Abstract
Pre-eclampsia (PE) is a hypertensive disorder of pregnancy associated with chronic inflammation, mitochondrial (mt) dysfunction and fetal demise. Natural Killer cells (NK cells) are critical for the innate immune response against tumors or infection by disrupting cellular mt function and causing cell death. Although NK cells can be stimulated by Tumor necrosis factor alpha (TNF-α), we don’t know the role of TNF-α on NK cell mediated mt dysfunction during PE. Our objective was to determine if mechanisms of TNF-α induced hypertension included activation of NK cells and multi-organ mt dysfunction during pregnancy. Pregnant rats were divided into 2 groups: normal pregnant (NP) (n=18) and NP +TNF-α (n=18). On gestational day 14, TNF-α (50ng/ml) was infused via mini-osmotic pump and on day 18, carotid artery catheters were inserted. Blood pressure (MAP) and samples were collected on day 19. TNF-α increased MAP (109 ± 2 vs 100 ± 2, p<0.05), circulating cytolytic NK cells (0.771 ± 0.328 vs.0.008 ± 0.003 % gated, <0.05) and fetal reabsorptions compared to NP rats. Moreover, TNF-α caused mtROS in the placenta (12976 ± 7038 vs 176.9 ± 68.04 % fold, p <0.05) and in the kidney (2191 ± 1027 vs 816 ± 454.7% fold, p<0.05) compared to NP rats. TNF-α induced hypertension is associated fetal demise, activation of NK cells and multi-organ mt dysfunction which could be mechanisms for fetal demise and hypertension. Understanding of the mechanisms by which TNF-α causes pathology is important for the use of anti-TNF-α therapeutic agents in pregnancies complicated by PE.
INTRODUCTION:
Pre-eclampsia is a multi-system disorder that is caused by sudden onset hypertension after 20 weeks of gestation. The organs that are affected by the disease include placenta, kidney, heart, brain and uterus. While we have made advances in understanding the pathophysiology of the disease, delivery of the fetus and the placenta remains the only treatment for this disease. There are multiple theories postulating the pathophysiology of pre-eclampsia, most of which revolve around the imbalance between anti-angiogenic factors in the maternal-placental interface 1–3. The complications of the disease is a significant contributor to maternal and perinatal morbidity and mortality worldwide. Maternal complications include end organ damage causing cerebrovascular disease, cardiovascular disease, renal, hepatic dysfunction and future cardiovascular disease. Fetal complications include fetal growth restriction, iatrogenic preterm birth, placental abruption and intrauterine fetal demise. Hence, understanding the pathophysiology of pre-eclampsia is important to enable use of targeted therapeutics to not only improve maternal outcomes but also in an attempt to prolong the gestation to improve fetal outcomes.
It is postulated that pre-eclampsia consists of a two stage model in which the first stage is due to a poorly perfused placenta3. This is associated with multiple factors such as increased Natural Killer cells, increased production of Angiotensin 1 receptor autoantibodies, increased oxidative stress and genetic factors. This hypoperfused placenta, leads to release of various circulating markers which causes endothelial dysfunction and produces the clinical manifestations of preeclampsia which is the second stage of the pathophysiology of pre-eclampsia4,5.
Tumor necrosis factor alpha (TNF-α) is a pro-inflammatory cytokine that can be produced by many immune cells and is increased in women with pre-eclampsia6,7. The median concentration of plasma TNF-α is increased by twofold in women with preeclampsia compared with that in normal third-trimester pregnancy 8. We have previously demonstrated that chronic infusion of TNF-α in pregnant rats, at a rates that mimics this observation in women with preeclampsia, results in a significant increase in blood pressure as well as increases circulating levels of TNF-α in treated rats compared to normal pregnant rats9. Natural Killer (NK) cells are stimulated by inflammatory cytokines such as TNF-α and are critical in host defense against intracellular stress, tumor or infection10. Cytolytic natural killer cells kill other cells by recognition of antibodies bound to the cellular surface or because of lack of MHCI molecules. We have previously shown the importance of NK cells in response to placental ischemia to cause hypertension and alterations in placental mitochondrial function11. Moreover, our laboratory has also previously shown an increase in mitochondrial reactive oxygen species (mtROS) and dysfunction to cause hypertension in response to placental ischemia 12. In addition, we have shown that infusing TNF-α into pregnant rats increased vasoconstrictor ET-1, mean arterial pressure (MAP) and decreased renal hemodynamics13. However, we do not know the effects of TNF-α on NK cells or mitochondrial function during pregnancy. Therefore, we hypothesized that TNF-α induced hypertension was associated with cytolytic NK cell activation and mitochondrial dysfunction thus contributing to the clinical pathology of pre-eclampsia. The objective of our study was to measure cytolytic NK cells and mitochondrial respiration and ROS in normal pregnant rats infused with TNF-α.
METHODS:
Female pregnant Sprague-Dawley rats purchased from Envigo (Indianapolis, IN) were used in the present study. Rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle with free access to standard rat chow and water. This study complied with guidelines and principles published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the University of Mississippi Medical Center’s Institutional Care of Animals and the Institutional Animal Care and Use Committee (IACUC). All first-time pregnant rats used were age (12–13 wk) and weight (250 g) matched. Pregnant rats were divided into two groups, the study group was infused with TNF-α and the control group consisted of normal pregnant rats.
