Highlights
-
•
Differences observed in all blood marker categories, from on-therapy until years later.
-
•
Differences observed in metabolic and blood cell markers, even pre-chemotherapy.
-
•
Blood markers were associated with cognition mainly years post-chemotherapy.
-
•
Structural brain metrics associated with cognition shortly and years post-treatment.
Keywords: Neuroimaging, Chemotherapy, Cognitive impairment, Biological markers, Breast cancer
Abstract
Breast cancer treatment can induce alterations in blood- and neuroimaging-based markers. However, an overview of the predictive value of these markers for cognition is lacking for breast cancer survivors.
This systematic review summarized studies of the last decade, using the PubMed database, evaluating blood markers, and the association between blood- or structural neuroimaging markers and cognition across the chemotherapy trajectory for primary breast cancer, following PRISMA guidelines.
Forty-four studies were included. Differences were observed in all blood marker categories, from on-therapy until years post-chemotherapy. Associations were found between cognitive functioning and (1) blood markers (mainly inflammation-related) during, shortly-, or years post-chemotherapy and (2) white and gray matter metrics in frontal, temporal and parietal brain regions months up until years post-chemotherapy. Preliminary evidence exists for epigenetic and metabolic changes being associated with cognition, only after chemotherapy.
This review demonstrated time-dependent associations between specific blood-based and structural neuroimaging markers with cognitive impairment in patients with breast cancer. Future studies are encouraged to include both neuroimaging- and blood markers (e.g. of neuronal integrity, epigenetics and metabolism) to predict long-term cognitive effects of chemotherapy.
Introduction
With 2.3 million new cases in 2020, female breast cancer is the most common cancer type worldwide [1]. Thanks to advances in cancer treatment, the number of survivors has grown remarkably and research has expanded, focusing on side effects of such treatments.
Cancer-related cognitive impairment (CRCI) is broadly reported, with a wide range (17–78%) of patients being affected, mainly in domains of memory, attention, psychomotor speed and executive functioning. CRCI can emerge before the start of therapy, during therapy and persist up to years after treatment (see recent reviews [2], [3], [4]).
While underlying mechanisms remain largely unknown, CRCI is hypothesized to be a complex interaction of vulnerability (i.e. mechanisms involved in DNA damage/repair or immune regulation [5]), cancer biology, aging, and both direct or indirect toxic treatment effects [2,6]. Some chemotherapeutic agents are known to cross the blood brain barrier (BBB), which can directly cause brain damage in areas important for cognitive functioning. However, given the complexity and duration of CRCI, it is unlikely that direct mechanisms of chemotherapy solely explain CRCI. Hence, indirect toxic mechanisms of chemotherapy also need to be considered to explain CRCI, such as cytokine-induced neuroinflammation and increased allostatic load in an individual [6]. Allostatic load, referring to the cumulative burden of chronic stress and live events potentially transitioning to a state in which stress response systems are repeatedly activated, which can for instance be evaluated with epigenetics and metabolomics [7].
To investigate the ongoing neurobiological influences of chemotherapy, neuroimaging studies have attempted to uncover the underlying neurobiology of CRCI more directly, via MRI and PET sequences. Both widespread structural and functional (e.g. metabolic) brain changes are observed in cancer patients. For reviews summarizing MRI studies, the reader is referred to recent literature [4,8]. Additionally, these studies show correlations between cognitive functioning and brain metrics, e.g. brain volume or white matter markers [8]. However, a comprehensive overview of markers obtained from different imaging modalities and their association with (i.e. possibly predictive value of) cognitive impairment after breast cancer treatment is currently missing.
In addition to the acquisition of neuroimaging, blood-based biomarkers provide a more affordable and easily accessible approach in identifying cancer patients who are possibly more susceptible for cognitive decline throughout time. Increased peripheral inflammation, hormonal deregulation, anemia, changes in epigenetic markers or telomeric length have all been observed after treatment with chemotherapy [1,3]. An overview of associations/correlations between cognitive impairments and specific circulating factors, cerebral spinal fluid constituents, and genetic polymorphisms in diverse cancer populations was provided by Castel and colleagues [9]. However, the existing literature on this topic has exponentially increased in the last years, for breast cancer patients specifically. Furthermore, it is unclear to what extent blood-based markers can have an added value to neuroimaging, to understand and prevent cognitive issues following chemotherapy.
A better understanding of biomarkers and mechanisms of CRCI is necessary to be able to identify and characterize subgroups of patients who are at risk for CRCI in a non-invasive and cost-effective way. The objective of this systematic review is thus to establish a comprehensive overview of studies from the past 10 years examining fluid-based biomarkers throughout the trajectory of treatment with chemotherapy for breast cancer, as well as cognitive sequelae related to these and neuroimaging biomarkers. More specifically, based on our systematic search we aim to unravel: (1) which fluid biomarkers differ throughout treatment with chemotherapy for breast cancer and (2) the time-dependency of associations between both fluid-based and markers derived from structural neuroimaging and (objective and self-report) cognition throughout treatment with chemotherapy.
Methods
Protocol
This review followed the latest PRISMA guidelines [10] and was registered with PROSPERO in April 2020 (CRD42020178498). A systemic literature search was performed using the PubMed database with date restriction from January 1st, 2010 to March 16th, 2020 with the latest search on March 16th, 2020.
Search strategy
Search strategy was based on four components: “breast cancer”, “fluid marker”, “imaging” and “cognition”. These subject headings were further elaborated by identifying synonyms and consulting medical descriptors, such as MeSH terms. The exact search term can be found in Appendix A.
Eligibility criteria
Eligibility criteria of studies were established according to the PICOS structure [10], following four criteria: (i) the study involved adult women scheduled for or treated with chemotherapy (i.e. in any phase or at any point in chemotherapeutic treatment) for primary breast cancer; (ii) cognitive outcome was assessed (neuropsychological tests or self-report questionnaires) and (iii) was linked with a (fluid- or imaging-) biomarker and (iv) full text written in English. Studies only using cognitive tasks inside the scanner or employing functional MR imaging were excluded.
Study selection
Two authors (G.S. and J.V.) independently assessed eligibility of the articles using the Rayyan system [11] in two stages: (i) studies were included, excluded or labelled as ‘maybe’ based on the extracted titles and abstracts, (ii) full-text of studies labelled as included and ‘maybe’ were assessed for inclusion criteria. Disagreement between the two reviewers was resolved through discussion, or if necessary, by a third independent reviewer (C.S.). Agreement between the two reviewers at the first step (title- and abstract-phase) was strong, κ = 0.877, 95% CI [.81,.94] (Appendix C), and after second review and discussion maximized to complete agreement. [12]
Data extraction
Data on authorship, publication year, number and age of participants, breast cancer stage of patients, administered chemotherapeutic agents or protocol, percentage of radiotherapy- and/or endocrine therapy- treated patients, menopausal state, specific characteristics of controls, timing of assessment, measured biomarkers, cognitive measures, questionnaires and main findings for cognition, blood and/or imaging markers as well as associations with cognition were extracted from each study.
Risk of bias
Risk of bias was assessed at study level according to the risk of bias QUADAS-2 tool [13] in Review Manager (RevMan) (The Cochrane Collaboration, 2014) for the following domains: patient selection, index test (biomarker), reference standard (cognitive assessment) and flow and timing. Risk of bias signaling questions along with study-specific risk of bias assessment can be found in Appendix B.
Results
Study selection
The screening process is shown in Fig. 1. Database search provided a total of 829 articles. From these, 768 articles were discarded for not meeting the inclusion criteria. Full text of the remaining 59 articles was examined in detail, from which 44 were included in the current review.
Fig. 1.
PRISMA flow chart.
Study and patient characteristics
Characteristics of the included studies are presented in detail in Appendix D. Twenty-five studies were conducted in North America, seven in Asia, 10 in Europe, one in Australia and one in South America. This review comprises a total of 3745 female participants of whom 1642 (44%) were breast cancer patients treated with chemotherapy (C+), 226 (6%) were breast cancer patients treated without chemotherapy (C-) and 1877 (50%) were healthy controls (HC). Most studies (n = 23/44) compared C+ patients with a HC group. Eleven studies only investigated C+ patients, four studies compared C+ with C- patients and six studies included both C- and HC control groups. Sample sizes of the included studies were generally small (n = 8–166, median = 24), with only two studies including more than 100 breast cancer patients.
