Abstract
Synonymous single nucleotide variants (sSNVs) are common in the human genome but are often overlooked. However, sSNVs can have significant biological impact and may lead to disease. Existing computational methods for evaluating the effect of sSNVs suffer from the lack of gold-standard training/evaluation data and exhibit over-reliance on sequence conservation signals. We developed synVep (synonymous Variant effect predictor), a machine learning-based method that overcomes both of these limitations. Our training data was a combination of variants reported by gnomAD (observed) and those unreported, but possible in the human genome (generated). We used positive-unlabeled learning to purify the generated variant set of any likely unobservable variants. We then trained two sequential extreme gradient boosting models to identify subsets of the remaining variants putatively enriched and depleted in effect. Our method attained 90% precision/recall on a previously unseen set of variants. Furthermore, although synVep does not explicitly use conservation, its scores correlated with evolutionary distances between orthologs in cross-species variation analysis. synVep was also able to differentiate pathogenic vs. benign variants, as well as splice-site disrupting variants (SDV) vs. non-SDVs. Thus, synVep provides an important improvement in annotation of sSNVs, allowing users to focus on variants that most likely harbor effects.
INTRODUCTION
The recent increase in accessibility of sequencing has facilitated a rise in precision medicine efforts focused on the interpretation of the effects of individual-specific genome variation (1). Genome-wide association studies (GWAS) have identified multiple variants marking specific phenotypes (2). However, the evaluation of variants in terms of their functional contributions to molecular pathogenicity mechanisms holds promise for both a better understanding of disease and drug discovery/optimization (3). SNVs (single nucleotide variants) are the most common variants in the human genome (4). Three types of SNVs are of particular interest—regulatory (i.e. changing the quantity/production of the gene product, e.g. transcription or splice site variants), non-synonymous (i.e. altering product protein sequence), and synonymous (i.e. variants in protein-coding regions that, due to the degeneracy of the genetic code, do not alter the protein sequence). Many computational tools have been developed to evaluate the functional effects of regulatory and non-synonymous variants (5,6). However, while an individual genome carries as many synonymous as non-synonymous SNVs (7), the former are often disregarded as functionally irrelevant. Still, sSNVs can cause disease (8) and affect gene function via multiple mechanisms, including binding of transcription factors (9), splicing (10), mRNA stability (11–13), co-translational folding (14–16), etc., as reviewed in our earlier work (17).
Existing methods for predicting sSNV effects are either (1) sSNV-specific tools, including SilVA (18), reg-SNP-splicing (19), DDIG-SN (20), TraP (21) and IDSV (22), or (2) general-purpose ones, including CADD (23,24), DANN (25), FATHMM-MKL (26), and MutationTaster2 (27). The number of computational sSNV effect predictors is limited in comparison to that of nsSNV (non-synonymous single nucleotide variant) effect predictors, as reviewed in (6,17). Partially, this paucity is due to the limited available experimental data evaluating variant effects, which could be used for training or testing of such methods. In fact, all existing predictors, except CADD and DANN, are trained using ‘pathogenic’ variants from databases such as Human Gene Mutation Database (HGMD) (28) and ClinVar (29). Here we note that ‘pathogenicity’ is not equivalent to ‘functional effect’ (30,31) and inferring variant-disease causality is complicated by this inequality. The experimental disease variant annotations are also often unreliable (17), as it is difficult to distinguish causative variants from simply associated ones. Moreover, the pathogenic label is inconsistent across databases, and possibly over time/database releases. Finally, even these labeled effect variants are few; even fewer are experimentally labeled neutral polymorphisms. Thus, predictors trained on these variants are likely insufficient to predict the effects of tens of millions of possible sSNVs in human genome.
Using positive-unlabeled learning (32–34), we inferred a subset of human sSNVs that could be used for training a predictor of sSNV molecular effect. We then developed, synVep (synonymous Variant effect predictor), a machine learning-based method for scoring putative effect for each possible human sSNV. synVep discriminated experimentally validated pathogenic sSNVs from randomly sampled common variants. Its predictions also displayed the expected trends (35) in evolutionary distances between orthologs, where the sSNVs corresponding to evolutionarily-close human relatives’ (e.g. chimp) reference nucleotide, have lower effect scores than those corresponding to the nucleotides of further away organisms (e.g. fruitfly). Furthermore, nucleotides that are not identified in any of the species evaluated here are deemed to have most effect when substituted into the human reference. However, many of the sSNVs that are not observed in the human population, tend to be scored very high (most effect), regardless of their appearance in other species.