The effect of TNF-α onBlood pressure measurement in normal pregnant rats
Recombinant, purified rat TNF-α (BioSource International, Morrisville, NC) was infused via mini-osmotic pump into NP rats at a rate of 50 ng/day for 5 days beginning on day 14 of gestation, as previously described by our laboratory 9,13.Arterial pressure was determined in all groups of rats on day 19 of gestation. Pregnant rats were catheterized on day 18 of gestation and catheter of V-3 tubing (SCI) was inserted into the carotid artery and exteriorized for direct monitoring of blood pressure. On day 19 of gestation, pregnant rats were placed in individual restraining cages and blood pressure measurements were recorded continuously for two 20-min periods after 30 min of stabilization using a pressure transducer (Cobe II Transducer CDX Sema). Rats were then anesthetized using isoflurane delivered by an anesthesia apparatus for blood and tissue collection. Pups and placentas were excised and weighed while dams were under anesthesia.
The effect of TNF-α on placental, renal and circulating natural killer cells
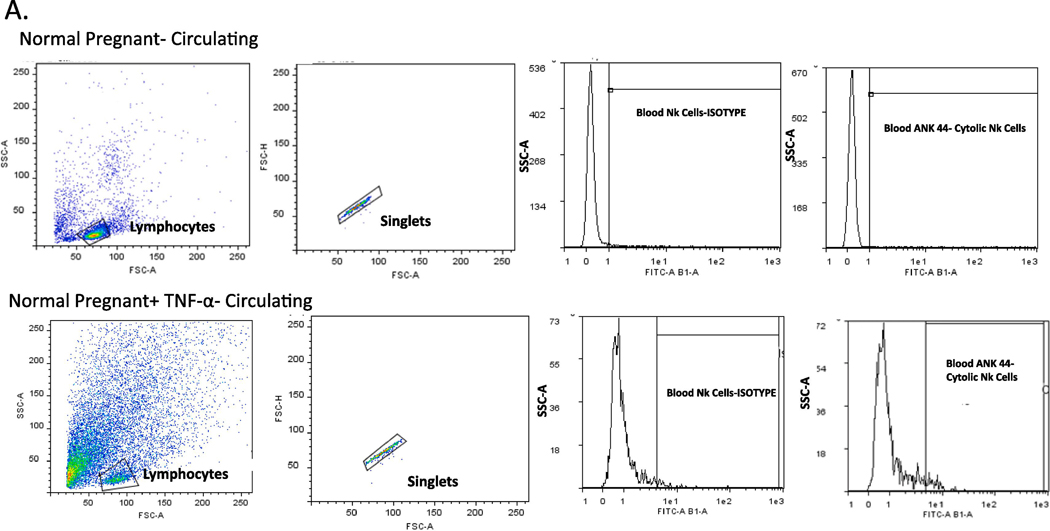
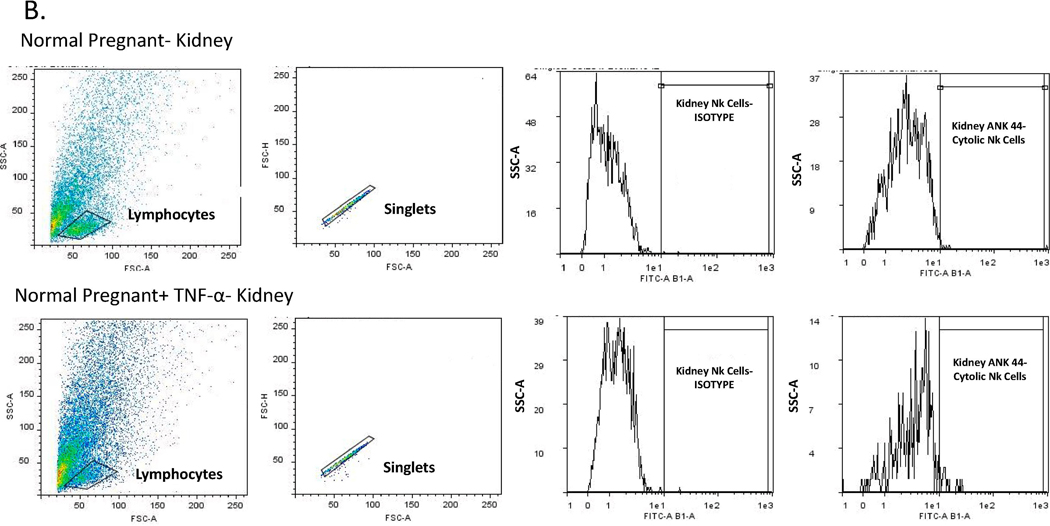
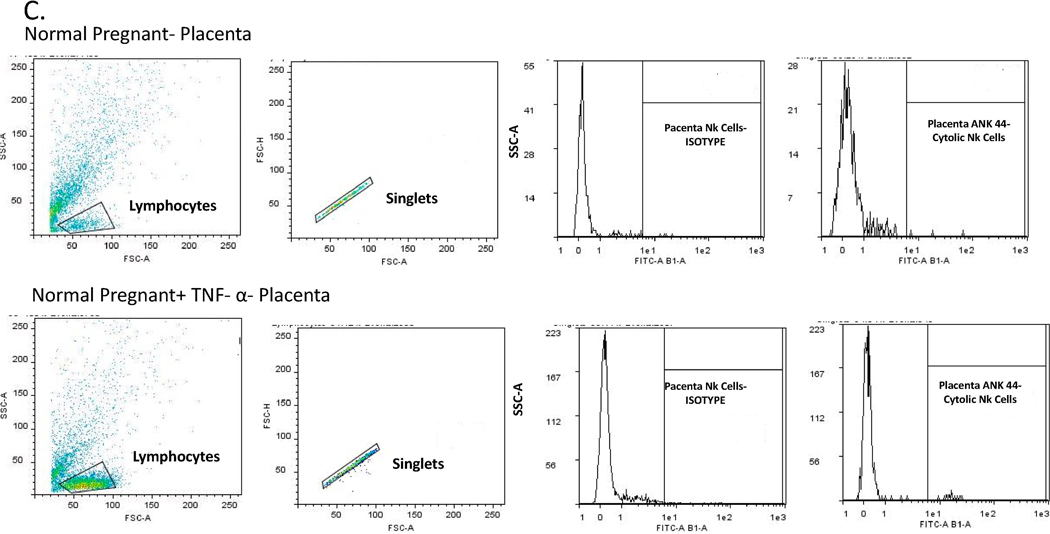
The placental, renal and circulating of NK cells were quantified by flow cytometry from placental and renal immune cells and peripheral blood mononuclear cells (PBMCs) isolated on gestational day 19 from Normal Pregnant (NP) rats and Normal pregnant rats with TNF-α infusion (NP + TNF-α). At the time of harvest, placenta, kidney and whole blood were collected and placental and renal lymphocytes and PBMCs were isolated by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY) according to the instructions of the manufacturer. For flow cytometric analysis, 1 × 106 cells were incubated for 30 min at 4°C with antibodies against rat Anti-Natural Killer Cell Activation Structures (ANK61) or rat Anti-Natural Killer Cell antibody (ANK44) (AbCam, Cambridge, MA). ANK61 is a killer cell activation structure that is expressed on all NK cells, while ANK44 is a marker of cytolytic activation in NK cells because it is only expressed on stimulated, cytotoxic NK cells 14. After washing, cells were labeled with secondary Fluorescein isothiocyanate (FITC; AbCam) antibody for 30 min at 4°C. As a negative control for each individual rat, cells were treated exactly as