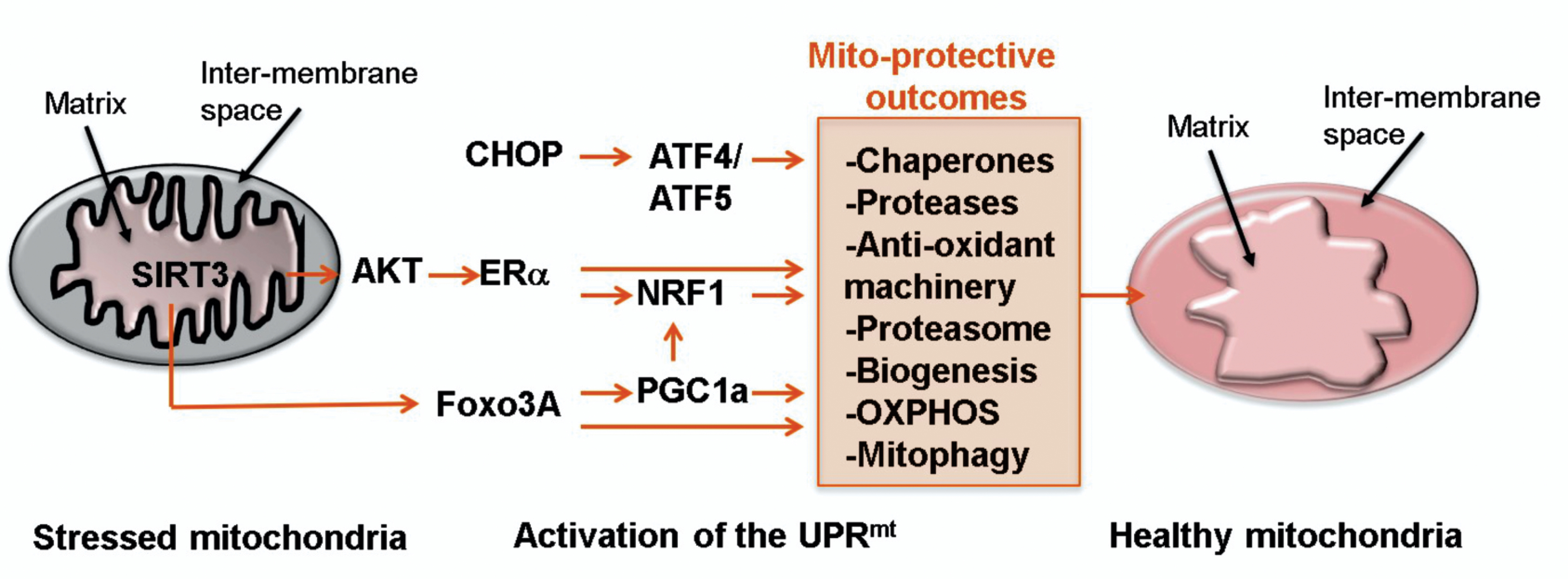
Diagram of the three axes of the mitochondrial unfolded protein response (UPRmt) that are activated upon stress (illustrated by orange star shapes) on the left side and resulting in several mitochondrial protective outcomes leading to restoration of healthy mitochondria (right).
The ER axis the UPRmt: coordinated regulation of mitochondrial metabolism and cytosolic proteostasis
In addition to the CHOP axis of the UPRmt, the ERα is activated upon accumulation of misfolded proteins and ROS in the mitochondria 1. ROS was found to be essential in the activation of the ERα under mitochondrial stress conditions, as treatment of cells with N-acetyl-cysteine (NAC) abolished the activation of the ERα 1. Further, ROS was necessary for the activation of AKT and in turn, AKT was necessary for the activation of the ERα 1. Down-stream of the activation of the ERα, as first reported by the Klinge group 41, the transcription factor NRF1 is up-regulated upon mitochondria stress 1. NRF1 is a key transcription factor implicated in mitochondrial biogenesis and metabolism 42,43. Further, the activity of the proteasome was also found to be up-regulated 1. The link between the proteasome and the ERα is intriguing but is entirely consistent with numerous reports that inhibition of the proteasome adversely affects the mitochondria 44–46. Therefore, in addition to the large number of genes regulated by the ERα that are implicated in cell cycle progression and proliferation, the transcriptional program regulated by the ERα includes genes implicated in mitochondrial biogenesis and cytosolic proteastasis. This latter set of genes appears to be transcribed however only when the ERα is phosphorylated by AKT.