A cross-sectional design was used in 19 studies, a prospective longitudinal design in 25 studies and one study used a combination of both designs. Assessments ranged from pre-surgery or -chemotherapy to 20 years post-chemotherapy.
Sociodemographic characterization revealed an average age at assessment of 52.93 (SD = 8.26), 52.07 (SD = 6.17) and 56.28 (SD = 10.23) years for the C+, C- and HC participants, respectively. Seventeen articles described the race/ethnicity of their participants, although the used definitions or categories differed. Most studies (n = 12) used white/Caucasian or non-white/African-American for describing ethnicity of their participants, resulting in 82% of these participants being white and 14% being non-white. Three studies described whether their participants were part of a minority group (not further specified), across which 32% of the participants were classified as such. Two studies mentioned Chinese or non-Chinese as ethnicity, across which 81% of the participants were Chinese. Most studies (n = 27) mentioned menopausal stage of participants at time of the assessment. Of those, in their respective groups, 51% of the C+ patients, 43% of the C- patients and 37% of the HC participants were postmenopausal, although menopausal status for this last group was underreported (only 16% known). Clinical characterization revealed that most C+ patients were treated with common standard-dose multi-agent chemotherapy regimens, containing docetaxel, epirubicin, doxorubicin, 5-fluorouracil, carboplatin, cyclophosphamide, methotrexate or paclitaxel, combined with radiotherapy and/or anti-hormone therapy. Twenty articles mentioned breast cancer stage; for the total of those C+ patients, 0.12% were in stage 0, 22% in stage I, 56% in stage II, 21% in stage III and 1% in stage IV. Of these 20 articles, six studies also reported breast cancer stage of C- patients: 19% were in stage 0, 66% in stage I and 15% in stage II. As expected, breast cancer stage of C- patients was generally lower than of C+ patients.
Risk of bias
The assessments of risk of bias of the included studies are shown in Appendix B. Of all included studies, 41% showed no risk of bias, 30% in one domain or unclear risk and 30% in two or more domains. High risk of bias was most common for the domain of patient selection (n = 18), followed by reference standard (n = 7). Most studies showing a high risk of bias in reference standard either used screening instruments which do not evaluate multiple cognitive domain scores, enabling domain-specific comparison to norm data [e.g., Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MOCA)] or did not interpret the cognitive results blinded for the results of the biomarker tests. Studies showing a high risk of bias in patient selection either applied an age restriction (e.g., only women aged 70+ years) or included participants based on cognitive cut-off scores (e.g., positive answer on at least a subset of questions), both leading to decreased representativeness of adult women with breast cancer (target population of this review).
Blood markers
Peripheral blood markers were independently analyzed in 20 studies and are categorized into six groups: (1) inflammatory mediators, (2) sex-and stress hormones, (3) blood cells/proteins, (4) metabolic-, (5) neuronal integrity- and (6) epigenetic markers (Table 1). No studies investigated CSF markers. Inflammatory mediators were most frequently examined, with IL-6 (n = 15), TNF-α/sTNFRII (n = 11) and CRP (n = 5) being the most abundant markers.
Table 1.
Blood markers showing differences for C+ patients (19 studies).
| Category marker | Investigated markers | Baseline | On-therapy | Short-term | Long-term | References |
|---|---|---|---|---|---|---|
| Inflammation | CRP, CRP M450, CXCL10, eotaxin, IFNγ, IGF-1, IL-1ra, IL-1β, IL-1β M450, IL-2, IL-4, IL-5, IL-6, IL-6 M450, IL-6R, IL-7, IL-8, IL-10, IL-10 M450, IL-12, IL-12p10, IL-13, IL-17, MCP-1, MIP-1 α, MIP-1β, TNF-α, RANTES, sTNFRI, sTNFRII | / | ↑↓ IL-6, ↑↓ IL-18, ↑↓ MCP-1, vs C-: ↑ IL-6, sTNFRII |
↑ IL-6, ↓ IL-12, IL-17, vs C- : ↑ sTNFRII, IL-1ra, CRP |
↑ IL-6, MCP-1, MIP-1β followed by ↓, ↓ IL-12, IL-17 vs C- : ↑ CRP, sTNFRII (followed by no differences), vs HC : ↑ IL-6, IL-1β, IL-2, IL-4, IL-8, IL-10, TNF-α |
[16,17,19,[23], [24], [25], [26],30] |
| Sex- and stress hormones | cortisol, oestradiol, estrogen, FSH, LH, progesterone | / | / | ↑ FSH, LH, ↓ oestradiol, |
/ | [15] |
| Blood- cells/proteins | albumin, blood cell concentration, blood coagulation markers, B cells, CD4+ T cells, CD8+ T cells, GLR, GM-CSF, G-CSF, granulocytes, Hb, HGF, monocytes, NK cells, PLR, SII, VEGF | vs cut-offs: ↓ Hb, vs HC: ↑ Hb |
↑ G-CSF, vs C-: ↑ VEFG |
↑ monocytes, ↓ B cells, CD4+ T cells |
↓ G-CSF, vs C- : ↑ GLR, PLR, SII, vs HC: ↑ blood coagulation markers |
[14,15,21,25,27] |
| Neuronal | Aβ−42, Aβ−40, BDNF, pNF-H, tau | / | ↑ pNF-H | / | / | [16,18] |
| Metabolism | cholesterol, creatinine, electrolytes, fasting blood glucose, homocysteine, LDH, liver function tests, rcSO2, triglycerides | vs cut-offs: ↑ LDH, vs HC: ↑ cholesterol, glucose |
/ | ↑ triglycerides; ↓ cholesterol |
/ | [14,15] |
| Epigenetics | whole blood oxidative DNA damage, methylation ratio in 880,965 CpG positions | / | / | Δ methylation ratio for 4 CpG positions | Δ methylation ratio for 2199 CpG positions vs. HC: increased oxidative DNA damage |
[21,22,28] |
Note. Short-term effects = assessment within one week to four months post-chemotherapy. Long-term effects = assessment within six months to 20 years post-chemotherapy.
↑: increased, ↓: decreased, Δ: change, Aβ: Plasma amyloid beta, BDNF: Brain-derived neurotrophic factor, C+: chemotherapy-treated patients, C-: chemotherapy-naïve patients, CRP: C-reactive protein, CXCL10: Interferon-inducible protein 10, FSH: Follicle-stimulating hormone, G-CSF: Granulocyte colony-stimulating factor, GLR: Granulocyte-to-lymphocyte ratio, GM-CSF: Granulocyte-macrophage colony-stimulating factor, HC: healthy controls, Hb: Haemoglobin, HGF: Hepatocyte growth factor, IFNγ: Interferon gamma, IGF-1: Insulin-like growth factor 1, IL: Interleukin, IL-1ra: Interleukin-1 receptor antagonist, LDH: Lactate dehydrogenase, LH: Luteinising hormone, MCP-1: Monocyte chemoattractant protein 1, MIP: Macrophage inflammatory protein, NK cells: Natural killer cells, PLR: Platelet-to-lymphocyte ratio, pNF-H: Phosphorylated neurofilament H, RANTES: Chemokine ligand 5, rcSO2: Regional cerebral tissue oxygen saturation. SII: Systemic immune-inflammation index, sTNFRI: Soluble tumor necrosis factor receptor I, sTNFRII: Soluble tumor necrosis factor receptor II, TNF-α: Tumour necrosis factor alpha, VEGF: Vascular endothelial growth factor.
Blood marker results are discussed in three sections: (1) pre-treatment and on-therapy findings, (2) post-chemotherapy findings of studies longitudinally investigating C+ patients alone and (3) post-chemotherapy differences between C+ patients and study-specific controls (C- or HC) (Table 1 and Fig. 2).
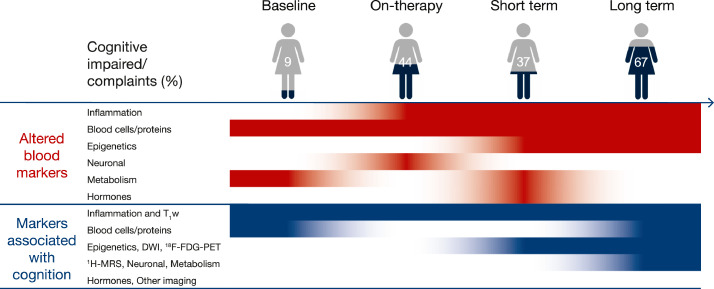
Fig. 2.
