Introduction
Despite the availability of a range of novel therapeutics to treat patients with moderate to severe inflamamtory bowel disease (IBD), a significant proportion are either primary or secondary nonresponders to these therapeutics. Recently, there have been a number of reports demonstrating that dual biologic therapy (DBT) combining mechanistically different biologics may be a potential therapeutic option in this patient population.1–6 There is, however, a paucity of data on the effectiveness and safety of DBT in general, and particularly combining tofacitinib, an oral, small-molecule Janus Kinase (JAK) inhibitor, with a biologic in patients with refractory IBD.
Methods
We conducted a retrospective institutional review board–approved review of Crohn’s disease (CD) or ulcerative colitis (UC) patients who were prescribed Tofacitinib in combination with a Biologic Therapy (TBT) at 2 IBD referral centers in the United States (Washington University in St. Louis School of Medicine, St. Louis, Missouri, and Brooke Army Medical Center, Fort Sam Houston, Texas). The detailed study design has been previously described.7 Patients were included if they had at least 1 follow-up visit by week 16 and excluded if tofacitinib was added for an active extraintestinal manifestation.
Clinical response was assessed by the Harvey-Bradshaw Index (HBI) for CD, the Simple Clinical Colitis Activity Index (SCCAI) for UC, or physician global assessment (PGA) for patients with ostomy at weeks 8, 16, 26, 39, and 52 (+/-4 weeks) after initiation of TBT. Clinical response was defined as either a decrease in HBI ≥3 and SCCAI ≥3 from baseline, whereas clinical remission was defined as HBI ≤4 and SCCAI ≤2, retrospectively on chart review.8 Endoscopic and radiographic disease activity were noted within 1 year before TBT and within 1 year of follow-up. Endoscopic/radiographic response was defined as a ≥1 reduction in Mayo endoscopic subscore (UC) or >50% reduction in Simple Endoscopic Score for Crohn’s Disease (SES-CD) or ≥50% reduction in the global simplified magnetic resonance index of activity (MARIAs) score (CD); endoscopic/radiographic remission was Mayo subscore of 0 or 1 (UC), SES-CD of 0 to 2 or global MARIAs score of <6 (CD).9, 10
The primary effectiveness outcome was clinical response at week 8 (end of induction therapy). The secondary effectiveness outcomes were clinical remission and corticosteroid-free clinical response/remission at week 8; clinical response/remission at weeks 16, 26, and at last documented assessment (LDA); endoscopic/radiographic response or remission; and normalization of CRP. Adverse events (AEs) were defined as serious if life-threatening, resulting in hospitalization, disability, or discontinuation of therapy. Changes in lipid profile following TBT was also assessed. Surgical complications of abdominal surgery were graded using the Clavien-Dindo Classification and Comprehensive Complication Index.
Nonparametric continuous and categorical variables were compared using Wilcoxon rank-sum and Fisher exact tests, respectively. Statistical tests were 2-sided, and P value of <0.05 was considered statistically significant. Stata version 16.0 (StataCorp, College Station, TX) was used for all analyses.
Results
Thirty-five IBD patients (25 UC, 10 CD) were included in this study, with a median follow-up of 4 months (interquartile range [IQR], 2.6–5.9). All patients were started on TBT because of lack of response to their current biologic, despite dose optimization. Twenty-one patients (60%) had failed 2 or more biologics before starting TBT, and all patients received an induction dose of 10 mg of tofacitinib twice daily (BID). Two-thirds of patients (20 of 30, 66.7%) who went on maintenance tofacitinib therapy were on 5 mg BID or 11 mg extended release (XR) daily dosing. The biologics combined with tofacitinib were vedolizumab (VDZ-TBT, n = 24, 68.6%), infliximab (IFX-TBT, 6, 17.1%), and ustekinumab (UST-TBT, 5, 14.3%; Table 1).
TABLE 1.