The ERα axis of the UPRmt was found to be a distinct axis from the CHOP axis of the UPRmt based on the findings that despite both axes being activated by the same mitochondria stress, inhibition of the ERα by shRNA did not affect the up-regulation of CHOP or its down-stream targets upon mitochondrial stress conditions 1. Conversely, inhibition of CHOP did not abolish the activation of the ERα and its down-stream targets 1.
The SIRT3 axis of the UPRmt: coordinated regulation of mitophagy, mitochondrial biogenesis as well as the anti-oxidant machinery.
Since luminal cells but not basal cells of the breast express the ERα, following the discovery of the ERα axis of the UPRmt, one pending question that arose was the mechanism by which ERα negative breast cancer cells survive elevated ROS in response to mitochondrial stress. This line of investigation led to the discovery of the SIRT3 axis 2,47. SIRT3 was found to regulate a distinct axis from the CHOP and ERα axes of the UPRmt, based on the observation that inhibition of either did not affect SIRT3 and its down-stream targets and vice versa 2.
The SIRT3 axis of the UPRmt orchestrates a multi-functional response aimed at reducing mitochondrial stress, which includes the activation of anti-oxidant SOD2 at the mRNA level through transcription by FOXO3A and also at the protein level through its deacetylation by SIRT3 2. The SIRT3 axis also regulates mitophagy of irreversibly damaged mitochondria as well as NRF1 indicating that both the ERα and the SIRT3 axes regulate mitochondrial biogenesis 2.
Importantly for the hypothesis presented in this perspective, inhibiting SIRT3 expression in basal cells that do not express the ERα leads to excessive ROS and cell death 2. In contrast, inhibition of SIRT3 in luminal ERα positive cells had limited effect on the survival of these cells under mitochondrial stress conditions 2.
The importance of this later observation arises from its potential impact on the fact that older women, who have lower level of SIRT3, tend to develop ERα positive breast cancer. In support of this possibility, the SIRT3 knockout mice develop exclusively ERα positive mammary tumors 27.
The integral view of the UPRmt and its potential role in defining breast cancer sub-type with age
These observations support the hypothesis that upon transformation and elevation in ROS in epithelial cells of the breast that lack SIRT3, the maintenance of the integrity of the mitochondria becomes dependent of the ERα axis of the UPRmt.
As the mitochondria are essential to produce metabolites to generate the building blocks of cellular mass: amino acids, lipids and nucleotides, despite the fact that the mitochondria of cancer cells are less efficient at producing ATP, their integrity must be preserved in order for a cancer cell to grow and proliferate. It is in this setting that the essential housekeeping function of the UPRmt may become mandatory. Further, by being composed of parallel axes, such multi-axes pathway allows for compensatory mechanism to maintain mitochondrial integrity despite the failure of one of the axis.
While the role of the CHOP axis is not clear in relation to breast cancer in elderly women, this axis appears to be activated very early during transformation and remains activated at all stages of tumor progression 48. Therefore, the CHOP axis may also play a general role in maintenance of the mitochondrial network without being specific for luminal or basal cells in the ductal tree of the breast.
The SIRT3 axis and metastasis: a potential mechanism that contributes to the more aggressive breast cancers in young women?.
