Abstract
Purpose:
To evaluate the effect of application of 3% air in helium cold atmospheric plasma jet, using an inexpensive device termed iCAP, in corneal scratch wound closure in vitro and the treatment of Pseudomonas aeruginosa (P. aeruginosa) keratitis in vivo.
Methods:
Thermal imaging to measure temperature of surfaces to which iCAP was applied and UV energy density delivered by iCAP were measured. Scratch wounds inflicted on in vitro cultures of a human corneal epithelial cell line were treated with iCAP and wound widths at various times post-application were measured. Rabbit eyes infected with P. aeruginosa were treated with iCAP and slit lamp biomicroscope examination conducted to determine corneal health outcomes 25h post infection. Corneal homogenates were plated on agar and viable bacterial colonies enumerated to determine the effect of iCAP on bacterial load in vivo in P. aeruginosa keratitis.
Results:
iCAP was shown to operate in the non-thermal regime and also shown to deliver much lower UV energy density than that necessary to cause harmful effects on ocular tissue. iCAP treatment significantly improved the rate of scratch wound gap closure in vitro in a human corneal epithelial cell line compared to controls. In vivo, iCAP treatment of P. aeruginosa keratitis infection in the rabbit eyes (N = 20) significantly reduced the incidence of corneal ulcer (P = 0.003) and corneal edema (P = 0.011) and significantly improved total cornea health (P = 0.034) compared to untreated (N = 10). Finally, in vivo iCAP treatment of P. aeruginosa keratitis infection in the rabbit eyes (N = 19) significantly reduced bacterial loads (P = 0.012) compared to untreated (N = 9).
Conclusion:
Our results strongly suggest that iCAP treatment was effective in improving corneal epithelial defect closure in vitro, reducing ulcer formation and decreasing inflammation in P. aeruginosa infected corneas in vivo and decreasing bacterial loads in P. aeruginosa infected corneas in vivo which led to improved overall cornea health outcomes in vivo. Further studies to investigate iCAP’s safety and efficacy against other infectious microbes responsible for causing ulcerative keratitis, with and without co-treatment with antimicrobial therapies are warranted.
Keywords: Cold atmospheric plasma, Pseudomonas aeruginosa, Bacterial keratitis, Corneal epithelial defect closure, Bacterial load reduction, Ulcer and inflammation reduction
1. Introduction
Ulcerative keratitis caused by infectious microbes (bacteria, fungi, amoebae and viruses) or due to eye trauma or chemical exposure is a medical problem of significant concern [1]. The most recent (2010) analyses of national healthcare databases indicate that keratitis accounts for approximately 930,000 annual Doctor’s office and outpatient clinic and 58,000 emergency department visits in the USA alone [2]. Of these, 76.5% result in antibiotic prescriptions for microbial keratitis caused by P. aeruginosa, S. aureus, coagulase-negative Staphylococcus, Streptococcus pneumoniae and viridians group Streptococci species [1,3–7]. Disease manifestation in ulcerative infectious keratitis, such as in P. aeruginosa keratitis, includes corneal ulcer, edema and/or hypopyon [3,6,8,9] which can result in major complications including elevated intraocular pressure, corneal thinning and perforation, and progression to endophthalmitis [6,10–12]. Consequently, clinical outcomes could be severe, including partial or complete vision loss, necessity for penetrating keratoplasty, corneal grafts, enucleation and evisceration [4,8,13]. Treatment costs for keratitis related codes are ~ $150 - $250 for Doctor’s office or outpatient clinic and ~ $500 - $700 for emergency department visits [2]. Thus, microbial keratitis, including keratitis caused by P. aeruginosa, accounts for a large ($175 million) financial burden in direct healthcare expenditures and is also estimated to consume over 250,000 hours of clinician time annually in the USA alone [2].
P. aeruginosa, in particular, is a formidable opportunistic pathogen which is responsible for severe infections of the eye, including keratitis and endophthalmitis [14]. Contact lens wear is one of the major factors that predispose the user to the development of P. aeruginosa keratitis [15–19]. Furthermore, this organism expresses a variety of virulence factors to aid disease progression such as various toxins and enzymes that induce severe inflammation and if left unchecked, lead to extensive ocular defects [20–22]. Moreover, recent susceptibility analyses suggest growing antibiotic resistance in Pseudomonas keratitis such that Cefazolin, Chloramphenicol, Vancomycin, Trimethoprim and Tetracycline are no longer viable choices to treat it [23]. Finally, even effective antibiotics such as Ceftazidime and Ciprofloxacin require lengthy treatment times (days to weeks) to overcome the infection and are typically not capable of helping regenerate ocular defects induced by the infection [24]. Thus, there is an urgent need for effective therapeutic approaches that can kill the infecting bacteria while simultaneously reducing ulceration and inflammation in order to lower the risk and severity of ocular defects that are an outcome of ulcerative infectious keratitis cases.
Cold atmospheric plasma (CAP) may represent a new potentially efficacious modality for the treatment of microbial keratitis. These plasmas are comprised of gentle jets of ionized gases maintained at atmospheric pressure and generated by application of high frequency high voltage through a dielectric barrier to gases such as air, argon, helium or mixtures of the above [25]. In such non-equilibrium plasmas, the degree of ionization is fractional and the ions are at near room temperature while the electrons stripped from the gas are at significantly higher temperatures. We have previously developed such a CAP jet comprised of helium gas with and without 3% air co-injected [26]. We and others have demonstrated that such CAP jets can effectively inactivate microbial species through the action of reactive oxygen and nitrogen species (RONS) that are abundantly delivered by these jets [26–31]. Moreover, it has also been shown that CAP jets do not induce bacterial resistance even after multiple application cycles [32]. Furthermore, CAP jets have also been studied in vitro, ex vivo and in vivo for their effects on corneal tissue and shown to be generally safe to apply with transient morphological and transcriptional changes that fade within a day [33–35]. Finally, in a recent investigation, a pure helium CAP jet was utilized to treat A. fumigatis keratitis in a rabbit eye model. Although the number of rabbits utilized was small, significant reduction in fungal loads was evident as a result of this pure helium CAP jet treatment [36]. However, in that study, slit lamp biomicroscope examination to determine the effect of CAP jet treatment on corneal health in the rabbit eyes was not conducted.
