Abstract
Chronic granulomatous disease (CGD) results from deficiency of nicotinamide adenine dinucleotide phosphate(NADPH) oxidase and impaired reactive oxygen species (ROS) generation. This leads to impaired killing of Aspergillus and, independently, a pathologic hyperinflammatory response to the organism. We hypothesized that neutrophil-derived ROS inhibit the inflammatory response to Aspergillus and that acute lung injury in CGD is due to failure of this regulation. Mice with gp91phox deficiency, the most common CGD mutation, had more severe lung injury, increased neutrophilinfiltration, and increased lung tumor necrosis factor (TNF) after Aspergillus challenge compared with wild-types. Neutrophils were surprisingly the predominant source of TNF in gp91phox-deficient lungs. TNF neutralization inhibited neutrophil recruitment in gp91phox-deficient mice and protected from lung injury. We propose that, in normal hosts, Aspergillus stimulates TNF-dependent neutrophil recruitment to the lungs and neutrophil-derived ROS limit inflammation. In CGD, in contrast, recruited neutrophils are the dominant source of TNF, promoting further neutrophil recruitment in a pathologic positive-feedback cycle, resulting in progressive lung injury.
Keywords: Aspergillus, chronic granulomatous disease, neutrophil, TNF, reactive oxygen species
Patients with chronic granulomatous disease display a pathologic hyperinflammatory response to Aspergillus that results in lung injury. In a mouse model, this is dependent on tumor necrosis factor production by neutrophils with impaired reactive oxygen species generation.
Chronic granulomatous disease (CGD) is a group of defects in NADPH oxidase, disrupting the major mechanism of non-mitochondrial superoxide anion generation and therefore all reactive oxygen species (ROS). The most common X-linked form is caused by mutations in CYBB, the gene encoding the gp91phox subunit [1, 2]. CGD is characterized by susceptibility to a narrow range of opportunistic pathogens, most often affecting the lungs, and results in considerable morbidity and premature death [3]. Notable among these microorganisms are the molds belonging to the Aspergillus genus, the most common cause of death in patients with CGD [3, 4].
Aspergillus molds are ubiquitous in the environment and humans inhale hundreds of spores daily [5]. Despite constant exposure, normal hosts clear the organism without developing disease [6]. In settings of immunodeficiency, Aspergillus conidia become metabolically active and germinate to form hyphae that penetrate the lung epithelium and cause invasive pneumonia. Patients with CGD exhibit 2 independent defects in response to Aspergillus: First, neutrophils display a well-defined impairment in oxidative killing [7], defective phagolysosomal alkalization leading to impaired protease activation [8], and impaired NETosis [9]. Second, CGD hosts generate a pathologically exaggerated inflammatory response to Aspergillus that is characterized by augmented neutrophilic infiltration [10], NF-κB activation [11], and increased inflammasome activation and interleukin 1β (IL-1β) release [12], resulting in acute lung injury independent of fungal killing [13]. This exaggerated inflammatory response to Aspergillus in CGD is incompletely understood but is clinically important. One manifestation is mulch pneumonitis, a potentially fatal form of acute respiratory distress syndrome, but not invasive pneumonia, that occurs after inhalation of dusts from mulch, compost, or leaves containing high concentrations of Aspergillus antigens [14, 15].
We have previously reported that, independent of their microbicidal role, neutrophils promote the maturation and efflux of monocyte-derived dendritic cells (DCs) from the lungs after challenge with killed Aspergillus hyphae, and neutropenia results in accumulation of tumor necrosis factor (TNF)–producing inflammatory DCs in the lungs after Aspergillus [16, 17]. The similarity between this exaggerated inflammatory phenotype in neutropenic mice and the clinical description of mulch pneumonitis led us to hypothesize that neutrophil-derived ROS inhibit inflammation after exposure to Aspergillus, and that acute lung injury in response to Aspergillus in CGD results from failure of this regulatory mechanism.
MATERIALS AND METHODS
Animals and In Vivo Procedures
Wild-type C57BL/6 and gp91phox-deficient mice on a C57BL/6 background [18] from The Jackson Laboratory were bred and maintained under pathogen-free conditions. Age- and sex-matched male and female mice 6–12 weeks old were used. TNF was neutralized by intraperitoneal injection of 300 μg of anti-TNF or isotype (clones XT3.11 and HRPN; BioXCell) at intervals noted in Figure 6 legend. Neutrophils were depleted using intraperitoneal injection of 400 μg of anti-Ly6G or isotype (clones 1A8 and 2A3; BioXCell) 1 day before Aspergillus challenge. Intratracheal injection, tissue harvest, bronchoalveolar lavage (BAL), bone marrow chimera, and histology were performed as described elsewhere [19–21].
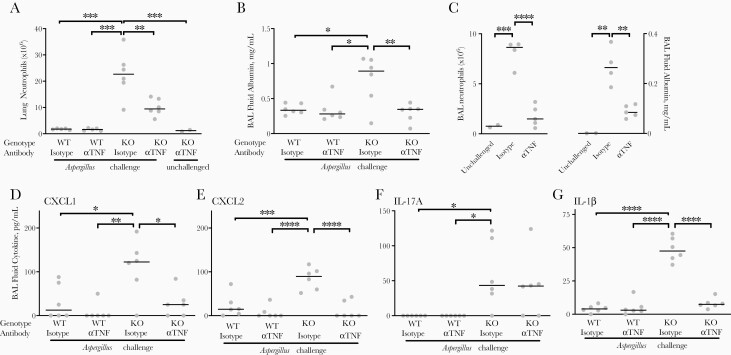
Figure 6.