In line with our earlier observations (17), we find that the variant frequency in the population is poorly correlated with the effect score; i.e. rare variants are about equally likely to have no effect on gene function as common variants (65% common versus 69% rare). synVep does not rely on conservation and is developed without an experimental or explicitly evolutionarily estimated gold-standard training/development set. Its success thus suggests the feasibility of a similar approach for the development of a training set for other variant types, e.g. nsSNVs or indels. We expect that synVep predictions will greatly contribute to our understanding of pathogenicity pathways and to the prioritization of synonymous variants in disease.
MATERIALS AND METHODS
Data collection
We extracted all 93 437 human protein-coding transcripts from the Ensembl BioMart (36) GRCh37 p.13 assembly (37) and discarded the ones containing unknown nucleotides, lacking a start/stop codon, or having patched (https://grch37.ensembl.org/Homo_sapiens/Info/Annotation) chromosome IDs. We then generated all possible sSNVs for the remaining 72 400 transcripts. We further used ANNOVAR (38) (installed 5 August 2019) to extract sSNVs in these transcripts, and their allele-count based frequencies, from the Genome Aggregation Database exome subset (gnomAD exome) (39). An sSNV present in gnomAD was labeled a singleton if it was seen in only one individual and otherwise labeled observed. Generated sSNVs were those in the set of all possible variants in the 72,400 transcripts that were not singleton or observed. Thus, we collected 4 160 063 observed, 3 438 470 singleton and 57 208 450 generated sSNVs (https://zenodo.org/record/4763256). Note that these correspond to 1 520 334 observed, 1 233 878 singleton and 21 314 668 generated sSNVs with unique genomic coordinates and reference/alternative alleles, i.e. in one transcript per gene.
To evaluate and compare the performance of our predictor to other predictors, we manually curated a dataset of 42 curated-effect sSNVs with known biological effects, including the 33 pathogenic variants from the Buske et al. study (18). We required that all sSNVs in this set were strongly associated with disease and that there was experimental evidence of their molecular effects. These 42 sSNVs (Supplementary Table S1) mapped to 170 transcript-based sSNVs and were excluded from model training throughout this manuscript.
Variant features
We collected 35 variant and sequence features (Supplementary Table S2), grouped into six categories: codon bias and autocorrelation (ten), protein structure (three), mRNA stability (eight), distance to regulatory factors (four), expression profile (three), and miscellaneous (seven). The reasons for selecting these features are described in our earlier paper (17). We further calculated the correlation of feature values across all sSNVs using the dython package (v0.6.7, https://github.com/shakedzy/dython), where correlations between continuous-continuous, continuous-categorical, and categorical-categorical features were computed using Pearson correlation (40), Cramer's V (41) and correlation ratio (42), respectively. Feature importance was obtained by calculating the average performance gain across all splits where the feature was present.
Transcript expression profiles
We downloaded the GTEx (43) ‘Transcript TPMs’ dataset (dbGaP Accession phs000424.v7.p2) and standardized the transcript expression across tissue samples. We then used the average expression of each transcript over all samples from the same tissue as the representative transcript expression for that tissue.
Calculations of some of the codon bias metrics described below require a reference set of coding sequences, which are typically a set of highest expressed transcripts (44). To identify these references, we collected the maximum expression values for all transcripts across the 53 tissues. We then selected the transcripts within the highest 1% expression per tissue. We also used log10 (minimum expression per tissue), log10 (median expression per tissue) and log10 (maximum expression per tissue) for each transcript as features.
Codon bias and autocorrelation
A variety of measures and/or their ‘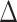 ’ form (difference in measure value after mutation versus value before mutation) are adopted as features to characterize the codon bias of transcripts (see Supplementary text for more details), including the Codon Adaptation Index (CAI, Supplementary text Equation S1) (44), Fraction of Optimal Codons (fracOpt, Supplementary text Equation S2) (45), Codon Usage Bias (CUB, Supplementary text Equation S3) (46), Intrinsic Codon Deviation Index (ICDI, Supplementary text Equation S4) (47), Synonymous Codon Usage Order (SCUO, Supplementary text Equation S5) (48), and tRNA Adaptation Index (tAI, Supplementary Equation S6) (49). The calculation of these values was performed in R (50) and is available as an R package in https://bitbucket.org/bromberglab/codonbiasmetrics/src/master/.