Baseline Characteristics and Adverse Events
| Baseline Demographics and Clinical Characteristics | N = 35 | ||
|---|---|---|---|
| Age in years, median (IQR) | 32 (26–39) | ||
| Male, n (%) | 17 (48.6) | ||
| Race, n (%) | Non-Hispanic white | 23 (65.7) | |
| African American/black | 9 (25.7) | ||
| Hispanic | 1 (2.9) | ||
| Asians | 2 (5.7) | ||
| Body mass index kg/m2, median (IQR) | 24.9 (22–29) | ||
| Smoking Status, n (%) N = 34 | Never smoker | 26 (76.5) | |
| Past or Current Smoker | 8 (23.5) | ||
| IBD type, n (%) | Ulcerative colitis | 25 (71.6) | |
| Crohn’s disease | 10 (28.6) | ||
| UC extent, n (%) N = 25 | E1: proctitis | 6 (23.0) | |
| E2: left-sided or distal (till splenic Flexure) | 17 (69.0) | ||
| E3: extensive UC (proximal/splenic Flexure) | 1 (4.0) | ||
| Not reported | 1 (4.0) | ||
| CD Montreal location, n (%) N = 10 | Ileal | 1 (10.0) | |
| Colonic | 1 (10.0) | ||
| Ileocolonic | 8 (80.0) | ||
| CD Montreal Phenotype, n (%) N = 10 | Nonstricturing/ nonpenetrating | 5 (50.0) | |
| Stricturing | - | ||
| Penetrating | 5 (50.0) | ||
| Number of prior biologics, median (IQR) | 2 (1–3) | ||
| Number of prior biologics failed, n (%) | 1 | 14 (40.0) | |
| 2 | 11 (31.4) | ||
| ≥3 | 10 (28.6) | ||
| Induction dosing, n (%) | 10 mg bid | 35 (100) | |
| Maintenance dosing, n (%) N = 30 | 5mg BID or 11mg daily | 20 (66.7) | |
| 10mg BID | 10 (33.3) | ||
| Biologic combined with Tofacitinib, n (%) | Infliximab | 6 (17.1) | |
| Ustekinumab | 5 (14.3) | ||
| Vedolizumab | 24 (68.6) | ||
| Pre-TBT CRP (mg/dL), Median (IQR) | 1.35 (0.5–11.6) | ||
| Pre-TBT clinical scores, median (IQR) | HBI (N = 9) | 10 (9–11) | |
| SCCAI (24) | 9 (7.5–10) | ||
| Pre-TBT endoscopic/ radiographic scores, median (IQR) | Mayo endoscopic subscore (N = 22) | 3 (2–3) | |
| SES-CD (N = 4) | 13.5 (8.5–21) | ||
| Global MARIAs (N = 6) | 3 (2–3) | ||
| Concurrent corticosteroid usage at combination start, n (%) | 16 (45.7) | ||
| Concurrent immunomodulator usage at combination start, n (%) | 2 (5.7) | ||
| Duration of IBD before starting tofacitinib in years, median (IQR) | 9 (3–16) | ||
| Follow up in months, median (IQR) | 4.0 (2.6–5.9) | ||
| Total exposure, Patient-years of follow up (PYF) | 14.9 | ||
| Adverse Events | |||
| No. patients with an adverse event, n (%) | 2 (5.7) | ||
| No. patients with a serious adverse event, n (%) | 1 (2.9) | ||
| Number of days to developing AEs, median [Range] | 32 [25– 85] | ||
| Adverse events | IBD type | Biologic | No. days to developing AEs |
| Clostridium difficile colitisa,b | UC | Infliximab | 25 |
| Candida esophagitisa | UC | Infliximab | 85 |
| Rash | UC | Vedolizumab | 32 |
aSame patient developed Clostridium difficile colitis and candida esophagitis at different time points.
bCategorized as serious AE because this infection led to hospitalization while on TBT.
