Abstract
The ribosomal DNA internal transcribed spacer sequences of 13 unrelated Encephalitozoon intestinalis isolates obtained from human immunodeficiency virus (HIV)-infected patients with intestinal microsporidiosis were analyzed by gene amplification and DNA sequencing. Among these isolates, we found only one genetic lineage which suggests that E. intestinalis may have a clonal distribution in HIV-infected patients.
In recent years, microsporidia have emerged as important opportunistic parasites in human immunodeficiency virus (HIV)-infected patients. Encephalitozoon intestinalis is the second most prevalent microsporidian species infecting humans (10, 21). This intestinal parasite is the cause of severe diarrheal illness and disseminated infections in HIV-infected patients (9, 16). Recent studies have also identified this pathogen in immunocompetent individuals (8, 18, 20). Humans remained the only recognized host of this agent until 1998, when Bornay-Llinares and colleagues described E. intestinalis from a variety of mammals (donkey, pig, dog, cow, and goat) in Mexico, suggesting the possibility that E. intestinalis infection is zoonotic in origin (1). In addition, this agent has recently been found in water, indicating that this human-pathogenic microsporidian may also be a waterborne pathogen (7). The epidemiology of E. intestinalis remains, however, poorly understood. To help elucidate the epidemiology of E. intestinalis, we wished to study the genetic diversity of E. intestinalis strains by analyzing the internal transcribed spacer (ITS) sequences of their ribosomal DNAs (rDNAs). This approach has proven successful in defining the genotype diversity within three microsporidian species in humans (Encephalitozoon cuniculi, Enterocytozoon bieneusi, and Encephalitozoon hellem) based on variation in the ITS sequences of their rDNAs and has helped us to study their zoonotic potential (4, 5, 11, 13, 14, 15, 19).
In this report, we used 13 stool specimens obtained over a 5-year period (1994 to 1998) from 13 unrelated HIV-infected patients with E. intestinalis seen in Paris, France (16, 17). Species-level identification of E. intestinalis was made by PCR with DNAs extracted from stools (n = 13) using a specific primer set of E. intestinalis small-subunit rDNAs as previously described (12). In this study, microsporidian DNAs were extracted from stored stool samples in potassium dichromate with a High Pure PCR Template Preparation kit (Boerhringer Mannheim, Meylan, France) by following the manufacturer's protocol for isolation of nucleic acids from yeast. Primers for PCR were chosen to amplify a 237-bp fragment that includes a portion of the small-subunit rDNA (107 bp), the entire ITS region (28 bp), and a segment of the large-subunit rDNA (102 bp). These primers do not amplify rDNA from E. cuniculi, E. hellem, or E. bieneusi. The forward primer EL1 (5′-CTA AGA TGA CGC AGT GGA CG-3′), complementary to positions 1 to 20, was designed by using the GenBank sequence of E. intestinalis (accession no. Y11611). The reverse primer EL2 (5′-CCC CAA GCG CTT CCG CTT CA-3′) was designed to be complementary to positions 218 to 237 of the GenBank sequence of E. intestinalis (accession no. Y11611). Amplification was done in a 50-μl reaction mixture including 2.5 μg of each primer/ml, 200 μmol of each deoxynucleoside triphosphate/liter, 75 mmol of Tris-HCl (pH 9.0)/liter, 20 mmol of (NH4)2SO4/liter, 0.01% Tween 20, 2 mmol of MgCl2/liter, and 2 U of Taq DNA polymerase (Goldstar; Eurogentec, Seraing, Belgium). After a denaturation of the DNA at 94°C for 10 mn, 40 cycles were run with a Hybaid (Teddington, Middlesex, United Kingdom) touchdown apparatus as follows: denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 30 s. A 10-min extension at 72°C was used after the 40 cycles. To detect inhibition of the amplification reactions, two different volumes of each DNA preparation were tested: 10 μl of the initial extract and a 10-fold dilution of released DNA. All positive samples were independently examined twice (DNA extraction and amplification). Each set of reaction mixtures included a negative control to ensure the absence of contamination of samples during analysis and a positive control represented by culture spores of E. intestinalis. To assess genetic diversity, DNA sequencing of amplified products was performed by automated means (ABI PRISM 377 system; Perkin-Elmer, Courtaboeuf, France). Both strands were sequenced with the primers used for the PCR. Sequences were edited with the Sequence Navigator (Perkin-Elmer) program and aligned using the Multalin program (2).
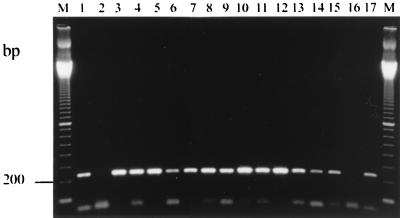
Using the DNA isolates as templates, amplified bands with primer set EL1-EL2 were of the expected size (237 bp) (Fig. 1). For five (38%) stool specimens, however, a 10-fold dilution of released DNA was necessary to remove PCR inhibitors (data not shown). The sequences of all PCR products were 100% identical with the corresponding published sequence of an E. intestinalis isolate (GenBank accession no. Y11611) (Table 1). This result suggests that all 13 isolates belong to the same genetic lineage.
FIG. 1.
Agarose gel analysis of PCR-amplified products using species-specific primers (EL1 and EL2) for E. intestinalis. Lanes: M, 100-bp DNA size marker; 1 and 17, positive controls; 2 and 16, negative controls; 3 to 15, stool specimens infected with E. intestinalis.
TABLE 1.
Alignment of the rDNA ITS sequences of Encephalitozoon species
| Encephalitozoon sp. | Host(s) | Sequence of the rDNA ITS regiona | GenBank accession no. |
|---|---|---|---|
| E. hellem | |||
| Genotype 1 | Parrots | TGTTGATTTGATTATT–––––––––TTGTGGGGATTTTTANTTTTTTAGTTTTTCT– | AF069064 |
| Genotype 2 | Humans | TGTTGATTTGATTGTTTGT––––GGTATTGAGAGTTTTTAGTTTTTT–––––TTCT– | AF110327 |
| Genotype 3 | Humans | TGTTGATTTGATTGTTTGTTTGTGGTATTGAGAGTTTTTAGTTTTTT–––––TTCT– | AF110328 |
| E. cuniculi | |||
| Strain I | Humans, rabbits, mice | TGTTGTTGTGTT–––––––––––––––TTGATGGATGTTTGTTTGTT–––––TGTGG | X98468, AJ005581 |
| Strain II | blue foxes, mice | TGTTGTTGTGTT–––––––––––––––TTGATGGAT––––GTTTGTT–––––TGTGG | NDb |
| Strain III | Humans, dogs, rodents, goats, sheep, swine, horses, foxes, cats | TGTTGTTGTGTT–––––––––––––––TTGATGGATGTTTGTTTGTTTGTT–TGTGG | X98466, L29560 |
| E. intestinalis | Humans | ––––––TTTGA––––––––––––GAGATTGGGGG––––GAATTTTTT–––––TGA–– | L20292, Y11611 |
The repeated GTTT sequences are underlined to demonstrate differences between genotypes and strains.
ND, not determined.
Previous studies showed that the ITS sequence was a main target for identifying gene polymorphism of microsporidia. For the two species E. cuniculi and E. hellem, a set of tetranucleotide repeats (5′-GTTT-3′) in the ITS has been found to vary among isolates from different hosts, resulting in the definition of three different genotypes for these parasites (Table 1) (5, 15). Also, heterogeneity in the ITS region has been described for E. bieneusi isolates. The heterogeneity is due to nucleotide substitutions in the ITS sequence, generating four distinct ITS types (11).
Although genetic homology between E. intestinalis isolates suggests that there is a possibility that all 13 patients contracted their infections from a common source, this possibility seems unlikely since the material was collected from the patients over a 5-year period and since the patients lived in different geographic areas. Our results are in agreement with those of two previous studies which examined the potential diversity of E. intestinalis isolates. In the first study, Del Aguila and colleagues showed at the antigenic level that eight isolates from five unrelated HIV-infected patients had very similar antigenic profiles by Western blotting (3). In the second study, three E. intestinalis isolates from two HIV-infected patients were analyzed (6). We determined that the ITS sequences of these isolates shared identical DNA sequences with our 13 isolates. This result reinforces the hypothesis of a clonal distribution of E. intestinalis in HIV-infected patients. However, this hypothesis needs to be extended to a larger number of E. intestinalis isolates, including those from other human, animal, and environmental sources, in order to better understand the epidemiology of E. intestinalis infection.
Acknowledgments
We acknowledge SIDACTION (Fondation pour la Recherche Médicale) and the Centre d'Etudes et de Recherche en Infectiologie for their financial support.
