Abstract
This paper describes a new chromogenic plate medium, CHROMagar Staph aureus (CHROMagar, Paris, France), for the identification of Staphylococcus aureus on the basis of colony pigmentation. The abilities of CHROMagar Staph aureus, thermostable nuclease (DNase), and mannitol salt agar (MSA) to identify S. aureus isolates (n = 114) and discriminate between S. aureus and coagulase-negative staphylococci (CoNS; n = 22) were compared. CHROMagar Staph aureus proved to be more sensitive and specific than DNase and MSA, allowing a reliable, simple, and rapid method for the identification of S. aureus isolates. All CoNS encountered in this study with the exception of S. chromogenes could be easily differentiated from S. aureus on this medium. The supplementation with 4 μg of oxacillin or methicillin per ml allowed simple identification of methicillin resistance in hospital-acquired S. aureus strains which show multiple-drug resistance profiles. Community-acquired methicillin-resistant S. aureus strains showing non-multi-drug resistance profiles require further evaluation on this new chromogenic medium. Methicillin or oxacillin resistance of all S. aureus isolates was confirmed by the detection of penicillin-binding protein 2a, encoded by the mecA gene, using the latex slide agglutination MRSA-Screen test (PBP 2′ Test, DR900M; Oxoid).
Staphylococcus aureus is one of the most frequently identified pathogens in clinical laboratories, while methicillin-resistant S. aureus (MRSA) is an important nosocomial pathogen (6). Considerable effort has been expended by numerous investigators (7–9) in the development of reliable media for differentiation of S. aureus from coagulase-negative staphylococci (CoNS). S. aureus has most frequently been associated with the coagulation of plasma, the fermentation of mannitol (mannitol salt agar [MSA]), the production of thermostable nuclease (DNase), egg yolk lipase hydrolysis (lipovitellin-salt-mannitol agar [LSM]), and the production of natural pigment (6).
Reliable and rapid methods to identify these organisms are crucial in any clinical laboratory. The tube coagulase test is regarded as the gold standard; however, variations in levels in plasma and incubation times and problems in interpretation can lead to misidentification. An alternative approach is to incorporate chromogenic substrates into a suitable isolation medium. Detection of the activities of specific bacterial enzymes, indicated by color change, negates the need for time-consuming and costly biochemical identification (10).
The present study examines the use of a new chromogenic plate medium, CHROMagar Staph aureus, for the identification of S. aureus and detection of MRSA. The criteria for medium evaluation included colony growth reaction, color reproducibility for the identification of S. aureus, and ease of color detection of MRSA when the medium was supplemented with methicillin or oxacillin. The study was divided into two parts. Part 1 compared the accuracy of reactions of S. aureus on CHROMagar Staph aureus, DNase, and MSA with the accuracy of reactions of CoNS. Part 2 compared the detection of MRSA on the new chromogenic medium when it was supplemented with 4 μg of methicillin or oxacillin per ml to detection on a standard Iso-Sensitest agar dilution screening plate containing the same concentration of methicillin. Methicillin or oxacillin resistance was confirmed by the detection of penicillin-binding protein 2a (PBP 2a) expressed by the mecA gene using the latex slide agglutination MRSA-Screen test (PBP 2′ Test, DR900M; Oxoid) (11, 12).
(This paper was presented in part at the IXth International Congress of Bacteriology and Applied Microbiology Meeting, Sydney, Australia.)
Bacterial cultures.
One hundred twenty-six significant staphylococcal isolates were examined at random upon isolation from clinical samples received for examination in the Department of Microbiology and Infectious Diseases at Concord Repatriation General Hospital, Concord, Australia. These included S. aureus (104 isolates), S. epidermidis (7 isolates), S. capitis (5 isolates), S. haemolyticus (3 isolates), S. warneri (2 isolates), S. hominis (2 isolates), S. cohnii (1 isolate), and S. simulans (1 isolate). These isolates represented clinical samples from sterile (blood, tissue, bone, eye) or nonsterile (sputum, superficial wound, urine) sites. In addition, 10 non-multi-drug-resistant community-acquired MRSA isolates were supplied from the bacteriophage-typing laboratory at Royal Prince Alfred Hospital, Sydney, Australia.
Culture media.