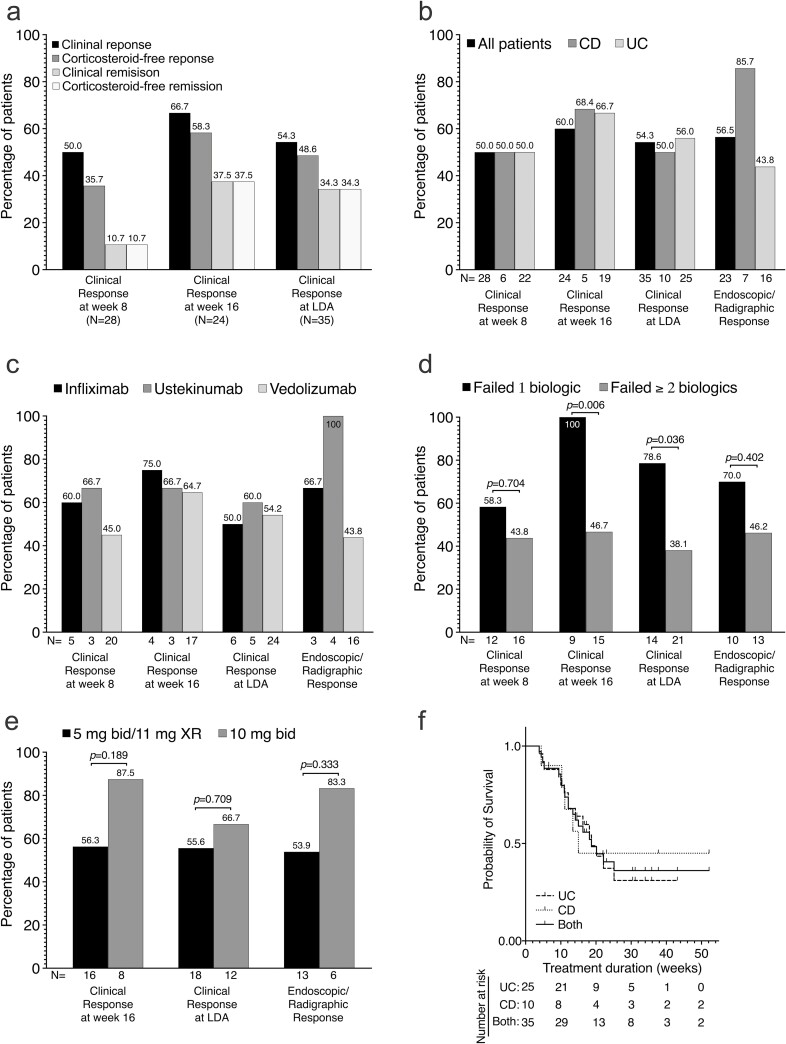
Twenty-eight patients had clinical assessments at week 8. Fourteen (50%) of these achieved clinical response at 8 weeks after TBT initiation, with 10 patients (35.7%) achieving corticosteroid-free clinical response and 3 patients (10.7%) in corticosteroid-free clinical remission (Fig. 1A). Clinical outcomes at week 16 and at last documented assessment are shown in Figure 1A. Of the 10 patients assessed at week 26, 9 (90%) achieved clinical response, with 7 (70%) in clinical remission.
FIGURE 1.
Effectiveness of tofacitinib plus biologic therapy in moderate to severe IBD—clinical, endoscopic/radiographic, and laboratory data. A, Bars showing the clinical response and remission, corticosteroid-free response and remission at week 8, week 16, and at last documented assessment. B and C, Bars showing the percentages of patients who had clinical response at week 8 and week 16, clinical response at last documented assessment, and endoscopic/ radiographic response (among those with abnormal endoscopy/radiography at baseline) while on TBT based on (B) type of IBD (CD vs UC) and (C) type of combinatorial therapy (IFX-TBT vs UST-TBT vs VDZ-TBT). N represents the total number of patients in each subgroup. D and E, Association between number of clinical and endoscopic outcomes and (D) number of prior failed biologics (1 biologic vs ≥2 biologics) and (E) maintenance dosing (5 mg BID/11 mg XR vs 10 mg BID). Bars represents proportion of patients in each outcome subgroup, and N represents the total number of patients in each subgroup. Proportions were compared using Fisher exact test, and P values are shown. F, Kaplan-Meier survivor curve of TBT persistence among UC (25), CD, (10) and all patients (both, 35) during the first year of treatment. Failure event was defined as withdrawal due to no response, loss of response, or adverse events. All patients still on TBT as at week 52 of treatment were censored. The median times on tofacitinib for UC, CD, and all patients (both) were 18.7, 15.0, and 18.7 weeks, respectively.
Pre- and on-TBT Mayo endoscopic subscores, SES-CD scores, and global MARIAs scores from images were available for 28 patients (21 UC, 7 CD). Of the 23 patients who had endoscopically or radiographically active disease at baseline, 13 (56.5%) achieved an endoscopic response, with 8 (34.8%) in endoscopic remission. Clinical and endoscopic response based on IBD type and combination biologic is shown in Figures 1B and 1C, respectively. Of the 10 patients who had abnormal CRP at baseline, 2 (20%) had a normal CRP by week 8 on TBT. Compared with those who had failed 2 or more biologics before starting TBT, patients who had previously failed 1 biologic were more likely to have clinical response at week 16 (P = 0.046) and at LDA (P = 0.036; Fig. 1D). There was no difference in clinical or endoscopic outcomes based of tofacitinib maintenance dosing (Fig. 1E). Tofacitinib plus biologic therapy was discontinued in 20 patients (57.5%), most frequently due to no response (15, 75.0%), loss of response (1, 5%), and other reasons (4, 20%). The median time to discontinuation of TBT was about 4.4 months (18.7 weeks; Fig. 1F).
Three AEs occurred in 2 patients (5.7%) while on TBT (Table 1). Both patients were male and had UC. One patient on IFX-TBT was hospitalized for Clostridium difficile infection and was the only serious AE (2.9%) in our cohort. He later developed candida esophagitis while on therapy. The second patient developed a rash while on VDZ-TBT. The median time to developing AEs was 32 days (range 25–85). Tofacitinib plus biologic therapy was not discontinued for any of these AEs, and overall, no patient discontinued TBT due to AEs. No DVT or herpes zoster reactivation or (major adverse cardiovascular event) MACE was reported.
One of 12 patients (7.14%) with normal lipid profile at baseline developed an abnormal profile at week 8 and 16, whereas 3 of 9 patients (33.3%) with abnormal baseline lipids reverted to a normal profile at 8 and 16 weeks. Seven UC patients (20%) underwent total proctocolectomy plus ileal pouch anal anastomosis (IPAA) and diverting ileostomy while on TBT due to failure of medical therapy. The median time to surgery was 3.5 months (IQR, 1.8–4.5). At least 1 Clavien-Dindo grade complication was reported in 4 (33.3%) patients. None had a grade 3 complication. Two other patients had IBD-related hospitalization: 1 was hospitalized for a CD flare, and the other was hospitalized for Clostridium difficile infection.
Discussion
Here, we showed that in a significantly refractory subset of IBD patients with a median disease duration of 8.5 years and nearly two thirds exposed to 2 or more prior biologics, 50% achieved clinical response on TBT at week 8, and 34.8% achieved endoscopic/radiographic remission. Only 5.7% had an AE. No single case of HZ, DVT, or MACE and no Clavien-Dindo complication of grade 3 or higher were reported among those who underwent surgery.
Recently, there has been an increasing number of reports on use of combination biologics and/or small molecules in refractory IBD cases.1–6 Most of these reports have been limited to DBT, with only 2 case series and 2 case reports reporting on TBT.3–6 Though the earlier reports have hinted at the safety and effectiveness of TBT, our report is the largest and most comprehensive case series demonstrating the effectiveness and safety of TBT for luminal disease in a subset of refractory IBD patients.
The overall clinical response (54.3%) rate and remission (34.3%) rate at last documented follow-up visits in our cohort were similar or slightly lower to what Yang et al (50% clinical response and 41% remission) and Kwapisz et al (73% clinical response) reported.1, 2 The endoscopic/radiographic response (56.5%) and remission (34.8%) induced by TBT in our patients were slightly higher than what other retrospective DBT studies have reported (Kwapisz et al, 44% with endoscopic/radiographic response; Yang et al, 43% with endoscopic response and 26% endoscopic remission). Differing baseline characteristics of patients and disparate clinical and endoscopic/radiographic assessment methods likely explain these slight differences.
The number of AEs in this cohort (5.7%) is lower than what Yang et al (13%) and Kwapicz et al (26.7%) reported. This AE rate is also lower than what we recently reported in a real-world tofacitinib monotherapy safety study (15.7%).7 No patient in our cohort developed HZV or DVT, both of which have been associated with JAK inhibitors. These findings suggest that the addition of tofacitinib to a biologic does not lead to new safety concerns beyond tofacitinib monotherapy, although larger studies with longer follow-up duration are necessary to adequately examine the long-term safety of TBT.
Our study is strong simply because it is the largest cohort study to date demonstrating the effectiveness and safety of TBT for luminal disease in a refractory IBD population from 2 large US IBD referral centers. However, the retrospective nature of disease activity assessment, the lack of a tofacitinib monotherapy control group, and the short follow-up duration are notable limitations of our study. Despite these limitations, our results are similar to other reports on DBT or combination of small molecule and biologics in refractory IBD patients.