To the best of our knowledge, this is the first study that investigates the effect of CAP treatment, using our inexpensive device, which we term iCAP, on P. aeruginosa keratitis in an in vivo rabbit eye model, both in terms of bacterial load enumeration as well as using slit lamp biomicroscope examination to determine early clinical outcomes following treatment.
2. Materials and Methods
2.1. iCAP device assembly
Our objective for this study was to pack our entire bench scale non-thermal plasma jet assembly, described in a previous publication [26], except for the gas cylinder(s), into a smaller, more portable, form factor. For this, as schematically depicted in Fig. 1, a PVM500 plasma driver (Information Unlimited, Amherst, NH, USA), a model MC-10SLPM-D/GAS:Helium, 5M, LIN digital mass flow controller (Alicat Scientific, Tucson, AZ, USA) and the 3D printed handheld plasma delivery nozzle were packed into a model 23YX16 hard shell ABS case (Seahorse Cases, La Verne, CA, USA) with sufficient soft foam to ensure resistance to mechanical shocks. The high voltage (HV) line from the plasma driver was attached to conductive copper tape wrapped securely around the end of a quartz tube of length 11.4cm and internal diameter 2mm using an insulted connector. The PVM-500 plasma driver applies an oscillating voltage of a sinusoidal waveform of frequency 20kHz and applied RMS current range from 38.77mA to 70.84mA over the applied RMS voltage range from 4.2kV to 6.6kV. The quartz tube acts as the dielectric barrier and enables ionizing the carrier gas (helium with 3% air) flowing through it. Thus, our iCAP device specifically belongs to the dielectric barrier discharge (DBD) plasma jet category. This quartz tube is securely placed in a 3D printed PLA housing with handles to allow a user to safely hold the plasma nozzle and easily direct the plasma jet emerging from the end of the quartz tube to the desired surface. Cutouts in the ABS case allow the power cord for the plasma driver to be plugged into a wall power outlet and tygon tubing from the gas cylinder to attach to the mass flow controller and then to the handheld plasma nozzle. The gas cylinder contains a custom gas mix comprised of 3% air in helium (Praxair, Danbury, CT, USA) which is fed at the rate of 2.35L/min into the plasma nozzle by the mass flow controller. The plasma driver enables application of high frequency and high voltage to the dielectric barrier (quartz tube) which in turn causes the 3% air in helium gas flowing through it to be ionized and emerge as a non-thermal plasma jet of length 2cm. Photographs of the iCAP device and its operation to produce non-thermal plasma jet are provided in the supplementary information (Fig. S1). Additional details of the experimental setup to measure the operating parameters of the PVM500 plasma driver within the iCAP device are provided in the supplementary information (Fig. S2).
Fig. 1.

Schematic depiction of the iCAP device that shows how the various components are connected, in order to generate the non-thermal plasma jet.
2.2. Thermal imaging of plasma jet and measurement of UV energy density delivered by iCAP
The handheld plasma nozzle was positioned perpendicular to a laboratory benchtop at a height of 2cm above the surface and the plasma jet generated by iCAP was then directed to the benchtop surface. Thermal images were captured using a thermal imager model Ti55FT (Fluke Corporation, Everett, WA, USA) at various applied voltages of 4.2, 5.8 and 6.6kV. In the same configuration, the plasma jet generated by iCAP was directed to a S120VC silicon photodiode sensor (Siemens USA, Washington D.C., USA) connected to a PM100D optical power and energy meter (ThorLabs, Inc., Newton, NJ, USA) such that the tip of the plasma jet touched the photodiode sensor. The energy density reported by the optical power and energy meter for 5min iCAP application to the sensor in the range from 200 – 400nm in 20nm increments for the various applied voltages of 4.2, 5.8 and 6.6kV were then recorded. We do not have the means to measure UV energy density below 200nm. Additional characterization of the 3% air in helium plasma jet using a bench scale assembly on which the iCAP is directly based and comprising plasma emission spectroscopy, amounts of H2O2 and NO delivered and in vitro bacterial species killing efficiency are described in our previous report [26].
2.3. In vitro corneal epithelial cell scratch wound healing assay
An SV40 immortalized human corneal epithelial cell line 10.014 pRSV-T (ATCC CRL-11515) was procured from ATCC (American Type Culture Collection, Manassas, VA, USA). This cell line was cultured in serum free keratinocyte media (ThermoFisher Scientific cat# 17005–042) (ThermoFisher Scientific, Waltham, MA, USA) containing 5ng/mL rh EGF, 0.05mg/mL bovine pituitary extract and 500ng/mL hydrocortisone in T25 cell culture flasks in a 5% CO2 incubator. It is necessary to pre-coat the T25 flasks with a mixture of 0.01mg/mL fibronectin, 0.03mg/mL bovine collagen type I and 0.01mg/mL bovine serum albumin to enable the cells to adhere to the surface and grow. Cells were sub-cultured 1:3 when they reached 80% confluence. Cells from passage numbers 5 – 7 were utilized in this study. The wells of a 24well cell culture plate were pre-coated with a mixture of 0.01mg/mL fibronectin, 0.03mg/mL bovine collagen type I and 0.01mg/mL bovine serum albumin and cells were added in each well at a seeding density of 50,000/well. The well plate was then transferred to the 5% CO2 incubator and allowed to incubate overnight to ensure that all cells adhered uniformly to the bottom of the wells in the well plate. Then, two scratch wounds per well were inflicted by firmly swiping a 200μL sterile pipette tip on the bottom of the wells in a X-pattern and the media was changed to remove loosened cells. The replacement media used was both serum and EGF free but contained bovine pituitary extract and hydrocortisone. The wells were divided into four treatment groups of four wells each (N = 4), namely, untreated, iCAP treated, 1mM N-acetylcysteine (NAC) treated and 1mM NAC and iCAP treated. The iCAP treatment groups were exposed to the iCAP jet comprised of 3% air in helium at a flow rate of 2.35L/min ionized by an applied voltage of 5.8kV for 1min per well. The well plate was transferred back to the 5% CO2 incubator. At periodic time intervals, digital micrographs, using a Nikon inverted microscope with a Sentech monochrome CCD camera, were collected. The images were opened in ImageJ (NIH, Bethesda, MD, USA) and analyzed for average scratch wound widths as a function of time after treatment.
2.4. In vivo Pseudomonas keratitis model and treatment with iCAP
The in vivo protocols utilized in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center (UMMC). This study is in full compliance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.4.1. P. aeruginosa culture and sample preparation
A clinical isolate of P. aeruginosa (ATCC # 19660) was streaked on sterile tryptic soy agar (TSA) and incubated at 37°C for 14 – 18h. Then, 10mL of sterile tryptic soy broth (TSB) was inoculated with a single colony of P. aeruginosa recovered from the plate. This was incubated with shaking (200rpm) at 37°C for 8 – 18h (overnight culture). A fresh 10mL sterile tube containing TSB was inoculated with 0.1mL of the above overnight culture and incubated with shaking (200rpm) at 37°C until the optical density (O.D.) at 600nm was approximately 0.2, which corresponds to 108 CFU/mL (subculture). This subculture was sequentially ten-fold serially diluted to obtain a diluted subculture containing 105 CFU/mL of P. aeruginosa.
2.4.2. Rabbit eye intrastromal injection with P. aeruginosa
A total of thirty female New Zealand White rabbits weighing 1.8 – 2.3kg and of average age 7 – 9 weeks were included in the study. The rabbits were anesthetized with a subcutaneous injection of a mixture of 35 – 50mg/kg ketamine and 2 – 10mg/kg xylazine (Henry Schein Animal Health, Dublin, Ohio, USA). One eye of each rabbit received 1 – 2 topical drops of proparacaine (0.5%; Bausch & Lomb, Bridgewater, New Jersey, USA). This was followed by intrastromal injection of 10μL of the diluted subculture of P. aeruginosa using a 30.5-gauge sterile needle. This procedure ensures that the actual amount of P. aeruginosa injected in the stroma is between 102 – 103 CFU.
2.4.3. Treatment with iCAP
Of the thirty rabbits inoculated with intrastromal injection of P. aeruginosa, ten rabbits were left untreated, while twenty rabbits were treated with iCAP. For this, prior to iCAP treatment 1 – 2 drops of topical proparacaine were applied to the infected eye and the rabbit was wrapped in a cloth/bunny snuggle while awake to prevent movement during iCAP treatment. The rabbit was then placed on an insulating rubber mat of thickness ~1inch on the surgical table and the iCAP jet was applied hand-held to the rabbit cornea ensuring that the tip of the jet always directly touched the cornea for the duration of the treatment. For each rabbit, a total of four iCAP treatments of duration 5min each at 4, 12, 16 and 18h post-infection were conducted by direct application of the iCAP plasma jet to the infected cornea of each rabbit using 3% air in helium and at an applied voltage of 6.6kV. This iCAP treatment regimen was selected from the results of a small in vivo pilot study that is described in the supplementary information (Fig. S3). Over the counter (OTC) artificial tears were applied if the eye appeared to be dry following each iCAP treatment.
2.4.4. SLE scoring and bacterial load enumeration
Immediately prior to 25h post-infection, a slit lamp biomicroscope (Topcon SL D, Kogaku Kikai K.K., Tokyo, Japan) was used to examine the infected but untreated and infected but iCAP treated eyes. Slit lamp examination (SLE) scores for the clinical parameters related to each anatomical site were assigned. Digital photographs of the eyes were captured. At 25h post-infection all rabbits were anesthetized with a subcutaneous injection of a mixture of 35–50 mg/kg ketamine and 2–10 mg/kg xylazine (Henry Schein Animal Health, Dublin, Ohio, USA) and then euthanized with an overdose (>390 mg per rabbit) of sodium pentobarbital (Fatal-Plus solution, Henry Schein Animal Health, Dublin, Ohio, USA) followed by bilateral pneumothorax induction. Each infected cornea, whether untreated or treated with iCAP, was extracted, coarsely chopped, placed in 3mL sterile phosphate buffered saline (PBS) and homogenized with an electric tissue homogenizer. The tissue homogenates were serially diluted, plated on TSA in triplicate and incubated overnight at 37°C until colonies appeared (12 – 16h). The bacterial loads in each infected cornea, whether untreated or treated with iCAP, were then enumerated based on these colony counts.
2.5. Statistical analysis
Data distribution normality was checked using an online Shapiro-Wilk test calculator (http://sdittami.altervista.org/shapirotest/ShapiroTest.html). For the in vitro HCE cell scratch wound healing experiment, an online one way ANOVA calculator with Bonferroni post-hoc test was utilized to compare the average wound widths of the various treatment groups at individual time-points (https://astatsa.com/OneWay_Anova_with_TukeyHSD/). For the in vivo Pseudomonas keratitis slit lamp examination results as well as the in vivo bacterial load results, an online Mann-Whitney-Wilcoxon rank sum test calculator (https://astatsa.com/WilcoxonTest/) with confidence interval set to 95% was used to compare the average SLE scores of the untreated to the iCAP treated rabbit eyes and separately to compare the average bacterial counts in the untreated to that in the iCAP treated rabbit eyes. Data is reported as means ± standard error of the mean (SEM). P value <0.05 was considered statistically significant.
3. Results
3.1. iCAP operates in the non-thermal regime and delivers minimal energy in the UV range
Thermal images of the surface to which the plasma jet produced by iCAP was directed using 3% air in helium gas mix at 2.35L/min at the three applied voltages of 4.2, 5.8 and 6.6kV are depicted in Fig. 2. It is clear that the temperature of the surface remains below 32°C which strongly suggests the non-equilibrium nature of the non-thermal plasma jet and lends confidence that it is safe to apply to living tissue without danger of excessive heating and thermal damage.
Fig. 2.

Thermal images suggest that iCAP operates in the non-thermal regime and the temperature of the surface (laboratory benchtop) to which the plasma jet is directed stays below 32°C even at the relatively high 6.6kV plasma driver application voltage.
Furthermore, energy densities of the optical energy delivered by iCAP using 3% in air helium gas mix are depicted in Fig. 3 which suggests that the non-thermal plasma produced by iCAP has minimal UV light co-production. For comparison, also in Fig. 3, the levels of UV energy required to cause mild photokeratitis (PK) are also depicted.[37] Note the logarithmic Y-axis scale and the finding that even at the relatively high applied voltage of 6.6kV, when the plasma jet appears at its most visually intense, the energy densities are much lower than that required to cause mild PK.
Fig. 3.
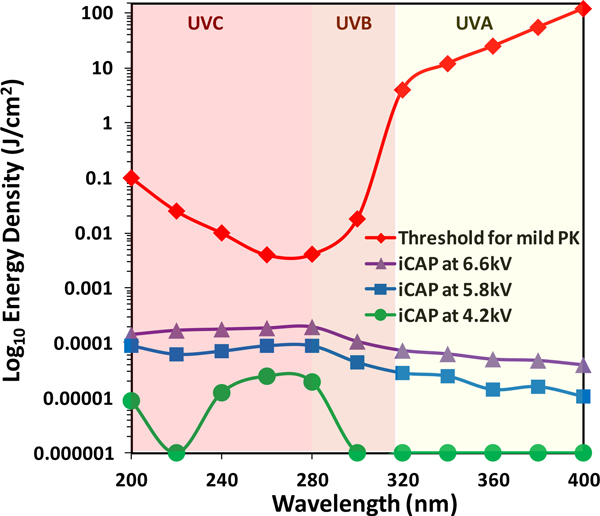
Optical power measurement suggests that iCAP may be inherently safe, in the context of the risk of causing mild PK, to utilize for the treatment of ulcerative keratitis since it delivers negligible UV energy density from 200 – 400nm. The threshold curve for mild PK has been adapted from reference # 37.
3.2. In vitro iCAP treatment improves the rate of closure of scratch wounds in a human corneal epithelial (HCE) cell line
Short (1min) treatment of scratch wounds inflicted in the human corneal epithelial (HCE) cell line with iCAP using 3% in air helium gas mix and at applied voltage of 5.8kV significantly improved the rate of wound closure compared to all other treatment groups as depicted in Fig. 4. In fact, NAC, which is a potent intracellular RONS scavenger greatly retarded wound healing even upon co-treatment with iCAP. This strongly suggests that the mechanism of action likely involves cellular signaling pathways evoked due to RONS delivery by iCAP. Quantitatively, after 21h, scratch wound gaps were reduced on an average by ~70% with iCAP treatment compared to a decrease of only ~35% for the untreated cells or the cells treated with iCAP in the presence of NAC.
Fig. 4.

(A) Representative photomicrographs showing that short (1 min) iCAP treatment significantly improves HCE cell scratch wound gap closure compared to controls. Wound margins are depicted by the yellow lines. (B) Quantitatively, 1min iCAP application decreased the scratch wound gap by ~70% in 21 hours, compared to untreated where the wound width only decreased by ~35%. NAC, a potent intra-cellular RONS scavenger, greatly hinders closure, strongly suggesting that NTP jet delivered RONS are responsible for improving scratch wound closure. n.s.=no significant difference, *p<0.05, **p<0.01. N=4 each treatment and time-point.
3.3. iCAP treatment significantly decreases the incidence of mucopurulent corneal ulcer and the severity of edema (swelling) in P. aeruginosa infected rabbit corneas leading to improved corneal health outcomes
Intrastromal P. aeruginosa inoculate injection into the rabbit corneas resulted in acute infection that was characteristic of Pseudomonas keratitis. Frank symptoms manifested beginning as early as a couple hours after inoculation, beyond which the infection progressed rapidly. Infected but untreated eyes displayed moderate to large mucopurulent ulcers accompanied by serious to severe swelling (edema) as depicted in Fig. 5(A).
Fig. 5.

(A) Example digital images showing that, if left untreated (top row), Pseudomonas keratitis in the rabbit eyes results in rapid disease progression culminating in moderate to large (red arrows) mucopurulent corneal ulcer formation and accompanying edema (swelling), but if treated with iCAP (lower row), ulcer formation is dramatically reduced or completely eliminated and edema is drastically lowered. (B) iCAP treatment results in significant reduction in the slit lamp examination (SLE) scores for edema and ulcer resulting in significant improvement in the total cornea SLE score.
In stark contrast, iCAP treatment had two very remarkable effects as also depicted in Fig. 5(A) – the incidence of corneal ulceration was dramatically reduced (by 76% on average compared to untreated) and the degree of swelling (edema) was sharply lowered (by 67% on average compared to untreated). The beneficial effects of iCAP were localized to the cornea as evident in the SLE scores at that anatomical site tabulated in Fig. 5(B). This in turn led to significantly improved overall corneal health (by 51% on average compared to untreated) as evident in the total cornea SLE score. The other anatomical sites, namely, the conjunctiva, the iris and the anterior chamber remained mainly unaffected (refer to supplementary information Table S1) i.e. iCAP treatment did not cause any additional harmful effects (on top of the infection) at these sites. This is expected, since the iCAP jet is directed to the cornea, the effector species (RONS) have short in vivo half lives and the iCAP jet does not come into contact with these other anatomical sites.