In cancer, SIRT3 has been reported both as an oncogene 49–53 and as a tumor suppressor 27,54–58, therefore creating confusion regarding its role in cancer. For instance, SIRT3 was reported to be decreased or absent in 87% of breast cancers and deleted in 20% of all human cancers and 40% of breast cancer 59. This reduction in SIRT3 leads to increase in ROS and the stabilization of HIF1α, which promotes a switch to glycolysis and contribute to the Warburg 59. Subsequently, our group also found that SOD2 levels are decreased in breast cancer upon activation of the oncogene Ras 2. Taken together, these results suggest that a moderate increase in ROS levels may be necessary for tumor initiation. Importantly however, deletion of SIRT3 gene was reported to be heterozygous suggesting that a selective pressure is taking place to maintain one copy of SIRT3 intact. Since the SIRT3 axis of the UPRmt induces NRF1, the antioxidant machinery and mitophagy to maintain mitochondrial integrity 2, the retention of one wild type copy of SIRT3 in cancer cells may be necessary for the activation of this axis of the UPRmt upon mitochondrial proteotoxic stress.
Therefore, the oncogenic function and tumor suppressor functions of SIRT3 may be reconcile by acting as a rheostat of ROS levels in cancer cells. Initially, SIRT3 and SOD2 levels decrease to up-regulate ROS and mediate the Warburg effect. However, under increased stress conditions, to avoid ROS levels to raise to excessive levels and induce cell death, the SIRT3 axis of the UPRmt would be activated to reduce ROS levels below a threshold that is compatible with mitochondrial function and maintain cell viability. In support of this idea, it has been reported that SIRT3 is overexpressed in highly metabolic tissues such as the heart 60,61, and in lymph node-positive breast cancer suggesting a need for SIRT3 during stress conditions in disease progression such as metastatic dissemination 62.
The idea that ROS levels in a cancer cells must be elevated but not to excess, a goldilocks-like phenomenon, is not without precedent. This proposed model is reminiscent of mitochondrial hormesis and the dual effect of ROS during aging. In the setting of aging, moderate levels of ROS are protective by activating cytoprotective responses however excessive ROS levels accelerate aging and decrease cell viability.
Importantly for this perspective however, age has never been considered in these studies. A diagram of the hypothetical dual role of SIRT3 is shown in figure 3. On one hand, SIRT3 is down regulated in older women and as stated above this decline may impose a selective pressure for the transformation of luminal cells due to their ability to rely on the ERα axis of the UPRmt to maintain mitochondrial fitness and cancer cell survival.
Figure 3.
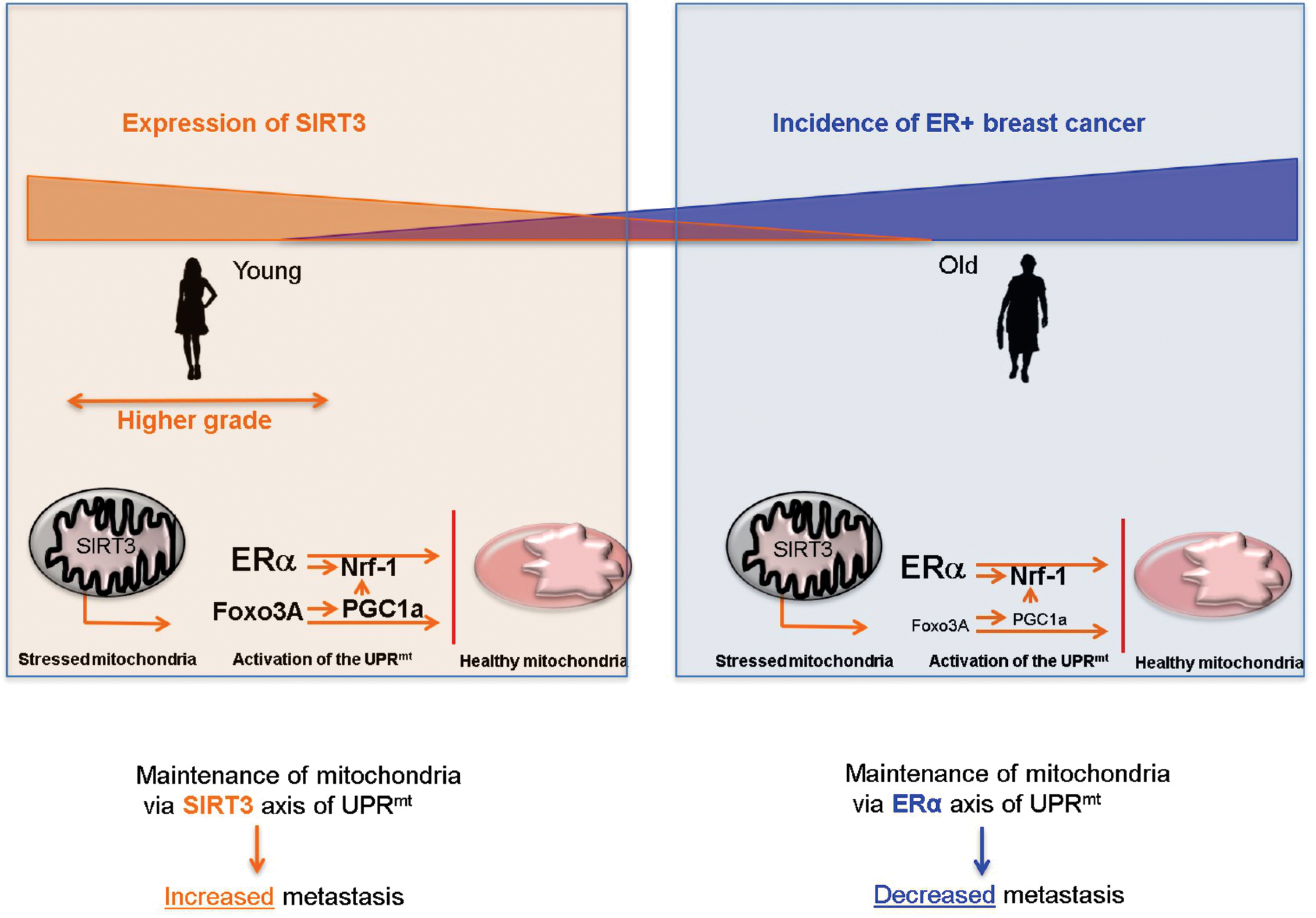
Hypothetical model of how young women (orange box) with high level of SIRT3 may maintain mitochondrial fitness through the SIRT3 axis of the UPRmt, and develop more aggressive tumors, while older women (blue box) rely on the ERα axis of the UPRmt and therefore develop mainly luminal and less aggressive breast cancers.
On the other hand, SIRT3 levels are high in both basal and luminal cells in young women. However, basal cells are more proliferative and invasive such that upon transformation of both cell types, over time basal cells overgrow the luminal cells resulting in a mainly basal cancer. In support of this possibility, we recently found using a gene signature of the SIRT3 axis of the UPRmt to inquire a large dataset of over 1800 breast cancer patients that the SIRT3 axis is significantly higher in triple negative breast cancer sub-type and inversely correlates with ERα status 63,64. Further, the SIRT3 axis signature was associated with increased rate of metastasis 63,64. Therefore, we hypothesize that these findings may contribute to the observation that young women tend to develop ERα negative breast cancers that are more aggressive (Fig. 3).
Concluding remarks:
The counterintuitive trend for older women to develop ERα positive breast cancers and younger women to develop ERα negative breast cancers remains a mystery. In this perspective, we propose a hypothesis that is centered on the critical importance to maintain mitochondrial function in cancer cells. One key pathway involved in the maintenance of the mitochondria is the UPRmt and both the ERα and SIRT3 have been shown to play key roles in this signaling cascade. Since SIRT3 declines with age and the gene signature of the SIRT3 axis of the UPRmt is associated with metastasis, higher level of SIRT3 may explain why younger women develop more aggressive tumors.
Acknowledgements:
This work was supported by NIH RO1AG059635 to D.G
Footnotes
Conflicts of interest: The authors confirmed they have no conflict of interest.
References
- 1.Papa L, Germain D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J Cell Sci 2011;124:1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol 2014;34:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenny TC, Hart P, Ragazzi M, et al. Selected mitochondrial DNA landscapes activate the SIRT3 axis of the UPRmt to promote metastasis. Oncogene 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenny TC, Germain D. mtDNA, Metastasis, and the Mitochondrial Unfolded Protein Response (UPRmt). Front Cell Dev Biol 2017;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenny TC, Manfredi G, Germain D. The Mitochondrial Unfolded Protein Response as a Non-Oncogene Addiction to Support Adaptation to Stress during Transformation in Cancer and Beyond. Front Oncol 2017;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenny TC, Germain D. mtDNA, Metastasis, and the Mitochondrial Unfolded Protein Response (UPR(mt)). Front Cell Dev Biol 2017;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell 2015;58:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab 2015;26:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose G, Dato S, Altomare K, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol 2003;38:1065–70. [DOI] [PubMed] [Google Scholar]
- 10.Albani D, Ateri E, Mazzuco S, et al. Modulation of human longevity by SIRT3 single nucleotide polymorphisms in the prospective study “Treviso Longeva (TRELONG)”. Age (Dordr) 2014;36:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker KJ, McClelland RA, Candlish W, Blamey RW, Nicholson RI. Heterogeneity of oestrogen receptor expression in normal and malignant breast tissue. Eur J Cancer 1992;28:34–7. [DOI] [PubMed] [Google Scholar]
- 12.Battersby S, Robertson BJ, Anderson TJ, King RJ, McPherson K. Influence of menstrual cycle, parity and oral contraceptive use on steroid hormone receptors in normal breast. Br J Cancer 1992;65:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd M, Hildebrandt RH, Bartow SA. Expression of the estrogen receptor gene in developing and adult human breast. Breast Cancer Res Treat 1996;37:243–51. [DOI] [PubMed] [Google Scholar]
- 14.Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol 1999;188:237–44. [DOI] [PubMed] [Google Scholar]
- 15.Walker RA, Martin CV. The aged breast. J Pathol 2007;211:232–40. [DOI] [PubMed] [Google Scholar]
- 16.Ruff M, Gangloff M, Wurtz JM, Moras D. Estrogen receptor transcription and transactivation: Structure-function relationship in DNA- and ligand-binding domains of estrogen receptors. Breast Cancer Res 2000;2:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupien M, Brown M. Cistromics of hormone-dependent cancer. Endocr Relat Cancer 2009;16:381–9. [DOI] [PubMed] [Google Scholar]
- 18.Bhat-Nakshatri P, Wang G, Appaiah H, et al. AKT alters genome-wide estrogen receptor alpha binding and impacts estrogen signaling in breast cancer. Mol Cell Biol 2008;28:7487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu Rev Med 2016;67:11–28. [DOI] [PubMed] [Google Scholar]
- 20.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011;13:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest 2008;118:3762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 2006;8:1765–74. [DOI] [PubMed] [Google Scholar]
- 23.Kitagishi Y, Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging (Review). Int J Mol Med 2013;31:511–5. [DOI] [PubMed] [Google Scholar]
- 24.Lombard DB, Alt FW, Cheng HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 2007;27:8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Nagasawa K, Munch C, et al. Mitochondrial Sirtuin Network Reveals Dynamic SIRT3-Dependent Deacetylation in Response to Membrane Depolarization. Cell 2016;167:985–1000 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Ren X, Gowda AS, et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis 2013;4:e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HS, Patel K, Muldoon-Jacobs K, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 2010;17:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010;40:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vargas-Ortiz K, Perez-Vazquez V, Diaz-Cisneros FJ, et al. Aerobic Training Increases Expression Levels of SIRT3 and PGC-1alpha in Skeletal Muscle of Overweight Adolescents Without Change in Caloric Intake. Pediatr Exerc Sci 2015;27:177–84. [DOI] [PubMed] [Google Scholar]
