Abstract
Combination therapy for treatment of multi-drug resistant bacterial infections is becoming common. In vitro testing of drug combinations under realistic pharmacokinetic conditions is needed before a corresponding combination is eventually put into clinical use. The current standard for design of such in vitro simulations for drugs with different half-lives is heuristic and limited to two drugs. To address that void, we develop a rigorous design method suitable for an arbitrary number of N drugs with different half-lives. The method developed offers substantial flexibility and produces novel designs even for two drugs. Explicit design equations are rigorously developed and are suitable for immediate use by experimenters. These equations were used in experimental verification using a combination of three antibiotics with distinctly different half-lives. In addition to antibiotics, the method is applicable to any anti-infective or anti-cancer drugs with distinct elimination pharmacokinetics.
Keywords: Mathematical modelling, In vitro simulation, Pharmacokinetics, Combination therapy, Multi-drug resistance
1. Introduction
Treatment of challenging bacterial infections by simultaneous use of two or more antimicrobial agents, known as combination therapy, has become common in recent years (Tamma et al. 2012, Tängdén 2014, Karakonstantis et al. 2020) as multi-drug resistant bacteria become increasingly prevalent (Jain et al. 2003, Saeed et al. 2007, Pop et al. 2008, Siwakoti et al. 2018, Wang et al. 2019). Whether combination of certain agents has synergistic or antagonistic therapeutic effects must be realistically assessed before use, for the safety and successful treatment of patients (Tamma et al. 2012, Tängdén 2014, Brochado et al. 2018). In vitro testing of the effectiveness of agent combinations under realistic pharmacokinetic conditions is useful before a corresponding combination is eventually put into clinical use (Layeux et al. 2010, Doern 2014, Karakonstantis et al. 2020).
To simulate in vitro the pharmacokinetics of two agents with distinct elimination half-lives, Blaser (1985) proposed a design method that has long been the standard for an experimental set-up which allows the concentration of each of the two agents in a common solution to follow its own exponential decline over time, with a corresponding half-life. While immediately useful for a combination of two agents, Blaser’s results cannot be used for combinations of three or more agents, a situation that is becoming increasingly more common with multi-drug resistant bacteria (Tamma et al. 2012, Tängdén 2014, Karakonstantis et al. 2020). Indeed, improved therapeutic effects have been observed from combination of ertapenem, meropenem, and colistin compared to double carbapenem alone (Karakonstantis et al. 2020), as well as from combining up to three of the following antibiotics: an aminoglycoside, an anti-pseudomonal beta-lactam, colistin, a fluoroquinolone, a macrolide, or rifampin (Tängdén 2014).
Given the growing importance of combination therapy entailing three or more agents, this paper presents the development and experimental validation of a general method for designing in vitro simulations of the pharmacokinetics of an arbitrary number, N, of antimicrobial agents in combination, each agent with its own distinct elimination half-life. Formulas are developed for the design of an in vitro simulation system that extends Blaser’s configuration and offer substantially higher flexibility even for the case of two agents. In addition, a novel configuration with some advantageous features and corresponding design formulas are developed.
In the rest of the paper we first present relevant theoretical background summarizing experiment design for in vitro simulation of the pharmacokinetics of two agents. Subsequently, in Materials and Methods we present the theoretical and experimental framework within which we develop and test the proposed experiment design method for N ≥ 3 agents. The results of the proposed approach are presented next in the form of explicit design equations. The design approach is illustrated experimentally on a hollow-fiber system (Bao et al. 1999, De Bartolo et al. 2009, Cadwell 2017). A discussion of the obtained theoretical and experimental results follows. Finally, conclusions are drawn and recommendations for further development are made.
2. Theoretical
2.1. Design of experiments for in vitro simulation of distinct pharmacokinetics for two agents in combination
While in vivo bacterial response to antimicrobial agents can be assessed in animal infection models, there are uncertainty and risk when translating data from experiments on animals to human treatment, due to differences between animals and humans in agent pharmacokinetics (Gloede et al. 2010, Tamma et al. 2012, Doern 2014). To address this issue through in vitro simulation, a hollow-fiber (HF) system (Fig. 1) can be used to assess the response of bacterial populations exposed to antimicrobial agents using clinically relevant dosing regimens, commensurate with agent pharmacokinetics in humans (Blaser et al. 1985, Bulitta et al. 2019). Bacteria are exposed to nutrients and agents but are restrained from leaving the hollow fiber cartridge.
Fig. 1.
