Dear Editor,
Jaw dystonia and myelopathy have been rarely described in breast carcinoma patients with anti-Ri/antineuronal nuclear antibody type 2 (ANNA-2) antibody. A high degree of suspicion can prompt appropriate workup and timely initiation of treatment, leading sometimes to a better outcome in such patients.
Here, we report a 52-year-old female who presented with a history of asymmetrical weakness in both the lower limbs. She initially noted weakness in her left lower limb, which progressed to the opposite leg in 10 days. Her weakness was progressive, and in the next 20–25 days she became bedridden. This was associated with urinary retention and constipation.
On general physical examination, a lump was detected in her right breast. Although it had not been her presenting complaint, the patient sponsored having this lump for the past 5 months. Her lower limb examination revealed spasticity, the power was of the Medical Research Council (MRC) grade 1/5. Deep tendon reflexes were 3+ at both the knee and ankle joints, while superficial plantar reflexes were equivocal. The remaining neurological and systemic examination was normal.
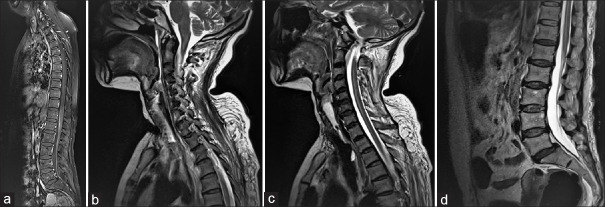
Magnetic resonance imaging (MRI) spine showed a T2 weighted hyperintensity extending from D-9 level till the conus medullaris [Figure 1a]. Positron emission tomography (PET) showed an avid lesion in the right breast (3.2 × 2.8 cm), mild thickening of the overlying skin, and nonavid right axillary lymph nodes (largest measuring 1.9 × 2.1 cm). The cerebrospinal fluid examination (CSF) showed cells: 7/μL (100% lymphocytes), protein: 89.1 mg/dL, glucose: 59 mg/dL; GeneXpert for tuberculosis was negative and did not show any atypical cells. Further, her serum paraneoplastic panel showed anti-Ri/ANNA-2 antibody, and no other paraneoplastic antibodies were detected.
Figure 1.
(a) MRI showing T2-weighted hyperintensities extending from D-9 to the conus, (b-d) Repeat MRI after 2 months showing T2-weighted hyperintensities extended to the cervicomedullary junction
The patient underwent modified radical mastectomy for her breast cancer. Histopathological examination showed invasive ductal carcinoma; immunochemistry revealed estrogen and progesterone receptors positive (95%, 20%), Her2neu was negative, and the pathological stage was TNM: P2 N1 Mx [Figure 2]. A tablet letrozole 2.5 mg once daily and trial of pulse therapy of intravenous methylprednisolone was given for 5 days, which led to mild improvement in muscle power (MRC grade 2/5). The patient left care against medical advice and was lost to follow-up for 2 months.
Figure 2.
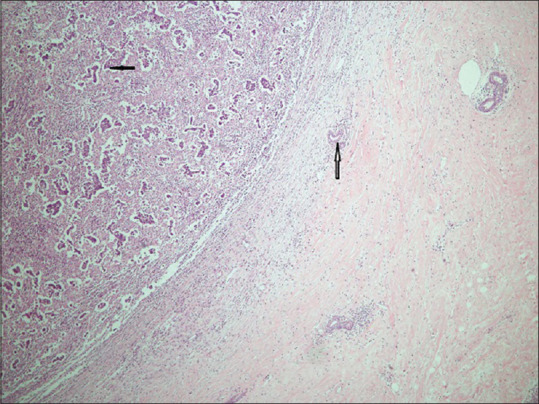
Histopathology of the breast showing invasive duct carcinoma with irregular ducts lined by atypical cells (bold arrow). Normal duct is seen on the right (hollow arrow) (Hematoxylin & Eosin ×200)
She again presented 2 months later with spastic quadriplegia (MRC grade 0/5). Repeat neuroimaging of the cord showed hyperintensities extending upwards to the cervicomedullary junction [Figure 1b-d]. Seven cycles of plasma exchange followed by intravenous immunoglobulin (2 gm/kg) and rituximab were given (1 gm each, 15 days apart), but there was no improvement in power.
A few days later, she developed jaw-closing oromandibular dystonia (Mallampatti Class IV: only hard palate visible) causing difficulty in maintaining oral hygiene and occasional tongue bite. Tablets trihexyphenidyl hydrochloride 2 mg thrice daily, tetrabenazine 25 mg twice daily, and clonazepam 0.25 mg thrice daily were given, after which her mouth opening increased slightly (Mallampatti Class III). She subsequently developed urosepsis associated with malnutrition, and after 4 months she succumbed to her illness.