3.4. iCAP treatment significantly decreases bacterial load in P. aeruginosa infected rabbit corneas
In addition to reducing inflammatory outcomes (edema and ulcer) of Pseudomonas keratitis, iCAP treatment was also found to reduce bacterial loads compared to untreated as depicted in Fig. 6. Although the mean reduction was a modest ~1.5log10CFU/cornea i.e. 96%, it was statistically significant (P = 0.012). Furthermore, while the range of bacterial load in the untreated eyes was narrowly distributed between ~8.4 – 7.6log10CFU/cornea, indicating the robust and uniform nature of the Pseudomonas keratitis rabbit eye model, the range of bacterial load in the iCAP treated eyes displayed broad variance. Two out of the twenty iCAP treated eyes were completely sterilized; a few eyes indicated partial disinfection while the rest of the eyes showed virtually no reduction in bacterial load. A detailed breakdown of bacterial loads in all untreated and iCAP treated eyes is provided in the supporting information (Table S2). This may be due to hand-held application of the plasma jet (as opposed to the use of a mechanical separator), and/or inherent inter-rabbit variability in response to iCAP treatment and/or may be related to hindered effectiveness of the plasma jet delivered RONS in accessing the embedded bacterial load within the complex biological matrix comprised of the diseased cornea and the mucopurulent exudate from the corneal ulcer. Longer and/or more frequent iCAP application may therefore be necessary to obtain more uniform and higher efficacy in bacterial load reduction in Pseudomonas keratitis.
Fig. 6.

iCAP application enables modest (~1.5log10CFU/cornea or ~96%) but statistically significant lowering of the bacterial load in Pseudomonas keratitis in vivo. Here, N = 9 untreated and N = 19 iCAP treated eyes. *p < 0.05.
4. Discussion
Cold atmospheric plasma (CAP) jet technology may be an attractive alternative or adjunct to standard antimicrobial therapy in the effective treatment of ocular surface infections e.g. bacterial keratitis. Our objective in designing our iCAP device was to ensure that readily available commercial components, or easily fabricated via 3D printed components be utilized in its construction. Our ultimate goal is that our iCAP device be reasonably inexpensive, easy and safe to utilize in order to find widespread acceptance and adoption by clinical practitioners for utilization at the point-of-diagnosis. Thus, to demonstrate feasibility, it was logical to package our previously reported bench scale non-thermal plasma jet assembly [26] into a relatively compact (15 ½ inches x 12 inches x 9 ½ inches) and relatively lightweight (18 lbs.) prototype iCAP assembly. The total cost of the iCAP prototype device reported herein (not including the cost of the custom 3% air in helium gas mix) is ~$1,500. The cost can be reduced further, in the future, by replacing the mass flow controller, with a gas delivery valve that is pre-calibrated for the required flow rate. Furthermore, the reliability of the iCAP device can be further improved, in the future, by replacing the current plasma driver with a custom design that utilizes higher quality electrical and mechanical parts while keeping its cost comparable to the currently utilized PVM500 plasma driver unit.
That the iCAP device is safe to utilize to treat ocular infections was evident in our observations that it does not produce harmful levels of UV light, nor does it heat the surface to which it is applied. This is in addition to several previous publications that underscore the suitability of such CAP jets for ocular treatment through in vitro, ex vivo and in vivo evidence [33–36,38]. Moreover, the observation that short iCAP application can improve the rate of HCE scratch wound closure in vitro, if translatable in vivo, would have significant benefits to infectious ulcerative keratitis patients and their attending clinicians alike. In particular, bacterial keratitis caused by pathogens such as P. aeruginosa, S. aureus and S. pneumoniae are characterized by the formation of corneal ulcers, which in turn, almost invariably progress to serious ocular defects that are especially difficult to address. This is due to the expression of virulence factors by these organisms that play an important role in microbial pathogenesis, invasion into corneal tissue and creation of ocular defects characteristic of these infections [39]. These virulence factors can be proteases or other hydrolytic enzymes [40–43] toxins [44] or proteins that improve adhesion [45] of the microbe to ocular tissue. Although antimicrobials may be effective in killing the keratitis causative microbe (unless antimicrobial resistant), they are generally not effective in preventing the formation of these ocular defects [23,24]. Co-treatment with anti-inflammatory therapeutics, such as steroidal agents may be effective in partially or completely addressing these ocular defects [41,46]. However, steroidal agents carry the risk of side effects such as hypersensitivity, localized toxicity leading to eyelid itching and swelling, elevated intraocular pressure (IOP), possibility of development of glaucoma, infrequent optic nerve damage and posterior subcapsular cataract formation. If the in vitro observation that iCAP can improve the rate of corneal defect closure stays true in vivo, then it would significantly reduce the burden of optical defects that are an invariable clinical outcome of ulcerative keratitis and may do so with less risk of side effects.
To the best of our knowledge, this is the first report of in vivo application of CAP jet to treat bacterial keratitis, specifically Pseudomonas keratitis. The only other study, that we are aware of, that investigated the application of such a CAP jet to treat infectious keratitis in vivo was for fungal (A. fumigatis) keratitis [36]. In that study, a pure helium non-thermal plasma jet was employed, with once daily 5min treatment over seven consecutive days, to treat rabbit eyes inoculated with A. fumigatis. The study reported significant efficacy of the non-thermal plasma jet in eradicating fungal loads in the eyes post-treatment. In contrast, our study was meant to be an acute phase study to investigate the early clinical outcomes within a day of iCAP jet treatment. We have previously demonstrated in vitro the ability of our iCAP jet to rapidly (<5min) and completely sterilize the bacterial species, E. cloacae and A. baumannii [26]. Others have provided strong evidence that the disinfection and sterilization ability of CAP jets both in vitro and in vivo can be extended to a variety of pathogenic species including bacteria [47,48], fungi [49–51] and viruses [52–54]. A general observation made is that the efficiency of killing these microbes with CAP is superior in vitro compared to in vivo [55]. Furthermore, even in vivo, microbes that are closer to the surface of the infected tissue are easier to kill with CAP compared to those that are in deeper layers of the tissue [55]. However, the growing consensus in the plasma medicine field is that infected tissue containing even deeply embedded microbes can be thoroughly sterilized if the dose, duration and applied power of the CAP jet is sufficient and the gas composition is appropriate [48,55]. In our study, the efficiency of killing P. aeruginosa in vivo in the rabbit eyes was found to be modest, but statistically significant. Moreover, there was significant inter-rabbit variance in the residual bacterial load after iCAP application. Given that our prototype iCAP is a relatively unoptimized system, and also that P. aeruginosa keratitis is accompanied by significant mucopurulent exudate from the corneal ulcer that can shield the embedded microbes from the RONS delivered by iCAP, the fact that we do obtain some degree of disinfection in vivo is quite encouraging. This observation clearly guides us towards one direction that our future research should take going forward, namely, optimization of the iCAP device to more efficiently kill keratitis causatives in vivo.