REFERENCES
- 1.Bornay-Llinares F J, da Silva A J, Moura H, Schwartz D A, Visvesvara G S, Pieniazek N J, Cruz-Lopez A, Hernandez-Jauregui P, Guerrero J, Enriquez F J. Immunologic, microscopic, and molecular evidence of Encephalitozoon intestinalis (Septata intestinalis) infection in mammals other than humans. J Infect Dis. 1998;178:820–826. doi: 10.1086/515356. [DOI] [PubMed] [Google Scholar]
- 2.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Aguila C, Croppo G P, Moura H, Da Siva A J, Leitch G J, Moss D M, Wallace S, Slemenda S B, Pieniazek N J, Visvesvara G S. Ultrastructure, immunofluorescence, Western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS. J Clin Microbiol. 1998;36:1201–1208. doi: 10.1128/jcm.36.5.1201-1208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis. 1996;22:557–559. doi: 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 5.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D C, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–421. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 6.Didier E S, Rogers L B, Orenstein J M, Baker M D, Vossbrinck C R, van Gool T, Hartskeerl R, Soave R, Beaudet L M. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J Eukaryot Microbiol. 1996;43:34–43. doi: 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 7.Dowd S E, Gerba C P, Pepper I L. Confirmation of the human pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol. 1998;64:3332–3335. doi: 10.1128/aem.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enriquez F J, Taren D, Cruz-Lopez A, Muramoto M, Palting J D, Cruz P. Prevalence of intestinal encephalitozoonosis in Mexico. Clin Infect Dis. 1998;26:1227–1229. doi: 10.1086/520278. [DOI] [PubMed] [Google Scholar]
- 9.Franzen C, Müller A, Hartmann P, Kochanek M, Diehl V, Fätkenheuer G. Disseminated Encephalitozoon (Septata) intestinalis infection in a patient with AIDS. N Engl J Med. 1996;21:1610–1611. doi: 10.1056/NEJM199611213352116. [DOI] [PubMed] [Google Scholar]
- 10.Kotler D P, Orenstein J M. Clinical syndromes associated with microsporidiosis. In: Wittner M, editor. The microsporidia and microsporidiosis—1999. Washington, D.C.: American Society for Microbiology; 1999. pp. 258–292. [Google Scholar]
- 11.Liguory O, David F, Sarfati C, Derouin F, Molina J M. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. J Clin Microbiol. 1998;36:1882–1885. doi: 10.1128/jcm.36.7.1882-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liguory O, David F, Sarfati C, Schuitema A R J, Hartskeerl R A, Derouin F, Modaï J, Molina J M. Diagnosis of infections caused by Enterocytozoon bieneusi and Encephalitozoon intestinalis using polymerase chain reaction in stool specimens. AIDS. 1997;11:723–726. doi: 10.1097/00002030-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Mathis A, Michel M, Kuster H, Müller C, Weber R. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology. 1997;114:29–35. doi: 10.1017/s0031182096008177. [DOI] [PubMed] [Google Scholar]
- 14.Mathis A, Breitenmoser A C, Deplazes P. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite. 1999;6:189–193. doi: 10.1051/parasite/1999062189. [DOI] [PubMed] [Google Scholar]
- 15.Mathis A, Tanner I, Weber R, Deplazes P. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int J Parasitol. 1999;29:767–770. doi: 10.1016/s0020-7519(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 16.Molina J M, Oksenhendler E, Beauvais B, Sarfati C, Jaccard A, Derouin F, Modaï J. Disseminated microsporidiosis due to Septata intestinalis in patients with AIDS: clinical features and response to albendazole therapy. J Infect Dis. 1995;171:245–249. doi: 10.1093/infdis/171.1.245. [DOI] [PubMed] [Google Scholar]
- 17.Molina J M, Chastang C, Goguel J, Michiels J F, Sarfati C, Desportes-Livage I, Horton J, Derouin F, Modaï J. Albendazole for treatment and prophylaxis of microsporidiosis due to Encephalitozoon intestinalis in patients with AIDS: a randomized double-bind controlled trial. J Infect Dis. 1998;177:1373–1377. doi: 10.1086/515268. [DOI] [PubMed] [Google Scholar]
- 18.Raynaud L, Delbac F, Broussolle V, Rabodonirina M, Girault V, Wallon M, Cozon G, Vivares C P, Peyron F. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J Clin Microbiol. 1998;36:37–40. doi: 10.1128/jcm.36.1.37-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinder H, Katzwinkel-Wladarsch S, Löscher T. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi. Parasitol Res. 1997;83:670–672. doi: 10.1007/s004360050317. [DOI] [PubMed] [Google Scholar]
- 20.Van Gool T, Vetter J C M, Weinmayr B, Van Dam A, Derouin F, Dankert J. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis. 1997;175:1020–1024. doi: 10.1086/513963. [DOI] [PubMed] [Google Scholar]
- 21.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]