In conclusion, these results suggest that the combination of tofacitinib with a biologic agent induces a significant clinical response and endoscopic/radiographic response without any new safety signals in a subset of IBD patients with active clinical symptoms despite biologic monotherapy.
Glossary
Abbreviations
- bid
twice daily
- CD
Crohn’s disease
- CRP
C-Rreactive protein
- DBT
dual biologic therapy
- DVT
deep venous thrombosis
- HZV
Herpes Zoster virus
- HBI
Harvey Bradshaw Index
- IBD
inflammatory bowel diseases
- IFX-TBT
iInfliximab plus tofacitinib
- IPAA
ileal pouch anal anastomosis
- IQR
iInterquartile range
- IRB
institutional review board
- JAK
Janus Kinase
- LDA
last documented assessment
- MACE
major adverse cardiovascular event
- MARIAs
simplified magnetic resonance index of activity
- mg
milligram
- MRE
magnetic resonance enterography
- PGA
physician global assessment
- PYF
patient-years of follow-up
- SCCAI
Simple Clinical Colitis Activity Index
- SES-CD
Simple Endoscopic Score for Crohn’s Disease
- TBT
tofacitinib plus biologic therapy
- UC
ulcerative colitis
- UST-TBT
ustekinumab plus tofacitinib
- VTE
venous thromboembolism
- VDZ-TBT
vedolizumab plus tofacitinib
- XR
extended release
Author Contribution: PD, AP, AK, and QAA conceived and designed the study. AK, QAA, and AP contributed to the acquisition of the data. QAA and PD analyzed data and drafted the initial manuscript. Interpretation of results and critical revisions was by RPR, MAC, AG, AG, MZ, and GC. All authors approved the final version of the manuscript.
Supported by: PD is supported by a Junior Faculty Development Award from the American College of Gastroenterology. MC is supported by DK109384, a Crohn’s and Colitis Foundation Daniel H Present Senior Research Award (Ref. 370763) and philanthropic support from the Givin’ It All for Guts Foundation (https://givinitallforguts.org) and the Lawrence C. Pakula MD IBD Research Innovation and Education Fund. AG is supported by the Lawrence C. Pakula, MD Advanced IBD Fellowship grant. Additional grant support for the REDCap database was provided by the Clinical and Translational Science Award (UL1 TR000448) and Siteman Cancer Center Support Grant (P30-CA091842).
Conflicts of Interest: PD has served as a consultant or advisory board member for Janssen, Pfizer, Celgene, Arena, and Prometheus and received research grants from Takeda. AG has served as a speaker for Janssen. RR is an advisor to Coloplast Corporation and has received research support from Gilead and Eli Lilly. MAC has consulted for Takeda, Pfizer, and Prometheus labs and has been on the speaker bureau for AbbVie, Pfizer, Takeda, and UCB and received research support from Incyte. All other authors have no conflicts to disclose.
References
- 1. Kwapisz L, Raffals LE, Bruining DH, et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2021;19:616–617. [DOI] [PubMed] [Google Scholar]
- 2. Yang E, Panaccione N, Whitmire N, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;51:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glassner K, Oglat A, Duran A, et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: A retrospective cohort study. J Dig Dis. 2020;21:264–271. [DOI] [PubMed] [Google Scholar]
- 4. Clark-Snustad KD, Singla A, Harper J, et al. Tofacitinib is safe and effective as monotherapy or in combination with biologic therapy in patients with Crohn’s disease. Gastroenterology. 2020;158:S-1208–S-1209. [Google Scholar]
- 5. Lee JA, Magavi PR, Konijeti GG. Treatment of ulcerative colitis and seronegative inflammatory spondyloarthritis with vedolizumab and tofacitinib. Inflamm Bowel Dis. 2020;26:e146. [DOI] [PubMed] [Google Scholar]
- 6. Le Berre C, Loeuille D, Peyrin-Biroulet L. Combination therapy with vedolizumab and tofacitinib in a patient with ulcerative colitis and spondyloarthropathy. Clin Gastroenterol Hepatol. 2019;17:794–796. [DOI] [PubMed] [Google Scholar]
- 7. Deepak P, Alayo QA, Khatiwada A, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020; S1542-3565(20)30913, -7. doi: 10.1016/j.cgh.2020.06.050. Accessed March 6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21:2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45:801–813. [DOI] [PubMed] [Google Scholar]
- 10. Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology. 2019;157:432–439.e1. [DOI] [PubMed] [Google Scholar]