This study is also, to the best of our knowledge, the first to comprehensively investigate the early clinical outcomes in Pseudomonas keratitis (or any bacterial keratitis) with CAP jet treatment using standard ophthalmologic SLE scoring methods. Our finding that the beneficial effects of iCAP are localized to the site of application, namely, the cornea, is extremely important from the standpoint of safety. The risk of bystander tissue damage has been shown to be negligible from the results of our study since the CAP jet is a highly localized, directed treatment modality. Furthermore, from the standpoint of clinical potential and utility, our study has clearly outlined, at least preliminarily, that iCAP appears to exert very powerful anti-inflammatory and anti-ulcer effects which may, in the future, make it the modality of choice to treat ulcerative keratitis at the point-of-diagnosis. The observation that iCAP’s in vivo efficacy in greatly reducing or eliminating ulcer formation and drastically reducing inflammation (edema or swelling) in a particularly severe form of ulcerative bacterial keratitis (Pseudomonas keratitis) is potentially very high, is also extremely encouraging. This observation also guides us in a second direction that our research should take going forward, namely, to investigate whether iCAP’s powerful anti-inflammatory and anti-ulcer effects are truly general for other bacterial and fungal keratitis causatives. Furthermore, it becomes important to investigate whether the early clinical outcomes of iCAP treatment in vivo for ulcerative keratitis described herein can be maintained long term such that the infection is fully eradicated and the ocular tissue completely healed without residual optical defects.
While the results of this work are encouraging, it should be noted that there are some limitations in our study. First, given its limited scope, it was not possible to include comparator groups of animals that were treated with standard antibiotics of choice that are known to be effective against Pseudomonas keratitis or a group of animals treated with a combination of standard antibiotics and iCAP. Second, given the severity of disease and the rapidity of progression in Pseudomonas keratitis, it was also not possible to investigate the effects of iCAP beyond a day as it would have been unethical to let the comparator group of rabbits, whose corneas were infected with P. aeruginosa but left untreated, suffer needlessly. Third, given the limited scope of our study, it was not possible to perform tissue histology, immunohistochemistry or conduct investigations into the molecular mechanisms of anti-inflammatory and anti-ulcerative action for iCAP. These limitations will be addressed in future investigations where we intend to greatly expand the scope of the study presented here.
5. Conclusions
In conclusion, for the first time, it has been shown, at least preliminarily, that our iCAP device has the potential to exert a powerful and sustained clinical impact on the effective treatment of Pseudomonas keratitis. Further optimization in the design, reliability, operational parameters and cost of our iCAP device may pave the way towards widespread clinical adoption of this seemingly powerful therapeutic modality to address ulcerative keratitis of infectious origin. Long term safety and efficacy studies will be crucially important in positioning our iCAP device for widespread patient and caregiver acceptance and utilization. In addition, comprehensive studies on the mechanism of action will be necessary to position our iCAP device for regulatory clearance and commercial insertion into ophthalmologic practice.
Supplementary Material
Acknowledgements
We thank Dr. Christi Parham, Dr. Alejandra Gonzalez and Dr. Ritwik Ghosh for helpful technical discussions on the results of the in vitro and in vivo iCAP study presented herein. We also thank Dr. Ashwin Balasubramanian, Ryan McMillen, Seth Berry and Rebecca Valencia for helpful technical discussions and support on iCAP device assembly.
Source of funding/grants
We gratefully acknowledge financial support (Grant # 1R43EY026824-01) for this study from the National Institutes of Health/National Eye Institute.
Footnotes
Ethical Statement
The in vivo protocols utilized in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center (UMMC). This study is in full compliance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Disclosure
None.
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC,; Smith SD, Whitcher JP, Margolis TP, Wong IG, Epidemiology of ulcerative keratitis in northern California, Arch. Ophthalmol. 128 (2010) 1022–1028. [DOI] [PubMed] [Google Scholar]
- [2].Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, Beach MJ, Estimated burden of keratitis – United States, 2010, Morbidity Mortality Weekly Rep. 63 (2014) 1027–1030. [PMC free article] [PubMed] [Google Scholar]
- [3].Deorukhkar S, Katiyar R, Saini S, Epidemiological feature and laboratory results of bacterial and fungal keratitis: A five-year study at a rural tertiary-care hospital in western Maharashtra, India, Singapore Med. J. 53 (2012) 264–267. [PubMed] [Google Scholar]
- [4].Green MD, Apel AJG,; Naduvilath T, Stapleton FJ, Clinical outcomes of keratitis, Clin. Exp. Ophthalmol. 35 (2007) 421–426. [DOI] [PubMed] [Google Scholar]
- [5].Laspina F, Samudio M, Cibils D, Ta CN, Fariña N, Sanabria R, Klauss V, de Kaspa H. Miño, Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: A 13-year survey in Paraguay, Graefes Arch. Clin. Exp. Ophthalmol. 242 (2004) 204–209. [DOI] [PubMed] [Google Scholar]
- [6].Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y, Bacterial keratitis: A prospective clinical and microbiological study, Br. J. Ophthalmol. 85 (2001) 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Varaprasathan G, Miller K, Lietman T, Whitcher JP, Cevallos V, Okumoto M, Margolis TP, Yinghui M, Cunningham ET, Trends in the etiology of infectious corneal ulcers at the F.I. Proctor foundation, Cornea 23 (2004) 360–364. [DOI] [PubMed] [Google Scholar]
- [8].Parmar P, Salman A, Kalavathy CM, Jesudasan CAN, Thomas PA, Pneumococcal keratitis: A clinical profile, Clin. Exp. Ophthalmol. 31 (2003) 44–47. [DOI] [PubMed] [Google Scholar]
- [9].Yildiz EH, Airiani S, Hammersmith KM, Rapuano CJ, Laibson PR, Virdi AS, Hongyok T, Cohen EJ, Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea 31 (2012) 1097–1102. [DOI] [PubMed] [Google Scholar]
- [10].Henry CR, Flynn HW Jr, Miller D, Forster RK, Alfonso EC, Infectious keratitis progressing to endophthalmitis: A 15-year study of microbiology, associated factors, and clinical outcomes, Ophthalmology 119 (2012) 2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].O’Neill EC, Yeoh J, Fabinyi DC, Cassidy D, Vajpayee RB, Allen P, Connell PP, Risk factors, microbial profiles and prognosis of microbial keratitis-associated endophthalmitis in high-risk areas, Graefes Arch. Clin. Exp. Ophthalmol. 252 (2014) 1457–1362. [DOI] [PubMed] [Google Scholar]
- [12].Zarei-Ghanavati S, Baghdasaryan E, Ramirez-Miranda A, Nguyen M, Yu F, Lee GJ, Deng SX, Elevated intraocular pressure is a common complication during active microbial keratitis, Am. J. Ophthalmol. 152 (2011) 575–581. [DOI] [PubMed] [Google Scholar]
- [13].Hoddenbach JG, Boekhoorn SS, Wubbels R, Vreugdenhil W, Van Rooij J, Geerards AJ, Clinical presentation and morbidity of contact lens-associated microbial keratitis: a retrospective study.” Graefes Arch. Clin. Exp. Ophthalmol. 252 (2014) 299–306. [DOI] [PubMed] [Google Scholar]
- [14].Eby AM, Hazlett LH, Pseudomonas keratitis, a review of where we’ve been and what lies ahead, J. Microb. Biochem. Tech. 8 (2016) 9–13. [Google Scholar]
- [15].Willcox MD, Management and treatment of contact lens-related Pseudomonas keratitis, Clin. Ophthalmol. 6 (2012) 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Robertson DM, Petroll WM, Jester JV, Cavanagh HD, The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: an overview, Eye Cont. Lens 33 (2007) 394–398; discussion 399–400. [DOI] [PubMed] [Google Scholar]
- [17].Robertson DM, Petroll WM, Jester JV, Cavanagh HD, Current concepts: contact lens related Pseudomonas keratitis, Cont. Lens Anterior Eye 30 (2007) 94–107. [DOI] [PubMed] [Google Scholar]
- [18].Fleiszig SM, Evans DJ, The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa, Clin. Exp. Optom. 85 (2002) 271–278. [DOI] [PubMed] [Google Scholar]
- [19].Fleiszig SM, The Glenn A. Fry award lecture 2005. The pathogenesis of contact lensrelated keratitis, Optom. Vis. Sci. 83 (2006) 866–873. [DOI] [PubMed] [Google Scholar]
- [20].Bruinsma GM, Rustema–Abbing M, de Vries J, et al. , Influence of wear and overwear on surface properties of etafilcon A contact lenses and adhesion of Pseudomonas aeruginosa, Invest. Ophthalmol. Vis. Sci. 43 (2002) 3646–3653. [PubMed] [Google Scholar]
- [21].Lyczak JB, Cannon CL, Pier GB, Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist, Microbes Infect. 2 (2000), 1051–1060. [DOI] [PubMed] [Google Scholar]
- [22].O’Callaghan RJ, Engel LS, Hobden JA, et al. , Pseudomonas keratitis: the role of an uncharacterized exoprotein, protease IV, in corneal virulence, Invest. Ophthalmol. Vis. Sci. 37 (1996) 534–543. [PubMed] [Google Scholar]
- [23].Mohammadpour M, Mohajernezhadfard Z, Khodabande A, Vahedi P, Antibiotic susceptibility patterns of pseudomonas corneal ulcers in contact lens wearers, Middle East Afr. J. Opthalmol. 18 (2011) 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK, Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment, SQU Med. J. 9 (2009) 184–195. [PMC free article] [PubMed] [Google Scholar]
- [25].Fridman A, Plasma Chemistry, 1st ed., Cambridge University Press, New York, 2008. [Google Scholar]
- [26].Parkey J, Cross J, Hayes R, Parham C, Staack D, Sharma AC, A battery powered, portable, and self-contained non-thermal helium plasma jet device for point-of-injury burn wound treatment, Plasma Processes Polym. 12 (2015) 1244–1255. [Google Scholar]
- [27].Laroussi M, Tendero C, Lu X, Alla S, Hynes WL, Inactivation of bacteria by the plasma pencil, Plasma Processes Polym. 3 (2006) 470–473. [Google Scholar]
- [28].Schütze A, Jeong JY, Babayan SE, Park J, Selwyn GS, Hicks RF, The atmospheric-pressure plasma jet: a review and comparison to other plasma sources, IEEE Trans. Plasma Sci. 26 (1998) 1685–1694. [Google Scholar]