CHROMagar Staph aureus was provided by the CHROMagar Company, Paris, France, and imported to Australia by DUTEC Diagnostics (a division of DUTEC Pty. Ltd., Sydney, Australia). The medium contained agar (15 g/liter), peptones (40 g/liter), NaCl (25 g/liter), and a proprietary chromogenic mix (3.5 g/liter). The medium was prepared as instructed by the manufacturer by avoiding heating at over 100°C. Methicillin or oxacillin (4 μg/ml) was added when the agar was cooled at 48°C. Each plate contained 20 ml of agar medium dispensed into 90-mm-diameter petri dishes. Horse blood (5%) agar (Oxoid-Columbia base-CM331; Oxoid, Victoria, Australia), MSA plates (Oxoid-CM85), and DNase (Oxoid-CM321) were prepared according to the manufacturer's instructions. S. aureus ATCC 25923 was used to monitor batch variability. Horse blood agar was used for organism viability testing.
Identification and susceptibility methods.
Gram stain reactions, morphology, reactions to catalase (3% [wt/vol] hydrogen peroxide, BDH-UN2014; Kilsyth, Victoria, Australia), mannitol salt fermentation, reactions to slide and tube coagulase (lyophilized rabbit plasma; bioMerieux, Marcy l'Etoile, France), and DNase activity were used for identification. API20 Staph galleries (bioMerieux) and STAPH-ZYM kits (Rosco, Taastrup, Denmark) were used for confirmatory species identification. On CHROMagar Staph aureus any growth appearing pink to mauve (Fig. 1) was interpreted as positive for the presence of S. aureus. Susceptibility of isolates was confirmed by agar plate dilution using a multipoint inoculation system (10). Alternatively, a disk agar diffusion method (National Committee for Clinical Laboratory Standards) using methicillin (5 μg) disks (OXOID) was also used (8). E-test strips (Australian Laboratory Services, Pty. Ltd., Sydney, Australia) confirmed the MICs for certain isolates (data not shown). S. aureus ATCC 25923 was used to monitor antibiotic potency for quality control. Testing was performed using a multipoint inoculating technique (MAST systems) as described previously (9). Briefly, each pin transferred 104 to 105 CFU of inoculum per ml to the surface of the culture medium. Moisture on the surfaces of agar plates was removed by air drying (lid kept ajar) at 35°C for 1 h prior to inoculation. All plates with inoculated medium were incubated at 30°C and at 35 to 37°C in ambient air. CHROMagar plates were incubated in the dark as instructed by the manufacturer. All S. aureus strains showing methicillin and oxacillin resistance were confirmed by the MRSA-Screen latex agglutination test. The test was performed in accordance with the manufacturer's instructions and has been described in previous studies (11, 12). Beta-lactamase enzyme testing was performed with cefinase disks (BBL, Cockeysville, Md.) as instructed by the manufacturer. For statistical analysis, CHROMagar Staph aureus agar results and those obtained with other conventional media were compared for sensitivity and specificity of the media.
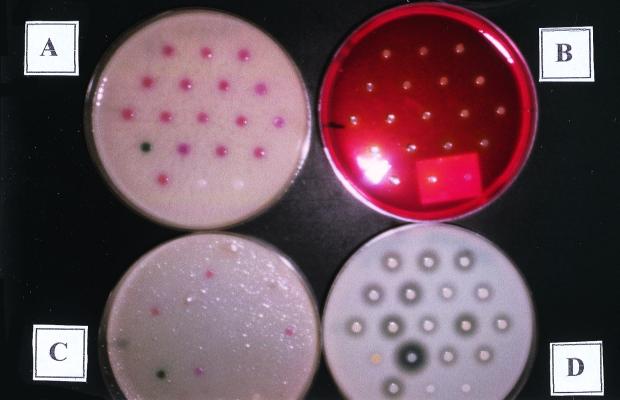
FIG. 1.
Comparisons of different agar media showing various reactions of 18 selected Staphylococcus isolates and one CoNS isolate mixed with enterococci. (A) CHROMagar Staph aureus with no antibiotic supplementation showing pink-to-red reactions for all S. aureus isolates. Row 4 spot 1 shows a blue color reaction for CoNS mixed with enterococci. Row 5 spots 2 and 3 show a white color for CoNS. (B) MSA showing yellow positive reactions and pink-to-red negative reactions of isolates. (C) CHROMagar Staph aureus supplemented with oxacillin showing the oxacillin (methicillin) resistance of isolates. The color of MRSA isolates is pink to red. (D) DNase agar showing clearing around positive isolates.
CHROMagar Staph aureus accuracy in detecting S. aureus.