Role of tumor necrosis factor (TNF) in mediating acute lung injury in gp91phox-deficient mice after Aspergillus challenge. Wild-type (WT) and gp91phox-deficient (KO) mice were treated with anti-TNF antibody (αTNF) or isotype control antibody and measurements were obtained on day 3. A, B, Total lung neutrophils, as determined with flow cytometry, and bronchoalveolar lavage (BAL) fluid albumin, as measured with enzyme-linked immunosorbent assay (ELISA). Antibodies were given 1 day before and 1 day after intratracheal challenge with nonviable Aspergillus germlings. C, BAL fluid neutrophils and albumin in gp91phox-deficient mice with antibodies administered only 1 day after intratracheal challenge with nonviable Aspergillus germlings, with measurements obtained on day 3. D–G, BAL fluid cytokines as measured with ELISA (antibodies as in A and B). In all plots, horizontal lines represent medians, and circles, individual animals. For all panels, groups differed significantly by 1-way analysis of variance. *P < .05, **P < .01, ***P < .001, and ****P < .0001 (Tukey multiple comparisons test). Abbreviations: IL-1β , interleukin 1β ; IL-17A, interleukin 17A.
Ethics Statement
Animal protocols were compliant with the National Research Council Guide for the Care and Use of Laboratory Animals, the US Animal Welfare Act, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Procedures were approved by the institutional animal care and use committees of the University of Virginia (protocol 3571), the University of Florida (201709979), and Brigham and Women’s Hospital (2019N000005).
Preparation and Administration of Killed Aspergillus
Aspergillus fumigatus (strain 13073; American Type Culture Collection) was grown on Sabouraud dextrose agar for 10–14 days, and conidia were harvested in 0.1% Tween 80 in phosphate-buffered saline (PBS) and filtered through sterile gauze. For live Aspergillus, conidia were counted with a hemocytometer and administered intratracheally at 3 × 107 conidia per 30 μL of saline. For killed Aspergillus, conidia were cultured in Roswell Park Memorial Institute 1640 medium (RPMI-1640) plus 10% fetal bovine serum overnight at 37°C in a shaking incubator, followed by resuspension in 70% ethanol when the culture contained primarily short hyphae. After 72 hours, fungal elements were washed and resuspended in PBS. The resulting germlings had a viability of <1:1.6 × 106 by serial dilution and culture. Fungal forms were counted with a hemocytometer and administered intratracheally at 9 × 105 germlings in 30 μL of saline, unless otherwise noted.
Flow Cytometry
Flow cytometry was performed as described elsewhere [20, 21] using antibodies outlined in supplementary material from BD Biosciences, eBioscience, or BioLegend. Intracellular staining was performed using Cytofix/Cytoperm solution with monensin (BD Biosciences). Data were acquired on a FACSCanto II or LSRFortessa using FACSDiva (version 8.0; BD Biosciences) and analyzed using FlowJo software (version 10.6.1; BD Biosciences). The number of each cell type was determined as the product of the percentage of the cell type and the total number of cells in the sample, using an automated cell counter (Countess; Invitrogen) or Accuchek counting beads (BD Biosciences). Fluorescent automated cell sorting was performed using a FACSAria Fusion flowcytometer. Ly6G+ cell selection was performed using anti-Ly6G magnetic beads, LS columns, and a quadroMACS magnet (Miltenyi). Slides containing selected cells were generated with a Cytospin centrifuge (Thermo Fisher) and stained with a modified Wright-Giemsa stain. For ex vivo assays, 1 × 105 Ly6G+cells per well in RPMI-1640 plus 10% fetal bovine serum were cultured in 96-well plates overnight.
Immunofluorescence
Two days after challenge with killed Aspergillus, mice were injected with 200 μg of brefeldin A and euthanized after 5 hours. Lungs were perfused with saline followed by 1% paraformaldehyde, D-leucine, periodate solution. Sections (3 μm) were cut and air dried, followed by incubation with 0.3% Triton X-100/10% horse serum in PBS for 30 minutes. After washing with PBS, anti-CD16/32 was added, followed by overnight incubation with anti-TNF antibody (rabbit polyclonal; Abcam) at 4oC. Sections were further incubated for 1.5 hours with phycoerythrin-labeled donkey anti-rabbit secondary and fluorescein isothiocyanate–labeled anti-neutrophil antibody (clone 7/4; Cedarlane). Sections were washed in PBS and mounted with ProLong Gold antifade agent with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher).
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assays for albumin (Bethyl Laboratories), CXCL1, CXCL2, IL-1β, interleukin 17 (IL-17), and TNF (R&D Systems) were performed according to the manufacturer’s instructions. Horseradish peroxidase was quantified using 2-component tetramethylbenzidine peroxidase substrate (KPL).
RNA Isolation and Reverse-Transcription Polymerase Chain Reaction
RNA was harvested using RNeasy Plus Mini Kit (Qiagen) and complementary DNA was synthesized using a reverse-transcription kit (Promega) per manufacturer’s instructions. Custom primer and probe combinations were generated (Sigma Aldrich) as described in the Supplementary Data. Reactions were performed using TaqMan Universal PCR Master Mix (Applied Biosystems) with a BioRad iQ5 thermal cycler.
Statistical Analyses
Data were analyzed using Prism software (version 8.0; GraphPad). Two groups were compared using Student t test, and 1-way analysis of variance with Tukey multiple comparisons test was used to analyze differences between ≥3 groups at a single time point. Comparisons of 2 groups over time or a range of inocula were achieved using 2-way analysis of variance with Sidak multiple comparisons test when groups were equal in size, or mixed-effects analysis when groups differed in size. Linear correlations between variables were quantified using Pearson correlation coefficient. Differences were considered statistically significant at P < .05. For multiple comparisons tests, multiplicity-adjusted P values are reported.
RESULTS
Increased Lung Injury in Response to Killed A. fumigatus in gp91phox-Deficient Mice
In immunocompetent hosts, Aspergillus conidia are cleared from the lungs before they express antigens detectable by innate immune receptors [6]. In contrast, in gp91phox-deficient mice, conidia swell and germinate, thereby unmasking immunostimulatory antigens. Immunocompromised hosts thus encounter both qualitatively and quantitatively different antigenic stimuli than do wild-type hosts. To compare innate immune responses to identical fungal antigenic stimuli between wild-type and gp91phox-deficient mice, we used ethanol-killed Aspergillus.
