Abstract
Background:
Co-occurrence of other autoimmune disorders (AID) and autoantibodies in patients with autoimmune demyelinating CNS disorders have not been studied previously in patients of Indian origin.
Objective:
To determine the frequency of concomitant autoimmune disorders, anti-nuclear antibody (ANA) and antithyroid antibody (ATAb) and to evaluate the impact on clinical course of disease.
Materials and Methods:
A total of 111 patients with MS and 152 patients with non-MS demyelinating disorders were included. Demographics, clinical course and disability were recorded. History of other autoimmune disorders (AIDs) in patients and first degree relatives was noted. Serum ANA and ATAb were tested.
Results:
Concomitant AIDs were seen in 21% of MS and 19% of non-MS patients. Autoimmune thyroid disease was most frequent and seen in 10.8% of MS and 6.6% of non-MS disorders. Frequency of ATAb was significantly higher among MS group (MS 25.5% vs non-MS 13.2% P = 0.04) but that of ANA was similar between the 2 groups (MS 19.8% vs non-MS 26.9% P = 0.17). A positive family history of autoimmune disorders was noted in 20% of MS and 15.1% of non-MS disorders. Clinical course was unaffected by presence of concomitant AID and autoantibodies.
Conclusion:
Cooccurrence of autoantibodies and AID are seen in a significant number of patients with MS and non-MS disorders and influences clinical management.
Keywords: Autoantibodies, autoimmune disorders, multiple sclerosis, non-MS disorders
INTRODUCTION
Recent epidemiological studies have described an increased susceptibility for people with one AID to have another disorder of autoimmune origin.[1] This increased relative risk of acquiring another AID may be due to a shared genetic susceptibility or an environmental trigger or both.[1] Several studies have shown an increased co-occurrence of multiple sclerosis (MS) with autoimmune thyroid disease,[2,3] psoriasis, and inflammatory bowel disease among others.[4] Other studies reported the association of thyroid function abnormalities and antithyroid antibodies in MS patients.[5] Aquaporin 4 IgG (AQP4 IgG) associated neuromyelitis optica spectrum disorder (NMOSD) is frequently associated with systemic autoimmune disorders, in nearly 20-30%. These include organ-specific disorders such as hypothyroidism, pernicious anemia, ulcerative colitis, myasthenia gravis, and idiopathic thrombocytopenic purpura; and non-organ-specific disorders such as systemic lupus erythematosus, antiphospholipid antibody syndrome, and Sjögren syndrome.[6] Approximately 40% of NMOSD may have coexisting antibodies without having an autoimmune illness.[7] These include ANA (43%), antibody against extractable nuclear antigen (ENA, 15%), SSA-A & B (10% & 3%), and rheumatoid factor (RA factor, 5%). Coexisting autoimmune disorders/autoantibodies are described to be less frequent among MOG-IgG associated disorders.[8] Among 50 patients with MOG-IgG associated disorders other AIDs were seen in 9% of patients and included in order of frequency Rheumatoid arthritis (RA), hypothyroidism (HT), Graves disease (GD), atopic dermatitis (AD), and atopic bronchial asthma (BA). Autoantibodies were detected in 42.2% with ANA being the most frequent (28%).
The coexistence of autoimmune disorders and autoantibodies in seronegative non-MS disorders is less well understood. The possibility of co-existing AID makes it important to investigate for the same among patients with MS, NMOSD, and other related disorders. Evidence of systemic disease or abnormal laboratory results suggestive of non-neurologic autoimmune diseases may impact treatment strategies and management.[9] In this background, the current study aimed to determine the frequency of concomitant autoimmune disorders, anti-nuclear antibody (ANA), and antithyroid antibody (ATAb) and to evaluate the impact on clinical course of disease.