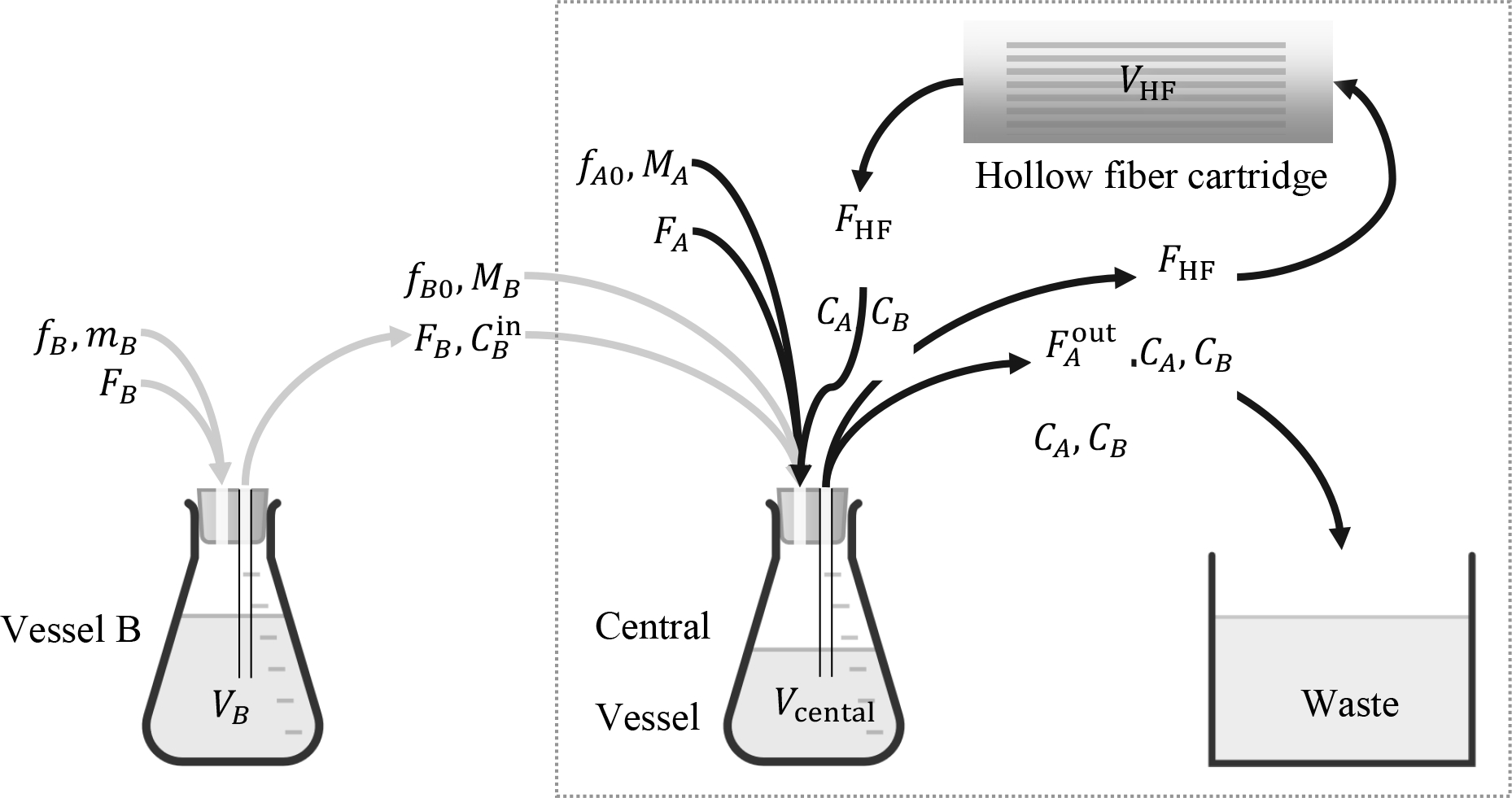
A hollow-fiber system for in vitro simulation of clinically relevant pharmacokinetics of antimicrobial agents. The system inside the dotted frame is suitable for a single agent, A; addition of vessel B in series with the central vessel renders it suitable for two agents, A, B. Volumetric flow rates FA, FB entering vessels B and central are sterile growth media. Mass flow rates fB, fB0, and fA0 refer to bolus injections mB, MB, MA of corresponding agents. With appropriate selection of (Blaser 1985), the entire system is suitable for handling simultaneously two agents, A and B, each following its own elimination pharmacokinetics as they go through the hollow-fiber cartridge. For corresponding j, the notation is as follows:
Fj = volumetric flow rate containing agent j = A, B.
FHF = internal circulation flow rate through the hollow fiber cartridge.
= volumetic flow rate from the central vessel to waste.
fj = volumetric flow rate of injection into vessel j containing agent j = B (Fig. 3).
fj0 = volumetric flow rate of injection into the central vessel, j = A, B.
Vj = liquid volume in vessel j = B.
Vcentral = liquid volume in central vessel.
VHF = liquid volume in hollow fiber cartridge.
Cj = concentration of agent j = A, B.
= concentration of agent j = B into the central vessel.
mj = mass of agent j = B in bolus injection to vessel j = B.
Mj = mass of agent j = A, B in bolus injection to central vessel.
Design of a hollow-fiber system for one or two agents, as shown in Fig. 1, refers to selecting the agent and broth flow rates as well as liquid volumes in each vessel, such that the profiles of CA(t) or {CA(t), CB(t)}, for one or two agents respectively, follow prescribed pharmacokinetics. Such pharmacokinetics typically corresponds to exponential decline of agent concentration over time after an initial peak, as captured by the following equation:
| (1) |
over time 0 ≤ t < P where kj is the elimination rate constant of agent j; Tj is the elimination time constant of agent j; is the elimination half-life of agent j; j = A or j = A, B for one or two agents, respectively. As explained by Blaser (Blaser 1985, Blaser et al. 1985) regarding the configuration shown in Fig. 1, the exponentially declining concentration profiles in eqn. (1) result from impulse bolus injections into two vessels rather than into one, as a single vessel, with a single time constant equal to liquid volume over total flow rate, would be inadequate for creating distinct concentration profiles for two agents with different half-lives. Note that the period, P between two successive injections is assumed by Blaser (1985) to be large enough to ensure that Cj(P) ≈ 0 at the time immediately before each new injection, as shown in Fig. 2.
Fig. 2.

Pharmacokinetic profile corresponding to periodic injections of an antimicrobial agent at times nP, n = 0, 1, 2, …, with subsequent exponential decline of agent concentration as shown in eqn. (1)).
A summary of the design procedure for the hollow-fiber system of Fig. 1, accommodating either one or two agents, is shown in Table 1 (input data to the design) and Table 2 (output data of the design).
Table 1.
Input data in the design procedure for the hollow-fiber system in Fig. 1 with one or two antimicrobial agents, following Blaser (Blaser 1985, Blaser et al. 1985)
| Parameter description | Parameter name | Agent, j |
|---|---|---|
| Half-life of antimicrobial agent j | A or {A, B} | |
| Period between two successive injections | P j | A or {A, B} |
| Peak concentration of antimicrobial agent j in the central vessel at injection time | A or {A, B} | |
| Liquid volume in combined central vessel and hollow-fiber cartridge |
Table 2.