Of 114 S. aureus isolates tested on CHROMagar Staph aureus, all grew and were identified chromogenically as S. aureus by a pink-to-mauve color change after 18 to 24 h of incubation. In contrast, DNase and MSA were successful in identifying S. aureus in 112 of 114 of isolates (98%). Of 22 CoNS tested on CHROMagar Staph aureus in this study, 21 isolates produced colorless or cream-colored colonies. One CoNS (S. chromogenes) produced a pale-pink pigment. This could not be easily differentiated from the pigment produced by S. aureus colonies. Mannitol was fermented in 8 of 22 CoNS isolates (5 S. capitis isolates, 1 S. simulans isolate, 1 S. chromogenes isolate, and 1 S. cohnii isolate) on MSA (36.5%), while 1 CoNS isolate (S. capitis) produced a positive reaction on DNase. Hence, in comparison to DNase and MSA, CHROMagar Staph aureus gave superior sensitivity and specificity for identification of S. aureus (Table 1).
TABLE 1.
Comparison of the levels of accuracy of reactions of S. aureus isolates on CHROMagar Staph aureus, DNase, and MSA and of coagulase testing of CoNS after 18 to 24 h of incubation
| Identification method | % of isolates showing positive reactions
|
Accuracy of medium in discriminating S. aureus and CoNS
|
||
|---|---|---|---|---|
| S. aureus (n = 114) | CoNS (n = 22) | Sensitivity (%) | Specificity (%) | |
| CHROMagar Staph aureus | 100 | 0a | 100 | 100 |
| DNase | 98.0 | 4.6 | 98.0 | 95.4 |
| MSA | 98.0 | 36.5 | 98.0 | 63.5 |
| Coagulaseb | 100 | 0 | 100 | 100 |
S. chromogenes produced a natural carotenoid (orange or red) pigment and gave a slightly pink color. The isolate was identified by API20 Staph.
All coagulase testing was confirmed by the standard tube method.
CHROMagar Staph aureus accuracy in detecting MRSA.
Following addition of methicillin and oxacillin (4 μg/ml) to the chromogenic medium, there was inhibition of all methicillin-sensitive strains, with full correlation to results from methicillin- and oxacillin-supplemented standard agar (Iso-Sensitest) dilution testing (Table 2). Multi-drug-resistant MRSA strains were reliably detected on the medium (100%) with similar color changes, and all were positive for PBP 2a. However, non-multi-drug-resistant community-acquired MRSA grew inconsistently on the chromogenic medium. Only 4 of 12 (30%) such isolates grew on the supplemented CHROMagar (Table 2). Different incubation temperatures (30, 35, and 37°C) did not affect this result (data not shown). All 12 strains were PBP 2a positive by the rapid MRSA-Screen latex test. The MICs (measured by E-test) for these isolates were not significantly different from those for the other MRSA tested. All methicillin-resistant CoNS grew as colorless or cream-colored colonies.
TABLE 2.
Detection of MRSA on CHROMagar Staph aureus when it was supplemented with 4 μg of methicillin per ml and at antibiotic susceptibility breakpointsa
| S. aureus typeb | No. of strains tested (n = 114) | % of strains showing positive colored growth on CHROMagar Staph aureus
|
% of strains showing resistance to the following antibiotics at the indicated susceptibility breakpoints (dilution in agar [μg/ml])
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without MET | With MET or OXc | MET (4) | OX (4) | PEN (0.12) | ERY (0.2) | TET (4) | GEN (1) | GEN (4) | GEN (8) | ||
| MSSA | 68 | 100 | 0 | 0 | 0 | 97e | 15 | 7 | 1 | 0 | 0 |
| HAMRSAd | 34 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CAMRSAd | 12 | 100 | 30 | 100 | 100 | 100 | 25 | 0 | 0 | 0 | 0 |
MET, methicillin; OX, oxacillin; PEN, penicillin; ERY, erythromycin; TET, tetracycline; GEN, gentamicin.
MSSA, methicillin-sensitive S. aureus; HAMRSA, hospital-acquired MRSA; CAMRSA, community-acquired MRSA.
Addition of 4 μg of methicillin or oxacillin per ml to the chromogenic medium. Similar results were obtained with both drugs.
Isolates were tested by the rapid MRSA-Screen latex agglutination test (PBP 2′ Test, DR900M; Oxoid) for the presence of PBP 2a.
Three isolates susceptible to penicillin were beta-lactamase enzyme negative.
In conclusion, CHROMagar Staph aureus achieved a higher sensitivity and specificity than those of two commonly used media, DNase and MSA, in identifying S. aureus isolates. The new chromogenic medium facilitated clear distinction between coagulase-positive and -negative staphylococci, permitting a reliable, simple, and rapid method of identification of S. aureus in this study. Results are encouraging. Further evaluation is needed with other species of CoNS not encountered or described in this study. The supplementation with oxacillin or methicillin allows nosocomial multi-drug-resistant MRSA to be detected, which was not the case with non-multi-drug-resistant community-acquired MRSA. Non-multi-drug-resistant community-acquired MRSA are of increasing clinical significance and represent a growing proportion of community-acquired S. aureus infections from outpatient clinics. They have been isolated from soft tissue, abscess, skin, blood, bone, eye, genital, and respiratory infections (1, 4). These do not represent nosocomial isolates which have spread in the community from hospitals, since most have susceptibility and phage-typing patterns (data not shown) distinct from those of multi-drug-resistant hospital MRSA isolates. Cases of community-acquired non-multi-drug-resistant MRSA have been reported in Chicago, Ill., Australia, and New Zealand (1, 5, 11). The cause of these organisms' growth anomaly on the test chromogenic medium remains unclear but may reflect active cotransportation of methicillin intracellularly with the chromogenic moiety. In the presence of methicillin or oxacillin, the chromogenically linked substrates may affect the cell membrane potential during permeation, leading to nonspecific membrane disorganization or induced cell death. Research is currently being undertaken to define these phenomena in these community-acquired non-multi-drug-resistant MRSA strains. Further evaluation of this new chromogenic medium with direct clinical specimens is needed before this medium can be used for routine direct screening for MRSA.
Acknowledgments
We thank Alison Vickery for supplying some strains for this study and Matthias Dorsch, Paul Attfield, Thusitha Gunasekera, and Andrew Boyd for discussions. The skilled support of Mohammad Siddique for medium preparation and the technical assistance of the Department of Microbiology and Infectious Diseases at Concord Repatriation General Hospital are recognized. We also thank the CHROMagar, Oxoid, DUTEC Diagnostics, and Rosco companies for supplying materials for this study.
REFERENCES
- 1.Collignon P. Increased incidence of methicillin-resistant strains of Staphylococcus aureus in the community. J Infect Dis. 1999;179:1592. doi: 10.1086/314788. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Collignon P, Gosbell I, Vickery A, Nimmo G, Stylianopoulos T, Gottleib on Behalf of the Australian Group on Antimicrobial Resistance T. Community-acquired methicillin-resistant Staphylococcus aureus in Australia. Lancet. 1998;352:145–146. doi: 10.1016/s0140-6736(98)85051-4. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Herold B C, Immergluck L C, Maranan M C, Lauderdale D S, Gaskin R E, Boyle-Vavra S, Leitch C D, Daum R S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 4.Kloos W E, Jorgensen J H. Staphylococci. In: Lennette E H, Balows A, Hausler W Jr, Truant J P, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 143–153. [Google Scholar]
- 5.Lally R T, Ederer M N, Woolfrey B F. Evaluation of mannitol salt agar with oxacillin as a screening medium for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;22:501–504. doi: 10.1128/jcm.22.4.501-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlino J, Gill R, Robertson G J. Application of lipovitellin-salt-mannitol agar for screening, isolation, and presumptive identification of Staphylococcus aureus in a teaching hospital. J Clin Microbiol. 1996;34:3012–3015. doi: 10.1128/jcm.34.12.3012-3015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlino J, Siarakas S, Robertson G J, Funnell G R, Gottlieb T, Bradbury R. Evaluation of CHROMagar Orientation for differentiation and presumptive identification of gram-negative bacilli and Enterococcus species. J Clin Microbiol. 1996;34:1788–1793. doi: 10.1128/jcm.34.7.1788-1793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 5th ed. 1993. Approved standard M2-A5. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 9.O'Brien F G, Pearman J W, Gracey M, Reley T V, Grubb W B. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak. J Clin Microbiol. 1999;37:2858–2862. doi: 10.1128/jcm.37.9.2858-2862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuther J W A. A simplified system for the identification of staphylococci by multipoint inoculation of test media. J Med Microbiol. 1968;22:179–182. doi: 10.1099/00222615-22-2-179. [DOI] [PubMed] [Google Scholar]
- 11.Van Griethuysen A, Pouw M, Van Leeuwen N, Heck M, Willemse P, Buiting A, Kluytmans J. Rapid slide latex agglutination test for detection of methicillin resistance in Staphylococcus aureus. J Clin Microbiol. 1999;37:2789–2792. doi: 10.1128/jcm.37.9.2789-2792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Leeuwen W B, van Pelt C, Luijendijk A, Verbrugh H A, Goessens W H F. Rapid detection of methicillin resistance in Staphylococcus aureus isolates by the MRSA-Screen latex agglutination test. J Clin Microbiol. 1999;37:3029–3030. doi: 10.1128/jcm.37.9.3029-3030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]