MATERIAL AND METHODS
Patient selection
Hundred and eleven patients with MS diagnosed by McDonald's 2017 criteria[10] and 152 patients with non-MS demyelinating disorders who were prospectively enrolled in the Mangalore demyelinating disease registry[11] were selected. Among 111 MS patients there were 93 with relapsing remitting (RR), 15 with secondary progressive (SP), and 3 with primary progressive (PP) MS [Table 1]. The non-MS demyelinating disorders included 40 aquaporin-4 antibody positive (AQP4-IgG +) NMOSD,[12] 41 myelin oligodendrocyte glycoprotein antibody positive (MOG-IgG+), and 71 seronegative patients [Table 1]. Blood samples from 46 age and gender matched healthy controls were included for analysis of antibody frequency for ANA and ATAb.
Table 1.
Demographic and clinical details
| Variables | MS cases (n = 111) | Anti AQP4 + Cases (n = 40) | Anti MOG + cases (n = 41) | Seronegative cases n = 71 |
|---|---|---|---|---|
| Phenotype | ||||
| RRMS | 93 | |||
| PPMS | 3 | |||
| SPMS | 15 | |||
| Non-MS disorders | ||||
| NMOSD | 35 | 14 | 40 | |
| RTM | 2 | 2 | 4 | |
| RON | 1 | 7 | 3 | |
| TM | 2 | 10 | 15 | |
| ON | 7 | 8 | ||
| ADEM | - | 1 | 1 | |
| Gender | ||||
| Female | 77 | 37 | 18 | 39 |
| Male | 34 | 3 | 23 | 32 |
| Age at disease onset (Mean & SD) | 27.5 ± 9.55 | 33.4 ± 12.6 | 22.7 ± 12.90 | 30.85 ± 14.48 |
| Disease duration years, median (range) | 6.0 (3.0-10.0) | 6.0 (3.0-14.0) | 4.0 (2.0-5.0) | 5.0 (3.0-8.0) |
| EDSS Median (range) | 2.5 (2.0-3.5) | 3.75 (2.5-7.8) | 2.00 (1.5-3.0) | 3.5 (2.5-6.0) |
MS = Multiple sclerosis, AQP4 = Aquaporin4, MOG = Myelin oligodendrocyte glycoprotein, RRMS = Relapsing remitting MS, PPMS = Primary progressive MS, SPMS = Secondary progressive MS, NMOSD = Neuromyelitis optica spectrum disorder, RTM = Recurrent transverse myelitis, RON = Recurrent optic neuritis, TM = Transverse myelitis, ON = Optic neuritis, ADEM = Acute disseminated encephalomyelitis, SD = Standard deviation, EDSS = Expanded Disability Status Scale
Data collection
Demographic details including gender, age at onset of disease, disease duration, and expanded disability status scale (EDSS) was obtained. A detailed history of other disorders of possible autoimmune origin were queried (using a previously validated questionnaire)[13] and confirmed (from medical records & prescriptions). Treatment history was recorded. Family history of AID in first degree relatives was also obtained.
Autoantibody testing
Patient sera in 1:100 dilution was tested for anti-nuclear antibody (blood collected prior to treatment with steroids) using indirect immunofluorescence (Euroimmun, Lubeck Germany) technique as per manufacturer's instructions. Anti-thyroid antibody including both thyroid peroxidase (TPO Ab) and thyroglobulin (TG Ab) were tested (1:10 serum dilution) in 102 MS, 136 non-MS (40 AQP4 IgG+, 41 MOG IgG+, 55 seronegative) patients and all healthy controls, using a commercial kit (Euroimmun, Lubeck, Germany). Tests were done by first author (CM) and results were independently verified by 2nd & 3rd coauthors (LP & AD). Presence of one or both ATAb was taken to be a positive test result. In addition thyroid functions including serum triiodothyronine (T3), thyroxine (T4), and thyroid stimulating hormone (TSH) levels were checked. Diagnosis of autoimmune disorders was made by other concerned specialists as per relevant criteria and patients were jointly managed with treating neurologist (LP). This study was approved by the institutional ethics committee and informed consent was obtained from all patients and healthy volunteers.