Schematic overview of maximum percentage of patients scoring cognitive impaired or those reporting complaints; blood markers showing alterations over time and blood and neuroimaging markers showing associations with cognitive score or complaints, all for chemotherapy-treated patients.
Note. Patients scoring cognitive impaired or reporting complaints were both defined based on (study-specific) norm scores of cross-sectional findings from studies included in this review [14,20,25,31,34,[37], [38], [39]]. Red = blood marker being altered at a given time point over the course of chemotherapy, blue = blood marker and/or neuroimaging metric showing an association with cognitive score at a given time point. White = no changes or association at a given time point. Fading of colors indicates transitioning to other time point, not strength of associations. Short-term = assessment within one week to four months post-chemotherapy. Long-term = assessment within six months to 20 years post-chemotherapy. DWI = diffusion weighted imaging, T1W = anatomical T1 weighted imaging, 1H-MRS = proton magnetic resonance spectroscopy, 18F-FDG-PET = metabolic fluor-18 fluorodeoxyglucose positron emission tomography.
Baseline and on-therapy effects for C+ patients
Compared to study-specific HCs or cut-off scores, C+ patients presented with higher values of metabolic markers (i.e. LDH [14], cholesterol and glucose [15]) pre-treatment. Additionally, lower Hb values were observed when compared to norm values [14], whereas another study reported higher Hb values compared to study-specific HCs [15]. During chemotherapy, increased levels of inflammatory markers, IL-6 [16] and sTNFRII [16], blood cells/proteins, G-CSF [17] and VEGF [16], and the neuronal marker pNF-H [18] were found, compared to baseline levels or C- patients. Additionally, the inflammatory markers IL-6, IL-18 and MCP-1 showed dynamic changes with increasing chemotherapy cycles, depending on the treatment regime [19].
Acute and chronic chemotherapy effects for C+ patients
With the exception of neuronal integrity markers, all blood markers showed acute changes for C+ patients (one week to four months after chemotherapy). For inflammatory markers, IL-6 levels increased [20], while IL-12 and IL-17 [17] decreased. Considering blood cells/proteins: monocyte levels increased, whereas B-cells and CD4+ T-cells decreased. [21] Other acute effects were observed for sex hormones, with increased FSH and LH and decreased oestradiol shortly after chemotherapy, as well as for metabolic markers, with increased triglycerides and decreased cholesterol levels [15]. Epigenetic changes included methylation ratio changes for four CpG positions, shortly post-chemotherapy [21].
Chronic effects (six months to two years) were observed less frequently, with increases persisting for some inflammatory markers (IL-6, MCP-1, MIP-1β), while most decreased (IL-12, IL-17, IL-6, MCP-1, MIP-1β) [17]. Changes in blood cells/proteins were also present, with decreases in G-CSF specifically [17]. Methylation ratios changed for 2199 CpG positions one year post-chemotherapy [22].
Acute and chronic group chemotherapy effects for C+ patients compared to controls
Other studies investigated differences in blood markers of chemotherapy-treated patients versus controls, for which acute effects (one to three months post-chemotherapy) were only observed for inflammatory markers. While some studies reported higher levels of cytokine concentrations in C+ patients (sTNFRII, IL-1ra, CRP), compared to C- shortly after chemotherapy [23,24], others did not observe differences (IL-1ra, IL-6, CRP [23], [24], [25]).
Chronic effects (six months to 20 years post-chemotherapy) were observed for inflammatory, blood cells/proteins and epigenetic markers. Higher levels of cytokines were found for C+ patients compared to HC (IL-6, IL-1β, IL-2, IL-4, IL-8, IL-10) [25,26] but not compared to C- patients, while others did differ between C+ and C- (CRP, sTNFRII [23,24]). This latter effect was observed six months after chemotherapy, but not one-year post-chemotherapy. Additionally, C+ patients showed higher levels of blood coagulation proteins compared to C- patients [25], and higher levels of blood cell ratio's compared to HC [27]. Lastly, increased long-term oxidative DNA damage was found in C+ patients when compared to HC [28].
Summary
In summary, differences in blood markers were found both when comparing post- to pre-chemotherapy levels and to healthy controls (see Fig. 2). Inflammatory markers, such as IL-6 and sTNFRII, were mainly examined and most consistently found to be higher or increase shortly after ending chemotherapy.
Imaging metrics
Regarding imaging metrics, the reader is referred to recent reviews summarizing structural neuroimaging findings [4,8,29]. Additionally, in Appendix D results are summarized from the included studies in this review.
Biomarkers associated with cognition in C+ patients
Associations between imaging-derived metrics and cognition were mostly investigated (n = 24), compared to those in blood markers (n = 21). No associations between CSF-based biomarkers and cognition were investigated.
Blood markers
Of the studies examining associations between blood markers (inflammatory, metabolic, epigenetic, hormones, blood cells/proteins, neuronal integrity markers) and cognition in chemotherapy-treated patients, 76% (16 studies) found significant associations (Table 2). All but two studies [19,31] investigated associations assessed at the same time. In the following sections, blood markers that were associated with both objective and self-reported cognitive functioning will be described according to the time point of the observed association, i.e. pre-treatment (baseline), during chemotherapy, shortly (one week to five months) or longer (six months to 20 years) post-chemotherapy.
Table 2.
Cognition-associated Blood Markers in C+ Patients (21 studies).
| Category marker | Cognition | Baseline | On-therapy | Short-term | Long-term | References |
|---|---|---|---|---|---|---|
| Inflammation | Objective | - IL-17 - CRP * |
- IL-1β, IL-4, IL-8, IL-12, sTNFRI - CRP * + IL-1β, IL-4, IL-7, IL-5, IL-8, IL-13, IL-17 |
Null finding : TNF-α | - IL-1β, IL-5, IL-6, IL-7, IL-8, IL-10, IFNγ, MCP-1, TNF-α - CRP * + IL-2, IL-4, IL-5, IL-7, IL-12, IL-17, MIP-1β, IGF-1 IL-1β, IL-2, IL-7, IL-8, IL-10, TNF-α (together with 3 neuronal markers, BMI, age and education) explained 71% of the variance in cognition Null findings : IL-6, sTNFRII |
[17,20,[23], [24], [25], [26],[31], [32], [33], [34], [35], [36]] |
| Self-report | / | - IL-1β, IL-6 + IL-4 Δ MCP-1 Null findings: IL-6, IL-10, MCP-1 and sTNFRII |
- IL-6, sTNFRII Null finding: TNF-α |
- IL-6 + IGF-1 Δ sTNFRII Null findings: CRP, IL-6, TNF- α |
[14,19,32] | |
| Sex and stress hormones | Objective | / | / | Null findings : oestradiol, progesterone | / | [15] |
| Blood cells/proteins | Objective | + G-CSF, GM-CSF | Null finding: albumin, hemoglobin, VEGF | Null findings: blood cell concentration, Hb | - GM-CSF, G-CSF, GRL, PLR, SII + G-CSF |
[15,17,27] |
| Self-report | Null finding: albumin, Hb | / | / | / | [14] | |
| Neuronal | Objective | / | Null finding: BDNF, pNF-H | / | Aβ−42, Aβ−40, tau (together with 13 cytokines, BMI, age and education) explained 71% of the variance in cognition Null finding: pNF-H |
[16,18,37] |
| Self-report | / | Null finding: pNF-H | / | / | [18] | |
| Metabolism | Objective | / | / | Null findings: cholesterol, glucose, triglycerides | - rcSO2 | [15,34] |
| Self-report | Null finding: creatinine, LDH | / | / | / | [14] | |
| Epigenetics | Objective | / | / | / | Δ methylation ratio changes in 56 CpG positions * | [22] |
| Self-report | / | / | Δ methylation ratio changes in CpG position cg16936953 | / | [21] |
Note. Short-term = assessment within one week to four months post-chemotherapy. Long-term = assessment within six months to 20 years post-chemotherapy.