described above except they were incubated with isotype control antibodies conjugated to FITC alone. Subsequently, cells were washed, fixed, and resuspended in 500 μl of Rosswell Park Memorial Institute (RPMI) medium and analyzed for single staining on a Gallios flow cytometer (Beckman Coulter, Brea, CA). Cells were gated in the forward and side scatter plot. Cells that stained as ANK61+ were designated as NK cells. Cells that stained as ANK44+ were designated as cNK cells. The percent of positive stained cells in the gated population above the negative control was collected for individual rats and the mean values for each experimental group were calculated.
The effect of TNF-α on Mitochondrial function
Intact placental and renal mitochondria were isolated by a differential centrifugation method. Briefly, placentas (2 placentas per rat) were rinsed Mito I buffer, minced with fine scissors, and homogenized using a glass-glass dounce homogenizer 12,13. Oroboros Oxygraph-2K was used to perform respiration measurements in the isolated mitochondria. Basal oxygen consumption was collected from the isolated mitochondria suspended in respiration buffer with no added exogenous substrates. Glutamate/malate (Complex I substrates) were added to collect state 2 respiration rate, and state 3 (coupled respiratory state) was measured by adding ADP. This was followed by leak/state 4 and uncoupled states, obtained by adding oligomycin and FCCP respectively. At the end of the experiment, non-mitochondrial oxygen consumption was measured by the addition of rotenone and antimycin A which was subtracted from all the measured states to correct for non-mitochondrial respiration. The measurements were taken using a sample volume of 30 μl in 2 ml of respiration buffer for placenta and 10ul in 2 ml of respiration for kidney. The collected data were normalized for the amount of protein and expressed as picomoles of oxygen consumed per second per milligram of mitochondrial protein. Hydrogen peroxide (H2O2) production was determined fluorometrically by using Amplex Red reagent.
Statistical analysis:
All data and results are presented as means ± SE. In the TNF-α arm of the study, an unpaired t-test was used when the 2 groups were compared. Analysis of Variance (ANOVA) was used for when more than 2 groups were compared, with a Bonferroni’s multiple comparisons test performed as post hoc analysis. A value of P <0.05 was considered statistically significant. A value of p <0.05 was considered statistically significant.
RESULTS:
The effect of TNF-α on hypertension and fetal demise in normal pregnant rats
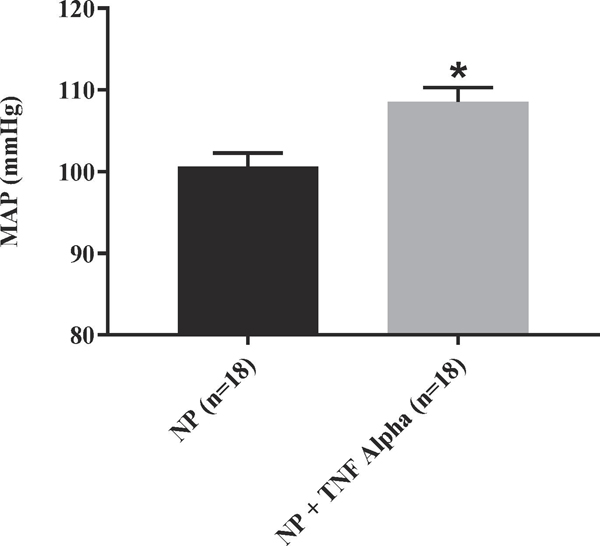
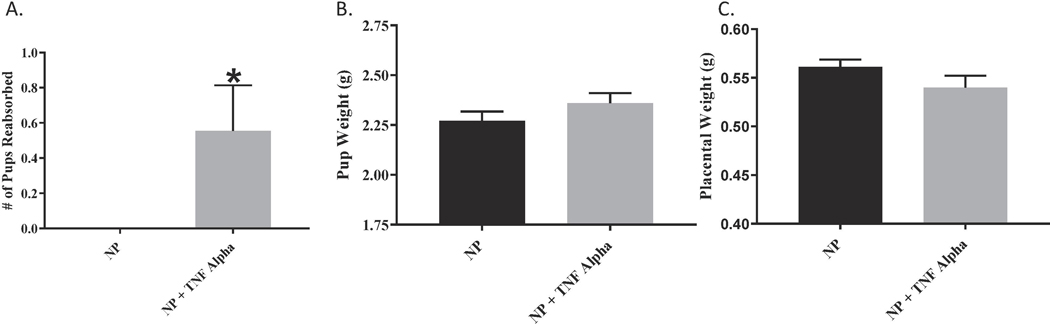
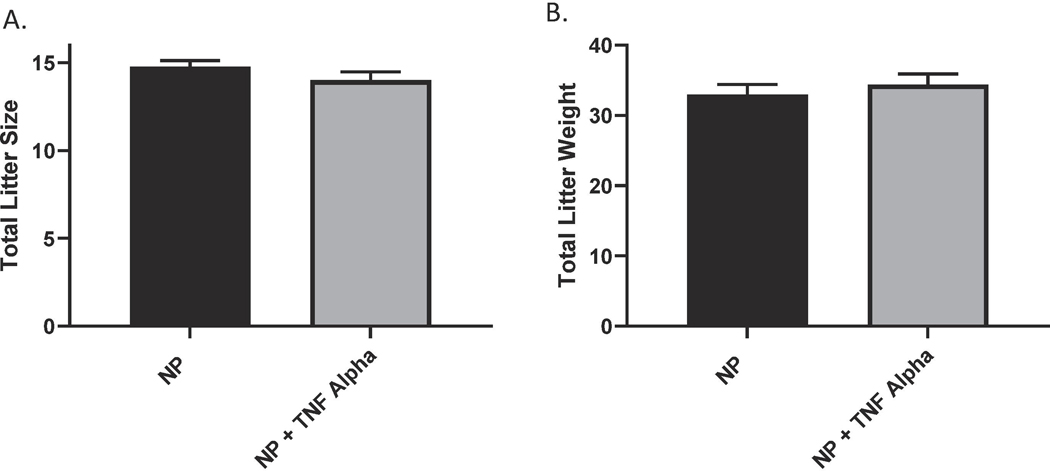
We have previously shown that chronic infusion of rat TNF-α increases blood pressure in normal pregnant rats 9. We were able to demonstrate that MAP was increased in the TNF-α treated rats as compared to the normal pregnant rats (109 ± 2 vs 100 ± 2, p<0.05, n=18). Figure. 1 Additionally, we noted an increase in pup reabsorptions in the TNF-α infused pregnant rats compared to reabsorptions in the normal pregnant group, Figure 2. However, there were no differences in weight of pups collected on gestation day 19 (2.3 ± 0.05 vs 2.4 ± 0.05) or their placental weight (0.6 ± 0.007 vs 0.5 ± 0.012) between the two groups; Figures 2 B-C. There was no significant differences in the total litter size (14.8 ± 0.34 vs 14.0 ± 0.49) between treatment groups or the total litter weight (32.9 ± 1.47 vs 34.4 ± 1.54), Figure 3.
Figure 1.
MAP was significantly elevated in TNF-α infused vs. normal pregnant rats (NP); p= <0.05
Figure 2.
(A) Fetal reabsorptions significantly increased in the TNF-α infused group as compared to the NP rats; p <0.05. However neither individual fetal weight (B) nor placental weight (C) were significantly different between NP and NP+TNF-α.
Figure 3.
There was no significant differences in total (A) litter size nor (B) litter weight between NP and NP+TNF-α.
The effect of TNF-α on placental, renal and circulating natural killer cells:
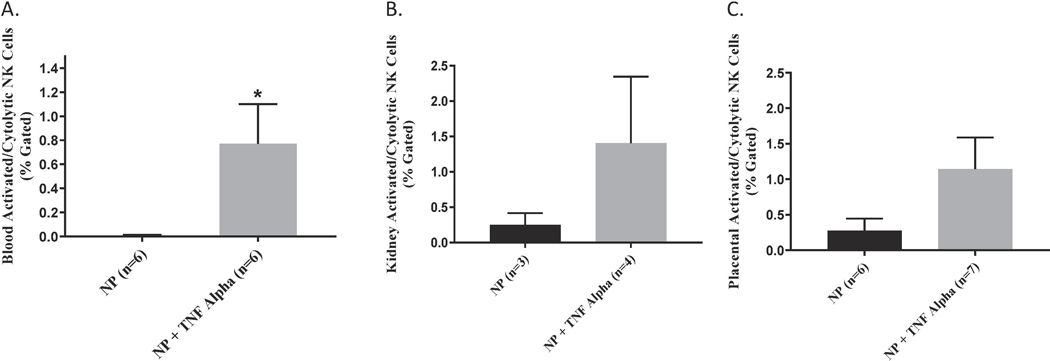
We measured total and activated (cytolytic) NK cells in the peripheral blood, placenta and kidney of pregnant rats in the two groups. Circulating activated (cytolytic) NK cells were significantly increased in the TNF-α infused rats (0.8 ± 0.33 vs 0.008 ± 0.003 % gated, *p<0.05, n=6, Figure 4A). Although not significant, activated NK cells were also greater in placenta (1.15 vs 0.28% gated) and kidney (1.40 vs 0.25% gated) of pregnant rats infused with TNF-α as compared to the normal pregnant group; Figure 4 B-C. Images of flow cytometry plots are provided for circulating, kidney, and placenta NK cells in Figures 5 A-C.