Statistical analysis
Statistical analyses were done using the IBM SPSS for Windows Version 21.0 (IBM Corp. Armonk, NY, USA) statistical package. Continuous variables between the groups was analyzed with the non-parametric Mann–Whitney U test. Analysis of categorical variables between the two groups was made with Chi square test. A P value of ≤0.05 was considered significant.
RESULTS
Autoantibodies and autoimmune disorders associated with MS
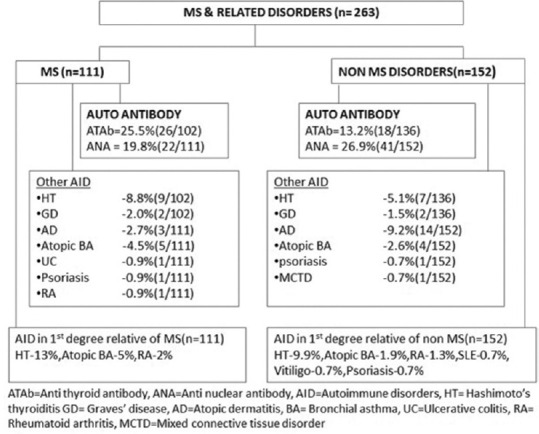
Among MS patients ATAb was detected in 25.5% (26/102), 42.3% (11/26) of whom had concomitant thyroid dysfunction. Additionally 3% (3/102) had hypothyroidism alone without ATAb. Thyroid dysfunction occurred in 8.1% of MS patients (9/111) before the onset of MS and 4.5% (5/111) patients during disease course. In all, 20% (22/111) of patients had a positive ANA test. Three patients tested positive for both ATAb and ANA without any other AID. Other autoimmune disorders associated with MS included atopic BA – 4.5% (5/111), AD – 2.7% (3/111), and one case each of ulcerative colitis, RA, and psoriasis (2.7%). Among first degree relatives of patients, the most common disorder seen was HT – 13% (14/111) followed by atopic BA – 5% (6/111) and RA – 2%(2/111).
Non-MS disorders
AQP4 IgG associated NMOSD
Twenty percent (8/40) had ATAb associated with AQP4 IgG + NMOSD. Among them 2 had accompanying thyroid dysfunction, one with GD that developed during the course of NMOSD and another with HT [Table 2, Figure 1]. Fifteen percent (6/40) had hypothyroidism unaccompanied by ATAb and 15% had ATAb without thyroid dysfunction. Among AQP4 IgG + patients 42% (17/40) tested positive for ANA. Other AID included two patients each with AD (5%) and atopic BA (5%). Four (10%) patients had first degree relatives with a diagnosis of HT and one relative (2.5%) had atopic BA.
Table 2.
Frequency of thyroid disorders and other autoimmune disorders in MS and related disorders
| Group (no) | ATAb + | HT (%) | GD (%) | Hypothyroidism (sans ATAb) | Other AID (%) |
|---|---|---|---|---|---|
| MS (102) | 26 (25.5%) | 9 (8.8%) | 2 (2%) | 3 (3%) | Atopic BA- 4.5% Atopic dermatitis - 2.7% Ulcerative colitis-0.9% Rheumatoid arthritis-0.9% Psoriasis-0.9% |
| AQP4IgG + (40) | 8 (20%) | 1 (2.5%) | 1 (2.5%) | 6 (15%) | Atopic dermatitis -5% Atopic BA -5% |
| MOGIgG + (41) | 5 (12.2%) | 3 (7.3%) | 1 (2.4%) | 6 (14.6%) | Atopic dermatitis -10% |
| Sero Negative (55) | 5 (9.1%) | 3 (5.5%) | 0 | 5 (9.1%) | Atopic dermatitis -11.3% Atopic BA -3% Psoriasis-1.4% MCTD-1.4% |
| Healthy controls (46) | 12 (26%) | 4 (8.7) | 0 | 0 |
ATAb = Anti thyroid antibody, HT = Hashimoto’s thyroiditis GD = Graves’ disease, BA = Bronchial asthma, MCTD = Mixed connective tissue disorder
Figure 1.
Autoantibodies and other autoimmune disorders in MS and related disorders
MOG IgG positive disorders
Anti-thyroid antibody was detected in 12.2% (5/41) among whom 3 patients had HT and one had GD. Fifteen percent (6/41) had hypothyroidism unaccompanied by ATAb. Ten percent of patients (4/41) had additionally AD. In 22% (9/41) of patients ANA was positive. Among family members, 12.1% of patients (5/41) reported AID namely HT (4) and psoriasis (1)
Seronegative non-MS disorders
Among seronegative patients 9.1% (5/55) had ATAb, 3 of whom had HT. In addition 9% (5/55) had primary hypothyroidism alone. Twenty one percent of patients (15/71) were positive for ANA. Other AID included AD in 11.3% (8/71), atopic BA in 3% (2/71), and one patient each had psoriasis and mixed connective tissue disorder. Family history (among first degree relatives) of other AID was present in 18.3% (13/71) and included HT (7), atopic BA (2), RA (2), and one patient each had relatives with SLE and vitiligo.
In the non-MS group other AIDs were diagnosed in 6% (9/152) prior to onset of atypical demyelinating disorder, 2% (3/152) at the time of diagnosis and 9.2% (14/152) during disease course.
We reviewed the treatments received by patients at the time they were tested for autoantibodies. None had received oral or parenteral steroids in the 6 months preceding blood testing. There was no significant difference in type of therapy given between patients with thyroid dysfunction/autoantibodies and those without, among MS and non-MS disorders [Supplementary Table 1]. We have compared the demographics, age at disease onset, duration, and disability (as measured by EDSS) among MS and non-MS groups based on the presence/absence of autoantibodies and found no statistical difference [Supplementary Tables 2 and 3].
Supplementary Table 1.
Treatment details
| a.) Treatment - MS Patients: | |||
|---|---|---|---|
|
| |||
| Drugs | With ATAb and thyroid disorder N = 30 | Without ATAb and Thyroid disorder N = 81 | P |
| MMF | 12 (40%) | 25 (30.9%) | 0.36 |
| Azathioprine | 2 (6.7%) | 15 (18.6%) | 0.12 |
| Dimethyl fumerate | 7 (23.3%) | 12 (14.8%) | 0.29 |
| Interferon beta | 2 (6.7%) | 6 (6.9%) | 0.89 |
| Rituximab | 5 (16.6%) | 14 (17.4%) | 0.93 |
| No treatment | 2 (6.7%) | 6 (7.5%) | 0.89 |
| Others (Glatira, teriflunomide, Ofatumumab) | 0 | 3 (3.7%) | |
|
| |||
| b.) Treatment – Non-MS patients: | |||
|
| |||
| Drugs | With ATAb and thyroid disorder N = 40 | Without ATAb and Thyroid disorder N = 112 | P |
| MMF | 19 (47.5%) | 43 (38.4%) | 0.31 |
| Azathioprine | 7 (17.5%) | 28 (25%) | 0.33 |
| Dimethyl fumerate | 0 | 1 (0.89%) | |
| Rituximab | 3 (7.5%) | 4 (3.57%) | 0.30 |
| No treatment | 11 (27.5%) | 36 (32.1%) | 0.58 |
Supplementary Table 2.