’ form (difference in measure value after mutation versus value before mutation) are adopted as features to characterize the codon bias of transcripts (see Supplementary text for more details), including the Codon Adaptation Index (CAI, Supplementary text Equation S1) (44), Fraction of Optimal Codons (fracOpt, Supplementary text Equation S2) (45), Codon Usage Bias (CUB, Supplementary text Equation S3) (46), Intrinsic Codon Deviation Index (ICDI, Supplementary text Equation S4) (47), Synonymous Codon Usage Order (SCUO, Supplementary text Equation S5) (48), and tRNA Adaptation Index (tAI, Supplementary Equation S6) (49). The calculation of these values was performed in R (50) and is available as an R package in https://bitbucket.org/bromberglab/codonbiasmetrics/src/master/.
These measures describe codon bias from different perspectives. CAI, fracOpt and CUB rely on a reference set of optimal codons, found in highly expressed genes (51). CAI computes the geometric mean of relative usage of a codon compared to the most frequently used codon for the same amino acid (44). fracOpt is the fraction of optimal codons in a sequence of a certain length. CUB weighs the frequency of amino acids in calculating codon bias. ICDI is independent of a reference set of genes (47). SCUO borrows the idea of entropy from Shannon information theory to describe codon usage bias of sequences (48). tAI focuses on translational efficiency by taking tRNA levels into account (49).
We also considered codon autocorrelation – a feature that has not yet been used by any sSNV predictors. In autocorrelated sequences, same codons cluster together, whereas they are separated in anticorrelated sequences (e.g. XXXYYY is more autocorrelated than XYXYXY, where X and Y are two different codons) (52). Cannarozzi et al. noted the association between codon autocorrelation and translation dynamics and proposed the tRNA pairing index (TPI) to describe a sequence's codon autocorrelation. Autocorrelated sequences benefit from rapid translation due to the recycling of isoaccepting tRNAs (52). However, we note that the significance of recycling is likely weaker if the interval between two issoaccepting codons is larger—a feature that is not accounted for in TPI. Therefore, we proposed a new measure, Codon Autocorrelation Measure (CAM, Supplementary Equation S7), to describe the variant-specific codon autocorrelation impact penalized by the distance between the synonymous codons.
Finally, we also introduced the change of frequency measure (CF, Supplementary text Equation S8), to describe the amount of impact on codon's frequency in a sequence due to the introduction of the variant.
Distance to regulatory and splicing sites
We used as features the distances to the nearest splice sites, transcription factor binding site (TFBS), RNA-binding protein (RBP) motif, and exonic splicing regulator (ESR). Their genomic coordinates were obtained from different sources as described below. We then computed the distance of a variant (in nucleotides) to all regulatory sites and selected the minimum value as the feature distance. We categorized these distances (d) into six categories as feature inputs: d = 0, 0 < d ≤ 3, 3 < d ≤ 5, 5 < d ≤ 10, 10 < d ≤ 20 and d > 20.
Genomic coordinates of regulatory regions were inferred as follows: (i) Splice sites were inferred from the ‘Genomic coding start’ and ‘Genomic coding end’ of all human protein-coding transcripts annotated in Ensembl BioMart GRCh37 p.13 assembly. (ii) We downloaded the Gene Transcription Regulation Database (GTRD, version 18.06) (53) and identified the genomic coordinates of TFBS, using hg38 to hg19 conversion via CrossMap (54) for correspondence with our transcript coordinates. (iii) We downloaded the ATtRACT database of RNA binding proteins and AssoCiated moTifs (55) and mapped the human RPB motifs to our set of transcript sequences. (iv) We also downloaded the supplementary data of Cáceres et al. (56) gold standard ESR motif set and mapped these to our transcripts.
Protein structure
We ran PredictProtein (57), a collection of tools for protein structure predictions, on all of the translated transcript sequences. We were particularly interested in protein secondary structure (PSS), residue solvent accessibility (SS) and disorder (PD) predictions; in PredictProtein, PROFphd (58) predicts PSS and SS, while Meta-disorder (MD) (59) predicts PD.
mRNA stability, structure and structural changes
We ran RNAfold (60) to predict (with calculation of partition function and base pairing probability matrix) the secondary structure and stability of all transcripts. We extracted the frequency of the structure with minimum free energy (MFE) in the structure ensemble, the free energy of the centroid structure, and its distance to the structure ensemble, as well as the local mRNA structure (strongly paired, strongly up/down -stream paired, weakly paired, weakly up/down-stream paired, or unpaired bases).