+: positive association, -: negative association, Δ: change, * association with cognition factor score measured over time, Aβ: Plasma amyloid beta, C+: chemotherapy-treated patients, CRP: C-reactive protein, G-CSF: Granulocyte colony-stimulating factor, GM-CSF: Granulocyte-macrophage colony-stimulating factor, IFNγ: Interferon gamma, IGF-1: Insulin-like growth factor 1, IL: Interleukin, LDH: Lactate dehydrogenase, MCP-1: Monocyte chemoattractant protein 1, MIP: Macrophage inflammatory protein, pNF-H: Phosphorylated neurofilament H, rcSO2: regional cerebral tissue oxygen saturation, sTNFRI: Soluble tumor necrosis factor receptor I, sTNFRII: Soluble tumor necrosis factor receptor II, TNF-α: Tumor necrosis factor alpha.
Objective cognition-associated blood markers
Pre-treatment effects were observed for inflammatory markers and blood cells/proteins. The cytokines IL-17 [17] and CRP [31] showed a negative association with processing speed and a combined scores of memory and processing speed respectively, while blood factors G-CSF and GM-CSF were positively associated with processing speed and executive functioning [17].
During chemotherapy, cognitive performance was both negatively [17,32,33] and positively [17,32] associated with inflammatory markers (e.g. IL-1β, IL-4), with mainly memory and processing speed domains being involved. The type of association depended on the measured cognitive domain. For instance, IL-1β was found to be negatively associated with processing speed on computerized tasks [17,32], while its association with attention span was positive [17]. Again, CRP levels during chemotherapy were negatively associated with global cognitive performance measured from pre- to two years post-chemotherapy [31]. No associations were found during chemotherapy between blood cells/proteins (i.e. albumin, Hb) [14], metabolic- (i.e. creatinine, LDH) [14] or a neuronal integrity indicator (i.e. pNF-H) [18] and global cognitive performance.
Regarding the studies using short-term assessments (one week to three months after treatment), no associations were found with objective cognitive performance, not with an inflammatory marker (TNF-α) [20], nor metabolic markers (cholesterol, glucose, triglycerides), blood cells/proteins (blood cell concentration, Hb), or hormones (i.e. oestradiol, progesterone) [15].
After a longer (six months to 20 years) interval since ending chemotherapy, inflammatory markers (e.g. IL-1β, IL-6, TNF-α) were both positively [17,34] and negatively [17,25] associated with global cognition [25,34] and specific cognitive domains (e.g. processing speed and memory) [17]. While most studies reported linear relationships (i.e. higher/lower cognitive performance was associated with a higher/lower inflammatory marker concentration), some showed non-linear relationships (i.e. high/low cognitive performance was associated with a specific inflammatory profile) [35,36]. Furthermore, one study found a negative association between CRP levels six months post-chemotherapy and a change in global cognitive functioning from pre- to six months post-chemotherapy. [31]. A few studies however reported the absence of significant associations between cytokines and memory [23,26], processing speed or executive functioning [23]. Secondly, a negative association was found between blood cells (GRL, PLR and SII) and global cognitive performance [27], while both negative (GM-CSF and G-CSF) and positive (G-CSF) associations were found between proteins related to blood cells and cognitive domains, such as memory and processing speed. [17]. Thirdly, an inverse association with the metabolic marker, rcSO2, and global cognition was reported in one study [34]. Fourthly, neuronal integrity- (i.e. Aβ−42, Aβ−40, tau) together with inflammatory markers (i.e. 13 cytokines) explained 71% of the variance in cognition in one study [37]. No such association was found with pNF-H, another neuronal integrity marker [18]. Lastly, increases in methylation ratios (as can be seen with gene ‘silencing’) were associated with lower memory scores one year after chemotherapy and with decreased memory scores from pre-to one year after post-chemotherapy, while no other domains (i.e. processing speed, attention and executive function) were found to be associated [22].
Self-Reported cognition associated blood markers
Baseline association between self-perceived cognitive functioning and metabolic markers (LDH, creatine) or blood cells/proteins (albumin, hemoglobin) were explored, but were not significant [14].
Two studies found cytokines to be both positively (IL-4) and negatively (IL-1β, IL-6) associated with self-perceived cognitive functioning during chemotherapy [19,32], while another study [16] found no such relationships. Additionally, changes in a cytokine (MCP-1) were negatively associated with changes in self-perceived cognitive functioning, while this was not the case for others (IL-6, IL-8) [19]. On-therapy association of self-perceived cognitive functioning with neuronal markers (pNF-H, BDNF) also did not show a relationship [16,18].
When evaluating short-term (three to four months post-chemotherapy) associations, two studies described a negative relationship between inflammatory markers (IL-6 [20] and sTNFRII [23], but no association with TNF-α [20]), while another study found a significant association between methylation ratio changes four months after chemotherapy and self-perceived cognitive decline [21].
Long-term effects (one to 1.5 years after chemotherapy) were explored in three studies, again focusing on inflammatory markers. While one study reported a negative association with IL-6 [24], this finding was not replicated by other groups [26,34]. Additionally, IGF-1 was positively associated with self-reported functioning, while this was not the case for CRP or TNF-a [34]. Lastly, declines in the inflammatory marker sTNFRII were positively associated with improvement on self-reported cognitive functioning [23].
Summary of cognition-associated blood markers
Relationships with objective cognitive domains, mostly processing speed and memory, were primarily investigated, showing that inflammatory markers (mainly cytokines) were most consistently associated, particularly during or years after ending chemotherapy. However, whether the association was positive, negative, linear or nonlinear depended on the specific cytokines and on the used method for assessing cognition. Associations with other biological indicators (i.e. blood cells/proteins, neuronal integrity- and metabolic markers) were almost exclusively found after longer time intervals. Relationships between blood markers and self-reported cognitive functioning were investigated to a lesser extent and were more heterogenous compared to objective cognition.
Imaging metrics
Of the studies exploring associations between neuroimaging-based markers and cognitive functioning in patients treated with chemotherapy, 67% (16 studies) found significant associations (Table 3). All but one study [40] assessed imaging and cognition on the same day. In the following section, neuroimaging metrics associated with both objective and self-reported cognitive functioning will be described according to the time point of the observed association, i.e. pre-treatment (baseline), during chemotherapy, shortly (one week to five months) or longer (six months to 20 years) post-chemotherapy.
Table 3.
Cognition-associated imaging metrics in C+ patients of the included studies (24 studies).
| Category | Cognition | Baseline | On-therapy | Short-term | Long-term | References |
|---|---|---|---|---|---|---|
| T1W | Objective | + GM density in frontal, temporal, occipital areas † | + Δ GM density in frontal gyrus * | + GM density in frontal, temporal, occipital areas, cerebellum † - GM density in hippocampus, cerebellum * Null finding † |
+ GM density in frontal areas * + Δ cortical thickness * + WM volume increase in corona radiata, SLF * - Δ WM volume reductions in corona radiata * + GM density in temporal areas, cerebellum, insula † Null finding † |
[28,41,[43], [44], [45],50,51] |
| Self-report | / | / | + GM density in frontal gyrus * | / | [55] | |
| DWI | Objective | Null finding † | / | + WM microstructure (FA) in parietal, temporal areas * + Δ WM microstructure (FA) in parietal, frontal, occipital areas * Null findings (FA) *, (FA, MD, RD and AD) † |
- WM microstructure (MD) * Null findings (MD) *, (FA) † |
[38,42,[46], [47], [48],52,53] |
| Self-report | / | / | + WM microstructure (FA) in frontal and parietal tracts * + WM microstructure (FA) in frontal and parietal tracts, corona radiata, cingulate gyrus and corpus callosum * |
Lower network attack tolerance * Null finding (FA) † |
[39,46,53,59] | |
| 18F-FDG-PET | Objective | / | / | / | + metabolism in orbital gyrus * | [54] |
| Self-report | / | / | - metabolism in frontal, temporal areas † | - metabolism in frontal, temporal areas † | [24] | |
| 1H-MRS | Self-report | / | / | / | - choline and myo-inositol concentrations in prefrontal cortex † | [57] |
| Other | Objective | / | / | iron deposition: Null finding † | / | [49] |
| Self-report | / | / | / | 99mTC-TRODAT-1 SPECT: Null finding † | [58] |
Note. Short-term = assessment within two weeks to five months post-chemotherapy. Long-term = assessment within six months to 10 years post-chemotherapy.