Figure 4.
(A) An increase in activated cNK cells noted in the circulation (p<0.05; (B) kidney and (C) placenta of the pregnant rats infused with TNF-α, however the increase in the placenta nor kidney reached statistical significance.
Figure 5.
Representative pictures of flow cytometry for activated NK cells in (A) circulating, (B) Kidney, and (C) Placenta.
The effect of TNF-α on renal Mitochondrial function during pregnancy:
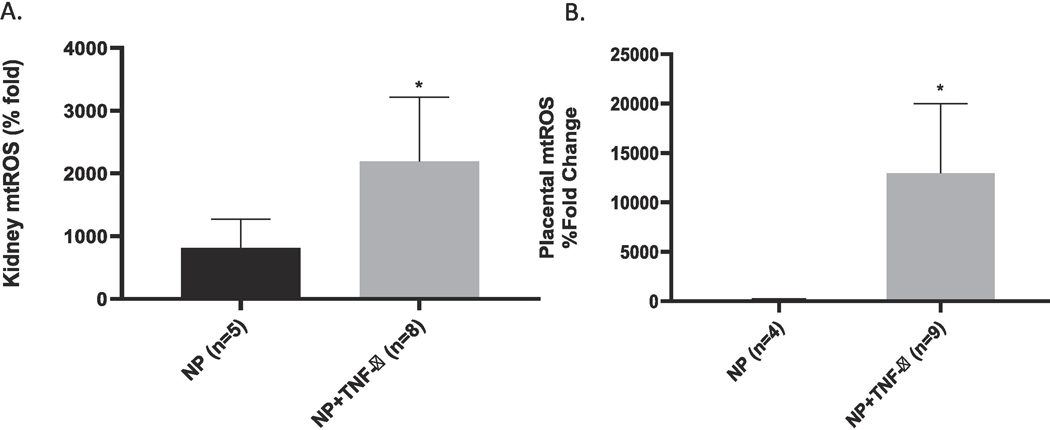
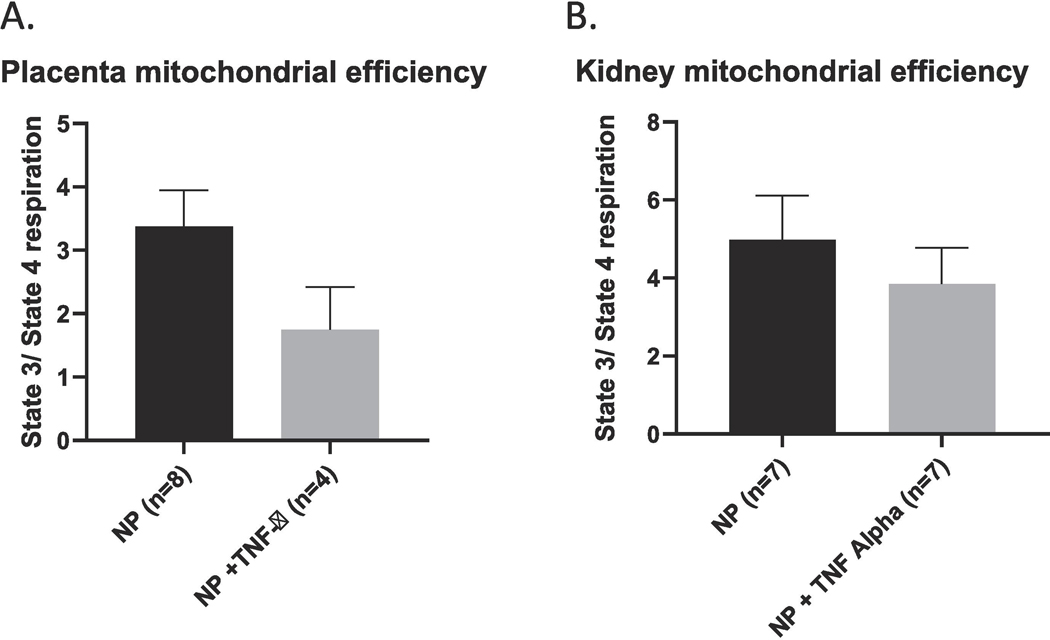
Mitochondrial reactive oxygen species (mtROS) was measured in isolated mitochondria from the placental and kidney. An increase in ROS production has been reported in normal gestation, but an excessive amount of ROS production is reported in pathological states such as PE.12 Therefore, an increase in mitochondrial ROS is indicative of impaired mitochondrial function. The mtROS was noted to be significantly increased in the placenta of rats infused with TNF-α (12976 ± 7038 vs 176.9 ± 68.04 %fold, *p <0.05, Figure 6A. Similarly, there was a significant increase in mtROS in the renal mitochondria of rats treated with TNF- α as compared to the normal pregnant group (2191 ± 1027 vs 816 ± 454.7 % fold, * p<0.05; Figure 6B. There were no differences noted in the different states of mitochondrial respiration from placenta of the NP rats compared to rats infused with TNF- α. Similarly, isolated renal mitochondria from pregnant rats infused with TNF-α showed no significant differences in the various states of respiration. We calculated the mitochondrial Respiratory Control Ratio (RCR), which is a measure of mitochondrial efficiency. Mitochondrial respiratory control encapsulates the main function of mitochondria: their ability to idle at a low rate yet respond to ADP by making ATP at a high rate, and, therefore, is an indicator of energy metabolism efficiency. Ratio (RCR) defined by the ratio of the oxidation rate in the presence of excess substrate and adenosine diphosphate (State 3) to the oxidation rate after ADP has been phosphorylated to a steady state concentration (State 4). A high RCR implies that the mitochondria have a high capacity for substrate oxidation and ATP turnover and a low proton leak. In contrast, a lower RCR ratio indicates less ATP is produced for a given consumption of oxygen and energy substrates. In our study a decrease in the RCR in both the placental and renal mitochondria were noted however this did not reach statistical significance Figure 7.
Figure 6.
Renal (NP(n=5) TNF (n=8)) and placental (NP (n=4) TNF (N=9) mtROS was increased in NP+TNF-α vs NP rats, (*p <0.05).
Figure 7.
RCR, a measure of mitochondrial efficiency, was noted to be lower but not significantly decreased in either the placenta or kidney of pregnant rats infused with TNF-α.
Discussion:
We have previously shown TNF to cause hypertension by decreasing renal hemodynamics and stimulating AT1-AA, sFlt-1 and endothelin9,13. In this study, we tested the hypothesis that an additional mechanism of TNF-α in causing the pathophysiology of PE would be to stimulate cytolytic NK cells to cause mitochondrial oxidative stress and dysfunction in the kidney and placenta during pregnancy.
The uterine natural killer cells play an important role in maintaining a normal pregnancy through angiogenesis at the maternal-fetal interface and are not cytolytic in nature 15. Activation of cytolytic NK cells leads to cytokine secretion, release of granzymes and perforins which cause mitochondrial dysfunction and cell death 16,17. In pre-eclampsia, there is an increase in the cytolytic NK cells and a decrease in the uterine natural killer cells 17,18. We have previously shown an important role for cytolytic NK cells to cause mitochondrial dysfunction in the placentas of RUPP rats compared to normal pregnant rats 18. Mitochondria have the ability to control cell survival and death, calcium homeostasis and a variety of intracellular processes 19. The mitochondrial function is largely dependent on the production of ATP and superoxide/hydrogen peroxide. Placental ischemia incites mitochondrial damage leading to a disruption in the electron transport chain (ETC) and release of free radicals 12. Although we have previously shown in the RUPP rat model of placental ischemia that reduction in mitochondrial respiration and increased mitochondrial ROS production in both placenta and kidney contribute to hypertension in response to placental ischemia12, the effect of TNF-α on mitochondria during pregnancy has not been examined. In our study, we were able to demonstrate that in response to TNF-α infusion, circulating cytolytic NK cells were significantly higher than in NP rats. In association with higher NK cells, both placental and renal mitochondrial ROS were significantly increased during TNF- α induced hypertension in pregnant rats. Interestingly, we were unable to demonstrate any significant differences in mitochondrial respiration in either renal or placental tissue. In addition, the respiratory control rate was decreased in placental and renal tissues however this wasn’t significant. Nevertheless this is suggestive of decreased mitochondrial efficiency in pregnant rats infused with TNF-α. Moreover, placental and renal cytolytic NK cells were increased but not significantly. Albeit those cytolytic NK from the circulation may not have yet completely infiltrated those tissues and this number may have been significantly higher with time. Our study confirms that indeed TNF-α activates cytolytic natural killer cells but also plays an important role to cause mitochondrial mtROS, independent of NK cells as a mechanisms of hypertension and fetal demise during pregnancy. Importantly we have recently published that CD4_ T cells from RUPP rats cause mt dysfunction, also independent of activated NK cells. It could be that TNF alpha is stimulating T cells or a factor stimulated in response to T cells that causes mt dysfunction20. However, T cells were not measured in this study but will be subject of further investigations into the mechanisms of mt dysfuction in responses to placental ischemia.
Natural killer cells are one of the earliest responders to arrive at target organs of inflammation. The role of NK cells in different organs is quite varied and yet to be fully understood 21. These cells have receptors on their surface which could either activate or inhibit their cytotoxic activity. NK cells function as soldiers in the innate immune system and are normally held in check when an inhibitory receptor binds to its ligand. NK cells are activated to kill cells that lack a signature of “self” Major Histocompatibility Complex class l antigen (MHC Class l), also called as the “missing self” MHC Class I theory of NK cell activation 22,23. These cells include tumor cells and host cells infected with viruses or other intracellular pathogens. Important to the “missing self” theory, in normal pregnancies, uterine NK cells in human placenta do not show killer activity but instead assist in establishing immunosuppression and tolerance to fetus allograft.
We have previously discussed the role of granzyme mediated ROS generation produced by cytolytic NK cells stimulated in response to placental ischemia. ROS production is an important signaling of the redox pathway and it controls physiological adaptation such as intracellular stress. However, ROS overproduction has been associated with endothelial dysfunction, vascular remodeling and end organ damage 24,25. For example, it is known that patients with renal allograft rejection have increased NK cell mediated cytotoxicity 26–28. Moreover, there are studies that demonstrate the role of mitochondrial dysfunction in patients with chronic renal allograft rejection 29,30. Although there is an increase in ROS, it does not have a significant impact on mitochondrial respiration which may be a product of functional “sparing” of mitochondria. Several studies have reported that TNF-α up-regulatory effect on HIF-1α was ROS mediated and independent of tissue hypoxia 31,32. HIF-1α has a protective effect against hypoxic events where it reduces mitochondrial oxygen consumption 33–36. This is an important cellular adaptation for survival and could be an explanation of the preserved mitochondrial respiration in the TNF-α infused rats. It is important to note that although there is no significant change in mitochondrial respiration in the TNF-α treated rats, the Respiratory Control Ratio (RCR) was decreased while mt ROS was increased, supporting the idea of inefficient oxygen utilization in the kidney of TNF alpha infused rats.
There have been other studies demonstrating conflicting responses concerning the effect of TNF-α especially in regard to organ function and mitochondrial sparing 37–39. Additional studies indicate TNF-α worsens cardiac function in patients with congestive heart failure and there is conflicting data that demonstrate the role of TNF-α in Doxorubicin mediated cardiotoxicity in cancer therapy 40. It has been shown that endogenous TNF-alpha production exerts a protective effect at the intracellular level against doxorubicin-induced cytotoxicity by up-regulating the mitochondrial anti-oxidant, MnSOD. 39,40. To determine if this was the case in our study, we measure MnSOD by western blot, which is a mitochondrial antioxidant that plays an important role in scavenging superoxide radicals (O−2). MnSOD was unchanged in our study. We measured other markers of renal injury and dysfunction. NGAL, which is a marker of acute kidney injury, which was not changed in pregnant rats treated with TNF-α compared to NP controls. KIM-1 is a marker of acute kidney injury specific to the proximal tubules and is detected as early at 24–28h after the insult has occurred 41. Similar to NGAL, there were no differences in plasma KIM-1 levels in the TNF-α pregnant rats.
TNF-α infusion causes renal vasoconstriction, reduces renal blood flow and has a natriuretic effect on the renal tubules 42,43. The oxygen consumption at the level of the kidneys is directly dependent on the sodium transport across the renal tubules 35. Therefore increased renal blood flow and glomerular filtration lead to increased oxygen consumption. In fact the kidney derives 95% of its energy through oxidative metabolism. Mitochondria are plentiful in both the proximal tubule and thick ascending limb, the site most significantly active in sodium transport and thus oxygen expenditure. However the coupling of transport and renal oxygenation in pathological states isn’t well described. In patients with acute renal injury where GFR and sodium transport are lower than normal healthy individuals, the oxygen consumption and extraction was increased 44. This suggests inefficient use of oxygen during Na transport, or other processes besides Na reabsorption, and supports the idea of dysfunction of mitochondrial utilization of oxygen for oxidative metabolism. This could be an alternative mechanism occurring in the kidney in this study in which mitochondrial respiration was maintained with TNF alpha during pregnancy 45. Nevertheless, further studies are necessary for us to better understand the effect that cytokines and immune cells have on not only renal function but renal oxidative phosphorylation and mitochondrial function even in presence of decreased GFR and RPF in order to better treat patients at the level of mitochondrial function and have a positive clinical outcome on their disease state.
There have been studies with several other ROS scavengers such as vitamin E and vitamin C with no proven benefit in the treatment of pre-eclampsia46,47. Other animal studies, such as the one from our laboratory has shown the use of MitoTEMPO, another such ROS scavenger to be beneficial in reducing hypertension 12. Mitochondrial supplements are commercially available and are routinely used in athletes to improve performance during long periods of exercise. Supplementing mitochondrial function improves respiration at the tissue level and could be a way to improve both renal and placental functions especially in the face of hypoxia, which is often associated with pre-eclampsia. However, it is unknown if these supplements or reduction of TNF mediated NK cell mitochondrial ROS will be beneficial during pre-eclampsia until new clinical trials are approved.
Our study has demonstrated that the role of TNF-α in pregnant rats causes NK cell mediated cytotoxicity in the placenta and kidneys and generates a significant amount of mtROS, however, sparing mitochondrial respiration. It is unknown if this sparing or protective effect of TNF-α in the mitochondria is a transient effect. Overall, both the mtROS and RCR data indicate that there is mt dysfunction in response to TNF during pregnancy, which ultimately may result in mt oxidative stress and reduced bioenergetics in the placenta and kidney. Clinically, understanding the role of TNF-α on the hemodynamics of pregnancy could play an important role in determining the timing of worsening clinical pathophysiology of pre-eclampsia. Although, delivery remains the only known treatment of pre-eclampsia, the use of novel therapeutic agents in pre-eclampsia, can prolong the gestation reduce neonatal morbidity and mortality. Understanding the role of TNF-α and its blockade in the different organ systems can also prevent the long term complications of pre-eclampsia.
Highlights.
TNF alpha causes hypertension and fetal demise during pregnancy
Renal and placental mitochondrial dysfunction occurs in response to elevated TNF alpha, which may be the mechanisms for hypertension and increased fetal death
Understanding the pathology caused by TNF alpha during pregnancy will provide a better understanding of the efficacy of TNF alpha inhibitors as potential therapeutics for preeclampsia.
Acknowledgements:
This study was supported by NIH grant RO1HD067541 (BL), NIH grants R00HL130456 and P20GM104357 (DCC), NIH P20GM121334 (BL, LMA), American heart association (AHA) early career award 19CDA34670055 (LMA)
Footnotes
DISCLOSURE STATEMENT: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Ahmed A, Rezai H, Broadway-Stringer S. Evidence-Based Revised View of the Pathophysiology of Preeclampsia. Adv Exp Med Biol. 2017;956:355–374. [DOI] [PubMed] [Google Scholar]
- 2.Karumanchi SA, Granger JP. Preeclampsia and Pregnancy-Related Hypertensive Disorders. Hypertension. 2016;67(2):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of Preeclampsia. Annual Review of Pathology: Mechanisms of Disease. 2010;5(1):173–192. [DOI] [PubMed] [Google Scholar]
- 4.Redman CWG, Sargent IL, Roberts JM. Immunology of Normal Pregnancy and Preeclampsia. Chesley’s Hypertensive Disorders in Pregnancy, 3rd Edition. 2009:129–142.
- 5.Redman CWG. Immunology of Preeclampsia. Seminars in Perinatology. 1991;15(3):257–262. [PubMed] [Google Scholar]
- 6.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor-Necrosis-Factor-Alpha Is Elevated in Plasma and Amniotic-Fluid of Patients with Severe Preeclampsia. Am J Obstet Gynecol. 1994;170(6):1752–1759. [PubMed] [Google Scholar]
- 7.Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38(2):89–93. [DOI] [PubMed] [Google Scholar]
- 8.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86(6):2505–2512. [DOI] [PubMed] [Google Scholar]
- 9.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52(6):1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barakonyi A, Miko E, Szereday L, et al. Cell death mechanisms and potentially cytotoxic natural immune cells in human pregnancies complicated by preeclampsia. Reprod Sci. 2014;21(2):155–166. [DOI] [PubMed] [Google Scholar]
- 11.Vaka VR, McMaster KM, Cornelius DC, et al. Natural killer cells contribute to mitochondrial dysfunction in response to placental ischemia in reduced uterine perfusion pressure rats. Am J Physiol Regul Integr Comp Physiol. 2019;316(5):R441–R447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaka VR, McMaster KM, Cunningham MW Jr., et al. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension. 2018;72(3):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMarca BD, Alexander BT, Gilbert JS, Ryan MJ, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5 Suppl A(Suppl A):S133–8. doi: 10.1016/j.genm.2008.03.013. PMID: ; PMCID: PMC2776712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elfarra J, Amaral LM, McCalmon M, et al. Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clin Sci (Lond). 2017;131(23):2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukui A, Yokota M, Funamizu A, et al. Changes of NK Cells in Preeclampsia. Am J Reprod Immunol. 2012;67(4):278–286. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald G, Shi LF, Velde CV, Lieberman J, Greenberg AH. Mitochondria-dependent and -independent regulation of granzyme B-induced apoptosis. J Exp Med. 1999;189(1):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields CA, McCalmon M, Ibrahim T, et al. Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy. Am J Physiol-Reg I. 2018;315(2):R336–R343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paupe V, Prudent J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem Bioph Res Co. 2018;500(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deer E, Reeve KE, Amaral LM, et al. CD4+ T Cells cause Renal & Placental Mitochondrial Oxidative Stress as mechanisms of hypertension in response to placental ischemia. American Journal of physiology. Renal Physiology. 2020. November. DOI: 10.1152/ajprenal.00398.2020. [DOI] [PMC free article] [PubMed]
- 21.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul S, Lal G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front Immunol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10(10):1713–1765. [DOI] [PubMed] [Google Scholar]
- 25.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. [DOI] [PubMed] [Google Scholar]
- 26.Uchida T, Ito S, Kumagai H, Oda T, Nakashima H, Seki S. Roles of Natural Killer T Cells and Natural Killer Cells in Kidney Injury. Int J Mol Sci. 2019;20(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10(8):1812–1822. [DOI] [PubMed] [Google Scholar]
- 28.Coffman T, Geier S, Ibrahim S, et al. Improved renal function in mouse kidney allografts lacking MHC class I antigens. J Immunol. 1993;151(1):425–435. [PubMed] [Google Scholar]
- 29.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93(21):11853–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, et al. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemia-reperfusion injury. Transplantation. 2003;76(1):50–54. [DOI] [PubMed] [Google Scholar]
- 31.Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1 alpha. Febs Lett. 2001;505(2):269–274. [DOI] [PubMed] [Google Scholar]
- 32.Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Jr., Reichner JS. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol. 2001;281(6):C1971–1977. [DOI] [PubMed] [Google Scholar]
- 33.Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab. 2006;3(3):150–151. [DOI] [PubMed] [Google Scholar]
- 34.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–197. [DOI] [PubMed] [Google Scholar]
- 35.Nourbakhsh N, Singh P. Role of Renal Oxygenation and Mitochondrial Function in the Pathophysiology of Acute Kidney Injury. Nephron Clin Pract. 2014;127(1–4):149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287(4):H1813–1820. [DOI] [PubMed] [Google Scholar]
- 37.Lien YC, Lin SM, Nithipongvanitch R, et al. Tumor necrosis factor receptor deficiency exacerbated Adriamycin-induced cardiomyocytes apoptosis: an insight into the Fas connection. Mol Cancer Ther. 2006;5(2):261–269. [DOI] [PubMed] [Google Scholar]
- 38.Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106(9):1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu ZW, Lin SQ, Wu WK, et al. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-kappa B pathways and mitochondrial protective mechanisms. Toxicology. 2008;247(2–3):133–138. [DOI] [PubMed] [Google Scholar]
- 40.Lou lD H, Singal PK Cytokines are not upregulated in adriamycin-induced cardiomyopathy and heart failure. Journal of Molecular and Cellular Cardiology 2004;Volume 36(Issue 5,):683–690. [DOI] [PubMed] [Google Scholar]
- 41.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen Y, Crowley SD. Renal Effects of Cytokines in Hypertension. Adv Exp Med Biol. 2019;1165:443–454. [DOI] [PubMed] [Google Scholar]
- 43.Shahid M, Francis J, Majid DSA. Tumor necrosis factor-alpha induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol-Renal. 2008;295(6):F1836–F1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh P, Ricksten SE, Bragadottir G, Redfors B, Nordquist L. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin Exp Pharmacol Physiol. 2013;40(2):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricksten SE, Bragadottir G, Redfors B. Renal oxygenation in clinical acute kidney injury. Crit Care. 2013;17(2):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, Group AS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354(17):1796–1806. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura YK, Omaye ST. Vitamin E-modulated gene expression associated with ROS generation. J Funct Foods. 2009;1(3):241–252. [Google Scholar]