Summary of design procedure given data in Table 1 for the hollow-fiber system in Fig. 1 with one or two antimicrobial agents, following Blaser (Blaser 1985, Blaser et al. 1985).
| Parameter description | Parameter name and design equation | Agent, j |
|---|---|---|
| Total volumetric flow rate out of central vessel, | ||
| Volumetric flow rate of B into and out of vessel B, FB |
FB, FA, VB are non-zero solutions of FB=kBVB |
|
| Volumetric flow rate of A into central vessel, FA | ||
| Liquid volume in vessel B, VB | ||
| Mass, Mj, of impulse bolus injection into the central vessel, approximated by a pulse of short duration Δt (Fig. 3) | A or {A, B} | |
| Mass, mB, of impulse bolus injection into the vessel B, approximated by a pulse of short duration Δt (Fig. 3) |
Note that fj0(t), j = A, B, and fB(t), fB0(t) in Fig. 1 refer to corresponding mass flow rate approximation of impulses by pulses of short duration Δt (Fig. 3) and of corresponding amplitudes, for appropriate bolus injections as follows:
| (2) |
| (3) |
where ϕ denotes pulse amplitude, as shown in Fig. 3, with specific ϕA0, ϕB0, ϕB referring to Fig. 1. These bolus injections may occur periodically at times nP, n = 0, 1, 2, …, in correspondence with Fig. 2.
Fig. 3.

Mass flow rate f(t) approximating a bolus impulse of mass m or M by a pulse of high amplitude ϕ and short duration Δt.
Finally, note that in the case of two agents, A, B, the agent with the smaller half-life must be accommodated in the central vessel, i.e. the experimental set-up should make certain that
| (4) |
because the formulas in Table 2 immediately lead to
| (5) |
which must be positive, thus implying the requirement in eqn. (4).
As already mentioned, the above method, summarized in Table 1 and Table 2, is suitable for up to two antimicrobial agents. In the rest of the paper we present the development of a general method that is suitable for an arbitrary number of agents, N, while also relaxing some of the restrictions placed in (Blaser 1985) and thus offering a continuum of novel designs, even for two agents.
3. Materials and Methods
3.1. Set-up for in vitro simulation of distinct pharmacokinetics for N agents
As Blaser (1985) argued, for the case of two agents, a vessel B in series with the central vessel must be included (Fig. 1) for an agent B to exhibit a concentration profile distinct from A as it goes through the hollow-fiber cartridge. Because Blaser’s design is limited to two agents, we develop here the following two new designs, suitable for any number of agents:
Starting with Fig. 1, which is suitable for two agents, Fig. 4 shows a configuration of a hollow-fiber system capable of handling three agents, A, B, C. In that configuration, a vessel for agent C is used in series with (i.e. feeding to) the central vessel, in the same fashion as vessel B. Four or more agents can be similarly handled by adding more vessels, all feeding to the central vessel. The central vessel in the configuration of Fig. 4 remains dedicated to handling agent A, through direct injection of bolus MA and broth inlet flow rate FA
Departing from the in-series configuration, we propose the novel experiment configuration shown in Fig. 5. In that configuration, injections of A, B, C are used into three corresponding vessels in parallel to each other. The outlet streams of these vessels are fed to the central vessel. Separate injections of A, B, C take place into the central vessel as well. We will demonstrate in the Results section that this configuration has some interesting properties that distinguish it from the in-series design.
Fig. 4.
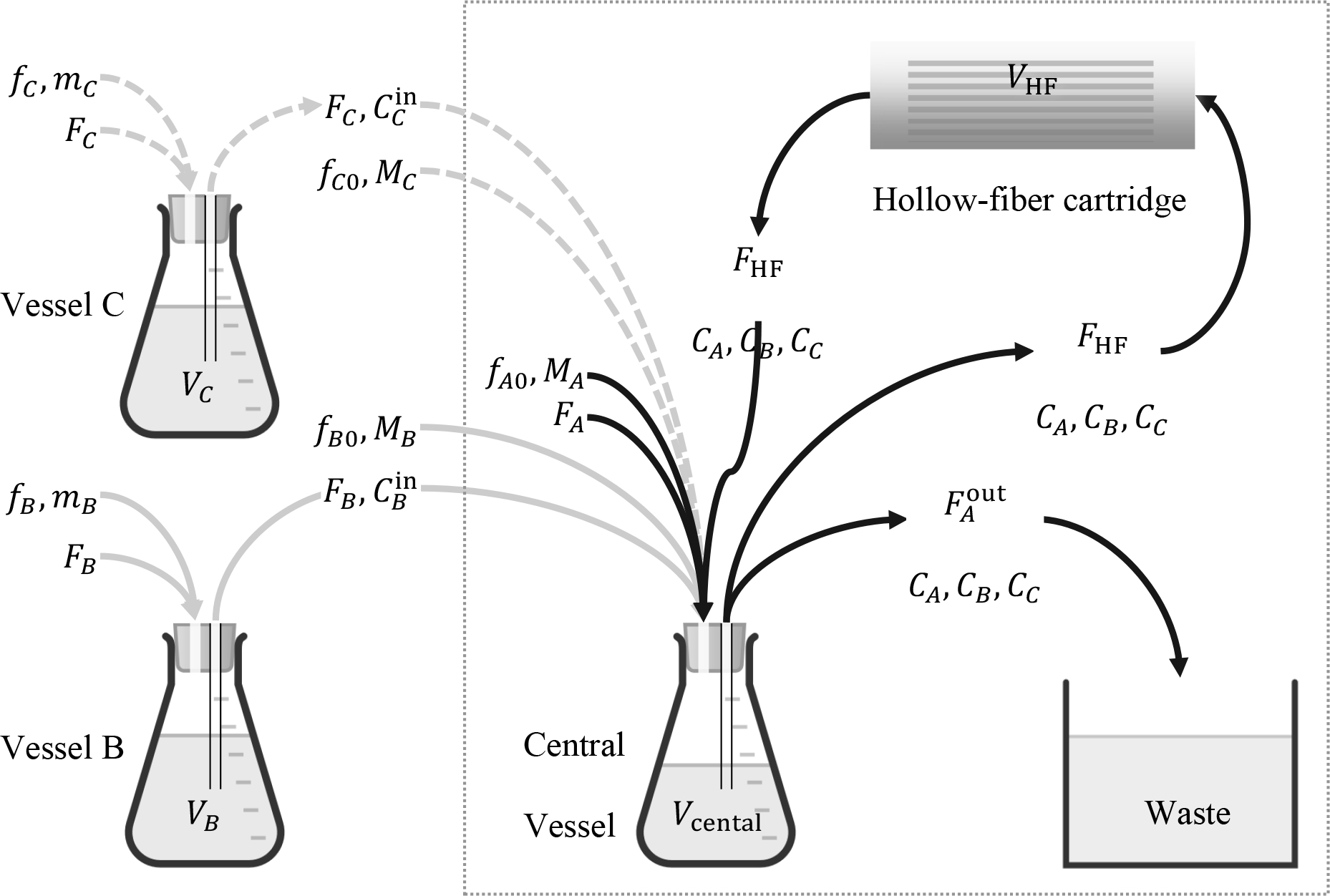
An in-series hollow-fiber system for in vitro simulation of three antimicrobial agents, A, B, C, each following its own elimination pharmacokinetics as it goes through the hollow-fiber cartridge. The notation followed is similar to Fig. 1.