+: positive association, -: negative association, Δ: change, *: whole-brain group imaging analysis, †: Region-of-interest group imaging analysis, AD: axial diffusivity, DWI: diffusion-weighted imaging, FA: fractional anisotropy, FDG: fluorodeoxyglucose, GM: gray matter, MD: mean diffusivity, MRI: magnetic resonance imaging, MRS: magnetic resonance spectroscopy, PET: positron emission tomography, R: radial diffusivity, SLF: superior longitudinal fasciculus, SPECT: single-photon emission computed tomography, T1W: T1-weighted MRI, VBM: voxel-based morphometry, WM: white matter.
Objective cognition-associated imaging metrics
Pre-treatment effects were found for volumetric assessments, with GM density in the frontal, temporal and occipital lobe being positively associated with both processing speed and memory [41], while no baseline association was found with diffusion tensor imaging (DTI) [42]. In the one study examining associations during chemotherapy, changes in GM density in the frontal gyrus were positively associated with changes in executive functioning [43].
Shortly (two weeks to five months) after chemotherapy, a positive association was found between GM volume in the frontal, temporal and occipital areas and processing speed and memory [41] and between GM density in the cerebellum and memory [44]. By contrast, another study reported no associations between short-term changes in GM reduction and changes in oral reading [45]. When investigating WM, a positive association was found between WM microstructural measures (i.e. fractional anisotropy (FA)) of parietal and temporal areas and attention and processing speed [46] and between changes in parietal, frontal and occipital WM tracts and changes in attention and memory [47]. Null findings were also reported between the same (i.e. FA) and additional measures (i.e. mean (MD), axial, radial diffusivity) of WM microstructure [42,48] or iron deposition [49] and cognitive performance.
Long-term effects (six months to 10 years post-chemotherapy) were explored in four studies. Positive associations were found between GM density and global cognitive functioning (frontal [28]) or memory (temporal area, cerebellum and insula [28]). Additionally, larger decreases in cortical thickness were associated with lower performance on memory tests [50], while no long-term association was found between hippocampal volume [51] and cognitive performance. Furthermore, increased WM volume in the corona radiata and superior longitudinal fasciculus was associated with a better performance for attention for younger patients, while WM volume reduction in the corona radiata was associated with worse memory scores for older patients. Additionally, a negative long-term association between WM injury (as estimated with MD) and executive function was found [38], whereas two studies found no long-term association between WM microstructure (MD, FA) [52,53] and performance on cognitive tests.
Only one study explored long-term associations with metabolic imaging markers, demonstrating a positive relationship between glucose consumption (FDG-PET) uptake in the orbital gyrus and global cognitive performance [54].
Self-Reported cognition-associated imaging metrics
No studies explored associations between imaging metrics and self-reported cognition either pre-or during chemotherapy.
Four studies investigated short-term (one to five months post-chemotherapy) associations. A positive association was found between GM density in the frontal gyrus and executive functioning [55]. Additionally, self-reported global cognitive functioning was positively associated with WM metrics (FA) in frontal and parietal tracts [46,56], as well as in the corona radiata, the cingulate gyrus and the corpus callosum [43]. Lastly, a negative association between glucose metabolism in the frontal and temporal cortex and memory functioning was found [24].
Long-term associations (six months to five years) were explored in five studies. Firstly, while based on diffusion-weighted imaging, one study showed an association between structural network efficiency and self-perceived global cognitive functioning [39], no long-term associations were found with changes in specific WM tracts [53]. Secondly, neurometabolic levels of choline and myo-inositol were negatively associated to subjective memory functioning [57]. Thirdly, the negative association between glucose metabolism in the frontal and temporal cortex and memory functioning observed shortly after ending chemotherapy, also remained after a long time interval [24]. Lastly, the only study employing SPECT did not find a long-term correlation between dopamine transporter binding ratio and self-reported global cognitive functioning [58].
Summary of cognition-associated imaging metrics
Associations with objective cognitive domains were more commonly explored compared to self-reported cognitive functioning. Across the structural brain imaging studies, mainly positive associations were found between better cognitive outcomes and imaging metrics representing neural integrity or volume. Both shortly and longer after ending chemotherapy, cognitive domains (mostly processing speed and memory) and self-perceived cognitive functioning were frequently associated with WM or GM metrics in frontal and temporal brain areas, across different imaging modalities.
Discussion
Our study was the first systematic review to explore blood and neuroimaging biomarkers associated with cognition in breast cancer patients in the last decade, as well as summarize chemotherapy-induced changes in blood markers. Our review shows that blood-based biomarkers are affected in patients with breast cancer over the course of a chemotherapeutic treatment, persisting up to years post-therapy. We suggest structural brain changes, inflammatory markers and blood/cells proteins to potentially provide more insights into individual susceptibility to cognitive decline at any timepoint, while the allostatic load markers of blood/brain metabolism, neuronal integrity or epigenetics could be more robust indicators of long-term cognitive effects of chemotherapy.
Blood and epigenetic alterations associated with chemotherapy
Our systematic review revealed that changes in blood-based biomarkers are observed before the initiation of therapy and can persist over the course of a treatment, until years after ending chemotherapy, both when comparing to norm-values or when investigating intrasubject changes.
Firstly, inflammatory markers and blood cells/proteins were found to be altered shortly until 20 years post-chemotherapy, with blood proteins even differing pre-chemotherapy. The exact mechanisms involved in the inflammatory response during cancer therapy are currently not fully understood. However, pro-inflammatory cytokine levels are known to be acutely induced as a result of tissue injury, for instance by tumor growth or after radiotherapy [60]. This review demonstrated that mediators of the acute inflammatory phase (such as IL-6) remain altered up until years after ending chemotherapy, making them interesting targets to investigate in the context of CRCI. Additionally, anemia is a common complication in patients with cancer, potentially contributing to the observed pre-chemotherapy differences in hemoglobulin levels, although inconsistent. Moreover, an “angiogenic switch” is considered a hallmark of tumor progression, consisting of development of angiogenic features and release of angiogenic factors [61]. As such factors are also involved in brain vascularization and neurogenesis, they serve as an additional interesting candidate to investigate chronic susceptibilities to adverse neurotoxic side-effects of cancer treatment. It must be mentioned the choice of our blood marker categories resulted in some markers (e.g. SII, T cells, B cells, ..) to be categorized as blood cell/proteins, while also contributing to immune responses. Therefore, these results combined underscore cancer ánd treatment effects on blood cells/proteins as well as the long-term inflammatory state present after treatment for cancer. Secondly, metabolic markers differed pre-chemotherapy and shortly post-chemotherapy. Some studies have shown a relationship between lipid metabolism and risk for cancer, potentially mediated by activation of inflammatory pathways [62], while whole-body metabolic influence on tumor characteristics requires further attention [63]. These baseline results emphasize the need to control for a subjects’ susceptibilities or comorbidities when investigating additional treatment effects on metabolic and/or blood cell markers. Thirdly, hormones were only altered shortly post-chemotherapy, potentially linked to chemotherapy-induced menopause [63]. Lastly, evaluation of epigenetic modifications can aid in cancer diagnosis and progression assessment and shows promise in the context of precision medicine [64]. Although rarely investigated, changes in methylation ratio's as well as increased oxidative DNA damage were found in patients, both acutely and chronically after chemotherapy. This emphasizes the potential of these markers beyond diagnosis.
These results confirm chemotherapy to have a strong impact on peripheral physiological function, up to years after finishing treatment. However, the reported results were very heterozygous in both timing and method of assessment, decreasing our ability to synthesize results. Although for instance inflammatory markers have received increasing attention, large scale longitudinal studies will remain necessary to disentangle intrinsic patient characteristics, such as risk factors and type of cancer, from treatment effects. Additionally, most studies focused on group differences, while individual thresholds are unclear and will need to be evaluated to ensure translation into clinical practice.
Biomarkers associated with cognition
The majority (i.e. 71%) of the included studies reported significant associations between one of the investigated biomarkers and cognition. While inflammatory markers and T1w structural imaging were associated with cognitive scores both before and over the entire trajectory of treatment, other markers associated in a time-varying matter and were mainly investigated in chronic settings.