We also used RNAsnp (61) to predict the variant-induced local secondary structure changes for all sSNVs. The ‘mode’ and ‘winsizeFold’ parameters should be assigned according to the length in nucleotides (L) of the input sequence. We assigned the parameters as follows: (i) for L ≤ 200, mod = 1 and winsizeFold = 100; (ii) for 200 < L ≤ 500, mod = 1 and winsizeFold = 200; (iii) for L > 500, mod = 2 and winsizeFold = 500. We recorded the local structure dissimilarity, global structural dissimilarity and their statistical significance (P-values).
Model construction
Classifier setup
We standardized all continuous features and label-encoded categorical features. We compared two classifiers for differentiating observed and generated variants: deep neural network (DNN) (62) and XGBoost (63); we selected XGBoost as the classification algorithm for its higher accuracy and speed (preliminary experiment described in Supplementary Text, Page 2). XGBoost is implemented in Python (v3.6.4) xgboost package (v0.8.2) integrated with sci-kit learn (0.20.3) (64) (https://xgboost.readthedocs.io/en/latest/python/python_api.html).
Balancing variant data by transcript
The generated set of sSNVs is much larger than the observed set, but the number of observed sSNVs per transcript varies greatly. Moreover, some classifier input features are transcript-specific. Thus, a predictor may ‘memorize’ transcripts that have more observed sSNVs, and preferentially assign its variants observed status, instead of finding variant-specific differences between observed and generated. To avoid this, we assigned sampling likelihood weights for the generated set, i.e. the sampling likelihood weight of a generated variant is the number of observed sSNVs in the corresponding transcript. In all further balancing of data sets, generated sSNVs were probabilistically added to the set on the basis of their weights. Thus, the number of generated sSNVs on a transcript that were selected for a particular training set was correlated with the number observed sSNVs on this transcript.
Positive unlabeled learning (PUL) to identify unobservable sSNVs
PUL is a semi- supervised approach applicable to scenarios where only positive data points are labeled and the rest can be positive or negative (32–34). We employed the modified version of PUL (34) to separate the generated sSNVs into unobservable and not-seen sets. To prevent overfitting, we adopted relatively conservative hyperparameters of XGBoost (100 trees [n_estimor], 5 maximum depth [max_depth], 30% of the features per tree [colsample_bytree], 30% subsamples per tree [subsample]). We left out from PUL a fraction of observed as a test set, aiming to reach <5% incorrect predictions for this set at the end of the PUL.
In one epoch of PUL, a classifier was trained to differentiate the observed sSNVs from the same number of unlabeled ones (generated; selected via transcript-based set balancing as described above). All unlabeled sSNVs, including the ones not used in training, were evaluated with the resulting model and the generated variants classified as observed (scoring below 0.5) were added to the not-seen pool. The PUL process was repeated until convergence (Supplementary text). sSNVs scoring >0.5 in prediction from the last PUL model were further excluded from our data set. One pitfall of this PUL strategy is that a fraction of the unlabeled samples may become positive (observed) with more sequencing in the future.
Differentiating the observable from not-seen using an intermediate model
We trained a model to differentiate the observable sSNVs from the not-seen sSNVs (termed intermediate model from here on). We excluded 10% (9274) of the common sSNVs (MAF > 0.01; excluded set) and all curated-effect sSNVs (170) from the construction of the intermediate model for testing and final model parameter optimization. We split the observed sSNVs into subsets of 9: 0.5: 0.5 size ratio for training (3 631 441 variants), validation and testing (201 746 variants each). We then randomly sampled the not-seen variants to match the observed validation and test set sizes; this left 47 923 258 not-seen sSNVs for training. We then up-sampled the 3.6M observed variants in the training set to create a balanced set of 47 923 258 observed and not-seen variants, each). We tuned the model hyperparameters by optimizing the F-score (Equation 3) of performance on the validation set and evaluated the resulting model on the test set.
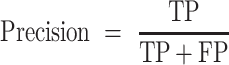 |
(1) |
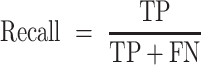 |
(2) |
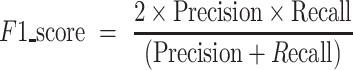 |
(3) |
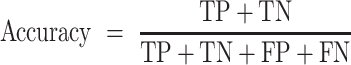 |
(4) |
where TP, TN, FP, FN are respectively, true positive, i.e. observed sSNVs predicted to be observed; true negative, not-seen sSNVs predicted to be not-seen; false positive, not-seen sSNVs predicted to be observed; false negative, observed sSNVs predicted to be not-seen.
Final model (synVep) training
We used the intermediate model to score the excluded common and curated-effect sSNVs, as well as all observed and not-seen sSNVs. Here we assumed that common variants should be enriched in no-effect/neutral variation. Based on the scores of excluded sSNVs, we defined effect and no-effect synVep development sets, where sSNVs (both observed and not-seen) scoring above the median of the curated-effect predictions were deemed effect; while sSNVs (both observed and not-seen) scoring below the median of the excluded common sSNV predictions were labeled no-effect. We thus collected 7 385 137 no-effect and 32 117 625 effect sSNVs.
We split the no-effect and effect sSNVs into subsets of 9:0.5:0.5 size ratio (in the same way as for the intermediate model) for training, validation and test sets (62 758 222:735 194:735 194 variants per set). We sampled equal numbers of effect sSNVs to match the no-effect sSNVs in validation and test sets. We trained the final model on the training set using the hyperparameters optimized (F-score; Equation 3) on the validation set. We finally evaluated the model on the test set. Note that none of the curated-effect, the excluded common sSNVs, or the ClinVar (described below) dataset variants were included in our model training.
Performance comparison with other predictors
For all comparisons with other predictors, we calculated the area under the receiver operating characteristic curve (auROC) using the pROC package (65) (v1.17.0.1), and the area under the precision recall curve (auPRC) using PRROC (66) (v1.3.1). Statistical significance of the differences between the auROC/auPRC of synVep and those of other predictors were tested using the pROC package (bootstrap method with the default settings, n = 2000; source code was modified to accommodate testing for auPRC).
Common/curated-effect dataset comparison
To evaluate synVep in comparison with other predictors, we used the 170 (transcript-based; 42 genomic coordinate-based) curated-effect sSNVs and the 9274 (transcript-based; 7957 genomic coordinate-based) excluded common sSNVs. Here, we again assumed that common variants should be enriched in no-effect/neutral variation.
Other predictors in this comparison included: CADD (phred-like scaled scores) (23), DANN (25), FATHMM-MKL (26), DDIG-SN (20) and EIGEN (67). EIGEN scores were collected using ANNOVAR (38) annotations; for other predictors, the scores were collected with default parameters as described in our earlier work (17). We did not include SilVA (18) or TraP (21) in this comparison because 33 of 42 of the curated-effect sSNVs are in their training sets.
Note that synVep scores are produced per variant per transcript, while other predictors use the genomic coordinates, i.e. one reference sequence per variant. For the purposes of our comparison, we randomly re-sampled each tool's predictions of the effect set (42 variant scores) to produce 170 scores. Furthermore, as the common sSNVs (putatively no-effect) outnumbered the effect set, we randomly sampled 170 common variant scores in 100 comparison iterations. For each sampling, we performed a one-sided permutation test (null hypothesis: mean of common variant scores is equal to mean of effect scores; alternative hypothesis: mean of common variant sores is lower than mean of effect scores) and recorded the P-value and the corresponding method accuracy (Equation 4).
We also computed the Spearman correlation across predictor scores and the Fraction of Consensus Binary Predictions (FCBP; i.e. the number of binarized predictions agreed upon by all predictors, divided by total number of predictions) (17). An effect/no-effect scoring threshold for the FCBP computation is required; we used the default value of score = 0.5 for DANN, FATHMM-MKL and DDIG-SN. For CADD, we used score = 15 as the threshold recommended by its online documentation (https://cadd.gs.washington.edu/info). As there was no recommended cutoff in the EIGEN publication (67), we selected the cutoff (score = 1.35) at the 75-percentile of EIGEN scores of 1000 randomly sampled observed sSNVs.
ClinVar dataset comparison
We downloaded all ClinVar (68) submissions from the FTP site (https://ftp.ncbi.nlm.nih.gov/pub/clinvar/) and identified the sSNVs among these. We only considered the sSNVs with the ