Abstract
Measles and rubella vaccinations are highly effective at reducing disease prevalence; however, logistic issues related to subcutaneous administration and vaccine wastage limit the extent of vaccination coverage. Microneedle (MN) patches can increase coverage by easing logistics through simplified administration and improved stability. This study demonstrates the thermostability of a bivalent measles and rubella vaccine MN patch. Rubella vaccine stability required pH buffering during drying; potassium phosphate buffer at neutral pH was optimal for both vaccines. Screening 43 excipients for their ability to retain potency during drying and storage yielded sucrose-threonine-potassium phosphate buffer formulation at pH 7.5 as an optimal formulation. MN patches made with this formulation had no significant loss of vaccine titer after one month and remained within a one log10 titer loss cutoff after 3 – 4 months at 5°C, 25°C and 40°C. Finally, these patches were shown to be immunogenic in juvenile rhesus macaques. This work demonstrates the potential for MN patches for measles and rubella vaccination to be removed from the cold chain, which is expected to decrease vaccine cost and wastage, and increase vaccination coverage.
Keywords: microneedle patch, measles and rubella vaccines, vaccine thermostability, stability outside cold chain, formulation
Graphical Abstract
Microneedle patches can simplify measles and rubella vaccination and thereby increase access to these vaccines, especially in low-resource settings. An optimized bivalent microneedle patch formulation enables measles and rubella vaccines to retain potency for one month, and remain within one log10 loss of titer for 3 – 4 months at 5°C, 25°C or 40°C.
1. Introduction
Measles and rubella (MR) elimination efforts rely on vaccines to protect the population, and vaccination coverage levels of over 95% are necessary to interrupt viral transmission by herd immunity [1]. However, vaccination rates for measles and rubella have stagnated over the past several years [2, 3], contributing to 142,300 measles deaths in 2018, and an estimated 100,000 children born with congenital rubella syndrome (CRS) in 2010 [3–6]. In 2012, the Measles and Rubella Initiative, a group of UNICEF, World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), the United Nations Foundation, and the American Red Cross, set regional elimination goals for 2015 [4, 7]. These goals were not achieved in part due to the logistical constraints of the current vaccination strategy to reach all populations needing vaccination [4].
In low-resource settings where most measles and rubella cases occur, MR vaccines are usually stored in lyophilized multi-dose vials and administered by subcutaneous administration. This requires vaccine administration by health care professionals and a robust cold chain to maintain refrigerated conditions needed for vaccine stability, which limits access to vaccination among populations in developing-country or hard-to-reach communities [8]. Before injection, the lyophilized vaccine vials must be correctly reconstituted, which requires additional expertise, and reconstitution errors have tragically led to many deaths [9]. The needle and syringe used to administer the vaccine must be properly discarded after use as biohazardous sharps waste to prevent injury to the patient, health care worker or others [10].
The need to maintain the cold chain is expensive and leads to further vaccine wastage. The current lyophilized vaccine is stable at 4°C for up to two years, and studies have shown stability for as much as two to four weeks at 37°C [11]. Breaks in the cold chain may lead to vaccine failures, possibly leading to disease outbreaks [12, 13]. Due to gaps in the cold chain, approximately half of measles vaccine vials had below the minimum dose potency when tested in Brazil [14] or Nigeria [15]. One review estimated that 75–100% of shipments are exposed to freezing temperatures [16]. Once reconstituted, within one hour, approximately half of the potency is lost if the vaccine is stored at 20°C, while almost all of the potency is lost if kept at 37°C [17, 18]. Therefore, vials must be discarded after 6 hours due to this loss of potency. The wastage factor or the number of vaccine doses wasted per dose administered is estimated at 3.4 for routine vaccination and 1.1 for supplemental immunization activities [19].
The World Health Organization (WHO) requires new MR vaccines technologies to withstand storage for one week at 37°C without loss of more than one log10 of titer [4, 20, 21]. Due to the complexity of administration, house-to-house vaccination campaigns are limited, despite their ability to increase vaccination coverage [22]. Therefore, in order to reach the next set of regional and global elimination goals, there is a need for a novel vaccination technology that is single dose, eliminates biohazardous sharps waste, is easily administered, and maintains thermostability outside the cold chain [17, 18, 23].
Microneedle (MN) patches provide a novel vaccine delivery method with the potential to increase vaccination coverage. MN patches consist of an array of water-soluble, solid, conically shaped needles measuring hundreds of microns in length and attached to a patch backing [24–27]. MNs are made of polymers, sugars, and other water-soluble, safe excipients that encapsulate the vaccine during storage and delivery [28]. Once MNs painlessly pierce the upper layers of skin, they fully dissolve, deliver the vaccine, and produce no sharps waste [29–33]. MN patches are available in single dose-single use packaging, reducing vaccine wastage and easing administration [18]. Once dissolved, the MN patches cannot be re-used, which increases the safety of the vaccinator and the patient and reduces the waste that must be moved from the vaccination site. Additionally, these patches can be administered by minimally trained personnel, again reducing costs associated with each vaccination and making vaccines better available in low-resource settings lacking health care infrastructure [18, 34, 35]. Finally, vaccines is incorporated into MN patches in a dried state, which can enable them to have improved thermostability compared with liquid vaccines without the need for reconstitution [36–40]. However, each vaccine that is incorporated into a MN patch requires its own formulation to stabilize the vaccine during patch manufacturing and storage [36, 41].
The current MR vaccine is packaged in lyophilized vials. These vials must be shipped between 2 and 8°C. Other methods of measles vaccination have employed carbon dioxide-assisted nebulization with bubble dryer (CAN-BD) and spray drying to develop stabilize the vaccine. CAN-BD creates a dry powder with about half the water content of lyophilized vaccines that can be delivered via aerosol into the lungs. Measles vaccine powder with myo-inositol and sugars saw a 0.6 log10 loss during storage for one week at 37°C [42, 43]. Spray drying can increase manufacturing throughput compared to lyophilized vials but must be reconstituted before administration. A combination of sucrose, trehalose, arginine, human serum albumin, and divalent cations stabilized measles vaccine for 8 weeks at 37°C for one log10 loss with no loss observed over that time at 4°C [44]. To our knowledge, no study has been performed to optimize the stability of both measles and rubella vaccines together outside the cold chain, and in general rubella vaccine stability has received much less attention compared to measles vaccine.
In previous work, MN patches were developed to deliver measles vaccine [17, 18, 23]. These patches were thermostable with one log 10 loss of titer after four months at 40°C and were immunogenic in cotton rats and rhesus macaques [35, 39]. As global measles eradication efforts ramp up, there is a focus to include rubella vaccinations as part of the measles vaccination campaigns [8]. To that end, novel formulations should be developed to incorporate both measles and rubella vaccines into a single MN patch. Recently, we presented data on use of the MR patch developed in this study, showing it to be immunogenic in infant rhesus macaques [45]. Here, we present a detailed study on the development of this MR patch that focuses on vaccine stability using a methodical screen to identify formulations that maintain stability of a bivalent MR patch during manufacturing and at elevated temperatures for at least one month. We assessed immunogenicity of the optimized MR patch in juvenile rhesus macaques to confirm results from in vitro screening. We also demonstrated that these patches exceed the WHO requirements for novel MR vaccines.
2. Results
2.1. pH and buffers
To optimize formulations for MR vaccine MN patches, we first wanted to determine the effect of pH on MR vaccine stability during drying, since pH is known to affect virus stability (Figure 1a and 1b). Potassium phosphate buffer was selected due to its ability to buffer across the selected pH range of 6.0 – 8.0. We found no significant loss of measles vaccine titer after drying over the pH range studied (Figure 1a, ANOVA, p=0.764). On the other hand, rubella vaccine titer was significantly lower after drying at low pH or in unbuffered water (ANOVA, p<0.0001). Drying rubella vaccine at pH 7.0 or 7.5 was significantly improved compared to drying in water but not statistically different from one another (Figure 1b, p=0.999 and 0.634 for measles and rubella, respectively). Drying at the optimal pH values of 7.0 or 7.5 resulted in no significant loss of titer for rubella vaccine but approximately one order of magnitude loss for measles vaccine.
Figure 1:
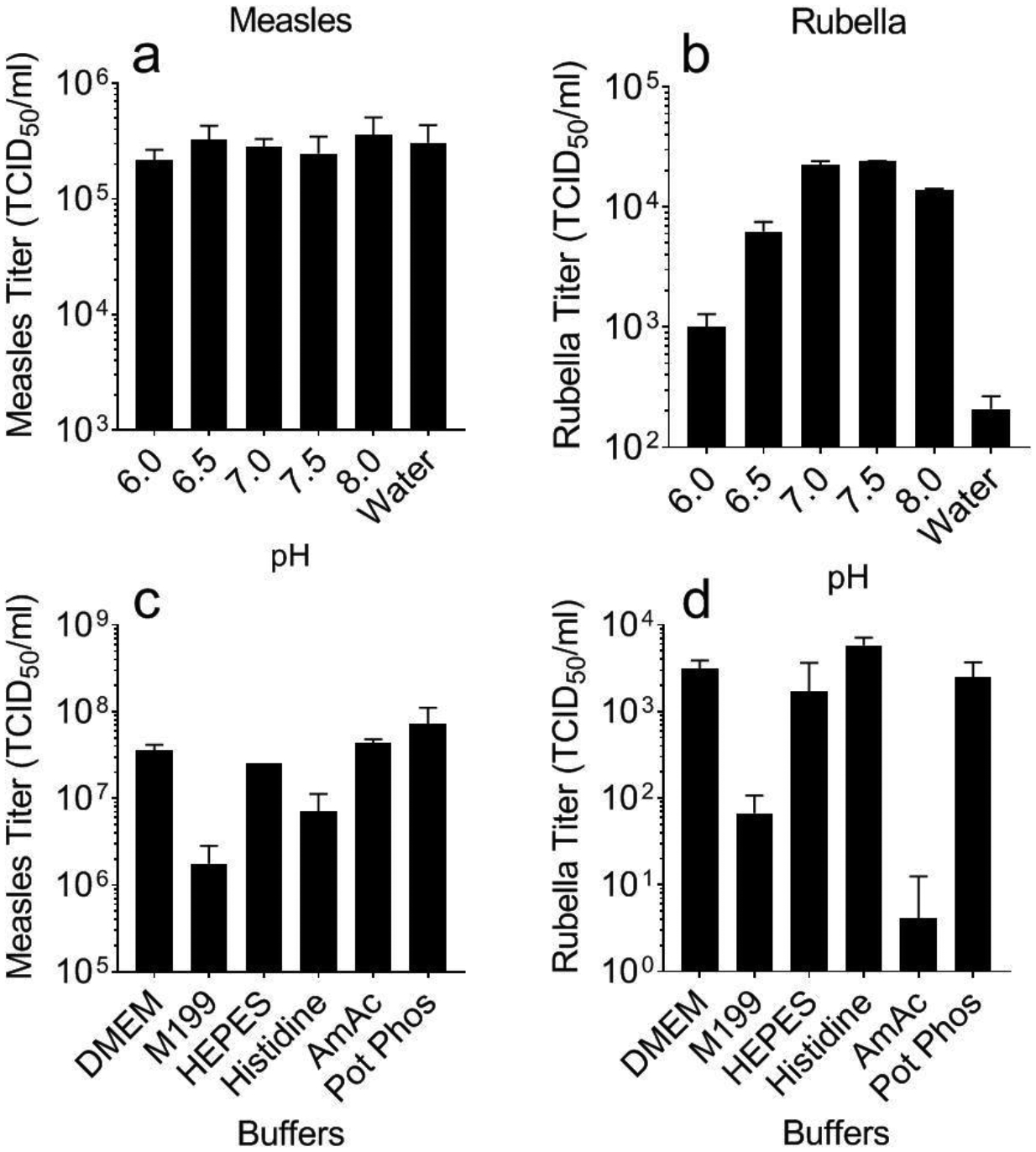
Effect of pH and buffer on measles and rubella vaccine titer after drying. Monovalent measles (a, c) or rubella (b, d) vaccine solutions were cast into Eppendorf tubes and dried as films for ~18 h at room temperature (20 – 25°C). pH was varied between 6.0 and 8.0 during drying in potassium phosphate buffer or in deionized water (a, b). Buffer composition was varied during drying at pH 7.5 (c,d). All formulations contained 10% sucrose and 1% CMC. Data are expressed as mean ± standard deviation based on 2–4 replicates each. Statistical analysis was performed by ANOVA, with p < 0.05 considered significant; specific findings from this analysis are reported in the text. DMEM: Gibco Dulbecco’s Modified Eagle Medium; M199: Gibco Media 199; HEPES: (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); AmAC: ammonium acetate buffer; Pot Phos: potassium phosphate buffer.
Although potassium phosphate buffer was effective, we next wanted to optimize the choice of buffers. Six buffers with buffering capacity around pH 7.0 and 7.5 were selected and screened for titer after drying (Figure 1c and 1d). For measles vaccine, M199 and histidine buffers had lower titer compared to the other four buffers (Figure 1c, ANOVA, p=0.031). For rubella vaccine, M199 and ammonium acetate buffers had lower titer upon drying (Figure 1d, ANOVA, p=<0.0001); a one-way ANOVA analysis comparing DMEM, HEPES, and potassium phosphate buffers showed that they were not significantly different in their ability to stability measles or rubella vaccines (p=0.408). Based on these data, potassium phosphate at a pH of 7.5 was selected as the buffer to use in subsequent experiments because it showed good results with both vaccines and is the buffer currently used in commercial MR vaccine-containing products [46].
2.2. Single excipients during drying
Forty-three excipients, such as sugars, amino acids, proteins, and salts, were selected from literature on the drying of proteins, vaccines and other biologics by lyophilization and other drying methods, and from ingredients lists of currently approved vaccines. Each excipient in potassium phosphate buffer (pH 7.5) was mixed with measles or rubella vaccine, and solutions were dried overnight in Eppendorf microcentrifuge tubes under room temperature vacuum and in the presence of desiccant. The remaining titer of rubella vaccine after drying (Figure 2, grey bars) shows that most excipients were able to maintain measurable level of titer, and a number of them maintained close to 100% potency. Measles vaccine, however, was more susceptible to damage due to drying, and many fewer excipients were able to maintain measles vaccine titer after drying (black bars). Excipients that maintained titer above the detection limit for both vaccines after drying were selected to move onto the next phase.
Figure 2:
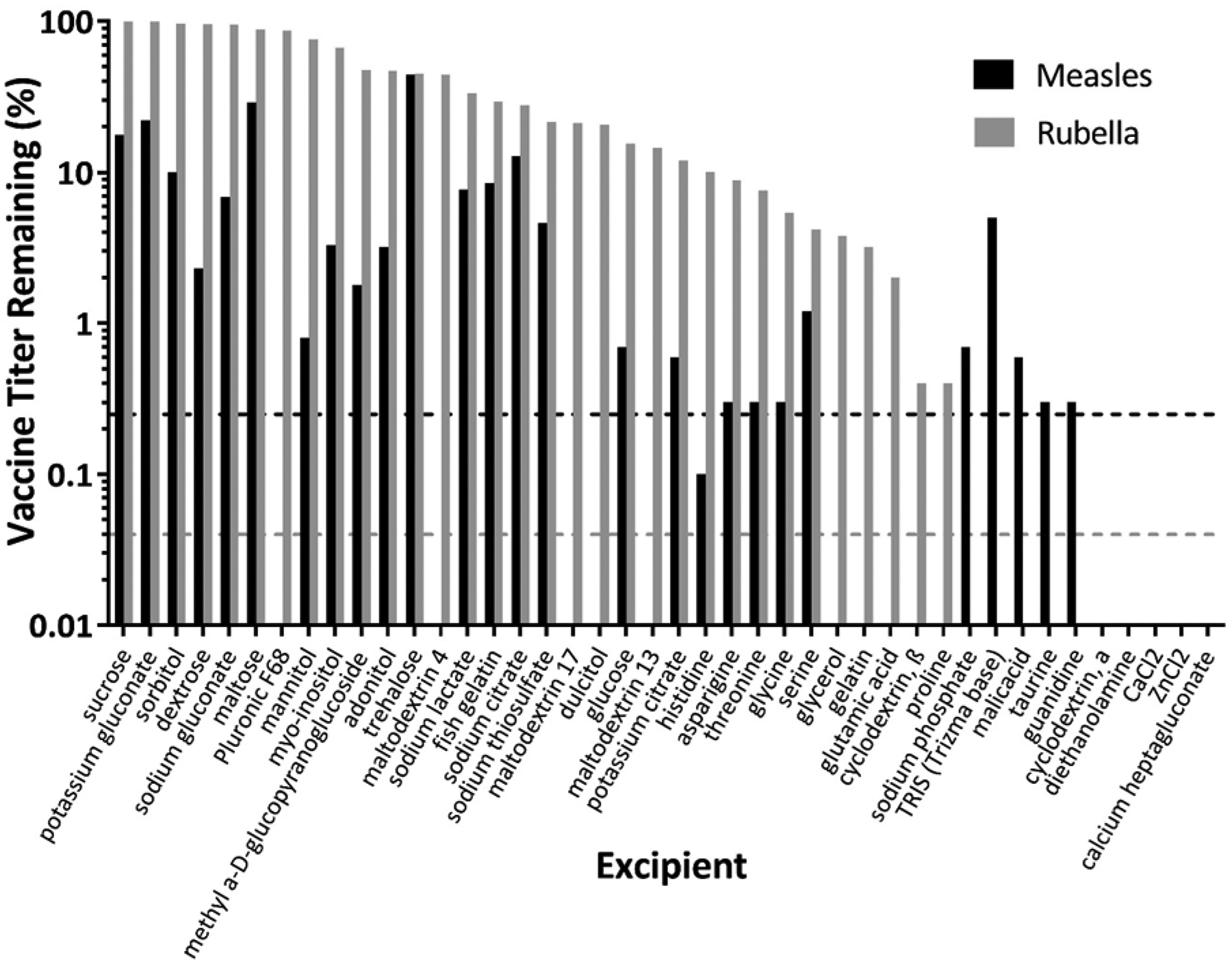
Effect of individual excipients on measles and rubella vaccine titer after drying. Individual excipients were mixed with monovalent measles or rubella vaccine samples and then dried as films for ~18 h at room temperature (20 – 25°C). All formulations contained 0.8 wt% potassium phosphate buffer (pH 7.5) and 1 wt% CMC. Vaccine titer was normalized to titer of liquid vaccine solutions before drying. The solid lines represent the lower detection limit for each assay (black for measles, grey for rubella). Data are expressed as mean ± standard deviation based on 2 replicates each. No statistical analysis was performed on these data.
2.3. Single excipients during storage
We next studied the effect of excipients on thermostability of MR vaccines during storage at elevated temperatures. Single excipients were mixed with measles or rubella vaccine, dried overnight at room temperature, and then stored at 40°C with desiccant for one week. After seven days, only three excipients had detectable measles vaccine titer: histidine, sucrose, and trehalose. For rubella vaccine, roughly two thirds of the excipients had some titer by day 7.
In this study, we are examining two sources of titer loss: drying and storage. While some excipients were effective at maintaining stability during drying (day 0), others were effective at maintaining stability during storage at elevated temperature (day 7). To separate the two effects and identify best candidates for stabilization during drying, titer at day 0 (Figure 3, black bar) was compared to the liquid control representing no loss of potency (Figure 3, dotted line) (Table S1 in Supporting Information (SI)). From this analysis, several excipients such as sorbitol, dextrose, and gluconate, were selected as stabilizers during drying. Other excipients were better at reducing titer loss during the storage at elevated temperatures. To assess vaccine titer loss during storage (independent of loss after drying), vaccine titer after one-week storage at 40°C was compared to the titer after just drying at day 0 (Figure 3, Table S1 in SI). This analysis identified the top excipients for drying as asparagine, maltose, sorbitol, potassium gluconate, dextrose, and sodium gluconate; the top storage excipients identified were histidine, sucrose, and trehalose.
Figure 3:
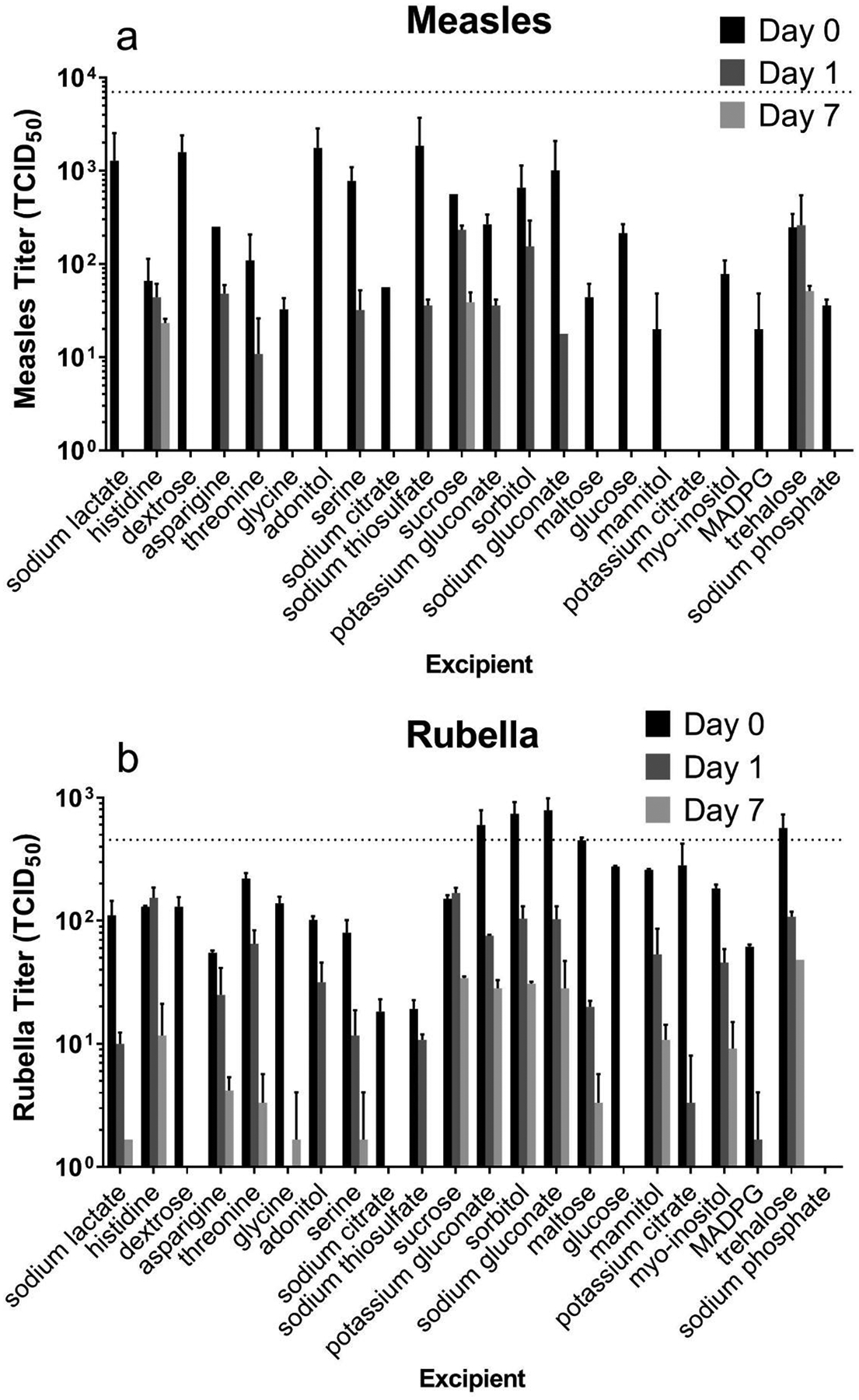
Effect of individual excipients on measles and rubella vaccine titer after drying and storage at 40°C for up to one week. Individual excipients were mixed with monovalent measles (a) or rubella (b) vaccine samples, dried as films for ~18 h at room temperature (20 – 25°C) and then stored at 40°C. All formulations contained 0.8 wt% potassium phosphate buffer (pH 7.5) and 1 wt% CMC. Day 0 represents stability after drying only. Titers were determined from 50 μl liquid or dried samples. The dotted lines indicate vaccine titer of liquid solutions before drying. Data are expressed as mean ± standard deviation based on 2 replicates each. No statistical analysis was performed on these data.
2.4. Excipient combinations
We hypothesized that by combining excipients, certain pairs of excipients would demonstrate better stability than either of the individual excipients alone. Stabilizers from the previous screen were selected for their ability to stabilize during drying and/or during storage. All stabilizers were combined in pairwise fashion and vaccine titers were compared at day 0 and day 7 after storage at 40°C to two controls: sucrose-threonine, which was the excipient formulation used in a previous study of measles vaccine MN patch stabilization [39] and the liquid control (Figure 4, dashed line).
Figure 4:
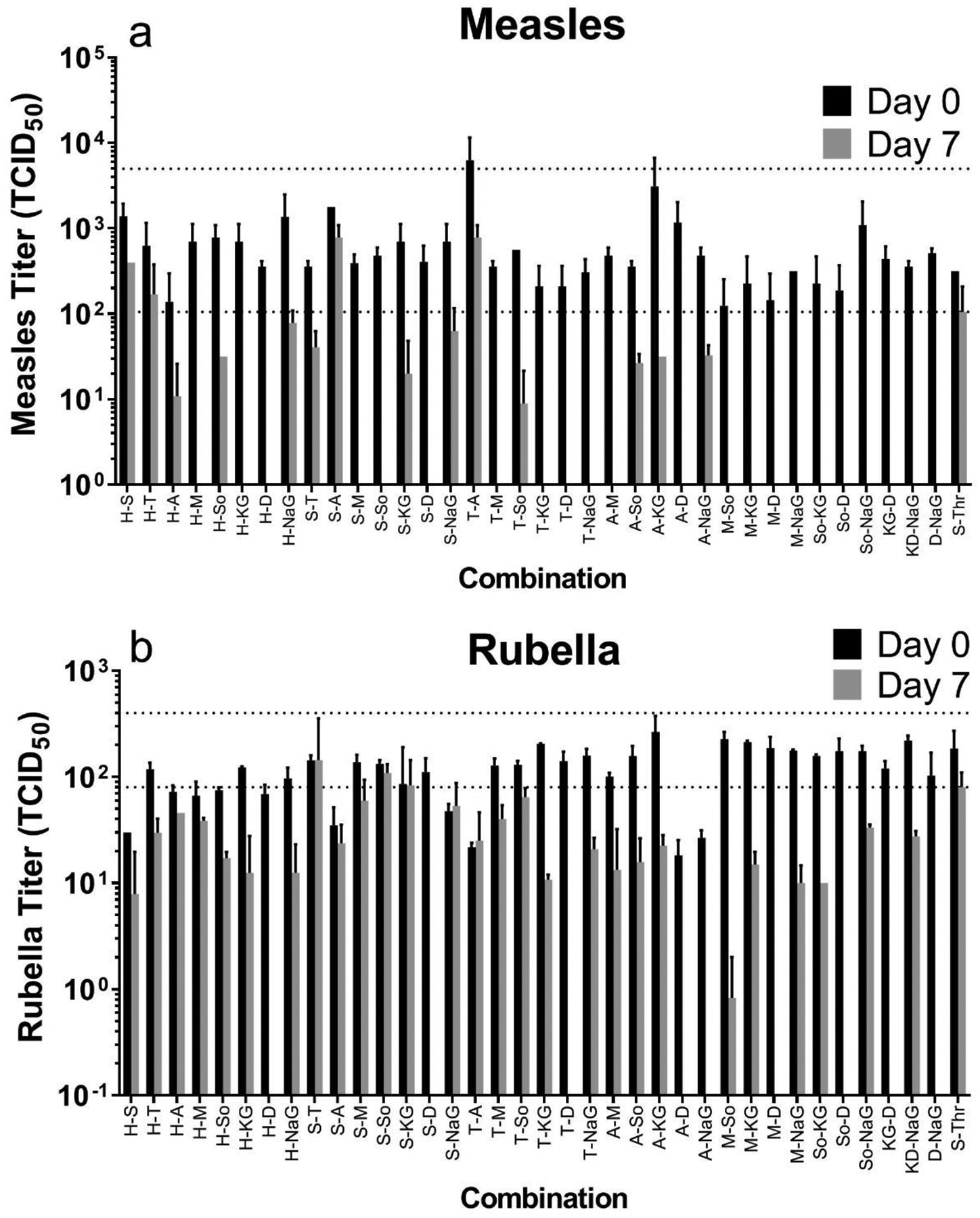
Effect of combinations of two excipients on measles and rubella vaccine titer after drying and storage at 40°C for one week. Combinations of two excipients were mixed with monovalent measles (a) or rubella (b) vaccine samples, dried as films for ~18 h at room temperature (20 – 25°C) and then stored at 40°C. All formulations contained 0.8 wt% potassium phosphate buffer (pH 7.5) and 1 wt% CMC. Titers were determined from 50 μl liquid or dried samples. The dotted lines indicate vaccine titer of liquid solutions before drying (upper line) and the dried sucrose-threonine formulation at Day 7 (lower line). Day 0 represents stability after drying only. Data are expressed as mean ± standard deviation based on 2 replicates each, which does not provide sufficient data for rigorous statistical comparison. However, we identified trends in the data for formulation selection using Student’s t-test (with p < 0.05 considered significant), as shown in Data tables S2 and S3. Abbreviation key can be found in Data tables S2 and S3.
Three excipient combinations – i.e., histidine- sucrose, histidine- sodium gluconate, trehalose- asparagine – showed no significant loss in titer between stock vs. day 0 (drying) and day 0 vs day 7 (storage) (Student’s t-tests, p>0.05, Tables S2 and S3 in SI). We also compared the remaining titer at day 7 to the titer of sucrose-threonine at day 7. Several combinations had higher titer for either measles or rubella vaccine, but no combinations had higher mean titers for both vaccines compared to sucrose-threonine. Taken together, this screening process identified combination excipient formulations that can retain potency of both measles and rubella vaccines for at least one week at 40°C.
2.5. Storage in microneedle patches
Until now, stability was studied by casting solutions into tubes. In the final study, we wanted to look at the stability of MR vaccines in MN patches over a range of temperatures. MN patches were fabricated with 10% sucrose, 3% threonine, and 1% CMC in potassium phosphate buffer at pH 7.5. Patches were stored at 5, 25, or 40°C with desiccant. After one month, no significant titer loss was noted at 5, 25 or 40°C for either vaccine (Figure 5, Student’s t-test, p>0.05). By the two-month time point, rubella vaccine titer was significantly lower than day 0 (Figure 5b, Student’s t-test, p=0.037, 0.0026, and 0.010 for 5°C, 25°C, and 40°C, respectively ), while measles vaccine remained stable for 3 months at 5°C and 25°C (Figure 5a, Student’s t-test, p>0.01), but significantly lost titer after 3 months at 40°C (Figure 5a, Student’s t-test, p=0.006). For the first time, these data show that a bivalent MR vaccine MN patch can be stable at elevated temperatures for at least one month. This well surpasses the WHO requirements for stability at 37°C for one week. Additional tests will be needed to more fully establish MR vaccine stability using larger sample sizes.
Figure 5:
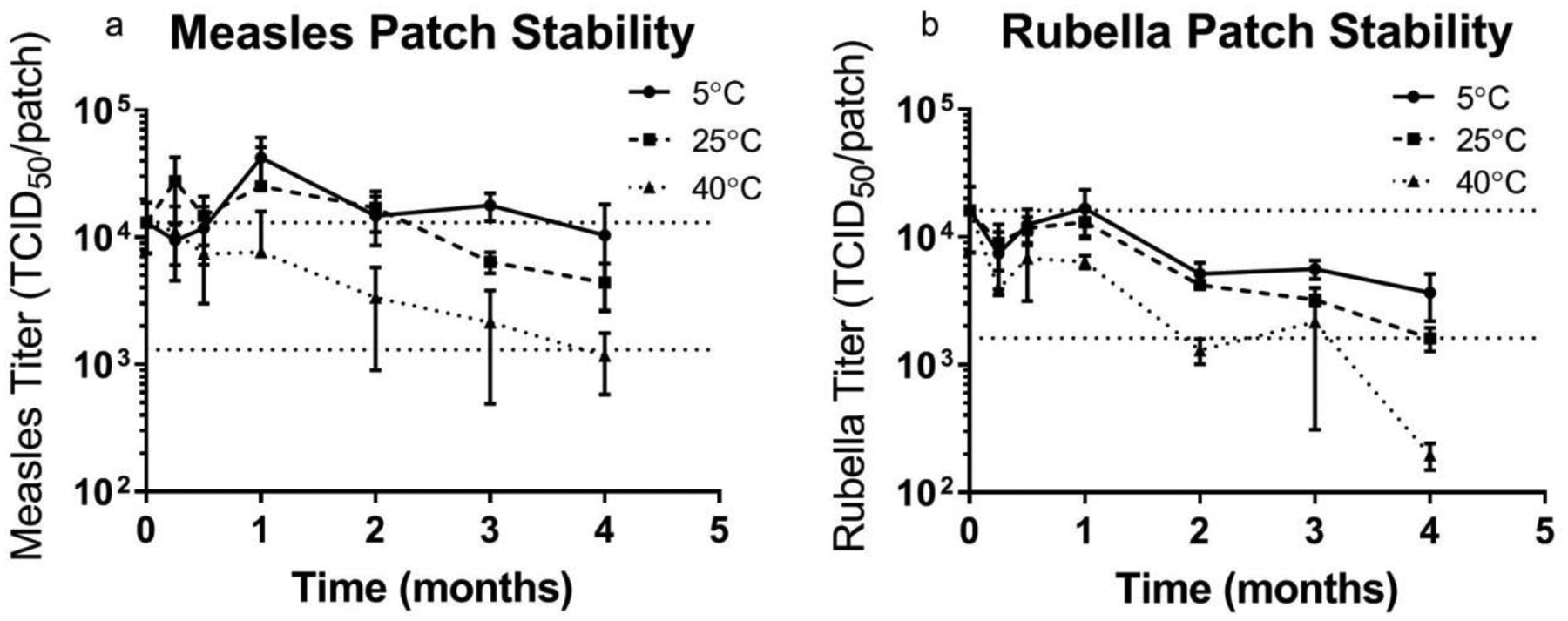
Stability of bivalent microneedle (MN) patches containing measles and rubella vaccines during extended storage. Patches were prepared using a formulation containing sucrose, threonine, and potassium phosphate buffer at pH 7.5 and then stored with desiccant at 5°C, 25°C or 40°C for up to 4 months. The upper horizontal dashed lines indicate vaccine titer of MN patches before storage and the lower horizontal dashed lines indicate vaccine titer equal to 10% of MN patch titer before storage. Data are expressed as mean ± standard deviation based on 4 replicates each. Statistical analysis was performed by Student’s t-test, with p < 0.05 considered significant; specific findings from this analysis are reported in the text.
Conventional lyophilized MR vaccines lose potency before expiration, i.e., often starting with a dose of ~104 TCID50 per 0.5 ml dose and remaining above 103 TCID50 per 0.5 ml dose before the end of the two-year shelf life [21, 46]. Guided by this analysis, both vaccines in the MR vaccine MN patch had less than a 10-fold drop in vaccine titer at 3 months of storage at all three temperatures, and both vaccines remained within one log10 of titer loss for 4 months at all temperatures other than rubella vaccine at 40°C.
2.6. Freeze thaw cycles
During vaccine transportation in the cold chain, vaccines may experience fluctuating temperatures, sometimes below 0°C. If packaged adjacent to ice packs, vaccines may be exposed to freezing conditions. To test vaccine stability in these cases, MN patches were exposed to one or three freeze-thaw cycles, where temperatures shifted from 5°C to −20°C every 90 min. The resulting titers were normalized by the titer for patches stored at 5°C during the same time (Figure 6). While measles vaccine appears to lose titer, there were no statistically significant differences in measles and rubella vaccine titer between the groups (ANOVA, p=0.25), indicating the MR vaccine MN patches may withstand the stresses of accidental freezing during storage.
Figure 6:
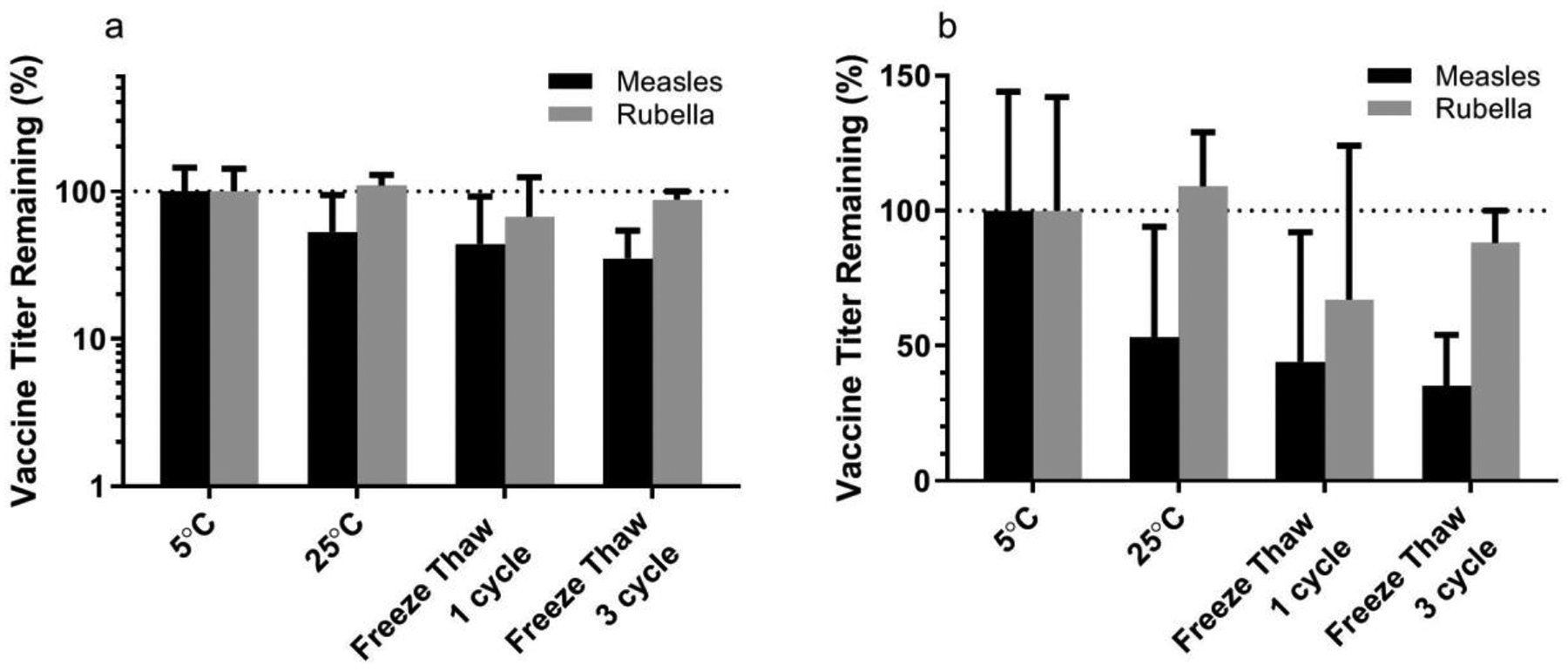
Stability of bivalent microneedle (MN) patches containing measles and rubella vaccines during freeze-thaw process presented with a (a) log-scale and (b) linear-scale y axis. Patches were prepared using a formulation containing sucrose, threonine, and potassium phosphate buffer at pH 7.5 and then cycled between frozen (−20°C) and thawed (5°C) conditions. The dashed lines indicate vaccine titer of MN patches stored at 5°C. Data are expressed as mean ± standard deviation based on 4 replicates each. Statistical analysis was performed by ANOVA, with p < 0.05 considered significant; specific findings from this analysis are reported in the text.
2.7. Rhesus macaques
While MR vaccine titer determined by TCID50 is expected to correlate with immunogenicity in vivo [47], we verified this expectation in this study by administering MR vaccine MN patches to rhesus macaques and comparing immune responses to those generated by conventional SC injection of the same MR vaccine at the same dose as the MN patch. Antigen-specific IgG titers were measured at the time of vaccination and one month after vaccination; there were no significant differences between IgG titers associated with MN patch vaccination and those associated with SC injection at either time point (Figure 7, Student’s t-test, p>0.22), although this study involved only eight animals and was not powered to find differences. This confirms that MR vaccination by MN patch using the optimized formulation was immunogenic.
Figure 7:
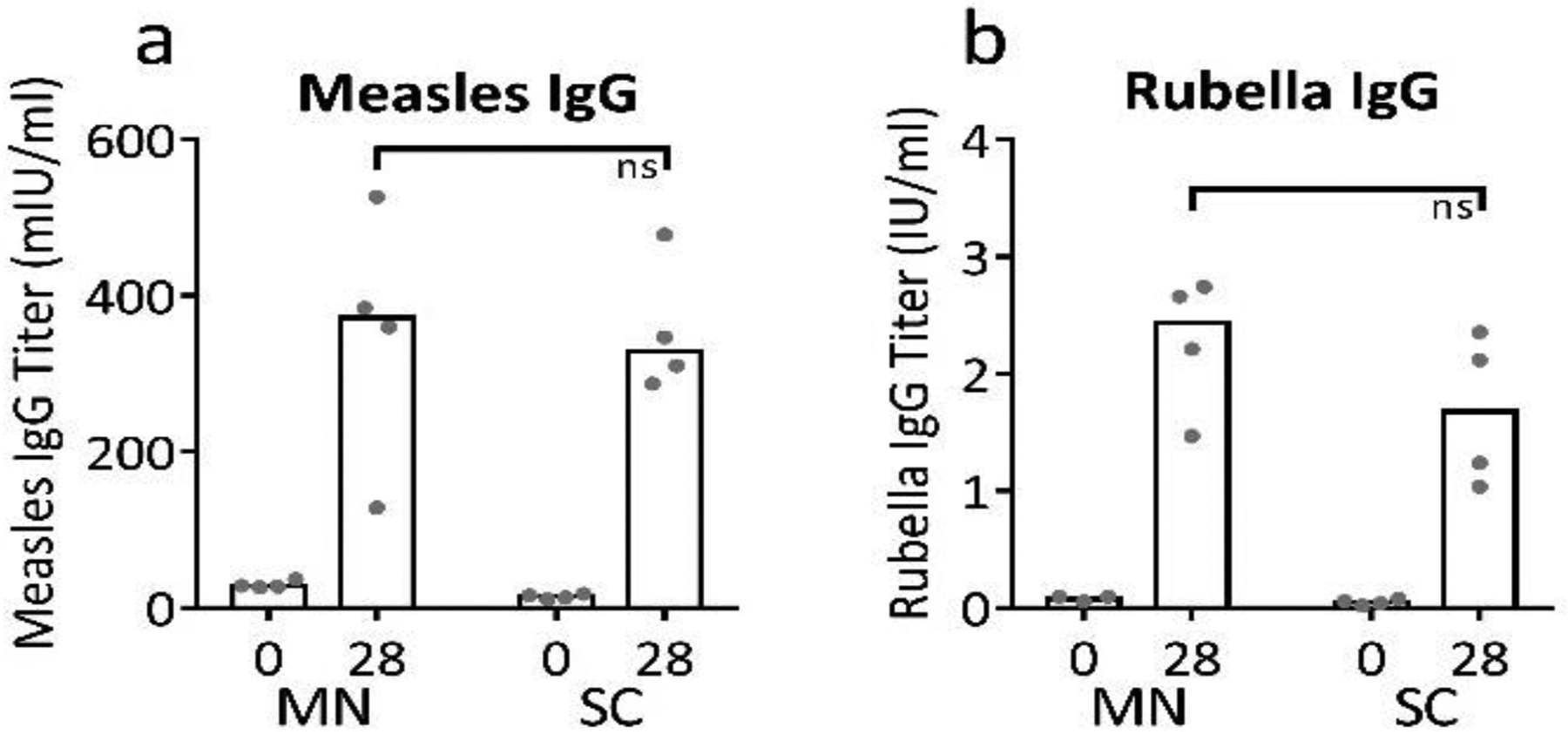
Immunogenicity of bivalent microneedle (MN) patches containing measles and rubella vaccines in non-human primates. Rhesus macaques were vaccinated by subcutaneous (SC) injection (n=4) or via MN patches formulated with sucrose, threonine, and potassium phosphate buffer at pH 7.5 (n=4). Serum IgG titers specific to measles (a) or rubella (b) were measured by ELISA. At 28 days post vaccination, titers between the two groups are not statistically different (Student’s t-test, p=0.95 and 0.22 for measles and rubella, respectively, ns = not significant). Data are expressed as bars to indicate the mean and with dots for individual rhesus macaques based on 4 replicates each.
3. Discussion
Licensed MR vaccines are safe and highly effective at prevention of disease. However, the current injection-based method to deliver the vaccines faces numerous challenges, limiting global access to the vaccine and hindering disease elimination efforts. One of the key challenges is the requirement to keep MR vaccine in the cold chain due to poor thermostability. In this study, we screened formulations that can provide increased thermostability that could allow MR vaccine MN patches to be taken at least partially out of the cold chain. To our knowledge, this is the first time that the thermostability of measles and rubella vaccines has been optimized together for the development of bivalent MN patches. Through our screening process, we identified a few different promising formulations, including one that we used in a prior vaccination study [45], and characterized that one in further detail to show that it kept both vaccines stable at elevated temperature for extended periods of time.
3.1. Effect of pH on stability
In the formulation screening process, we considered potency loss due to drying and due to storage. When we screened drying of the vaccines at different pH values and in different buffers, the presence of a buffer was shown to be extremely important to maintain rubella vaccine titer during drying but was less important for measles vaccine. The E1 and E2 proteins on the rubella virus membrane undergo a conformation shift between pH 5 and 6 [48, 49]. Once exposed to a low pH environment, the rubella virus shows a marked reduction in infectivity [48]. Additionally, the pH of a solution drops as water is removed through crystallization during freezing or evaporation during drying [50]. The decrease in pH has been shown to influence protein conformations and result in denaturation [51, 52]. These observations are consistent with our finding that buffering at neutral pH is critical to maintaining titer of rubella vaccine during drying.
3.2. Effect of excipients on stability
In the next part of the screen, we studied the effects of various excipients to stabilize MR vaccines during drying and storage. During drying and storage, one of the key stresses on the proteins is the removal of the protein’s hydration shell [53]. Sugars, such as sucrose, trehalose, and gluconate, can provide hydrogen bonding to the protein and thereby serve as water substitutes during drying [53, 54]. Depending on their side chains, amino acids can stabilize proteins in the dry state through a variety of mechanisms, including ionic interactions, reducing exposed hydrophobic regions, filling of void volumes, and slowing molecular dynamics [55, 56]. We further demonstrated that solutions with two excipients outperformed individual excipients during drying and storage. This is consistent with other literature which shows co-solutes are beneficial in protein stability [38, 39, 56, 57]. While CMC was included in formulations to provide mechanical strength to the MNs, we did not specifically study the possible impact of CMC on vaccine stability. We found that the sucrose-threonine-potassium phosphate formulation provided the greatest degree of stabilization, but there were other excipient combinations that similarly stabilized measles and rubella vaccines, which gives formulators greater flexibility when designing formulations.
3.3. Significance of a thermostable measles and rubella vaccine microneedle patch
MN patches offer many advantages over current needle and syringe delivery technology. MR elimination and possible future eradication efforts rely on high vaccination coverage to meet their goals; however, these efforts are hindered by reliance on lyophilized vaccines that require cold-chain storage and expert reconstitution and administration. MN patches remove many barriers to vaccination in low-resource setting in both routine and campaign modes of administration, because they can be administered without reconstitution or injection by trained heath care personnel, generate no biohazardous sharps waste, are painless and, as shown in this study, can be formulated for thermostability at least partially outside the cold chain.
Here, we demonstrated the development of a bivalent MR vaccine MN patch which had no significant loss in measles or rubella vaccine titer at 5°C, 25°C or 40°C for measles vaccine after 1 month of storage. Noting that conventional MR-containing vaccines experience up to a one log10 loss of potency during their shelf life (e.g., from 104 TCID50 to 103 TCID50), titer loss for both measles and rubella vaccines in this study were within the one log10 loss window after 3 months of storage at` 5°C, 25°C or 40°C and were also within one log10 titer loss for 4 months for both vaccines at all temperatures other than rubella vaccine at 40°C. This level of stability exceeds WHO requirements for less than one log10 loss after just one week at 37°C. While the stability levels shown here may not allow for complete removal of MR vaccine MN patches from the cold chain, they do suggest that patches could be transported in a controlled temperature chain [58], in which, for example, the ‘last mile’ of distribution may be conducted outside of the cold chain. During a vaccination campaign, patches could be refrigerated during transport and storage from manufacturer to central distribution points to district level sites. However, during the final phase when vaccinators go house-to-house or to remote location, patches could be stored at ambient temperature without the need for refrigeration using heavy, bulky ice packs. This contrasts with current lyophilized vaccines that must constantly be kept on ice even when accessing remote locations.
Thermostable MR vaccine MN patches may allow for a different vaccine vial monitor (VVM) to be used. Current lyophilized measles or MR vaccines are manufactured with a VVM14 for medium stability. This medium stability monitor allows for 14 days at 37°C, 90 days at 25°C, or 3 years at 5°C [59, 60]. We have demonstrated that MN patches could be stored at 40°C for more than three months with less than one log10 loss of titer, indicating that VVM 30 could be used. If VVM30 were used, vaccine could be stored for longer times at mid to high temperatures, thereby reducing vaccine wastage.
3.4. Limitations of the study
This study used stocks of measles or rubella vaccine viruses that were propagated using Vero cells instead of the standard substrates that are used in vaccine manufacturing: chick embryo cells for measles vaccine and human diploid lung fibroblasts for rubella [46]. This may give the vaccines a different stability profile and require different formulations for optimal performance. This study also only followed stability for 4 months. Future studies should assess stability for up to 2 years, which is a typical shelf life for MR vaccine-containing products [21]. While MR vaccine MN patches were shown to be immunogenic in rhesus macaques, it was a small study and only used freshly made patches. Future studies should demonstrate that MR vaccine patches are immunogenic after extended storage, although immunogenicity is expected based on measured TCID50 values. Another limitation is that these screening studies, as well as the animal study, had relatively small numbers of replicates that were not statistically powered, indicating that future studies will need to be performed with larger sample sizes. In addition, we were only able to report IgG titers and do not have data on neutralizing antibody levels, which are more closely correlated with protective efficacy of the vaccination. Finally, although a number of studies have shown measles or MR vaccine patches to be immunogenic and protective in rodents and non-human primates [35, 39, 45], future studies will be needed in human subjects, such as those planned to occur in the near future [23].
4. Conclusion
To eliminate and possibly eradicate measles and rubella, greater vaccination coverage is necessary. The global health community has called for improved vaccine delivery systems that can ease administration, are thermostable, and do not require hypodermic needles. To this end, we developed MN patches for MR vaccination that are simple to administer, avoid the dangers of hypodermic needles, and maintain thermostability outside the cold chain. To optimize thermostability, we studied the effects of pH and buffers on vaccine stability during drying, finding that potassium phosphate buffer at pH 7.5 to be optimal. Next, 43 excipients were screened and narrowed down based on their ability to maintain titer during drying and short-term storage, and the top individual excipients were then combined and tested as pairs. Among the top-performing formulations, we selected sucrose-threonine-potassium phosphate as the optimal formulation for the MN patches and tested for long-term stability. After one month of storage, there was no significant loss in measles or rubella vaccine titer at 5, 25 or 40°C, although additional studies with larger sample sizes are warranted to more fully establish the stability profile and immunogenicity in non-human primates. After three months at all three temperatures, both vaccines lost less than one log10 of titer, exceeding the WHO requirement for one log10 loss after one week. The MR vaccine patches were also not susceptible to significant loss during multiple freeze thaw cycles. Furthermore, these patches generated robust immune responses comparable to a subcutaneous injection. In conclusion, MN patches for MR vaccination can be formulated for thermostability enabling at least partial removal from the cold chain, which can enable more widespread MR vaccination coverage.
5. Experimental Section/Methods
Vaccines.
MR vaccines were prepared as described previously [35]. Briefly, stocks of monovalent measles or rubella vaccine viruses (generously provided by Serum Institute of India, Pune, India) were added to confluent flasks of Vero cells (ATCC, Manassas, VA) with Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Grand Island, NY) and 2% fetal bovine serum (FBS, Gibco) and incubated for five days at 37°C. Cell suspensions were freeze-thawed and centrifuged to remove the cellular debris. Measles vaccine titers were approximately 7 log10 TCID50/ml, and rubella vaccine titers were approximately 5 log10 TCID50/ml (TCID50 refers to the median tissue culture infectious dose that infects 50% of cells in a standard assay [61]). Vaccine aliquots were stored at −80°C until use.
Vaccine formulation and storage.
MR vaccines were formulated into casting solutions to be used in MN patch fabrication. All excipients were purchased from Sigma Aldrich (St. Louis, MO). Measles vaccine was used as prepared at a titer of 7 log10 TCID50/ml. Rubella vaccine was concentrated using Vivaspin filters (Sartorius, Goettingen, Germany) with a 300 kDa molecular weight cut off until the volume decreased ten-fold. In screening experiments, measles or rubella vaccine was mixed with excipient solutions to achieve final casting solution concentrations of 10% w/v excipient and 1% w/v carboxyl-methylcellulose (CMC) in buffer. We selected 10% w/v concentration for excipients because our experience indicates that this concentration can usually fully embed vaccine in the dried excipient matrix, without increasing casting solution viscosity to the point where the casting process is difficult or causing overfilling of the microneedle mold cavities that results in deposition of excipient, as well as vaccine, in the patch backing layers, which is undesirable. If the solubility of the excipient was lower than 10%, the maximum solubility was used instead. For pH and buffer screens, casting solutions containing 10% sucrose and 1% CMC were used.
For screening pH, buffers, and excipients, 50 μl of casting solutions of different formulations were cast into Eppendorf microcentrifuge tubes using a micropipette. These samples were dried overnight under vacuum with desiccant at room temperature (20–25°C), leaving behind a thin film of solids at the bottom of the tube after evaporation of the water. The following day, samples were packaged in sealed aluminum pouches (Oliver-Tolas Healthcare, Grand Rapids, MI) with desiccant and placed in stability chambers (Caron, Marietta, OH) at 5, 25, or 40°C. Buffer concentrations were 300 mM HEPES, 300 mM histadine, 150 mM ammonium acetate, 50 mM (0.8% w/v) potassium phosphate. DMEM and M199 were used at 1x strength as commercially available.
Microneedle patch preparation.
MN patches were prepared as previously described [38, 39]. MN patches consisted of 100 conical MNs measuring 650 μm in height and distributed over a 1 cm2 area. First, MN patch molds were prepared, as previously described [62]. Then 30 μl of casting solution was cast onto the PDMS molds. The solution was dried into the tips of the mold cavities under vacuum to form the MNs, and excess solution was removed. A second cast of 28% w/v poly-vinyl alcohol (PVA, Acros Organics, Geel, Belgium) and 21% w/v sucrose was added to the molds to form the patch backing. Following two days of drying at room temperature with desiccant, patches were demolded and packaged with desiccant as described above for storage in stability chambers at 5, 25, or 40°C. For freeze thaw cycles, patches were stored at alternating temperatures of 5 or −20°C for 1.5 hours each. After drying, the composition of the MN patches correlated with the excipient concentrations in the casting solution. For example, the 10% sucrose – 3% threonine – 0.8% potassium phosphate – 1% CMC casting solution yielded dry MNs with a composition of 67.5% w/v sucrose, 20.3% w/v threonine, 5.4% w/v potassium phosphate and 6.8% w/v CMC. This assumes that the PVA and sucrose in the second cast forming the patch backing do not mix with the first cast, which is probably incorrect and there is also some amount of PVA and sucrose in the MNs as well.
Measles infectivity assay.
Samples were reconstituted in 1 mL DMEM. Tenfold dilutions of the sample were added to Vero cells seeded on 48 well plates with DMEM with 2% FBS. After five to seven days of incubation, plates were incubated with crystal violet, and titers were counted using the Spearman and Karber algorithm [61].
Rubella infectivity assay.
To determine the rubella vaccine titer, samples were reconstituted in 1mL DMEM [63]. If the sample contained measles vaccine, anti-measles IgG (EMD, Millipore, Billerica, MA) was incubated at a 1:500 dilution with the sample for 1 hour at 37°C. Then, tenfold dilutions of the sample were incubated on Vero cells and incubated for one hour at 37°C followed by the addition of a mixture of DMEM, 50% avicel (FMC BioPolymer, Newark, DE), and 2% FBS. After three to five days, cells were fixed in cold methanol, incubated with E1 antibody (CDC, in-house), HRP-conjugated antibody, and precipitating TMB (Clinical Science Products, Mansfield, MA). An ELISPOT analyzer (CTL, Cleveland, OH) was used to count the foci.
Rhesus macaques.
Seronegative juvenile rhesus macaques (2 years of age, approximately 5 kg) with no previous exposure to measles or rubella and that has not previously been vaccinated against measles or rubella (seronegative status was confirmed by virus neutralization assays, data not shown) were randomly assigned to be vaccinated with subcutaneous (SC) injection or MN patch. In both groups, vaccinations were administered on the back between the shoulder blades. For MN patch vaccination, hair was removed with clippers and depilatory cream. Then patches were applied to the skin, held with light pressure for 30 s, and left on the skin for 15 min to allow for MN dissolution. Serum samples were collected via serum separator tubes (Becton, Dickson, and Company, Franklin Lakes, NJ). Serum IgG titers were measured with ELISA kits: Virion/Serion IgG Measles Kit and Virion/Serion IgG Rubella Kit (Würzburg, Germany). All animal protocols were approved by the Institutional Animal Use and Care Committee of the Centers for Disease Control and Prevention (Protocol 2322ROTMONC Animal Welfare Assurance number A-4365–01) and Georgia Institute of Technology (Protocol A14098, Animal Welfare Assurance number D16–00474 [A-3822–01]).
This study was carried out after completion of the MR patch optimization in vitro to confirm immunogenicity in animals. We chose rhesus macaques as an accepted model for MR vaccination in a primate. We also chose to use a small cohort of juvenile rhesus macaques to provide initial data in preparation for the follow-on study in a larger cohort of infant rhesus macaques that has been published separately [45]. Further details about the animal study in terms of the ARRIVE guidelines is shown in Supporting Information.
Statistical analysis.
MR vaccine titers are reported as mean ± standard deviation. Sample size of 2–4 was used as indicated in text and figure legends. ANOVA was used to differentiate among the buffer and pH samples and among the freeze thaw cycles. Student’s t-test was used in storage studies to compare fresh liquid samples to dried samples or dried samples to stored samples. IgG titers were compared using Student’s t-test. In all cases, p values of less than 0.5 were considered significant. Calculations were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgements
The authors acknowledge Donna Bondy for administrative assistance, Miraj Desai for experimental assistance, and Ryan Johnson for assistance with the rhesus macaques. This work was support by grants from the Bill and Melinda Gates Foundation (Investment ID SOL-1156167 and INV-003561). Jessica Joyce was also funded through the National Institutes of Health Cell and Tissue Engineering Training Grant and National Science Foundation Graduate Research Fellowship (Grant No. DGE-1148903).
Mark Prausnitz is an inventor of patents licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products (including Micron Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Jessica C. Joyce, Georgia Institute of Technology, Wallace H. Coulter Department of Biomedical Engineering, 314 Ferst Drive NW, Atlanta, GA 30332
Marcus L. Collins, Centers for Disease Control and Prevention, Viral Vaccine Preventable Diseases Branch, 1600 Clifton Rd. M/S C22, Atlanta, GA 30333.
Paul A. Rota, Centers for Disease Control and Prevention, Viral Vaccine Preventable Diseases Branch, 1600 Clifton Rd. M/S C22, Atlanta, GA 30333.
Mark R. Prausnitz, Georgia Institute of Technology, Wallace H. Coulter Department of Biomedical Engineering, 314 Ferst Drive NW, Atlanta, GA 30332 Georgia Institute of Technology, School of Chemical and Biomolecular Engineering, 311 Ferst Drive NW, Atlanta, GA 30332.
References
- 1.Orenstein WA and Gay NJ, The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis, 2004. 189 Suppl 1: p. S27–35. [DOI] [PubMed] [Google Scholar]
- 2.Midterm Review of the Global Vaccine Action Plan 2016, Strategic advisory group of experts on immunization. [Google Scholar]
- 3.Perry RT, et al. , Progress Toward Regional Measles Elimination — Worldwide, 2000–2014. Morbidity and Mortality Weekly Report (MMWR), 2015. 64 (44): p. 1246–1251. [DOI] [PubMed] [Google Scholar]
- 4.Measles and Rubella Global Strategic Plan 2012–2020 Midterm Review. 2016, World Health Organization. [Google Scholar]
- 5.Grant GB, D. S, Dumolard L, Kretsinger K, Reef SE, Progress Toward Rubella and Congenital Rubella Syndrome Control and Elimination — Worldwide, 2000–2018, in MMWR Morb Mortal Wkly Rep. 2019. p. 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vynnycky E, et al. , Using Seroprevalence and Immunisation Coverage Data to Estimate the Global Burden of Congenital Rubella Syndrome, 1996–2010: A Systematic Review. PLoS One, 2016. 11(3): p. e0149160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Measles and Rubella Strategic Plan 2012–2020. 2012, World Health Organization [Google Scholar]
- 8.Goodson JL, et al. , Research priorities for global measles and rubella control and eradication. Vaccine, 2012. 30(32): p. 4709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statement regarding findings of joint investigation of 15 deaths of children in Nachodokopele village, Kapoeta East County in South Sudan. 2017, World Health Organization; UNICEF. [Google Scholar]
- 10.Durrheim DN and Goodson JL, Time for an immunisation paradigm shift. Transactions of The Royal Society of Tropical Medicine and Hygiene, 2017. 111(2): p. 41–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strebel P, Papania M, and Halsey N, Vaccines, Plotkin S and Orenstein W, Editors. 2004, Philadelphia: Saunders. [Google Scholar]
- 12.Hales CM, et al. , Measles Outbreak Associated With Low Vaccine Effectiveness Among Adults in Pohnpei State, Federated States of Micronesia, 2014. Open Forum Infect Dis, 2016. 3(2): p. ofw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breakwell L, et al. , Measles Outbreak Associated with Vaccine Failure in Adults--Federated States of Micronesia, February-August 2014. MMWR Morb Mortal Wkly Rep, 2015. 64(38): p. 1088–92. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira SA, et al. , Re-evaluation of the basic procedures involved in the storage of measles vaccine in public health units of the municipality of Niteroi, State of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop, 1993. 26(3): p. 145–9. [DOI] [PubMed] [Google Scholar]
- 15.Fowotade A, et al. , Measles vaccine potency and sero-conversion rates among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. J Immunoassay Immunochem, 2015. 36(2): p. 195–209. [DOI] [PubMed] [Google Scholar]
- 16.Matthias DM, et al. , Freezing temperatures in the vaccine cold chain: A systematic literature review. Vaccine, 2007. 25(20): p. 3980–3986. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin MM, et al. , Perspective on Global Measles Epidemiology and Control and the Role of Novel Vaccination Strategies. Viruses, 2017. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adhikari BB, et al. , Assessing the Potential Cost-Effectiveness of Microneedle Patches in Childhood Measles Vaccination Programs: The Case for Further Research and Development. Drugs R D, 2016. 16(4): p. 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dayan GH, et al. , Cost-effectiveness of three different vaccination strategies against measles in Zambian children. Vaccine, 2004. 22(3–4): p. 475–84. [DOI] [PubMed] [Google Scholar]
- 20.Global Vaccine and Immunization Research Forum: New technologies to support measles elimination. 2016; Available from: http://www.who.int/immunization/research/forums_and_initiatives/gvirf/Plenary5_Measles.pdf?ua=1.
- 21.Temperature sensitivity of vaccines. 2006: World Health Organization. [Google Scholar]
- 22.Linkins RW, et al. , Evaluation of house-to-house versus fixed-site oral poliovirus vaccine delivery strategies in a mass immunization campaign in Egypt. Bull World Health Organ, 1995. 73(5): p. 589–95. [PMC free article] [PubMed] [Google Scholar]
- 23.Prausnitz MR, et al. , A microneedle patch for measles and rubella vaccination: a game changer for achieving elimination. Current Opinion in Virology, 2020. 41: p. 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demuth PC, et al. , Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv Funct Mater, 2013. 23(2): p. 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JW, Park JH, and Prausnitz MR, Dissolving microneedles for transdermal drug delivery. Biomaterials, 2008. 29(13): p. 2113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling MH and Chen MC, Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater, 2013. 9(11): p. 8952–61. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan SP, et al. , Dissolving polymer microneedle patches for influenza vaccination. Nature medicine, 2010. 16(8): p. 915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonificio A, et al. , Fabrication of cell culture-derived influenza vaccine dissolvable microstructures and evaluation of immunogenicity in guinea pigs. Vaccine, 2015. 33(25): p. 2930–8. [DOI] [PubMed] [Google Scholar]
- 29.Arya J and Prausnitz MR, Microneedle patches for vaccination in developing countries. J Control Release, 2016. 240: p. 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Pilar Martin M, et al. , Local response to microneedle-based influenza immunization in the skin. mBio, 2012. 3: p. e00012–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YC, Park JH, and Prausnitz MR, Microneedles for drug and vaccine delivery. Advanced drug delivery reviews, 2012. 64(14): p. 1547–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prausnitz MR, Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu Rev Chem Biomol Eng, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Quan FS, et al. , Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol, 2010. 84(15): p. 7760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman JJ, et al. , Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine, 2014. 32(16): p. 1856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edens C, et al. , Measles vaccination using a microneedle patch. Vaccine, 2013. 31(34): p. 3403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, et al. , Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release, 2011. 152(3): p. 349–55. [DOI] [PubMed] [Google Scholar]
- 37.Levin A, et al. , An economic evaluation of thermostable vaccines in Cambodia, Ghana and Bangladesh. Vaccine, 2007. 25(39–40): p. 6945–57. [DOI] [PubMed] [Google Scholar]
- 38.Mistilis MJ, et al. , Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res, 2017. 7(2): p. 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edens C, et al. , A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine, 2015. 33(37): p. 4712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrdoljak A, et al. , Induction of broad immunity by thermostabilised vaccines incorporated in dissolvable microneedles using novel fabrication methods. J Control Release, 2016. 225: p. 192–204. [DOI] [PubMed] [Google Scholar]
- 41.Landscape Analysis: Trends in vaccine availability and novel vaccine delivery technologies: 2008–2025. 2008: PATH and World Health Organization [Google Scholar]
- 42.Burger JL, et al. , Stabilizing formulations for inhalable powders of live-attenuated measles virus vaccine. J Aerosol Med Pulm Drug Deliv, 2008. 21(1): p. 25–34. [DOI] [PubMed] [Google Scholar]
- 43.Kisich KO, et al. , Dry powder measles vaccine: particle deposition, virus replication, and immune response in cotton rats following inhalation. Vaccine, 2011. 29(5): p. 905–12. [DOI] [PubMed] [Google Scholar]
- 44.Ohtake S, et al. , Heat-stable measles vaccine produced by spray drying. Vaccine, 2010. 28(5): p. 1275–1284. [DOI] [PubMed] [Google Scholar]
- 45.Joyce JC, et al. , A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques. The Journal of infectious diseases, 2018. 218(1): p. 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MMR II (Measles, Mumps, and Rubella Virus Vaccine Live) Merck Package Insert. [Google Scholar]
- 47.Griffin DE and Pan C-H, Measles: Old Vaccines, New Vaccines, in Measles: Pathogenesis and Control, Griffin DE and Oldstone MBA, Editors. 2009, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 191–212. [Google Scholar]
- 48.Katow S and Sugiura A, Low pH-induced conformational change of rubella virus envelope proteins. J Gen Virol, 1988. 69 (Pt 11): p. 2797–807. [DOI] [PubMed] [Google Scholar]
- 49.Mauracher CA, et al. , pH-dependent solubility shift of rubella virus capsid protein. Virology, 1991. 181(2): p. 773–7. [DOI] [PubMed] [Google Scholar]
- 50.Williams-Smith DL, et al. , Changes in apparent pH on freezing aqueous buffer solutions and their relevance to biochemical electron-paramagnetic-resonance spectroscopy. Biochem J, 1977. 167(3): p. 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pikal-Cleland KA, et al. , Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. Arch Biochem Biophys, 2000. 384(2): p. 398–406. [DOI] [PubMed] [Google Scholar]
- 52.Pikal-Cleland KA and Carpenter JF, Lyophilization-induced protein denaturation in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. J Pharm Sci, 2001. 90(9): p. 1255–68. [DOI] [PubMed] [Google Scholar]
- 53.Arakawa T, et al. , Factors affecting short-term and long-term stabilities of proteins. Advanced Drug Delivery Reviews, 2001. 46(1): p. 307–326. [DOI] [PubMed] [Google Scholar]
- 54.Crowe JH, Carpenter JF, and Crowe LM, The role of vitrification in anhydrobiosis. Annu Rev Physiol, 1998. 60: p. 73–103. [DOI] [PubMed] [Google Scholar]
- 55.Bozorgmehr MR and Monhemi H, How Can a Free Amino Acid Stabilize a Protein? Insights from Molecular Dynamics Simulation. Journal of Solution Chemistry, 2015. 44(1): p. 45–53. [Google Scholar]
- 56.Forney-Stevens KM, Bogner RH, and Pikal MJ, Addition of Amino Acids to Further Stabilize Lyophilized Sucrose-Based Protein Formulations: I. Screening of 15 Amino Acids in Two Model Proteins. J Pharm Sci, 2016. 105(2): p. 697–704. [DOI] [PubMed] [Google Scholar]
- 57.Stärtzel P, et al. , Freeze Drying of l-Arginine/Sucrose-Based Protein Formulations, Part I: Influence of Formulation and Arginine Counter Ion on the Critical Formulation Temperature, Product Performance and Protein Stability. Journal of Pharmaceutical Sciences, 2015. 104(7): p. 2345–2358. [DOI] [PubMed] [Google Scholar]
- 58.Organization, W.H. Controlled temperature chain (CTC). August 28, 2020]; Available from: https://www.who.int/immunization/programmes_systems/supply_chain/ctc/en/index1.html.
- 59.Measles-containing Vaccines (Measles, MR, MMR) 2015: Médecins Sans Frontières [Google Scholar]
- 60.Vaccine vial monitor (VVM) assignments for different WHO-prequalified vaccines and their proper handling. 2014: World Health Organization. [Google Scholar]
- 61.Hierholzer JC and Killington RA, Virus isolation and quantitation, in Virology Methods Manual, Kangro HO and Mahy BW, Editors. 1996, Academic Press: London. p. 25–46. [Google Scholar]
- 62.Chu LY, Choi SO, and Prausnitz MR, Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. Journal of pharmaceutical sciences, 2010. 99(10): p. 4228–38. [DOI] [PubMed] [Google Scholar]
- 63.Chen MH, et al. , An indirect immunocolorimetric assay to detect rubella virus infected cells. J Virol Methods, 2007. 146(1–2): p. 414–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