Fig. 5.

An in-parallel hollow-fiber system for in vitro simulation of three antimicrobial agents, A, B, C, each following its own elimination pharmacokinetics as it goes through the hollow-fiber cartridge.
3.2. Ensuring well-mixed conditions for the central vessel / hollow-fiber cartridge
It is noted that the flow rate FHF of the fluid circulating out of the central vessel through the hollow-fiber cartridge and back is assumed to be high enough to ensure that the system comprising the central vessel and hollow-fiber cartridge is well mixed with uniform concentration of each agent throughout. This ensures that all bacteria are exposed to approximately the same concentrations of agents at any time t A quantitative criterion that determines the range of values of FHF that are high enough to ensure well-mixed conditions is developed in the Results section.
3.3. Design tasks for in-series and in-parallel configurations
The in-series and in-parallel configurations shown in Fig. 4 and Fig. 5, respectively, require selection of corresponding growth media and agent flow rates as well as liquid volumes in each vessel, such that they can generate exponentially decaying concentrations Cj(t) with different half-lives, t1/2j, j = A, B, C, … (eqn. (1)). The settings for the corresponding design problems are shown in Table 3 and Table 4. Formulas for completing the system design are subject to analysis. The results of such analysis are presented in the Results section.
Table 3.
Input data in the design procedure for the in-series hollow-fiber system in Fig. 4 with three (or more) antimicrobial agents
| Parameter description | Parameter name | Agent, j |
|---|---|---|
| Half-life of antimicrobial agent j | A, B, C, … | |
| Period between two successive injections | P j | A, B, C, … |
| Peak concentration of antimicrobial agent j in the central vessel at injection time | A, B, C, … | |
| Liquid volume in each vessel | V j | A, B, C, … |
| Initial conditions in central vessel | Cj(0) | A, B, C, … |
Table 4.
Input data in the design procedure for the in-parallel hollow-fiber system in Fig. 5 with three antimicrobial agents
| Parameter description | Parameter name | Agent, j |
|---|---|---|
| Half-life of antimicrobial agent j | A, B, C, … | |
| Period between two successive injections | P j | A, B, C, … |
| Peak concentration of antimicrobial agent j in the central vessel at injection time | A, B, C, … | |
| Liquid volume in all vessels | V j | A, B, C, … |
| Liquid volume in combined central vessel and hollow-fiber cartridge | A, B, C, … | |
| Initial conditions in central vessel | Cj(0) | A, B, C, … |
3.4. Mathematical model for in-series hollow-fiber system
Assuming constant volumes VB, VC, … and negligible flow rate for fj compared to Fj mass balance on agent j, j = B, C, … around each corresponding vessel in Fig. 4 yields
| (6) |
where j = B, C, ….
Similarly, mass balance on all agents j = A, B, C, … around the combined central vessel of effective volume
| (7) |
yields
| (8) |
| (9) |
where j = B, C, ….
| (10) |
and the effective volume of the central vessel along with the hollow fiber cartridge and the connecting tubes, is assumed to be a well mixed system for high enough circulation rate FHF, as we show rigorously in APPENDIX A.
The design task entails use of the above mathematical model along with the input data in Table 3 to determine the functions fj0(t), j = A, B, C, …; the functions fj(t), j = A, B, C, …; and values for the parameters Fj, j = A, B, C, ….
3.5. Mathematical model for in-parallel hollow fiber system
Assuming constant volumes VA, VB, VC, … and negligible flow rate for fj compared to Fj, mass balance on agent j, j = A, B, C, … around each corresponding vessel in Fig. 5 yields eqn. (6) for j = A, B, C, …. Similarly, mass balance on all agents j = A, B, C, … around the combined central vessel yields
| (11) |
where j = A, B, C, … and
| (12) |
is the effective volume of the central vessel along with the hollow fiber cartridge and the connecting tubes, assumed to be a well mixed system for high enough circulation rate FHF, as we show rigorously in APPENDIX A.
The design task entails use of the above mathematical model along with the input data in Table 4 to determine the functions fj0(t), j = A, B, C, …; the functions fj(t), j = A, B, C, …; and values for the parameters Fj, j = A, B, C, ….
3.6. Laplace transforms
Development of the proposed experiment design method using the preceding mass balance equations along with the specifications of the design is greatly facilitated by use of Laplace transforms (Spiegel 1965) which turn all differential equations of the mathematical problem into algebraic equations that can be easily manipulated to produce the results sought.
3.7. Antimicrobial agents
Levofloxacin powder was acquired from Johnson and Johnson Pharmaceutical Research and Development, LLC. Ceftazidime powder were obtained from Chem-Impex International (Wood Dale, IL). Meropenem powder was purchased from TCI America (Portland, OR).A stock solution in sterile water has been prepared ahead of time and stored in −70°C. Prior to each experimental study, the antimicrobial agents were thawed and diluted to the appropriate concentrations.
