Abstract
Using the PCR assay, we found a high prevalence of TT virus (TTV) DNA in saliva and semen from patients who were seropositive for TTV. This finding suggests that the presence of TTV in body fluids other than serum may affect the routes of viral transmission.
Recently, the genome from a novel DNA virus, termed the TT virus (TTV), was isolated from the serum of patients with posttransfusion non-A-G hepatitis by using representational difference analysis (6, 8). TTV is an unenveloped single-stranded DNA virus, with an isopycnic density of 1.31 to 1.32 g/ml in CsCl (8). The TTV genome has two possible open reading frames, capable of encoding 770 and 202 amino acids. Due to the genome structure and its banding in buoyant density gradient centrifugation, TTV might be most closely related to Circoviridae among the known animal virus families (3, 4, 10). The TTV sequence was detected in sera and liver tissues from liver disease patients, suggesting that TTV might be responsible for a part of acute and chronic liver disease of unknown etiology (2, 8). On the other hand, it has been reported that TTV infection does not induce significant liver damage (5). Members of our group recently reported that TTV infection is widespread in the general population worldwide and suggested that TTV may be a common DNA virus in humans (1). This implies that the routes of TTV transmission may differ from the routes of hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatitis G virus (HGV) transmission. The presence of virus in body fluids other than serum, such as saliva and semen, may affect the routes of viral transmission. Accordingly, we analyzed whether TTV is present in extravascular compartments.
Paired samples of serum, saliva, and semen were obtained from 10 drug addicts (all males, ranging in age from 18 to 54 years), either intravenous drug users or heroin smokers. Four of them were coinfected with HCV, but no evidence of HBV, HGV, or human immunodeficiency virus infections was obtained by PCR assays. Saliva and semen were collected in sterile tubes and stored at −30°C until use. Informed consent was obtained from the participants in this study. DNA was extracted from 100 μl of serum, saliva, and semen, respectively, using a nucleic acid extraction kit (SepaGene RV-R; Sanko Junyaku Co., Ltd., Tokyo, Japan). The semen was diluted to twice its original volume with phosphate-buffered saline and used for DNA extraction. TTV DNA was amplified by PCR as described previously (1). In brief, the thermocycler was programmed first to preheat at 95°C for 10 min to activate AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.), followed by 55 cycles consisting of 94°C for 20 s, 60°C for 20 s, and 72°C for 30 s using a Perkin-Elmer 9600 or 9700 thermal cycler. The sequences of the TTV-specific primers were 5′-GCTACGTCACTAACCACGTG-3′ (T801, sense primer; nucleotides 6 to 25) and 5′-CTBCGGTGTGTAAACTCACC-3′ (T935, antisense primer; nucleotides 185 to 204; B = G, C, or T) as designed by Takahashi et al. (9) in the 5′ end region of the TA278 isolate. The PCR products were detected by electrophoresis on 2% agarose gels, stained with ethidium bromide, and photographed under UV light. The sizes of the PCR products were estimated according to the migration pattern of a 50-bp DNA ladder (Pharmacia Biotech, Uppsala, Sweden). All PCR assays were performed in duplicate to confirm specificity. To characterize the nucleotide sequence of isolated TTVs, amplicons were purified using a QIAquick gel extraction kit (Qiagen Inc., Chatsworth, Calif.). The recovered PCR products were subjected to direct sequencing from both directions using the ABI Prism dye terminator cycle sequencing ready reaction kit (Perkin-Elmer). Sequences of amplified DNA were determined by using a sequencer (ABI model 373A; Applied Biosystems, Foster City, Calif.).
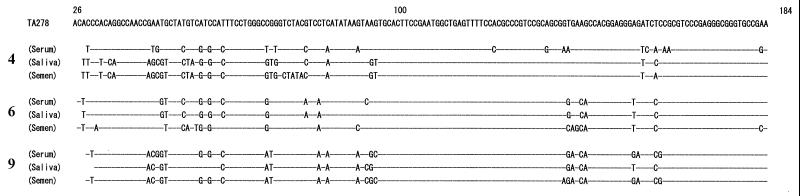
As a result, TTV DNA was detected in the serum of 10 (10 of 10 [100%]), the saliva of 7 (7 of 9 [78%]), and the semen of 6 (6 of 10 [60%]) individuals (Table 1). In contrast, HCV RNA was detected in the saliva and semen of only one (20%) of five patients who were seropositive for HCV RNA. Elevated serum alanine aminotransferase was found in five patients coinfected with HCV and TTV but not in the remaining patients infected only with TTV. To verify that the products amplified by PCR were of TTV origin, sequence analyses were performed in three cases. The results revealed that although the nucleotide sequences of TTV DNA from serum, saliva, and semen had sequence variations among them, all had a high level of similarity to the TA278 isolate, which is the prototype of TTV (Fig. 1).
TABLE 1.
Detection of TTV DNA in serum and body fluids from 10 male drug addicts and comparison with HCV RNA detection
| Case no. | Age (yr) | Detection of:
|
|||||
|---|---|---|---|---|---|---|---|
| TTV DNA
|
HCV RNA
|
||||||
| Serum | Saliva | Semen | Serum | Saliva | Semen | ||
| 1 | 54 | + | + | + | + | − | − |
| 2 | 53 | + | NDa | + | + | ND | + |
| 3 | 48 | + | + | − | + | − | − |
| 4 | 46 | + | + | + | + | + | − |
| 5 | 38 | + | + | + | + | − | − |
| 6 | 33 | + | + | + | − | − | − |
| 7 | 33 | + | − | − | − | − | − |
| 8 | 24 | + | − | − | − | − | − |
| 9 | 23 | + | + | + | − | − | − |
| 10 | 18 | + | + | − | − | − | − |
ND, not done.
FIG. 1.
Comparison of nucleotide sequences of TTV recovered from paired samples of serum, saliva, and semen from three individuals (cases 4, 6, and 9).
Our results indicate that TTV DNA, besides being present in blood, can also be found frequently in saliva and semen. It was reported previously that TTV viremia is widespread in the general population worldwide, since the prevalence among patients, including healthy populations, was found to be more than 70% (1). Such an extremely high prevalence of TTV infection in the general population suggests that TTV may be transmissible not only through blood but also by a nonparenteral route. Indeed, Okamoto et al. reported that TTV was excreted in the feces, thereby suggesting that TTV is transmitted not only parenterally but also nonparenterally by a fecal-oral route (7). In addition to the feces, TTV would be transmitted by saliva and semen. The high degree of similarity between the paired samples of serum-, saliva-, and semen-derived TTV sequences suggests that the virus detected in saliva and semen originated from the same source as the virus found in serum. Sequence analysis of TTV DNA from serum, saliva, and semen showed little variation among the samples examined. It is known that despite TTV being a DNA virus, the TTV sequence has a wide range of divergence (1, 4, 8).
In conclusion, a high prevalence of TTV DNA in saliva and semen from patients infected with TTV was found by PCR assay. This finding suggests that body fluids other than serum may be significant vehicles for the nonparenteral transmission of TTV, although the infectivity of TTV DNA-positive body fluids remains to be demonstrated.
Acknowledgments
We thank Takeshi Kurata for his continuous encouragement during this study and Hideo Naito and Naoto Aiba for their kind cooperation.
This study was supported in part by Grants-in-Aid for Science Research of the Ministry of Education, Science and Culture of Japan and the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Abe K, Inami T, Asano K, Miyoshi C, Masaki N, Hayashi S, Ishikawa K-I, Takebe Y, Win K M, El-Zayadi A R, Han K-H, Zhang D Y. TT virus infection is widespread in the general populations from different geographic regions. J Clin Microbiol. 1999;37:2703–2705. doi: 10.1128/jcm.37.8.2703-2705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology. 1998;28:839–842. doi: 10.1002/hep.510280335. [DOI] [PubMed] [Google Scholar]
- 3.Miyata H, Tsunoda H, Kazi A, Yamada A, Khan M A, Murakami J, Kamahora T, Shiraki K, Hino S. Identification of a novel GC-rich 113-nucleotide region to complete the circular, single-stranded DNA genome of TT virus, the first human circovirus. J Virol. 1999;73:3582–3586. doi: 10.1128/jvi.73.5.3582-3586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mushahwar I K, Erker J C, Muerhoff A S, Leary T P, Simons J N, Birkenmeyer L G, Chalmers M L, Pilot-Matias T J, Dexai S M. Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc Natl Acad Sci USA. 1999;96:3177–3182. doi: 10.1073/pnas.96.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naoumov N V, Petrova E P, Thomas M G, Williams R. Presence of a new described human DNA virus (TTV) in patients with liver disease. Lancet. 1998;352:195–197. doi: 10.1016/S0140-6736(98)04069-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto H, Akahane Y, Ukita M, Fukuda M, Tsuda F, Miyakawa Y, Mayumi M. Fecal excretion of a nonenveloped DNA virus (TTV) associated with posttransfusion non-A-G hepatitis. J Med Virol. 1998;56:128–132. [PubMed] [Google Scholar]
- 8.Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Iizuka H, Miyakawa Y, Mayumi M. Molecular cloning and characterization of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol Res. 1998;10:1–16. [Google Scholar]
- 9.Takahashi K, Hoshino H, Ohta Y, Yoshida N, Mishiro S. Very high prevalence of TT virus (TTV) infection in general population of Japan revealed by a new set of PCR primers. Hepatol Res. 1998;12:233–239. [Google Scholar]
- 10.Takahashi K, Ohta Y, Mishiro S. Partial ∼2.4kb sequence of TT virus genome from eight Japanese isolates: diagnostic and phylogenetic implications. Hepatol Res. 1998;12:111–120. [Google Scholar]