Demographics and clinical characteristics based on the presence and absence of ANA
| MS cases (n = 111) | NMO-IgG + cases (n = 40) | MOG -IgG + cases (n = 41) | Seronegative cases (n = 71) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| positive | negative | P | positive | negative | P | positive | negative | P | positive | Negative | P | |
| Male/Female | 6/16 | 28/61 | 0.7 | 1/16 | 2/21 | 0.74 | 3/6 | 20/12 | 0.11 | 6/9 | 26/30 | 0.51 |
| Age at onset (mean ± stdv) | 25.1 ± 10.2 | 28.1 ± 9.4 | 0.40 | 36 ± 14.3 | 31.5 ± 11.1 | 0.29 | 20.8/± 12.8 | 23.2 ± 13.2 | 0.24 | 31.6 ± 15.9 | 30.6 ± 14.25 | 0.83 |
| EDSS Median (range) | 2.75 (1-3.5) | 2.5 (2.0-3.5)f | 0.54 | 3.5 (2.75-8.5) | 4 (2.0-7.0) | 0.69 | 2.0 (1.5-2.0) | 2.0 (1.5-3.0) | 0.23 | 4.5 (2.0-7.5) | 3.0 (2.6-5.5) | 0.32 |
| Disease duration in years Median (range) | 6.0 (2.0-8.5) | 6 (3.00-10.00) | 0.41 | 4.0 (1.5-14.00) | 7.00 (5.00-14.00) | 0.22 | 4.00 (0.87-5.5) | 4.0 (2.0-6.0) | 0.50 | 4.5 (2.75-8.00) | 5.00 (3.00-8.00) | 0.34 |
Supplementary Table 3.
Demographics and clinical characteristics based on the presence and absence of anti-thyroid antibody
| MS cases (N = 102) | NMO-IgG + cases (n = 40) | MOG IgG + cases (n = 41) | Seronegative cases (n = 55) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| positive | negative | P | positive | negative | P | positive | Negative | P | positive | Negative | P | |
| Gender Male/Female |
8/18 | 22/54 | 0.86 | 1/7 | 2/30 | 0.54 | 2/3 | 21/15 | 0.43 | 1/4 | 22/28 | 0.29 |
| Age at onset | 28.2 ± 9.4 | 27.3 ± 9.3 | 0.47 | 30.25 ± 6.7 | 34.3 ± 13.6 | 0.30 | 23.8 ± 16.5 | 22.5 ± 12.6 | 0.8 | 33.8 ± 19.8 | 30.9 ± 13.7 | 0.74 |
| EDSS median (range) | 2 (1.5-3.5) | 2.5 (2.0-3.5) | 0.26 | 5.75 (2.1-9.5) | 3.5 (2.1-6.5) | 0.49 | 2.0 (1.25-6.75) | 1.5 (1.0-2.0) | 0.20 | 4.5 (2-5.5) | 3.0 (1-4.75) | 0.64 |
| Disease duration in years median (range) | 4.0 (3.0-8.00) | 5.00 (2.0-10.0) | 0.96 | 6.0 (3.5-7.75) | 7.00 (3.00-14.7) | 0.84 | 5.0 (2.5-9.5) | 4.00 (2.00-6.0) | 0.63 | 6.0 (3.5-7.5) | 4.00 (2.5-7.0) | 0.38 |
Frequency of ATAb was significantly higher among MS group (MS 25.5% vs non-MS 13.2% P = 0.04). Frequency of ANA was similar between the 2 groups (MS 19.8% vs non-MS 26.9% P = 0.17). Among non-MS disorders, frequency of ANA positivity among patients was significantly higher among AQP4IgG + NMOSD (AQP4 IgG + 42.5% vs MOG IgG + 22% P = 0.04; AQP4 IgG + 42.5% vs seronegative 21% P = 0.017). Five percent of MS patients and 8.5% of non-MS patients had both ANA and ATAb (MS 5% vs non-MS 8.5% P = 0.36). Speckled pattern was the most common ANA pattern seen in 50% of MS and 44% of non-MS disorders who were positive for the test and there was no gender or age bias detected for the same (data not shown).
Healthy controls
Among 46 healthy controls there were 12 patients who tested positive for ATAb (26%). Among them 4 had concomitant (asymptomatic) hypothyroidism. Antinuclear antibody testing revealed that 2 (4.3%) were positive among healthy controls.
DISCUSSION
Autoimmune CNS demyelinating disorders are heterogeneous conditions with a varied clinical course, associated biomarkers and treatment modalities. The shared genetic susceptibility with other autoimmune disorders raises the possibility of cooccurrence of these disorders in MS and related disorders. While several studies outside the country have addressed the coexistence of other AID and the impact on disease course, we are for the first time reporting the same based on data from our registry from India.
In our study concomitant AIDs were seen in 21% of MS and 19% of non-MS disorders. Among the MS cohort autoimmune thyroid disease was most commonly seen followed by atopic BA (4.5%) and AD (2.7%). Our study like others[14] showed that a number of patients had ATAb without concomitant thyroid dysfunction. Among MS patients 3% (3/102) had hypothyroidism unaccompanied by ATAb which we have labelled as non immune thyroid disease. It may be argued that in some of the latter, treatment for thyroid dysfunction and or disease modifying therapy may have influenced antibody detection. Non-immune thyroid disease has however been reported in approximately 9% of patients with MS.[15] Among non-MS disorders, AQP4 IgG and MOG IgG disorders have been previously studied for the associated AID and auto-antibodies.[6,8] In our study AD was most frequent (9.2%) followed by autoimmune thyroid disorder (6.6%) and atopic BA (2.6%). Frequency of ATAb was significantly increased among MS patients when compared to non-MS disorders (p = 0.01). Frequency of ANA was similar between the 2 groups, though ANA frequency among non-MS disorders was significantly higher in AQP4 IgG + NMOSD. Presence of autoimmune disorders and or autoantibodies did not influence the clinical course of the disease as mentioned in some other studies.[16] A positive family history of autoimmune disorders was noted in both MS (20%) and non-MS (15.1%) patients. Familial autoimmune disorders are not uncommon and have been reported for most autoimmune disorders including MS.[17,18]
We found subclinical hypothyroidism among some healthy controls accompanied by ATAb among them. Previous studies in coastal south western India have shown asymptomatic hypothyrodism and high prevalence of ATAb among healthy and predominantly women volunteers.[19] In our study there were many similarities between MS and non-MS disorders including the co-occurrence of autoimmune disorders that targeted thyroid gland, auto-antibodies being detected in the absence of clinical disease and a positive family history of autoimmunity. Presence of concomitant AID did not influence the clinical course of underlying demyelinating disorder.
It is important to investigate for other AID from the point of mitigation of comorbidities such as underlying thyroid dysfunction in the case of autoimmune thyroid disease. Further the presence of autoantibodies, other AID and a positive family history of AID lends support to the presumed autoimmune nature of the neurological illness This is particularly true for seronegative non-MS disorders. The detection of ANA in our patient cohort was not associated with any collagen vascular disorder throughout the course of observation in the study and probably reflects a shared genetic susceptibility.
There were several limitations to our study. Our patient numbers were small in each subgroup. We have tested only two auto-antibodies among patients and relied on clinical and treatment history for co-existence of other autoimmune disorders. Recall bias may have influenced details about history of AID among family members. A larger population study is also warranted to understand whether concomitant autoimmune disorders exceeded expectation for the general population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sloka S. Observations on recent studies showing increased co-occurrence of autoimmune diseases. J Autoimmun. 2002;18:251–7. doi: 10.1006/jaut.2002.0588. [DOI] [PubMed] [Google Scholar]
- 2.Sloka JS, Phillips PW, Stefanelli M, Joyce C. Co-occurrence of autoimmune thyroid disease in a multiple sclerosis cohort. J Autoimmune Dis. 2005;9:2–9. doi: 10.1186/1740-2557-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munteisa E, Canob JF, Flores JA, Martinez-Rodrigueza JE, Miretb M, Roquera J. Prevalence of autoimmune thyroid disorders in a Spanish multiple sclerosis cohort. Eur J Neurol. 2007;14:1048–52. doi: 10.1111/j.1468-1331.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 4.Marie RN, Reider N, Cohen J, Stuve O, Sorensen PS, Cutter G, et al. A systematic review of the incidence and prevalence of autoimmune diseases in multiple sclerosis. Mult Scler J. 2015;21:282–93. doi: 10.1177/1352458514564490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long Y, Zheng Y, Chen M, Zhang B, Gao C, Shan F, et al. Serum thyroid-stimulating hormone and anti-thyroglobulin antibody are independently associated with lesions in spinal cord in central nervous system demyelinating diseases. PLoS One. 2014;9:e100672. doi: 10.1371/journal.pone.0100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer A, Elsone L, Appleton R, Jacob A. A review of current literature and a guide to the early diagnosis of autoimmune disorders associated with neuromyelitisoptica. Autoimmunity. 2014;47:154–61. doi: 10.3109/08916934.2014.883501. [DOI] [PubMed] [Google Scholar]
- 7.Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitisoptica and non organ-specific autoimmunity. Arch Neurol. 2008;65:78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]
- 8.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoiili K, et al. MOG-IgG in NMO and related disorders: A multicentric study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment response and long term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzimab versus interferon beta 1a as first line treatment for patients with relapsing remitting multiple sclerosis: A randomized controlled phase 3 trial. Lancet. 2012;380:1819–28. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 10.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 11.Pandit L, Mustafa S, Kunder R, Shetty R, Misri Z, Pai S, et al. Optimizing the management of neuromyelitisoptica and spectrum disorders in resource poor settings: Experience from the Mangalore demyelinating disease registry. Ann Indian Acad Neurol. 2013;16:572–6. doi: 10.4103/0972-2327.120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitisoptica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malli C, Pandit L, D’Cunha A, Mustafa S. Environmental factors related to multiple sclerosis in Indian population. PLoS One. 2015;10:e0124064. doi: 10.1371/journal.pone.0124064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durelli L, Oggero A, Verdun E, Isadoro G, Barbero P, Bergamasco B, et al. Thyroid function and anti thyroid antibodies in MS patients screened for Interferon treatment. A multicenter study. J Neurol Sci. 2001;193:17–22. doi: 10.1016/s0022-510x(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 15.Niederwieser G, Buchinger W, Bonelli RM, Berghold A, Reisecker F, Költringer P, et al. Prevalence of autoimmune thyroiditis and non-immune thyroid disease in multiple sclerosis. J Neurol. 2003;250:672–5. doi: 10.1007/s00415-003-1053-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Zhong Y, Wang Y, Dai Y, Qiu W, Zhang L, et al. Neuromyelitisoptica spectrum disorders without and with autoimmune diseases. BMC Neurol. 2014;14:162. doi: 10.1186/s12883-014-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cárdenas-Roldán J, Rojas-Villarraga A, Anaya JM. How do autoimmune diseases cluster in families? A systematic review and meta-analysis. BMC Med. 2013;11:73. doi: 10.1186/1741-7015-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzlef O, Alamowitch S, Sazdovitch V, Chillet P, Joutel A, Tournier-Lasserve E, et al. Autoimmune diseases in families of French patients with multiple sclerosis. Acta Neurol Scand. 2000;101:36–40. doi: 10.1034/j.1600-0404.2000.101001036.x. [DOI] [PubMed] [Google Scholar]
- 19.Cyriac T, Chellappa PM, Sinnet PR, Immanuel A. Prevalence of hypothyroidism and its association with anti-thyroid peroxidase antibody among adult sea food consuming population attending a tertiary health care center in Kerala. Int J Biomed Adv Res. 2015;6:648–55. [Google Scholar]