3.8. Hollow fiber experiment
The target half-lives and maximum concentrations simulated for meropenem (Drusano and Hutchison 1995), ceftazidime (Leroy et al. 1984), and levofloxacin (Fish and Chow 1997) are shown in Table 5. Each drug was dosed at time 0 h. Meropenem and ceftazidime were dosed again at approximately 16 h. Each dose was given 30 minutes to ensure ample mixing in the system. Samples were taken at approximately 1, 2, 3, 4, 6, 8, 17, 18, 19, 20, 22 and 24 h. The liquid volume of the system comprising the central vessel and the hollow-fiber cartridge (eqn. (7)) was VA = 180 ml with Vcentral = 60 and VHF = 120 ml. The liquid volumes in vessels A and B as well as the injection periods PA, PB, PC are also shown in Table 5. The values of CA(0), CB(0), CC(0) were equal to zero. Antimicrobial agent concentrations in the samples were assayed using validated methods by liquid chromatography tandem mass spectroscopy (LC-MS/MS) (Tam et al. 2017, Zhou et al. 2017).
Table 5.
Values of pharmacokinetic parameters and vessel volumes for experiment design
| Agent, j | (h) | Vj (ml) | Pj (h) | |
|---|---|---|---|---|
| Meropenem (A) | 1 | 10 | 180 | 16 |
| Ceftazidime (B) | 2.5 | 10 | 270 | 16 |
| Levofloxacin (C) | 8 | 10 | 396 | 24 |
Note that the target elimination half-lives mimic human pharmacokinetics. An arbitrary Cmax was used as a proof-of concept. The Cmax observed in humans after clinical dosing can be achieved by a proportional adjustment of the drug amount to inject in the model.
4. Results
4.1. Circulation rates for the central vessel / hollow-fiber cartridge system to be well mixed
It is intuitively clear that a high circulation flow rate FHF from the central vessel through the hollow-fiber and back (Fig. 4) creates in effect a well mixed system comprising the central vessel and the hollow-fiber cartridge, with effective volume VA as shown in eqn. (7). However, it is not immediately obvious how high the flow rate FHF should be, to be high enough for reasonable application of the “well mixed” assumption. This question is addressed rigorously in APPENDIX A, where we show that values of FHF about 20–30 times the value of are practically sufficient. Derivation of this results employs the Lambert function, which has emerged to be of widespread applicability in engineering problems (Kesisoglou et al. 2021).
4.2. Design method for in vitro simulation of distinct pharmacokinetics for N agents combined: In-series configuration
Given the data in Table 3 and using the mass balances and Laplace transforms as described in Materials and Methods, we show in APPENDIX B that the functions fj0(t), j = A, B, C, … and fj(t), j = B, C, … can be selected to be impulses of corresponding magnitudes Mj and mj i.e.
| (13) |
and
| (14) |
Formulas for values of the parameters mj, j = B, C, …, and Mj, j = A, B, C, …, as well as for all flow rates Fj, j = A, B, C, …, are shown in Table 6. All proofs for the results in Table 6 are shown in APPENDIX B.
Table 6.
Summary of design procedure given data in Table 3 for the in-series hollow-fiber system in Fig. 4 with three or more antimicrobial agents
| Parameter description | Parameter name and design equation | Agent, j |
|---|---|---|
| Total volumetric flow rate out of central vessel, | ||
| Volumetric flow rates, Fj of j into and out of vessel j | Fj = Vj kj | B, C, … |
| Volumetric flow rate, FA of A into central vessel | ||
| Mass, Mj of bolus injected into the central vessel as pulse over short time Δt | A, B, C, … | |
| Mass, mj of bolus injected into the vessel j as pulse over short time Δt | B, C, … |
4.3. Design method for in vitro simulation of distinct pharmacokinetics for N agents combined: In-parallel configuration
Similarly, given the data in Table 4 and using the mass balances and Laplace transforms as described in Materials and Methods, we show in APPENDIX C that the functions fj0(t), j = A, B, C, … and fj(t), j = A, B, C, … can be selected to be impulses of corresponding magnitudes Mj and mj i.e.
| (15) |
| (16) |
Formulas for values of the parameters mj and Mj, j = A, B, C, …, as well as for all flow rates Fj, j = A, B, C, …, are shown in Table 7. All proofs for the results in Table 7 are shown in APPENDIX C.
Table 7.
Summary of design procedure given data in Table 4 for the in-parallel hollow-fiber system in Fig. 5 with three or more antimicrobial agents
| Parameter description | Parameter name and design equation | Agent, j |
|---|---|---|
| Volumetric flow rates, Fj, of agent j into and out of vessel j | Fj = Vj kj | A, B, C, … |
| Total flow rate out of central vessel, | ||
| Mass, Mj of bolus injection into the central vessel as pulse over short time Δt | A, B, C, … | |
| Mass, mj, of bolus injection into vessel j as pulse over short time Δt | A, B, C, … |
4.4. Experiment design resulting from application of developed in-series design method
Using design specifications as shown in Table 5 and with experiment parameters as discussed in the Hollow fiber experiment section, we obtained the following values (Table 8) of the design parameters (Table 6) involved in the in-series experiment configurations (Fig. 4) tested in our laboratory.
Table 8.
Values of design parameters for in-series configuration of experiment set-up
| Agent, j | Fj | Mj (μg) | mj (μg) | |
|---|---|---|---|---|
| Meropenem, A | 0.26 | 2.08 | 1800 | NA |
| Ceftazidime, B | 1.25 | 1800 | 2700 | |
| Levofloxacin, C | 0.57 | 1800 | 12600 |
4.5. Experiment outcomes
Measurements of agent concentration over time and corresponding model predictions are shown in Fig. 6 for the in-series configuration.
Fig. 6.

Measurements of concentrations Cj(t) for three antimicrobial agents and comparison with model predictions for the in-series experimental set-up shown in Fig. 4.