- [29].Wu X-Q, Wang S-G, Han L, et al. , Sterilizing effect of atmospheric pressure plasma jet on microbes, Wei Sheng wu xue bao 45 (2005) 312–314. [PubMed] [Google Scholar]
- [30].Park BJ, Lee D, Park J-C, et al. , Sterilization using a microwave-induced argon plasma system at atmospheric pressure, Phys. Plasmas 10 (2003) 4539–4544. [Google Scholar]
- [31].Kvam E, Davis B, Mondello F, Garner AL, Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage, Antimicrob. Agents Chemother. 56 (2012) 2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matthes R, Assadian O, Kramer A, Repeated applications of cold atmospheric pressure plasma does not induce resistance in Staphylococcus aureus embedded in biofilms, GMS Hyg. Infect. Control 9 (2014) Doc17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brun P, Brun P, Vono M, Venier P, Tarricone E, Deligianni V, et al. , Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma, PLoS One 7 (2012) e33245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rosani U, Tarricone E, Venier P, Brun P, Deligianni V, Zuin M, et al. , Atmospheric-pressure cold plasma induces transcriptional changes in ex vivo human corneas, PLoS One 10 (2015) e0133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alhabshan R, Belyea D, Stepp MA, Barratt J, Grewal S, Shashurin A, Keidar M, Effects of in-vivo application of cold atmospheric plasma on corneal wound healing in New Zealand white rabbits, Int. J. Opthalmic. Pathol. 2 (2013) 1000118. [Google Scholar]
- [36].Nikmaram H, Kanavi MR, Ghoranneviss M, Balagholi S, Ahmadieh H, Roshandel D, Amini M, Cold atmospheric pressure plasma jet for the treatment of Aspergillus keratitis, Clin. Plasma Med. 9 (2019) 14–18. [Google Scholar]
- [37].Sliney DH, Ultraviolet radiation effects upon the eye: Problems of dosimetry, Rad. Protec. Dosimet. 72 (1997) 197–206. [Google Scholar]
- [38].Nejat F, Nabavi N-S, Nejat M-A, Aghamollaei KJ, Safety evaluation of the plasma on ocular surface tissue: An animal study and histopathological findings, Clin. Plasma Med. 14 (2019) 1000084. [Google Scholar]
- [39].Lakhundi S, Siddiqui R, Khan NA, Pathogenesis of microbial keratitis, Microbial Pathogenesis 104 (2017) 97–109. [DOI] [PubMed] [Google Scholar]
- [40].Thibodeaux BA, Caballero AR, Marquart ME, Tommassen J, O’Callaghan RJ, Corneal virulence of Pseudomonas aeruginosa elastase B and alkaline protease produced by Pseudomonas putida, Curr. Eye Res. 32 (2007) 373–386. [DOI] [PubMed] [Google Scholar]
- [41].Marquart ME, Monds KS, McCormick CC, Dixon SN, Sanders ME, Reed JM, McDaniel LS, Caballero AR, O’Callaghan RJ, Cholesterol as treatment for pneumococcal keratitis: cholesterol-specific inhibition of pneumolysin in the cornea, Invest. Ophthalmol. Vis. Sci. 48 (2007) 2661 – 2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marquart ME, Caballero AR, Chomnawang M, Thibodeaux BA, Twining SS, O’Callaghan RJ, Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions, Invest. Ophthalmol. Vis. Sci. 46 (2005) 3761–3768. [DOI] [PubMed] [Google Scholar]
- [43].Reed JM, O’Callaghan RJ, Girgis DO, McCormick CC, Caballero AR, Marquart ME, Ocular virulence of capsule-deficient Streptococcus Pneumoniae in a rabbit keratitis model, Invest. Ophthalmol. Vis. Sci. 46 (2005) 604–608. [DOI] [PubMed] [Google Scholar]
- [44].Marquart ME, O’Callaghan RJ, Infectious keratitis: secreted bacterial proteins that mediate corneal damage, J. Opthalmol. 2013 (2013) 369094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Panjwani N, Pathogenesis of Acanthamoeba keratitis, Ocul Surf. 8 (2010) 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McCormick C, Caballero A, Tang A, Balzli C, Song J, O’Callaghan R, Effectiveness of a new tobramycin (0.3%) and dexamethasone (0.05%) formulation in the treatment of experimental Pseudomonas keratitis, Curr. Med. Res. Opin. 6 (2008) 1569–1575. [DOI] [PubMed] [Google Scholar]
- [47].Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A, Vasilets VN, Ayan H, Friedman G, Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria, 4 (2007) 370–375. [Google Scholar]
- [48].Scholtz V, Pazlarova J, Souskova H, Khun J, Julak J, Nonthermal plasma – A tool for decontamination and disinfection, Biotech. Advances 33 (2015) 1108–1119. [DOI] [PubMed] [Google Scholar]
- [49].Akishev Y, Grushin M, Karalnik V, Trushkin N, Kholodenko V, Chugunov V, et al. , Atmospheric-pressure, nonthermal plasma sterilization of microorganisms in liquids and on surfaces, Pure Appl. Chem. 80 (2008) 1953–1969. [Google Scholar]
- [50].Soušková H, Scholtz V, Julák J, Kommová L, Savická D, Pazlarová J, The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge, Folia Microbiol. 56 (2011) 77 – 79. [DOI] [PubMed] [Google Scholar]
- [51].Daeschlein G, Scholz S, Von Woedtke T, Niggemeier M, Kindel E, Brandenburg R, et al. , In vitro killing of clinical fungal strains by low-temperature atmospheric-pressure plasma jet, IEEE Trans. Plasma. Sci. 39 (2011) 815 – 821. [Google Scholar]
- [52].Ehlbeck J, Schnabel U, Polak M, Winter J, Von Woedtke T, Brandenburg R, et al. , Low temperature atmospheric pressure plasma sources for microbial decontamination, J. Phys. D Appl. Phys. 44 (2011) 013002. [Google Scholar]
- [53].Yasuda H, Miura T, Kurita H, Takashima K, Mizuno A, Biological evaluation of DNAdamage in bacteriophages inactivated by atmospheric pressure cold plasma, Plasma Processes Polym. 7 (2010) 301–308. [Google Scholar]
- [54].Alshraiedeh N, Alkawareek M, Gorman S, Graham W, Gilmore B, Atmospheric pressure, nonthermal plasma inactivation of MS2 bacteriophage: effect of oxygen concentration on virucidal activity J. Appl. Microbiol. 115 (2013) 1420–1426. [DOI] [PubMed] [Google Scholar]
- [55].Ermolaeva SA, Varfolomeev AF, Y Chernukha M, Yurov DS, Vasiliev MM, Kaminskaya AA, et al. , Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds, J. Med. Microbiol. 60, 2011, 75–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