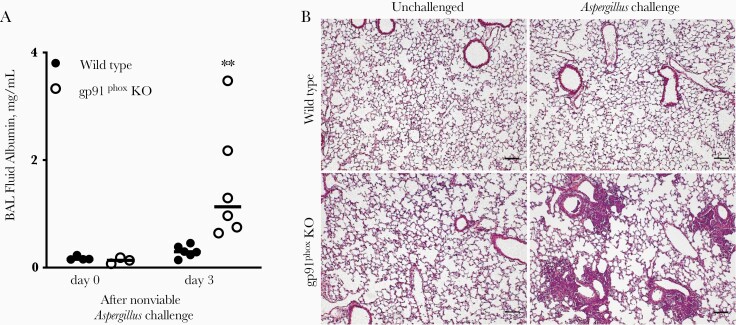
We began by characterizing lung injury induced by Aspergillus in gp91phox-deficient mice compared with wild-type mice. After intratracheal challenge with killed Aspergillus, gp91phox-deficient mice, but not wild-type mice, developed a marked increase in albumin in the BAL fluid, evidence of loss of integrity of the alveolar-capillary barrier and a measure of acute lung injury (Figure 1A) [22]. Histologic assessment supported this finding, with filling of alveoli surrounding small airways by leukocytes in gp91phox-deficient, but not wild-type, mice (Figure 1B). This linked the exaggerated inflammatory response to Aspergillus in gp91phox-deficient mice to increased lung injury.
Figure 1.
Lung injury in wild-type and gp91phox-deficient (gp91phox-knockout [KO]) mice in response to Aspergillus fumigatus. Wild-type and gp91phox-KO mice were inoculated with nonviable A. fumigatus germlings intratracheally on day 0. A, Albumin content in bronchoalveolar lavage (BAL) fluid was measured using enzyme-linked immunosorbent assay. **P < .01 (mixed-effects analysis). Horizontal lines represent medians, and circles, individual animals; data are representative of 3 independent experiments. B, Lungs were fix inflated with 2% paraformaldehyde followed by paraffin embedding and hematoxylin-eosin staining. Representative sections shown are taken from lungs of wild-type and gp91phox-KO mice untreated (left panels) and on day 3 after challenge (right panels) (original magnification ×40; scale bar represents 100 μm). Images shown are representative of 5 animals challenged with Aspergillus.
Increased Leukocyte Accumulation in gp91phox-Deficient Mice After Aspergillus
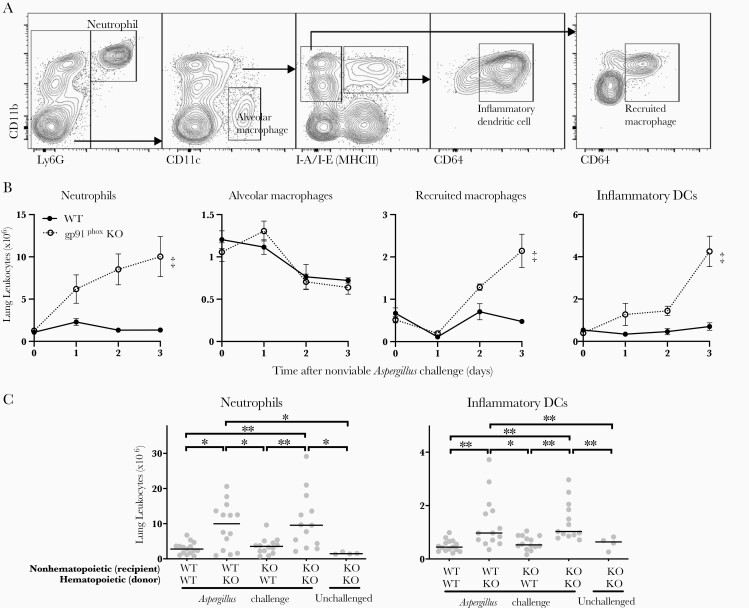
We next compared the accumulation of leukocytes in the lungs of gp91phox-deficient and wild-type mice after intratracheal challenge with Aspergillus. We found a >7-fold accumulation of neutrophils in gp91phox-deficient mice compared with wild-type mice after Aspergillus challenge (Figure 2A and 2B). There were also smaller increases in the recruitment of monocyte-derived inflammatory DCs and recruited macrophages in gp91phox-deficient animals over the first 3 days after challenge (Figure 2B). Alveolar macrophages declined beginning on day 2 but did not differ significantly between wild-type and gp91phox-deficient animals.
Figure 2.
Accumulation of leukocytes in the lungs of gp91phox-deficient (gp91phox-knockout [KO]) mice after Aspergillus challenge. A, Representative flow cytometry identification of neutrophils, alveolar macrophages, inflammatory dendritic cells (DCs), and recruited macrophages in single-cell suspension of whole lung after intratracheal challenge with nonviable Aspergillus germlings, gated on CD45+ cells (not shown). B, Time course of accumulation of leukocyte subsets in wild-type (WT) and gp91phox-KO mouse lungs after Aspergillus challenge on day 0. Data represent means with standard errors of the mean for 5 per group per time point and are representative of 2 independent experiments; ‡P < .001 (2-way analysis of variance [ANOVA]). C, Lung leukocytes 3 days after challenge with nonviable Aspergillus germlings in bone marrow chimera, using WT and gp91phox-KO mice. Horizontal lines represent medians, and circles, individual animals. P < .001 by 1-way ANOVA for both cell types. *P < .05; **P < .01 (Tukey multiple comparisons test). Data shown are pooled from 3 independent experiments.
Similar to patients with CGD, gp91phox-deficient animals have impaired NADPH oxidase signaling in both hematopoietic and nonhematopoietic cells, and the observed phenotype is not necessarily attributable to leukocyte ROS production [23]. To assess this, we examined the lung leukocyte response to Aspergillus in bone marrow chimeric animals. After reconstitution with gp91phox-deficient bone marrow, wild-type mice displayed similar exaggerated lung neutrophil and inflammatory DC accumulation as gp91phox-deficient mice, whereas wild-type or gp91phox-deficient mice reconstituted with wild-type bone marrow failed to show the same level of neutrophil and inflammatory DC accumulation (Figure 2C). This indicated that the exaggerated inflammatory response in gp91phox-deficent mice after Aspergillus challenge was due to loss of NADPH oxidase activity in hematopoietic cells.
Neutrophils and Enhanced TNF Response in gp91phox-Deficient Animals
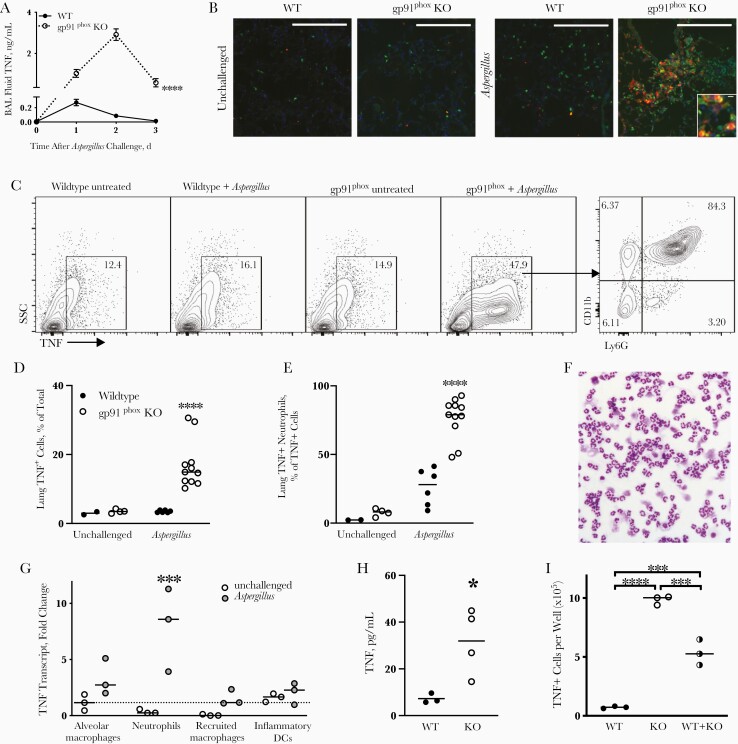
TNF is essential to host defense against Aspergillus [24] and, in the context of neutropenic invasive aspergillosis, is produced mainly by recruited monocyte-derived inflammatory DCs [16]. Compared with wild-type mice, which displayed a small elevation of BAL fluid TNF on the first day after Aspergillus challenge, we found a marked elevation of TNF in gp91phox-deficient mice (Figure 3A). To define the cellular source of TNF in gp91phox-deficient mice, we used immunofluorescence microscopy (Figure 3B). We detected rare neutrophils in lungs of uninfected animals and marked infiltration of neutrophils after challenge with ethanol-killed Aspergillus in gp91phox-deficient compared with wild-type animals. Surprisingly, in gp91phox-deficient lungs, but not those in wild-type mice, TNF was colocalized with neutrophils (Figure 3B and Supplementary Figure 1).
Figure 3.
Lung tumor necrosis factor (TNF) production by neutrophils in gp91phox-deficient (gp91phox-knockout [KO]) mice after Aspergillus challenge. Wild-type (WT) and gp91phox-KO mice were inoculated intratracheally with nonviable Aspergillus germlings on day 0. A, TNF protein concentration in bronchoalveolar lavage (BAL) fluid was measured with enzyme-linked immunosorbent assay (ELISA). Data represent means with standard errors of the mean for 4–6 mice per group per time point. ****P < .001 (mixed-effects analysis). B, Immunofluorescence images of lungs of WT and gp91phox-KO mice, unchallenged or 2 days after Aspergillus challenge. Green represents neutrophils (7/4 antigen); red, TNF; blue, nuclei(DAPI) (original magnification ×40; [×100 for inset]; large scale bars represent 100 µm; small scale bar, 10 µm); data are representative of 2–3 animals per group. C, Representative intracellular staining for TNF in lungs from WT and gp91phox-KO mice (gp91phox) untreated and 2 days after Aspergillus challenge (gated on CD45+ cells) Numbers shown are percent of CD45+ cells except far right panel is percent of TNF+ cells. Abbreviation: SSC, side scatter. D, E, Summary data from flow cytometry shown in C. Data shown are pooled from 4 independent experiments; ****P < .001 (mixed-effects analysis). F, Representative DiffQuick stain of Cytospin preparation of Ly6G+ cells isolated from single-cell suspensions of gp91phox-KO mouse lungs on day 2 after Aspergillus challenge by positive magnetic selection (original magnification ×20). G, TNF transcript in sorted lung leukocytes in gp91phox-KO mice 2 days after Aspergillus challenge. Quantitative reverse-transcription polymerase chain reaction of >50 000 cells from each population was normalized to TNF messenger RNA expression in alveolar macrophages from untreated animals. P < .01 (2-way analysis of variance [ANOVA]); ***P < .001 (Sidak multiple comparisons test). Abbreviation: DCs, dendritic cells. H, Positively selected Ly6G+ cells from single-cell suspensions of WT or gp91phox-KO mouse lung on day 2 after Aspergillus challenge were cultured ex vivo. TNF was measured by ELISA in supernatant after overnight culture. *P = .03 (t test). I, The number of TNF+ neutrophils was determined with flow cytometry after overnight culture. P < .001 (1-way ANOVA); ***P < .001 and ****P < .0001 (Tukey multiple comparisons test). In D, E, and G–I, horizontal lines represent medians, and circles, individual animals.
To quantify this observation, we used intracellular cytokine staining for TNF in lungs of wild-type and gp91phox-deficient mice after intratracheal Aspergillus challenge. On day 2 after challenge, gp91phox-deficient animals displayed an increase in TNF-positive cells compared with wild-type and unchallenged animals (Figure 3C and 3D). TNF was intracellular and not membrane or receptor bound, since unpermeabilized samples showed little TNF staining (Supplementary Figure 2A). The majority (median, 79%) of TNF-positive cells in gp91phox-deficient animals were CD11b+Ly6G+ neutrophils (Figure 3C and 3E and Supplementary Figure 2B, 2C, and 2F). When sorted, these cells displayed typical neutrophil morphology (Figure 3F). CGD neutrophils were also a major cellular source of TNF at an earlier time point and after challenge with live conidia, with smaller contributions from alveolar macrophages and monocyte-derived cells (Supplementary Figure 2D–2F).
TNF receptors are rapidly internalized after binding soluble ligand. We thus assessed whether intracellular TNF detected in neutrophils represented internalized exogenous ligand or de novo expression of TNF. We isolated purified populations of neutrophils, alveolar macrophages, inflammatory DCs, and recruited macrophages (as in Figure 2A) from lungs of gp91phox-deficient animals and quantified TNF transcript. Neutrophils contained markedly greater TNF transcript than other cell types analyzed after Aspergillus challenge (Figure 3G), but TNF transcript was upregulated to a lesser extent in all examined cell populations. Furthermore, when neutrophils were isolated from lungs of Aspergillus-challenged mice, TNF was increased in supernatant of gp91phox-deficient neutrophils compared with those from wild-type mice after overnight ex vivo culture (Figure 3H). Similarly, after overnight culture, neutrophils from gp91phox-deficient animals, but not from wild-type mice, continued to stain positive for intracellular TNF (Figure 3I).
Because small numbers of wild-type neutrophils can enhance fungal killing by CGD neutrophils [25], we also assessed whether a similar phenomenon may occur relating to inhibition of TNF production, but coculture of equal numbers of wild-type and gp91phox-deficient neutrophils did not appreciably affect their production of TNF (Figure 3I). These experiments indicate that neutrophils in gp91phox-deficient animals synthesize and secrete TNF protein; however, this does not exclude the possibility that TNF produced from other cell types may also be relevant. These data taken together with the large number of neutrophils in lungs of gp91phox-deficient mice after challenge with Aspergillus (Figure 2B), lead us to conclude that neutrophils are the primary source of TNF in gp91phox-deficient mice after Aspergillus challenge.
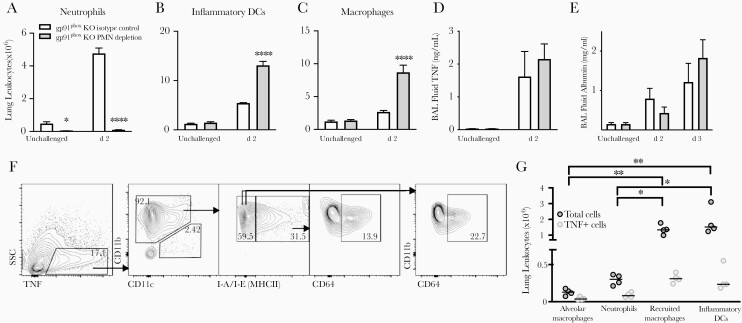
Neutrophil Depletion and TNF Production by Monocyte-Derived Leukocytes
In wild-type neutropenic mice with invasive aspergillosis, monocyte-derived DCs are the main cellular source of TNF in infected lungs [16, 17]. This contrasts with gp91phox-deficient hosts, in which neutrophils are the predominant source. To probe this interplay in gp91phox-deficient mice, we assessed the effect of neutrophil depletion by administering anti-Ly6G antibody and found accumulation of monocyte-derived inflammatory DCs and macrophages, similar to the prior report in neutropenic wild-type animals (Figure 4A–4C). Neutrophil-depleted gp91phox-deficient animals had similar levels of BAL fluid TNF on day 2 after injury (Figure 4D), and no significant difference in lung injury on either day 2 or 3 after Aspergillus challenge (Figure 4E). The cellular source of TNF in neutrophil-depleted gp91phox-deficient mice was monocyte-derived DCs and macrophages, similar to the source in neutropenic wild-type mice (Figure 4F and 4G). Thus, in the context of neutropenia, monocyte-derived leukocytes are the main source of TNF in both wild-type and CGD hosts.
Figure 4.
Effect of neutrophil depletion on lung tumor necrosis factor (TNF) production in gp91phox-deficient (gp91phox-knockout [KO]) mice after Aspergillus challenge. Gp91phox-KO mice were treated with anti-Ly6G (PMN depletion) or isotype control antibody 1 day before intratracheal challenge with nonviable Aspergillus germlings. A–C, Number of lung neutrophils, inflammatory dendritic cells (DCs), and macrophages as determined with flow cytometry. Bar graphs represent means with standard errors of the mean for 3–4 animals per group; data shown are representative of 3 independent experiments. *P < .05; ****P < .001 (mixed-effects analysis). D, E, Bronchoalveolar lavage (BAL) fluid TNF and albumin concentrations by enzyme-linked immunosorbent assay. Data represent means with standard errors of the mean for 4 mice per group per time point. There were no significant differences between isotype control or PMN depletion groups for TNF or albumin. F, Intracellular staining for TNF in lung leukocytes 2 days after nonviable Aspergillus challenge (gated on CD45+ cells) in neutrophil-depleted gp91phox-KO mouse. Percentages shown for TNF+ cells (left panel) represent percentage of total CD45+ cells; all other percentages, percentage of TNF+ cells. Abbreviation: side scatter,SSC. G, Summary data of total and TNF+ lung leukocyte populations in neutrophil-depleted gp91phox-KO mice 2 days after Aspergillus challenge. Horizontal lines represent medians, and circles, individual animals. P < .01 by 1-way analysis of variance for both cell types; *P < .05 and **P < .01 by Tukey multiple comparisons test.
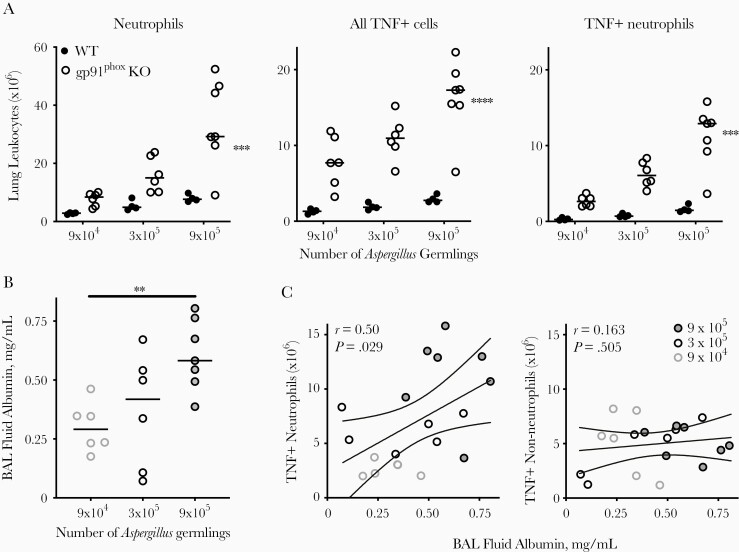
Recruitment of TNF-Producing Neutrophils and Mediation of Lung Injury
To connect neutrophil-derived TNF to lung injury after Aspergillus, we challenged animals with 3 different inocula of killed Aspergillus and quantified the recruitment of TNF-producing leukocytes and the extent of lung injury. As expected, the numbers of lung neutrophils, total TNF-producing cells, and TNF-producing neutrophils increased with increasing Aspergillus inocula in both wild-type and gp91phox-deficient mice, and this effect was more pronounced in the latter (Figure 5A). The extent of lung injury in gp91phox-deficient mice also increased with increasing Aspergillus inocula (Figure 5B), and was correlated significantly with the number of lung TNF-producing neutrophils but not the number of non-neutrophil TNF-producing cells (Spearman ρ, 0.49 vs 0.14; Figure 5C). The association between lung TNF-producing neutrophils with lung injury in gp91phox-deficient mice is consistent with, but not direct evidence for, a causal role for these cells in mediating lung injury.
Figure 5.
Correlation between lung tumor necrosis factor (TNF)–producing neutrophils and lung injury in gp91phox-deficient mice (gp91phox-knockout [KO]) after Aspergillus challenge. A, Number of lung neutrophils, TNF-positive (TNF+) cells, and TNF+ neutrophils on day 2 after challenge with different inocula of nonviable Aspergillus germlings. ***P < .001 and ****P < .0001 for differences between groups and differences between inocula by mixed-effects analysis. B, Bronchoalveolar (BAL) fluid albumin, as measured with enzyme-linked immunosorbent assay. **P < .01 for association of BAL fluid albumin with inoculum (1-way analysis of variance). C, Association between TNF+ neutrophils and non-neutrophil leukocytes with BAL fluid albumin in gp91phox-KO mice. Linear correlation (solid line) and 95% confidence intervals (dashed lines) are shown; r is the Pearson correlation coefficient. In A and B, horizontal lines represent medians, and circles, individual animals. Data are pooled from 2 independent experiments.
In neutropenic wild-type mice with invasive aspergillosis, TNF production by monocyte-derived DCs resulted in a positive feedback cycle that caused further recruitment of TNF-producing DCs [16]. We hypothesized an analogous positive feedback cycle in gp91phox-deficient mice, wherein production of TNF by lung neutrophils mediates further recruitment of TNF-producing neutrophils, leading to progressive lung injury. We tested this using TNF neutralization in wild-type and gp91phox-deficient mice in response to intratracheal Aspergillus challenge. TNF neutralization in gp91phox-deficient (but not wild-type) mice resulted in diminished recruitment of neutrophils to the lungs in response to Aspergillus challenge (Figure 6A), which was associated with attenuated lung injury (Figure 6B).
A similar pattern was observed when TNF neutralization was delayed for 24 hours after Aspergillus challenge (Figure 6C). In gp91phox-deficient hosts, TNF neutralization significantly reduced CXCL1 and CXCL2 in BAL fluid (Figure 6D and E), chemokines essential to lung neutrophil recruitment in aspergillosis [24, 26]. Interestingly, TNF neutralization did not influence BAL fluid IL-17 (Figure 6F), suggesting that TNF mediates the recruitment of neutrophils via CXC chemokines but independently of IL-17. Finally, TNF neutralization also attenuated BAL fluid IL-1β, indicating that TNF is proximal toNLRP3 activation, a key mechanism in inflammatory lesions in CGD [12]. Taken together, these data provide evidence for an amplification loop in lungs of gp91phox-deficient hosts in which neutrophil-derived TNF in response to Aspergillus perpetuates further recruitment of TNF-producing neutrophils and mediates progressive lung injury.
DISCUSSION
Patients with CGD are profoundly susceptible to both invasive aspergillosis and to acute lung injury in response to the fungus [14]. In the clinical context, infection and pathologic response occur concurrently; injury that occurs as a result of invasive infection is difficult to discern from injury resulting from aberrant inflammation. By using nonviable Aspergillus germlings, we sought to specifically assess mechanisms that underlie susceptibility of CGD hosts to fungal acute lung injury, and we identified neutrophil-derived TNF as the key mediator driving an amplification loop resulting in Aspergillus-mediated lung injury.
Normal hosts generate no detectable cytokine response to inhalation of small numbers of Aspergillus conidia, owing to a combination of epitope-masking in resting conidia and rapid mucociliary clearance [6]. In response to large inocula, normal hosts generate a wave of TNF that resolves within 48–72 hours [27] and is protective against infection, since neutralization of TNF in immunocompetent mice and in humans promotes invasive aspergillosis [24, 28]. TNF production after inhaled Aspergillus is exaggerated in both immunocompetent animals that are repeatedly challenged with the fungus and in neutropenic hosts; in both of these settings, TNF is produced almost exclusively by recruited monocyte-derived cells [16, 29]. In neutropenia, TNF neutralization is harmful as it disrupts further recruitment of monocyte-derived DCs, which are critical to host defense against Aspergillus [16].
In the current study, we report a similar exaggerated TNF response in a CGD model that is necessary to the lung injury induced by the fungus, but we establish neutrophils as the primary source of TNF and show that neutrophil-derived TNF drives further neutrophil recruitment. We further found that, similar to findings in neutropenic wild-type mice, neutrophil depletion in gp91phox-deficient mice leads to compensatory TNF production by monocyte-derived inflammatory cells, resulting in a similar extent of lung injury. Importantly, TNF neutralization protected animals with CGD against lung injury and inhibited further neutrophil recruitment, implicating this cytokine in the pathologic inflammatory response to Aspergillus in CGD.