5. Discussion
5.1. Validation of design equations through experimental outcomes
The aim of the experiments performed was to validate the predictions of the design described in section 4.4, for three agents with significantly different half-lives. The experimental outcomes shown in Fig. 6 validate these predictions, as data points follow the concentration profiles anticipated by the design, with only small deviations. Note that levofloxacin measurements are more noisy than those of meropenem and ceftazidim. Whether there are systematic factors for this will be subject of future investigation.
5.2. Feasibility constraints of in-series design for N agents
Given that mj > 0, j = B, C, …, in Table 6, the design equation
| (17) |
immediately places the constraint
| (18) |
on the selection of agent A, among A, B, C,…, for the central vessel.
In addition, because FA > 0 the design equation
| (19) |
in Table 6 implies
| (20) |
Combined eqns. (18) and (20) define the feasible area for the design parameters of the in-series configuration.
Note that eqn. (18) is the generalization of eqn. (4), stated by Blaser (1985) for 2 agents.
5.3. Comparison of proposed in-series designs to prior design for two agents
When the in-series design for N agents, summarized in Table 6, is applied to N = 2 agents, it offers additional flexibility compared to the design summarized in Table 2, in a number of ways, as follows:
- While Blaser’s (1985) two-agent design (Table 2) requires that
the N-agent design in Table 6 can begin with any given set of volumes Vj (Table 3) as long as the constraints in eqns. (18) and (20) are satisfied. This adds an important degree of freedom to the proposed design. For two agents, A, B, it is interesting to visualize the feasible area of VA, VB given kA, kB; with kA > kB suggested by the N-agent design in Table 6, as shown in Fig. 7. That figure includes Blaser’s (1985) two-agent design (Table 2) which is based on eqn. (21).(21) The injection period, Pj, for each antimicrobial agent in all vessels does not have to be large enough to ensure that Cj(nP) ≈ 0, n = 0, 1, 2, …. Therefore, the values of Cj(0), j = A, B, C, …, are added to input data shown in Table 3.
- Unlike Blaser (1985), the in-series design in Table 6 does not require that
because there is flexibility in the ratios of vessel volumes. Of course, Cj(t) and Cin(t) share the same exponential decline rate kj, as explained in APPENDIX B. To help visualize the situation for two agents, Fig. 8 shows feasible values of the ratio
for feasible values of the ratio in (0, 1). That figure includes Blaser’s (1985) two-agent design (Table 2) which is a single horizontal line at . To visualize the situation in yet another way, Fig. 9 shows the profiles of both and CB(t) resulting from Blaser’s (1985) design in (Table 2) and from the in-series design for N = 2 in Table 6 for a few feasible design choices on VB satisfying (Fig. 7).
Fig. 7.

Feasible area (shaded, extending upwards) for given for the in-series design procedure of Table 6 applied to N = 2 agents. Note the design curve according to Blaser (Blaser 1985), following eqn. (21).
Fig. 8.

Feasible area (shaded, extending upawrds) for given for the in-series design procedure of Table 6 applied to N = 2 agents. Note the fixed value at 1 according to Blaser (Blaser 1985).
Fig. 9.

Profile of for the system in Fig. 1 with specifications VA = 100 ml, , ; , , and design parameters {FA, FB, VB, MA, MB, mB} computed according to Blaser (Blaser 1985) (Table 2) and Table 6 for 2 agents. The values of {MA, MB, mB} are {1000, 1000, 1500} μg for all designs.
We also note that in-parallel designs may result in profile differences between and Cj(t) in an entirely similar fashion.
5.4. Feasibility constraints and flexibility of in-parallel design for N agents
Given that mj > 0, j = A, B, C, …, in Table 7, the design equation
| (22) |
immediately places the constraint , j = A, B, C, …, which results in the design constraint
| (23) |
The great importance of eqn. (23) for the flexibility of the in-parallel method is that large enough volumes Vj can always be found, regardless of the values of kj, j = A, B, C, …, of V0 and of any preferable ratios of any one vessel volume over another. How large is large enough can be visualized in the case of two agents, for which eqn. (23) becomes
| (24) |
| (25) |
The feasible volume corresponding to eqns. (24) and (25) is shown in Fig. 10.
Fig. 10.

Feasible space for , , according to eqns. (24) and (25). Note that for the same plot can be used by swapping A with B, because of symmetry.
5.5. Sensitivity and robustness
A key approximation inherent in the configuration of the HF system studied is the well-mixed vessel assumption for high enough circulation flow rates (section 4.1). The experimental results shown in Fig. 6 validate this assumption, as the theoretically anticipated spikes in concentrations Cj at bolus injection times and subsequent exponential declines (continuous lines in Fig. 6) are closely followed by experimental data points in the same figure. A small lag that can be observed is due both to the approximation of bolus injection impulse by a short-duration pulse and to the circulation flow rate creating well-mixed but possibly not perfectly-mixed conditions.
The proposed design also proved robust to additional small deviations of experimental conditions from ideal, such as small discrepancies between inlet and outlet flow rates for each vessel – a result of pumping precision limitations – and small fluctuations of liquid volume in each vessel.
Application of the proposed design in future experimental studies would certainly provide valuable data to assess its range of applicability and robustness. Furthermore, because the proposed method can produce designs in a broad continuum, as illustrated by Fig. 7, Fig. 8, and Fig. 10, an open issue to be further investigated is which of these designs have the most appealing characteristics, both theoretically and in specific applications.
5.6. Extensions of the proposed method
The approach taken to develop the design equations presented in sections 4.2 and 4.3 is not confined to the system configurations shown or to the exponential decline pharmacokinetics considered, but could conceivably be extended to different configurations (e.g. multi-compartment studies) or to different pharmacokinetic patterns. While pertinent questions would have to be resolved in detail for such cases, this paper’s approach of writing dynamic mass balances and performing the design algebraically in the Laplace domain would be immediately applicable. This can be the subject of future investigations.
6. Conclusions
A general method was developed to design an in vitro model for simultaneous simulation of the kinetics of an arbitrary number of N drugs with different half-lives. The method developed entails two possible configurations: (a) An in-series configuration, which generalizes for N drugs Blaser’s two-drug design (Blaser 1985) and offers additional flexibility even for two drugs, and (b) an in-parallel configuration, which is new and offers different flexibility compared to the in-series configuration. Corresponding design equations were developed for sizing and operation of each configuration (Table 6 and Table 7). The in-series design equations were used for experimental verification using a combination of three antibiotics with distinctly different half-lives (meropenem, ceftazidime, and levofloxacin). Additional experiments in the future would further test the range of applicability of the proposed method. While experimental verification in this work involved antibiotics, the method is applicable to any anti-infective and anti-cancer drugs with distinct elimination pharmacokinetics. In addition, the approach to developing the proposed method lends itself to extensions for cases of different in vitro configurations (e.g. simulating multi-compartment systems) or different pharmacokinetic patterns. Finally notions of optimality among feasible designs can be investigated and optimal designs sought.
With increasing importance of in vitro simulation of the kinetics of an arbitrary number of drugs in combination, the methods developed here are an important new tool for the design of such in vitro models.
Highlights.
Development of a general method to design an in vitro model for simultaneous simulation of the kinetics of an arbitrary number of N drugs with different half-lives
The method developed entails two possible configurations: (a) An in-series configuration, and (b) an in-parallel configuration
Corresponding design equations for sizing and operation of each configuration are rigorously developed and are suitable for immediate use by experimenters
These equations were used for experimental verification using a combination of three antibiotics with distinctly different half-lives (meropenem, ceftazidime, and levofloxacin).
With increasing importance of in vitro simulation of the kinetics of an arbitrary number of drugs in combination, the methods developed here are an important new tool for the design of such in vitro models
Acknowledgments
The Institute of Allergy and Infectious Diseases of the National Institutes of Health under award (grant number R01AI140287) supported the research reported in this publication. 100% of the project costs were financed with Federal money. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding sources had no involvement in study design; in the collection, analysis and interpretation of data, nor in the writing of the report nor in the decision to submit the article for publication.
7. APPENDIX A. High circulation rate implies a well-mixed system
Mass balance for agent A around the central vessel (Fig. 4) immediately yields
| (-26) |
where
| (-27) |
is the time needed for liquid drawn from the central vessel to circulate in plug flow at rate FHF through the hollow-fiber cartridge volume VHF
Taking Laplace transforms of the above eqn. (-26) and solving for yields
| (-28) |
where tilde denotes the Laplace transform of a corresponding function of time. The above eqn. (-28) implies that CA(t) will include a weighted sum of exponentials of the form , where pn are the poles of namely the roots of the transcendental algebraic equation
| (-29) |
It can be shown that the solution of the above eqn. (-29) can be expressed in terms of the Lambert function as
| (-30) |
where
| (-31) |
and Wn(z) is the Lambert function of order n = −1, 0, 1, 2, …, defined as a solution of the algebraic equation xex = z Based on the properties of Lambert functions, it is straightforward to show that all pn have negative real part for n = −1, 0, 1, 2, …, therefore CA(t) remains bounded at all times. In addition, all pn are complex, except p0. More importantly, Fig. 11 shows that for comparable values of VHF and Vcentral, if r is high enough, the real parts of all complex-valued pn are much larger than the magnitude of p0 suggesting that the corresponding terms exp(pnt), n ≠ 0 will decay much faster than exp(p0t) so as to be negligible. Therefore, for high enough r, the effective volume of the combined system comprising the central vessel and the hollow-fiber cartridge will be
| (-32) |
as shown in Fig. 11.
Fig. 11.

Real and imaginary parts (top and bottom, respectively) of modes , n = −1, 0, 1, 2, …, in eqn. (-30) in terms of circulation ratio . Note that for r greater than about 30, the real part of qn, n = −1, 0, 1, 2, … suggests that the decay of all , n ≠ 0 is so much faster than the decay of as to be negligible. Furthermore, for r greater than about 30, the imaginary part of qn, n = −1, 0, 1, 2, … suggests that no appreciable oscillations are going to appear in , n ≠ 0 as all oscillation frequencies , n = −1, 1, 2, … are comparable to the exponential decay rates .
Similar arguments can be made for the agents B, C, … going through the hollow-fiber system shown in Fig. 4. Starting with mass balance
for agent j = B, C, … one can immediately get
| (-33) |
whose denominator is exactly the same as that of in eqn. (-28).
The proof for the system shown in Fig. 5 is entirely similar and omitted for brevity.
8. APPENDIX B. Proof of eqns. in Table 6
Agent A:
Taking Laplace transform of eqn. (8) yields
| (-34) |
Now, because the concentration of agent A flowing out the central vessel must follow eqn. (1) for j = A it follows that
| (-35) |
Comparison of the numerators in eqn. (-34) and (-35) immediately implies that
| (-36) |
which suggests that therefore fA0(t) must be an impulse of magnitude :
| (-37) |
where
| (-38) |
Similarly, comparison of the denominators in eqn. (-34) and (-35) immediately implies
| (-39) |
Agent j = B, C, ….
Following the same approach as above for agent j, j = B, C, …, one can take Laplace transform of eqn. (9) to get
| (-40) |
Because the concentration of agent j flowing out the central vessel must follow eqn. (1) for j = B, C, …, it follows that
| (-41) |
Comparison of eqns. (-40) and (-41) immediately implies that the term (s + kj) must be introduced into and the term eliminated from the denominator of the right side of eqn. (-40). To accomplish this, a simple and experimentally practical choice is to select
| (-42) |
| (-43) |
and to require that
| (-44) |
The exponential decline in eqn. (-42) implies that the appropriate profile of over time can be easily constructed by setting
| (-45) |
The values of the parameters αj and Mj, needed to complete the above design of and fj0, can be easily determined by matching like powers of s in eqn. (-44). This, combined with eqn. (-43) yields
| (-46) |
and combined with eqn. (-42) yields
| (-47) |
The above profile for can be easily created by the corresponding to an injection of bolus
| (-48) |
into vessel j = B, C, ….