Firstly, pro-inflammatory blood markers (e.g. IL-1β, IL-4, IL-6, CRP) were associated with cognitive decline or complaints over the entire course of a chemotherapy trajectory, until 10 years post-chemotherapy. This could suggest inflammation as a potential contributor to the indirect neurotoxicity of chemotherapy. Although the mechanisms by which peripheral inflammation interferes with neural processing in humans are not fully established, there is a body of preclinical research that suggests ongoing crosstalk between the immune system and the brain [65]. For instance, cytokines are known to be able to cross the blood brain barrier, thereby increasing permeability for chemotherapeutic agents and facilitating neuroinflammation and central neurotoxicity [66]. Consequently, cytokines can induce the process of sickness behavior, including feelings of malaise and fatigue, which could partly explain associations with cognitive outcomes as well [67]. Associations found years post-chemotherapy, suggest that these inflammatory markers may also be associated with chronic or delayed CRCI. The study of Starkweather et al. (2016) gives some initial evidence for this hypothesis by showing negative associations between CRP levels during chemotherapy and cognitive functioning from pre- to two years post-chemotherapy. However, many cytokines were both positively and negatively associated with cognitive decline. As inflammatory markers are expected to be interdependent and interact in networks [68], linear statistical models are potentially an oversimplification of the underlying time-dependent biological processes. Only two studies evaluated nonlinear relationships, showing that indeed, significant nonlinear biomarker-behavior relationships exist in cancer survivors [36,37]. Future studies are therefore encouraged to take into account inflammatory profiles (i.e. composite scores, longitudinal measures) of subjects instead of individual or cross-sectional measured markers. Epigenetic alterations associated both shortly and years after chemotherapy with cognition, consequently being potential blood markers for chronic CRCI. By contrast, associations between cognitive performance and other blood markers (i.e. blood cells/proteins, neuronal integrity- and metabolic markers), occurred less frequently and exclusively years post-chemotherapy, although the same blood markers did show alterations throughout the trajectory of treatment with chemotherapy. This underscores that, while cancer and its treatment induce peripheral psychological function changes, not all changes are individually informative for patients their cognitive outcomes. Nonetheless, especially for evaluating long-term chemotherapeutic effects, (mainly inflammatory) blood markers provide an easily accessible and valuable add-on for neuroimaging to investigate further. The question whether certain blood values are earlier precursors for cognitive decline and neuroimaging features than others, should be addressed in future longitudinal studies.
Secondly, our review revealed studies exploring imaging-based features consistently found white matter tracts and gray matter volume/density in frontal, temporal and parietal areas to be associated with cognitive outcomes, both shortly and years after chemotherapy for breast cancer. Comparable to inflammatory measures for blood markers, structural T1w neuroimaging was mainly investigated and showed consistent positive associations of brain volume/density with cognitive score, in line with previous research [8]. Consequently, there is evidence that such brain volume/density brain changes are associated with the phenomenon of CRCI as well as cognitive impairment in general. A limited number of studies explored associations with brain metabolism or metabolites, showing some initial evidence for relationships with self-perceived cognitive functioning from a few months until five years post-chemotherapy. However, since pre- and on-therapy associations were almost not investigated for other imaging modalities, it is difficult to disentangle imaging markers sensitive to specific CRCI effects or cognition in general.
Interestingly, some included studies evaluated both blood and neuroimaging markers. These studies showed for instance that higher TNF-α(-receptor) levels measured shortly or years after chemotherapy were associated with lower insular [16] and hippocampal volume [35], suggesting that chemotherapy-induced inflammation might directly or indirectly contribute to microstructural brain changes. Additionally, higher cytokine levels measured before and one year after treatment were associated with neural hypermetabolism in breast cancer patients treated with chemotherapy (not in patients who were chemotherapy naïve). This is consistent with the possibility that an initial inflammatory response could set up a cascade with long-term impact on brain metabolism and potentially cognitive outcomes. However, the exact biological underpinnings of these brain changes remain unknown. Furthermore, it is important to note that none of these human studies could demonstrate causality, which future preclinical work could investigate more in detail.
Finally, as expected, CSF markers were not investigated in this population so far. Previous studies of patients with acute lymphoblastic leukemia (ALL) receiving (intrathecal and intravenous) methotrexate, demonstrated robust links between cognitive domains, such as working memory or verbal abilities, and modified CSF components, such as fatty acids, phospholipids, and even tau protein. However, these patients receive diagnostic lumbar punctures, while this invasive procedure is not applied for breast cancer patients. Future research will unravel whether CSF-extracted markers could be of added value for diagnostic and prognostic purposes of CRCI in patients with cancer involving the brain or spinal cord. Meanwhile, less invasive blood and neuroimaging markers still require further exploration in other cancer populations such as breast cancer.
Limitations of existing studies and future directions
Several shortcomings of the included studies in this review should be noted. First, the included studies of this review are very heterogenous with respect to assessment approach, both regarding neuropsychological tests and questionnaires. For instance, for the assessment of attention, more than 10 different tests and for self-perceived cognitive functioning, 14 different questionnaires were used. Although most studies applied batteries of tests, some only used a screening instrument to assess global cognitive functioning, such as MMSE and MOCA, which are known to be rather insensitive for CRCI [69,70]. Furthermore, cognitive impairment criteria widely varied between studies. This could explain the wide range of C+ patients (i.e. 1–67%) demonstrating CRCI after chemotherapy. A standardized, uniform approach for cognitive assessment is thus needed. The International Cognition and Cancer Task Force (ICCTF) recommended criteria to assess cognitive impairment (i.e. two test scores ≤ −1.5 SD from the normative mean, or a single test score ≤ −2 SD below the mean) and a core set of neuropsychological tests adequate for the phenomenon of CRCI [71], which future studies are encouraged to consult. Additionally, the Cancer Neuroscience Initiative Working Group (CNIWG) recently recommended using, at a minimum, the Cognitive Function Short Form 8a for measurement of self-reported CRCI (sr-CRCI) to increase scientific rigor and enable meta-analyses in the future [72].
Secondly, the included studies widely vary in demographic variables, including age, ethnicity, education and menopausal state, and in the extent to which these were taken into account. Age has been found to moderate the effects of chemotherapy on CRCI, with older patients being more prone to neurodegenerative processes and CRCI [2,73]. However, a recent population-based study did not support that hypothesis, showing similar cognitive profiles among older cancer patients (mean 74 years) and individuals who remained cancer free [74]. By consequence, generalizing results across different age populations could yield misleading results and warrants further investigation. As a result of different age restrictions (some studies only included patients older than 60 or 70 years), menopausal state also showed great variance, with studies including either only premenopausal, only postmenopausal females or the combination of both. Lastly, education level of participants was rarely reported. Since both menopausal status and education have showed moderating effects on cognitive outcomes [2,6], generalization of the encountered results requires caution.
A third shortcoming concerns the small sample sizes of the included studies (n = 8–166, median = 24). Consequently, the statistical power may have been limited. Although it is time consuming and costly to acquire large cohorts for brain imaging studies, aggregation of imaging datasets of different sites (e.g. with the use of phantom scans) could provide a way to overcome these practical and financial challenges [75]. Additionally, with the establishments of ‘biobanks’, blood biomarkers could be investigated in larger cohorts of cancer patients, increasing sensitivity to the potential subtle alterations.
The present review has focused on associations between blood or imaging biomarkers and cognitive functioning, only in chemotherapy-treated patients. Consequently, it remains to be investigated whether the found associations are unique for chemotherapy-treated patients compared to chemotherapy-naïve patients, or rather reflect the contribution of other treatments (e.g. anti-hormone, radiotherapy) or cancer characteristics. Additionally, not all studies included detailed descriptive statistics, including mean values and confidence intervals for biomarkers. For these reasons, and together with the heterogeneity of the included studies, pooling of data for a meta-analysis was not possible, which would be extremely valuable to determine weighting of the individual biomarkers to the phenomenon of CRCI.