Breast cancer–related paraneoplastic disease (PND) with positive anti-Ri/ANNA-2 antibody manifesting as myelopathy, though described in the literature, is quite rare. Pittock et al., reported 34 patients with anti-Ri/ANNA-2 antibody suffering from different malignancies, of which 9 patients had breast malignancy and 1 had myelopathy.[1] Another large series of 56 patients with breast cancer–related paraneoplastic neurologic disease showed 5 patients had anti-Ri/ANNA-2 autoantibody, and only 1 manifested as myelopathy.[2]
PND is an immune response against the neurons triggered by malignancy. Anti-Ri/ANNA-2 IgG binds to two highly conserved neuron-specific RNA-binding protein antigens (molecular masses 55 kDa and 80 kDa) that are distributed in the nervous system, encoded by Nova-1 and Nova-2 genes, respectively.[1,3]
PND associated with anti-Ri/ANNA-2 antibody manifests with a broad spectrum of neurological syndromes such as opsoclonus and/or myoclonus, cerebellar syndromes, brainstem encephalitis, torsional nystagmus, cranial neuropathy, seizures, axial rigidity and spasms, myelopathy, peripheral neuropathy, and Lambert–Eaton syndrome.[1] Jaw Dystonia causing trismus is rarely described in the literature.[4,5,6]
MRI spine in paraneoplastic myelopathy may show symmetric, longitudinally extensive involvement, or gray matter changes.[7] We found only two cases of longitudinally extensive myelitis reported for anti-Ri/ANNA-2 with breast malignancy with some clinical response.[8]
MRI brain may show abnormal signal changes in the temporal area, hemispheric white matter, insular cortex, and pons.[4] It was hypothesized that jaw-closing dystonia and spasticity without the Babinski sign was attributed to the selective involvement of brainstem tegmentum, propriospinal connections, and descending bulbospinal upper motor neurons, leading to signs of disinhibition or excitatory phenomena.[4] Histopathologically, it was also demonstrated as damage to antigen-containing inhibitory fibers innervating the brainstem motor nuclei by CD8+ lymphocytes.[4]
Treatment for paraneoplastic jaw dystonia and myelopathy with anti-Ri/ANNA-2 antibody involves surgical excision as the first step in management. Next begins, a trial of immunosuppressive medications that consists of steroids, intravenous immunoglobulin, plasma exchange, cyclophosphamide, mycophenolate mofetil, azathioprine, hydroxychloroquine, methotrexate, and rituximab.[2,9] For paraneoplastic jaw dystonia trial of trihexyphenidyl hydrochloride, clonazepam, and botulinum toxin can be tired in combination with the above drugs.[4] Some of the patients reported amelioration of jaw dystonia to this treatment.[4] The patient in our case had a heartening response initially, the poor outcome may be attributable to the absence of any treatment for 2 months while the patient was lost to follow up.
In conclusion, it is impossible to overstate the importance of a thorough general physical and systemic examination while investigating a case of progressive myelopathy, especially in the case of females. A high degree of suspicion combined with clinical acumen may uncover uncommon culprits like paraneoplastic syndrome as a source of the neurologic decline. Awareness regarding these rare manifestations of breast malignancy as myelopathy and jaw dystonia among clinicians is highly desirable as early diagnosis and prompt treatment may be essential for improving outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: Paraneoplastic accompaniments. Ann Neurol. 2003;53:580–7. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BL, Zalewski NL, Degnim AC, McKeon A, Flanagan EP, Pittock SJ, et al. Breast cancer-related paraneoplastic neurologic disease. Breast Cancer Res Treat. 2008;167:771–8. doi: 10.1007/s10549-017-4566-0. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau J, Posner JB. Neurologic paraneoplastic antibodies (anti-Yo; anti-Hu; anti-Ri): The case for a nomenclature based on antibody and antigen specificity. Neurology. 1994;44:2241–6. doi: 10.1212/wnl.44.12.2241. [DOI] [PubMed] [Google Scholar]
- 4.Pittock SJ, Parisi JE, McKeon A, Roemer SF, Lucchinetti CF, Tan KM, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri) Arch Neurol. 2010;67:1109–15. doi: 10.1001/archneurol.2010.209. [DOI] [PubMed] [Google Scholar]
- 5.Freydl E, Thier K, Schur S, Oberndorfer S. P15.01 Anti-Ri syndrome presenting with bilateral CN VI palsy and jaw dystonia. Neuro Oncol. 2016;18:75–6. [Google Scholar]
- 6.Bekircan-Kurt CE, Temucin C, Elibol B, Saka E. ’Jaw clenching’ in anti-Ri-antibody-associated paraneoplastic syndrome. Parkinsonism Relat Disord. 2013;19:132–3. doi: 10.1016/j.parkreldis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan EP, McKeon A, Lennon VA, Kearns J, Weinshenker BG, Krecke KN, et al. Paraneoplastic isolated myelopathy: Clinical course and neuroimaging clues. Neurology. 2011;76:2089–95. doi: 10.1212/WNL.0b013e31821f468f. [DOI] [PubMed] [Google Scholar]
- 8.Patrice KA, Saylor D, Williams T, Mowry E, Newsome E, Probasco J. A rare cause of myelopathy: Anti-Ri (ANNA-2) associated paraneoplastic myelopathy. Neurology. 2016;86:6.148. [Google Scholar]
- 9.Leypoldt F, Eichhorn P, Saager C, Münchau A, Lewerenz J. Successful immunosuppressive treatment and long-term follow-up of anti-Ri-associated paraneoplastic myelitis. J Neurol Neurosurg Psychiatry. 2006;77:1199–200. doi: 10.1136/jnnp.2005.086140. [DOI] [PMC free article] [PubMed] [Google Scholar]