9. APPENDIX C. Proof of eqns. in Table 7
Taking Laplace transform of eqn. (9) for the symbols used in Fig. 5 yields
| (A-1) |
Now, because the concentration of agent j flowing out the central vessel must follow eqn. (1) for j = A, B, C, …, it follows that
| (A-2) |
Comparison of eqns. (A-1) and (A-2) immediately implies that
| (A-3) |
In entirely similar fashion as in APPENDIX B, one gets
| (A-4) |
| (A-5) |
and
| (A-6) |
Footnotes
Conflict of interest
All the contributing authors have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10 References
- Bao L, Liu B and Lipscomb G (1999). “Entry mass transfer in axial flows through randomly packed fiber bundles.” AIChE J. 45(11): 2346–2356. [Google Scholar]
- Blaser J (1985). “In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives.” J. Antimicrob. Chemother: 125–130. [DOI] [PubMed] [Google Scholar]
- Blaser J, Stone B and Zinner S (1985). “Two compartment kinetic model with multiple artificial capilary units.” J. Antimicrob. Chemother 15: 131–137. [DOI] [PubMed] [Google Scholar]
- Brochado A, Telzerow A, Bobonis J, Banzhaf M, Mateus A, Selkrig J, Huth E, Bassler S, Beas J, Zietek M, Ng N, Foerster S, Ezraty B, Py B, Barras F and Savitski M (2018). “Species-specific activity of antibacterial drug combinations.” Nature: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL and Hoover JL (2019). “Generating Robust and Informative Nonclinical In Vitro and In Vivo Bacterial Infection Model Efficacy Data To Support Translation to Humans.” Antimicrobial Agents and Chemotherapy 63(5): e02307–02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell J (2017). “The hollow fiber infection model: Principles and practice.” Arch. Clin. Microbiol 08. [Google Scholar]
- De Bartolo L, Salerno S, Curcio E, Piscioneri A, Rende M, Morelli S, Tasselli F, Bader A and Drioli E (2009). “Human hepatocyte functions in a crossed hollow fiber membrane bioreactor.” J. Biomater 30(13): 2531–2543. [DOI] [PubMed] [Google Scholar]
- Doern CD (2014). “When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing.” J. Clin. Microbiol 52(12): 4124–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano GL and Hutchison M (1995). “The pharmacokinetics of meropenem.” Scandinavian journal of infectious diseases. Supplementum 96: 11–16. [PubMed] [Google Scholar]
- Fish DN and Chow AT (1997). “The Clinical Pharmacokinetics of Levofloxacin.” Clinical Pharmacokinetics 32(2): 101–119. [DOI] [PubMed] [Google Scholar]
- Gloede J, Scheerans C, Derendorf H and Kloft C (2010). “In vitro pharmacodynamic models to determine the effectof antibacterial drugs.” J. Antimicrob. Chemother 65: 186–201. [DOI] [PubMed] [Google Scholar]
- Jain A, Roy I, Gupta M, Kumar M and Agarwal S (2003). “Prevalence of extended-spectrum β-lactamase-producing Gram-negative bacteria in septicaemic neonates in a tertiary care hospital.” J. Med. Microbiol 52(5). [DOI] [PubMed] [Google Scholar]
- Karakonstantis S, Kritsotakis E and Gikas A (2020). “Pandrug-resistant Gram-negative bacteria: a systematic review of current epidemiology, prognosis and treatment options.” J. Antimicrob. Chemother 75(2): 271–282. [DOI] [PubMed] [Google Scholar]
- Kesisoglou I, Singh G and Nikolaou M (2021). “The Lambert function should be in the engineering mathematical toolbox.” Computers & Chemical Engineering 148: 107259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layeux B, Taccone F, Fagnoul D, Vincent J and Jacobs F (2010). “Amikacin Monotherapy for Sepsis Caused by Panresistant Pseudomonas aeruginosa.” Antimicrob. Agents Chemother 54(11): 4939–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A, Leguy F, Borsa F, Spencer GR, Fillastre JP and Humbert G (1984). “Pharmacokinetics of ceftazidime in normal and uremic subjects.” Antimicrobial Agents and Chemotherapy 25(5): 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, Mitchell S, Kandel R, Schreiber R and D’Agata E (2008). “Multidrug Resistant Gram Negative Bacteria in a Long Term Care Facility: Prevalence and Risk Factors.” J. Am. Geriatr. Soc 56(7). [DOI] [PubMed] [Google Scholar]
- Saeed S, Naim A and Tariq P (2007). “A study on prevalance of multi-drug-resistant gram negative bacteria.” Int. J. Biol. Biotech 4(1): 71–74. [Google Scholar]
- Siwakoti S, Subedi A, Sharma A, Baral R, Bhattarai N and Khanal B (2018). “Incidence and outcomes of multidrug-resistant gram-negative bacteria infections in intensive care unit from Nepal- a prospective cohort study.” Antimicrob. Resist. Infect. Control 7(114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel MR (1965). Laplace Transforms, McGraw-Hill. [Google Scholar]
- Tam VH, Chang K, Zhou J, Ledesma K, Phe K, Gao S, Bambeke F, Sánchez-Díaz A, Zamorano L, Oliver A and Cantón R (2017). “Determining β-lactam exposure threshold to suppress resistance development in Gram-negative bacteria.” J. Antimicrob. Chemother 72(5): 1421–1428. [DOI] [PubMed] [Google Scholar]
- Tamma P, Cosgrove S and Maragakis L (2012). “Combination therapy for treatment of infections with gram-negative bacteria.” J. Clin. Microbiol 25(3): 450–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tängdén T (2014). “Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria.” Upsala J. Med. Sci 119(2): 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wei H, Zhao Y, Shang L, Di L, Lyu C and Liu J (2019). “Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China.” Bosn. J. Basic. Med. Sci 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tran B and Tam V (2017). “The complexity of minocycline serum protein binding.” J. Antimicrob. Chemother 72(6): 1632–1634. [DOI] [PubMed] [Google Scholar]