Independent of their microbicidal activity, ROS act as second messengers that regulate inflammation [30]. Mice with deletion of gp91phox, p47phox, or p40phox subunits of NADPH oxidase have enhanced production of multiple inflammatory cytokines and exaggerated leukocyte infiltration into the lungs in response to nonviable Aspergillus or zymosan [10, 11, 31, 32]. The mechanisms of lung injury in response to fungal antigens in CGD have not been studied extensively, and attention has focused on the role of mononuclear phagocytes and T helper 17 (Th17) immunity. Defects in monocyte and macrophage autophagy have been shown to lead to inflammasome activation in these cells [12, 33]. Consistent with this role for mononuclear phagocytes in initiating lung injury, restoration of NADPH oxidase in CD68+ cells alone is sufficient to abrogate pathologic inflammation in response to Aspergillus [34]. Downstream, IL-1β derived from mononuclear phagocytes has been shown to mediate IL-17responses in lung γδ T cells [12, 35], the latter controversially linked to dysregulated T-cell tryptophan metabolism [35, 36].
Our current study adds to this literature by establishing CGD neutrophils as a cell-autonomous driver of lung injury. We provide evidence that, once neutrophils arrive in the lungs, they perpetuate further neutrophil recruitment via release of neutrophil-derived TNF, as well subsequent induction of neutrophil-chemotactic chemokine ligands, including CXCL1 and CXCL2. We provide further evidence that neutrophil-derived TNF is necessary to production of IL-1β but not IL-17. TNF may lead to increased IL-1β through downstream activation of the inflammasome; however neutrophil-derived proteases can also process extracellular pro–IL-1β into the active form [37], and therefore inhibition of neutrophil recruitment may contribute to decreased IL-1β after TNF neutralization.
The identification of neutrophils as a source of TNF was an unexpected finding, because neutrophils are not a significant source for this cytokine in other contexts. Nearly all literature on TNF and neutrophils concerns the role of TNF in neutrophil priming, migration, and cell death, but we found an article reporting production of TNF by neutrophils isolated from 2 patients with CGD in response to lipopolysaccharide [38]. The normal interaction between TNF and ROS may nevertheless provide insights into our findings: TNF-mediated activation of NF-κB induces the expression of gp91phox [39], which, in turn, can inhibit NF-κB activity and control inflammation [40]. The loss of ROS-mediated NF-κB inhibition may upregulate TNF in neutrophils in the absence of NADPH oxidase after Aspergillus challenge. Another mechanism that may contribute to perpetuation of lung injury in this model relates to TNF-mediated neutrophil apoptosis [41, 42]. Resistance of CGD neutrophils to apoptosis [43, 44], and consequent impaired efferocytosis by mononuclear phagocytes [45, 46], may remove a negative feedback mechanism that would otherwise dampen inflammation. Finally, since the polymorphonuclear subset of myeloid-derived suppressor cells are indistinguishable from conventional neutrophils by flow cytometry [47] and can inhibit inflammation by a ROS-dependent mechanism [48], a reduction of this population may be postulated to explain the pathologic inflammation in mice with CGD.
The present work has several implications for future research. The potential benefit of controlling the hyperinflammatory response has been studied in patients with CGD colitis receiving the IL-1 receptor antagonist, anakinra. Anakinra was well tolerated in small case series, with some patients showing marked improvement in severity of disease, particularly early in the course of therapy [12, 49]. Small case series have also included patients with CGD-associated granulomatous colitis treated with the anti-TNF antibody, infliximab. These reports have described improved bowel inflammation but increased incidence of infectious complications associated with TNF inhibition [50, 51]. Our study provides a rationale for studying the administration of anti-TNF therapy to patients with CGD and life-threatening mulch pneumonitis. The risk of infection in mulch pneumonitis would require close scrutiny in the context of a study but may be mitigated by concomitant antifungal therapy. Finally, as noted above, the mechanism by which neutrophils of CGD hosts generate TNF is of substantial interest, because specific inhibition of such mechanisms may offer novel therapeutic targets without broad inhibition of TNF.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the University of Virginia Flow Cytometry Core for assistance with fluorescent automated cell sorting and McClinchey Histology Labs for assistance with lung histology.
Author contributions. R. E. C, T. J. B., and B. M. designed the experiments. R. E. C., with help from K. R. M., A. M. B., M. D. B., Y. S., and Z. Z., performed all experiments. R. E. C. analyzed and interpreted the data. R. E. C. and B. M. wrote the manuscript, and K. R. M., A. M. B., M. D. B., Y. S., and T. J. B. edited it.
Financial support. This work was supported by the National Institutes of Health (grants HL136903 to R. E. C.; HL33391 to T. J. B.; and EB024501, AI135128, and AI117397 to B. M.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dunogué B, Pilmis B, Mahlaoui N, et al. Chronic granulomatous disease in patients reaching adulthood: a nationwide study in France. Clin Infect Dis 2017; 64:767–75. [DOI] [PubMed] [Google Scholar]
- 2. Winkelstein JA, Marino MC, Johnston RB Jr, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine 2000; 79:155–69. [DOI] [PubMed] [Google Scholar]
- 3. Marciano BE, Spalding C, Fitzgerald A, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015; 60:1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumental S, Mouy R, Mahlaoui N, et al. Invasive mold infections in chronic granulomatous disease: a 25-year retrospective survey. Clin Infect Dis 2011; 53:e159–69. [DOI] [PubMed] [Google Scholar]
- 5. Codina R, Fox RW, Lockey RF, DeMarco P, Bagg A. Typical levels of airborne fungal spores in houses without obvious moisture problems during a rainy season in Florida, USA. J Investig Allergol Clin Immunol 2008; 18:156–62. [PubMed] [Google Scholar]
- 6. Aimanianda V, Bayry J, Bozza S, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009; 460:1117–21. [DOI] [PubMed] [Google Scholar]
- 7. Gazendam RP, van Hamme JL, Tool AT, et al. Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. J Immunol 2016; 196:1272–83. [DOI] [PubMed] [Google Scholar]
- 8. Henriet SS, Jans J, Simonetti E, et al. Chloroquine modulates the fungal immune response in phagocytic cells from patients with chronic granulomatous disease. J Infect Dis 2013; 207:1932–9. [DOI] [PubMed] [Google Scholar]
- 9. Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol 2011; 127:1243–52.e7. [DOI] [PubMed] [Google Scholar]
- 10. Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med 1997; 185:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Segal BH, Han W, Bushey JJ, et al. NADPH oxidase limits innate immune responses in the lungs in mice. PloS One 2010; 5:e9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Luca A, Smeekens SP, Casagrande A, et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A 2014; 111:3526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Segal BH, Veys P, Malech H, Cowan MJ. Chronic granulomatous disease: lessons from a rare disorder. Biol Blood Marrow Transplant 2011; 17:S123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqui S, Anderson VL, Hilligoss DM, et al. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin Infect Dis 2007; 45:673–81. [DOI] [PubMed] [Google Scholar]
- 15. Ameratunga R, Woon ST, Vyas J, Roberts S. Fulminant mulch pneumonitis in undiagnosed chronic granulomatous disease: a medical emergency. Clin Pediatr (Phila) 2010; 49:1143–6. [DOI] [PubMed] [Google Scholar]
- 16. Park SJ, Burdick MD, Brix WK, et al. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 2010; 185:6190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SJ, Burdick MD, Mehrad B. Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect Immun 2012; 80:1759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollock JD, Williams DA, Gifford MA, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 1995; 9:202–9. [DOI] [PubMed] [Google Scholar]
- 19. Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med 2007; 175:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barletta KE, Cagnina RE, Burdick MD, Linden J, Mehrad B. Adenosine A2B receptor deficiency promotes host defenses against gram-negative bacterial pneumonia. Am J Respir Crit Care Med 2012; 186:1044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barletta KE, Cagnina RE, Wallace KL, Ramos SI, Mehrad B, Linden J. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J Immunol Methods 2012; 375:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matute-Bello G, Downey G, Moore BB, et al. ; Acute Lung Injury in Animals Study Group . An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011; 44:725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roy J, Galano JM, Durand T, Le Guennec JY, Lee JC. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J 2017; 31:3729–45. [DOI] [PubMed] [Google Scholar]
- 24. Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol 1999; 162:1633–40. [PubMed] [Google Scholar]
- 25. Rex JH, Bennett JE, Gallin JI, Malech HL, Melnick DA. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infect Dis 1990; 162:523–8. [DOI] [PubMed] [Google Scholar]
- 26. Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol 1999; 163:6086–94. [PubMed] [Google Scholar]
- 27. Brieland JK, Jackson C, Menzel F, et al. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect Immun 2001; 69:1554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc 2008; 83: 181–94. [PubMed] [Google Scholar]
- 29.. Fei M, Bhatia S, Oriss TB, et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A 2011; 108:5360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014; 20:1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellson CD, Davidson K, Ferguson GJ, O’Connor R, Stephens LR, Hawkins PT. Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med 2006; 203:1927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Endo D, Fujimoto K, Hirose R, et al. Genetic phagocyte NADPH oxidase deficiency enhances nonviable Candida albicans-induced inflammation in mouse lungs. Inflammation 2017; 40:123–35. [DOI] [PubMed] [Google Scholar]
- 33. Oikonomou V, Moretti S, Renga G, et al. Noncanonical fungal autophagy inhibits inflammation in response to IFN-γ via DAPK1. Cell Host Microbe 2016; 20:744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grimm MJ, Vethanayagam RR, Almyroudis NG, et al. Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J Immunol 2013; 190:4175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 2008; 451:211–5. [DOI] [PubMed] [Google Scholar]
- 36. Maghzal GJ, Winter S, Wurzer B, Chong BH, Holmdahl R, Stocker R. Tryptophan catabolism is unaffected in chronic granulomatous disease. Nature 2014; 514:E16–7. [DOI] [PubMed] [Google Scholar]
- 37. Afonina IS, Müller C, Martin SJ, Beyaert R. proteolytic processing of interleukin-1 family cytokines: variations on a common theme. Immunity 2015; 42:991–1004. [DOI] [PubMed] [Google Scholar]
- 38. Hatanaka E, Carvalho BT, Condino-Neto A, Campa A. Hyperresponsiveness of neutrophils from gp 91phox deficient patients to lipopolysaccharide and serum amyloid A. Immunol Lett 2004; 94:43–6. [DOI] [PubMed] [Google Scholar]
- 39. Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 2006; 281:5657–67. [DOI] [PubMed] [Google Scholar]
- 40. Trevelin SC, Dos Santos CX, Ferreira RG, et al. Apocynin and Nox2 regulate NF-κB by modifying thioredoxin-1 redox-state. Sci Rep 2016; 6:34581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J Immunol 2000; 165:435–41. [DOI] [PubMed] [Google Scholar]
- 42. Dyugovskaya L, Polyakov A, Ginsberg D, Lavie P, Lavie L. Molecular pathways of spontaneous and TNF-α-mediated neutrophil apoptosis under intermittent hypoxia. Am J Respir Cell Mol Biol 2011; 45:154–62. [DOI] [PubMed] [Google Scholar]
- 43. Fadeel B, Ahlin A, Henter JI, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood 1998; 92:4808–18. [PubMed] [Google Scholar]
- 44. Kasahara Y, Iwai K, Yachie A, et al. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood 1997; 89:1748–53. [PubMed] [Google Scholar]
- 45. Sanmun D, Witasp E, Jitkaew S, et al. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am J Physiol Cell Physiol 2009; 297:C621–31. [DOI] [PubMed] [Google Scholar]
- 46. Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 2009; 113:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004; 172:989–99. [DOI] [PubMed] [Google Scholar]
- 49. Hahn KJ, Ho N, Yockey L, et al. Treatment with anakinra, a recombinant IL-1 receptor antagonist, unlikely to induce lasting remission in patients with CGD colitis. Am J Gastroenterol 2015; 110:938–9. [DOI] [PubMed] [Google Scholar]
- 50. Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-α blockade in chronic granulomatous disease-related colitis. Clin Infect Dis 2010; 51:1429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deffert C, Olleros ML, Huiping Y, et al. TNF-α blockade in chronic granulomatous disease-induced hyperinflammation: patient analysis and murine model. J Allergy Clin Immunol 2011; 128:675–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.