- 30.Palacios OM, Carmona JJ, Michan S, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebert AS, Dittenhafer-Reed KE, Yu W, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 2013;49:186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 2020;21:421–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oakes SA. Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am J Pathol 2020;190:934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldridge JE, Horibe T, Hoogenraad NJ. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One 2007;2:e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS One 2007;2:e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinus RD, Garth GP, Webster TL, et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem 1996;240:98–103. [DOI] [PubMed] [Google Scholar]
- 37.Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem 2007;76:701–22. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. Embo J 2002;21:4411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr Biol 2016;26:2037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teske BF, Fusakio ME, Zhou D, et al. CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis. Mol Biol Cell 2013;24:2477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 2008;22:609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem 2006;97:673–83. [DOI] [PubMed] [Google Scholar]
- 43.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 2008;88:611–38. [DOI] [PubMed] [Google Scholar]
- 44.Myeku N, Duff KE. Targeting the 26S Proteasome To Protect Against Proteotoxic Diseases. Trends Mol Med 2018;24:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deger JM, Gerson JE, Kayed R. The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration. Aging Cell 2015;14:715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim J, Yue Z. Neuronal aggregates: formation, clearance, and spreading. Dev Cell 2015;32:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenny TC, Hart P, Ragazzi M, et al. Selected mitochondrial DNA landscapes activate the SIRT3 axis of the UPR(mt) to promote metastasis. Oncogene 2017;36:4393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenny TC, Hart P, Ragazzi M, et al. Selected mitochondrial DNA landscapes activate the SIRT3 axis of the UPRmt to promote metastasis. Oncogene 2017;36:4393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George J, Nihal M, Singh CK, Zhong W, Liu X, Ahmad N. Pro-Proliferative Function of Mitochondrial Sirtuin Deacetylase SIRT3 in Human Melanoma. J Invest Dermatol 2016;136:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi J, Koh E, Lee YS, et al. Mitochondrial Sirt3 supports cell proliferation by regulating glutamine-dependent oxidation in renal cell carcinoma. Biochem Biophys Res Commun 2016;474:547–53. [DOI] [PubMed] [Google Scholar]
- 51.Alhazzazi TY, Kamarajan P, Joo N, et al. Sirtuin-3 (SIRT3), a novel potential therapeutic target for oral cancer. Cancer 2011;117:1670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SH, Ozden O, Jiang H, et al. Sirt3, mitochondrial ROS, ageing, and carcinogenesis. Int J Mol Sci 2011;12:6226–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei L, Zhou Y, Dai Q, et al. Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis 2013;4:e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Wang WY, Cao LP. SIRT3 inhibits cell proliferation in human gastric cancer through down-regulation of Notch-1. Int J Clin Exp Med 2015;8:5263–71. [PMC free article] [PubMed] [Google Scholar]
- 55.Dong XC, Jing LM, Wang WX, Gao YX. Down-regulation of SIRT3 promotes ovarian carcinoma metastasis. Biochem Biophys Res Commun 2016;475:245–50. [DOI] [PubMed] [Google Scholar]
- 56.Allison SJ, Milner J. SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways. Cell Cycle 2007;6:2669–77. [DOI] [PubMed] [Google Scholar]
- 57.Marfe G, Tafani M, Indelicato M, et al. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J Cell Biochem 2009;106:643–50. [DOI] [PubMed] [Google Scholar]
- 58.Desouki MM, Doubinskaia I, Gius D, Abdulkadir SA. Decreased mitochondrial SIRT3 expression is a potential molecular biomarker associated with poor outcome in breast cancer. Hum Pathol 2014;45:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finley LW, Carracedo A, Lee J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell 2011;19:416–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 2009;119:2758–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 2008;28:6384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashraf N, Zino S, Macintyre A, et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer 2006;95:1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kenny TC, Gomez ML, Germain D. Mitohormesis, UPR(mt), and the Complexity of Mitochondrial DNA Landscapes in Cancer. Cancer Res 2019;79:6057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kenny TC, Craig AJ, Villanueva A, Germain D. Mitohormesis Primes Tumor Invasion and Metastasis. Cell Rep 2019;27:2292–303 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