In this review, we observed i) blood cells/proteins being altered from before until years after ending chemotherapy, ii) inflammatory and epigenetic changes being induced on- or shortly after chemotherapy and persisting chronically and iii) metabolic markers and hormones being altered shortly after chemotherapy for cancer and iv) imaging and blood-based markers being associated with cognitive scores, on different time points throughout the trajectory of chemotherapy. Specifically, metrics from diffusion-weighted and metabolic imaging, blood markers of metabolism, neuronal integrity or epigenetics were mainly associated with cognitive scores post-chemotherapy, primarily when more impairment was observed in this population. While other markers, such as structural imaging, inflammatory markers, or blood cells/proteins, were associated with cognitive scores even before start of chemotherapy, up until years after ending treatment. Based on these results, we provide some indications for future time-specific biomarker assessments in patients treated for cancer. More specifically, evaluating structural brain changes, inflammation and blood/cells proteins could provide more insight into individual susceptibility to cognitive decline at any timepoint, while markers of blood/brain metabolism, neuronal integrity or epigenetics are more robust to evaluate (long-term) cognitive effects of chemotherapy to include in future research. Furthermore, future research needs to investigate relationships between peripheral markers and central nervous system neurobiological mechanisms in both preclinical studies and in vivo studies; i.e. translational projects. Additionally, while this review summarized evidence on several individual blood/neuroimaging markers that were associated with cognitive outcomes, future multiparametric studies evaluating a combination of biomarkers will be valuable to disentangle loadings of the individual markers, to further guide the investigation of valuable non-invasive biomarkers for clinical implementation.
Conclusion
This systematic review investigated biomarkers of cancer-related cognitive impairment in chemotherapy-treated breast cancer patients. We provided evidence for chemotherapy to alter several peripheral blood markers, and highlighted existing associations between blood markers, neuroimaging measures and CRCI during treatment, up to years after treatment. Especially for evaluating long-term associations, blood markers could provide for a more easily accessible alternative or add-on marker to neuroimaging. Yet, more homogeneous, and large-scale studies applying the recommendations of the ICCTF and CNIWG concerning cognitive assessment and impairment criteria, are advised to validate the neurocognitive predictive value of these measures in the future. Finally, the time-dependency of associations needs to be clarified, to provide the required information on potential future therapeutic targets.
CRediT authorship contribution statement
Gwen Schroyen: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition. Julie Vissers: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing. Ann Smeets: Supervision, Validation, Writing – review & editing. Céline R. Gillebert: Supervision, Validation, Writing – review & editing. Jurgen Lemiere: Supervision, Validation, Writing – review & editing. Stefan Sunaert: Supervision, Validation, Writing – review & editing. Sabine Deprez: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. Charlotte Sleurs: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors have no conflict to declare.
Funding
This work was supported by the Leuven Cancer Institute (LKI), Stichting tegen Kanker, Research Fund KU Leuven and the Kinderkankerfonds Leuven.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101297.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ahles T.A., Root J.C. Cognitive effects of cancer and cancer treatments. Annu. Rev. Clin. Psychol. 2018;14(1):425–451. doi: 10.1146/annurev-clinpsy-050817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sousa H., Almeida S., Bessa J., Pereira M.G. The developmental trajectory of cancer-related cognitive impairment in breast cancer patients: a systematic review of longitudinal neuroimaging studies. Neuropsychol. Rev. 2020;30(3):287–309. doi: 10.1007/s11065-020-09441-9. [DOI] [PubMed] [Google Scholar]
- 4.Lange M., Joly F., Vardy J., et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019;30(12):1925–1940. doi: 10.1093/annonc/mdz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleurs C., Madoe A., Lagae L., et al. Genetic modulation of neurocognitive development in cancer patients throughout the lifespan: a systematic review. Neuropsychol. Rev. 2019;29(2):190–219. doi: 10.1007/s11065-019-09399-3. [DOI] [PubMed] [Google Scholar]
- 6.Ahles T.A., Saykin A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidi J., Lucente M., Sonino N., Fava G.A. Allostatic Load And Its Impact On Health: A Systematic Review. Psychother. Psychosom. 2021;90(1):11–27. doi: 10.1159/000510696. [DOI] [PubMed] [Google Scholar]
- 8.Li M., Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: a systematic review. Neurosci. Biobehav. Rev. 2018;92:304–317. doi: 10.1016/j.neubiorev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Castel H., Denouel A., Lange M., Tonon M.C., Dubois M., Joly F. Biomarkers associated with cognitive impairment in treated cancer patients: potential predisposition and risk factors. Front Pharmacol. 2017;8:138. doi: 10.3389/fphar.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan - a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHugh M.L. Interrater reliability: the kappa statistic. Biochem. Med. 2012;22(3):276–282. doi: 10.11613/bm.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting P.F. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8):529. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Aaldriks A.A., Giltay E.J., Le Cessie S., et al. Prognostic value of geriatric assessment in older patients with advanced breast cancer receiving chemotherapy. Breast. 2013;22(5):753–760. doi: 10.1016/j.breast.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y., Tuluhong D., Shi Z., et al. Postchemotherapy hippocampal functional connectivity patterns in patients with breast cancer: a longitudinal resting state functional MR imaging study. Brain Imaging Behav. 2019;14(5):1456–1467. doi: 10.1007/s11682-019-00067-x. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins V., Thwaites R., Cercignani M., et al. A feasibility study exploring the role of pre-operative assessment when examining the mechanism of “chemo-brain” in breast cancer patients. Springerplus. 2016;5(1):1–11. doi: 10.1186/s40064-016-2030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon D.E., Cohen R., Chen H., et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J. Neuroimmunol. 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natori A., Ogata T., Sumitani M., Kogure T., Yamauchi T., Yamauchi H. Potential role of pNF-H, a biomarker of axonal damage in the central nervous system, as a predictive marker of chemotherapy-induced cognitive impairment. Clin. Cancer Res. 2015;21(6):1348–1352. doi: 10.1158/1078-0432.CCR-14-2775. [DOI] [PubMed] [Google Scholar]
- 19.Janelsins M.C., Mustian K.M., Palesh O.G., et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support. Care Cancer. 2012;20(4):831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chae J.W., Ng T., Yeo H.L., et al. Impact of TNF-α (rs1800629) and IL-6 (rs1800795) polymorphisms on cognitive impairment in asian breast cancer patients. PLoS ONE. 2016;11(10) doi: 10.1371/journal.pone.0164204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao S., Hu Q., Kerns S., et al. Impact of chemotherapy for breast cancer on leukocyte DNA methylation landscape and cognitive function: a prospective study. Clin. Epigenet. 2019;11(1):45. doi: 10.1186/s13148-019-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang G.S., Mi X., Jackson-Cook C.K., et al. Differential DNA methylation following chemotherapy for breast cancer is associated with lack of memory improvement at one year. Epigenetics. 2019;15(5):499–510. doi: 10.1080/15592294.2019.1699695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz P.A., Bower J.E., Kwan L., et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav. Immun. 2012;30(Suppl):S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomykala K.L., Ganz P.A., Bower J.E., et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7(4):511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardy J.L., Stouten-Kemperman M.M., Pond G., et al. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2017;13(1):15–26. doi: 10.1007/s11682-017-9728-5. [DOI] [PubMed] [Google Scholar]
- 26.Apple A.C., Schroeder M.P., Ryals A.J., et al. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage Clin. 2018;20:110–118. doi: 10.1016/j.nicl.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Der Willik K.D., Koppelmans V., Hauptmann M., Compter A., Ikram M.A., Schagen S.B. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 2018;20(1):135. doi: 10.1186/s13058-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conroy S.K., Mcdonald B.C., Smith D.J., et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res. Treat. 2012;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu R., Du M., Ren J., et al. Chemotherapy-induced grey matter abnormalities in cancer survivors: a voxel-wise neuroimaging meta-analysis. Brain Imaging Behav. 2020;15(4):2215–2227. doi: 10.1007/S11682-020-00402-7. 2020 154. [DOI] [PubMed] [Google Scholar]
- 30.Chae J.W., Ng T., Yeo H.L., et al. Impact of TNF-α (rs1800629)and IL-6 (rs1800795) polymorphisms on cognitive impairment in Asian breast cancer patients. PLoS ONE. 2016;11(10) doi: 10.1371/journal.pone.0164204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starkweather A., Kelly D.L., Thacker L., Wright M.L., Jackson-Cook C.K., Lyon D.E. Relationships among psychoneurological symptoms and levels of C-reactive protein over 2 years in women with early-stage breast cancer. Support. Care Cancer. 2016;25(1):167–176. doi: 10.1007/s00520-016-3400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung Y.T., Ng T., Shwe M., et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann. Oncol. 2015;26(7):1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams A.M., Shah R., Shayne M., et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol. 2017;314:17–23. doi: 10.1016/j.jneuroim.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson B.W., Craft M.A., Carlson J.R., et al. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. GeroScience. 2018;40(3):325–336. doi: 10.1007/s11357-018-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesler S., Janelsins M., Koovakkattu D., et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun. 2013;30 Suppl(0):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henneghan A.M., Palesh O., Harrison M., Kesler S.R. Identifying cytokine predictors of cognitive functioning in breast cancer survivors up to 10 years post chemotherapy using machine learning. J. Neuroimmunol. 2018;320:38–47. doi: 10.1016/j.jneuroim.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henneghan A., Haley A.P., Kesler S.R. Exploring relationships among peripheral amyloid beta, tau, cytokines, cognitive runction, and psychosomatic symptoms in breast cancer survivors. Biol. Res. Nurs. 2019;22(1):126–138. doi: 10.1177/1099800419887230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stouten-Kemperman M.M., De Ruiter M.B., Koppelmans V., Boogerd W., Reneman L., Schagen S.B. Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2014;9(2):275–284. doi: 10.1007/s11682-014-9305-0. [DOI] [PubMed] [Google Scholar]
- 39.Kesler S.R., Watson C.L., Blayney D.W. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol. Aging. 2015;36(8):2429–2442. doi: 10.1016/j.neurobiolaging.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen B.T., Jin T., Patel S.K., et al. Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res. Treat. 2018;172(2):363–370. doi: 10.1007/s10549-018-4911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lepage C., Smith A.M., Moreau J., et al. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3(1):1–10. doi: 10.1186/2193-1801-3-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen B.T., Ye N., Wong C.W., et al. Effects of chemotherapy on aging white matter microstructure: a longitudinal diffusion tensor imaging study. J. Geriatr. Oncol. 2019;11(2):290–296. doi: 10.1016/j.jgo.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Chen H., Lv Y., et al. Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Sci. Rep. 2018;8(1):13801–13807. doi: 10.1038/s41598-018-32257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrier J., Viard A., Levy C., et al. Longitudinal investigation of cognitive deficits in breast cancer patients and their gray matter correlates: impact of education level. Brain Imaging Behav. 2018;14(1):226–241. doi: 10.1007/s11682-018-9991-0. [DOI] [PubMed] [Google Scholar]
- 45.Chen B.T., Sethi S.K., Jin T., et al. Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res. 2018;20(1):38. doi: 10.1186/s13058-018-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deprez S., Amant F., Yigit R., et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum. Brain Mapp. 2010;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deprez S., Amant F., Smeets A., et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J. Clin. Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 48.Mo C., Lin H., Fu F., et al. Chemotherapy-induced changes of cerebral activity in resting-state functional magnetic resonance imaging and cerebral white matter in diffusion tensor imaging. Oncotarget. 2017;8(46):81273–81284. doi: 10.18632/oncotarget.18111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen B.T., Ghassaban K., Jin T., et al. Subcortical brain iron deposition and cognitive performance in older women with breast cancer receiving adjuvant chemotherapy: a pilot MRI study. Magn. Reson. Imaging J. 2018;54:218–224. doi: 10.1016/j.mri.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henneghan A., Rao V., Harrison R.A., et al. Cortical brain age from pre-treatment to post-chemotherapy in patients with breast cancer. Neurotox. Res. 2020;37(4):788–799. doi: 10.1007/s12640-019-00158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apple A.C., Ryals A.J., Alpert K.I., et al. Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. NeuroImage Clin. 2017;14(C):685–691. doi: 10.1016/j.nicl.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Ruiter M.B., Reneman L., Boogerd W., et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum. Brain Mapp. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Billiet T., Emsell L., Vandenbulcke M., et al. Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging Behav. 2017;12(1):64–77. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 54.Boles Ponto L.L., Menda Y., Magnotta V.A., et al. Frontal hypometabolism in eldery breast cancer survivors determined by [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET): a pilot study. Int. J. Geriatr. Psychiatry. 2014;30(6):587–594. doi: 10.1002/gps.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mcdonald B.C., Conroy S.K., Smith D.J., et al. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav. Immun. 2012;30(Suppl):S117–S125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li T.Y., Chen V.C.H., Yeh D.DC., et al. Investigation of chemotherapy-induced brain structural alterations in breast cancer patients with generalized q-sampling MRI and graph theoretical analysis. BMC Cancer. 2018;18(1):1211. doi: 10.1186/s12885-018-5113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kesler S.R., Watson C., Koovakkattu D., et al. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain Imaging Behav. 2012;7(4):501–510. doi: 10.1007/s11682-013-9228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitor T., Kozasa E.H., Bressan R.A., et al. Impaired brain dopamine transporter in chemobrain patients submitted to brain SPECT imaging using the technetium-99m labeled tracer TRODAT-1. Ann. Nucl. Med. 2019;33(4):269–279. doi: 10.1007/s12149-019-01331-2. [DOI] [PubMed] [Google Scholar]
- 59.Li X., Chen H., Lv Y., et al. Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Sci. Rep. 2018;8(1):13801–13807. doi: 10.1038/s41598-018-32257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pyter L.M., Cochrane S.F., Ouwenga R.L., Patel P.N., Pineros V., Prendergast B.J. Mammary tumors induce select cognitive impairments. Brain Behav. Immun. 2010;24(6):903–907. doi: 10.1016/j.bbi.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/J.CELL.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Mazzuferi G., Bacchetti T., Islam M.O., Ferretti G. High density lipoproteins and oxidative stress in breast cancer. Lipids Heal. Dis. 2021;20(1):1–13. doi: 10.1186/S12944-021-01562-1. 2021 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan H.G.M., Houédé-Tchen N., Yi Q.L., et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J. Clin. Oncol. 2005;23(31):8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 64.Pasculli B., Barbano R., Parrella P. Epigenetics of breast cancer: biology and clinical implication in the era of precision medicine. Semin. Cancer Biol. 2018;51:22–35. doi: 10.1016/J.SEMCANCER.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Santos J.C., Pyter L.M. Neuroimmunology of behavioral comorbidities associated with cancer and cancer treatments. Front. Immunol. 2018;9(JUN):1195. doi: 10.3389/fimmu.2018.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wardill H.R., Mander K.A., Van Sebille Y.Z.A., et al. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int. J. Cancer. 2016;139(12):2635–2645. doi: 10.1002/ijc.30252. [DOI] [PubMed] [Google Scholar]
- 67.Kelley K.W., Bluth R.M., Dantzer R., et al. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17(Suppl):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 68.Carr E.J., Dooley J., Garcia-Perez J.E., et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nat. Immunol. 2016;17(4):461–468. doi: 10.1038/ni.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyers C.A., Brown P.D. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006;24(8):1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 70.Cerulla Torrente N., Navarro Pastor J.B., de la Osa Chaparro N. Systematic review of cognitive sequelae of non-central nervous system cancer and cancer therapy. J. Cancer Surviv. 2020;14(4):464–482. doi: 10.1007/s11764-020-00870-2/Published. [DOI] [PubMed] [Google Scholar]
- 71.Wefel J.S., Vardy J., Ahles T., Schagen S.B. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 72.Henneghan A.M., Van Dyk K., Kaufmann T., et al. Measuring self-reported cancer-related cognitive impairment: recommendations from the cancer neuroscience initiative working group. JNCI J. Natl. Cancer Inst. 2021 doi: 10.1093/JNCI/DJAB027. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lange M., Heutte N., Rigal O., et al. Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist. 2016;21(11):1337–1348. doi: 10.1634/theoncologist.2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Willik K.D., Hauptmann M., Jóźwiak K., et al. Trajectories of cognitive function prior to cancer diagnosis: a population-based study. JNCI J. Natl. Cancer Inst. 2020;112(5):480–488. doi: 10.1093/JNCI/DJZ178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deprez S., de Ruiter, Bogaert S., Peeters R., Belderbos J., De Ruysscher D., Schagen S., Sunaert S., Pullens P., Achten E. Multi-center reproducibility of structural, diffusion tensor, and resting state functional magnetic resonance imaging measures. Neuroradiology. 2018;60(6):617–634. doi: 10.1007/s00234-018-2017-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.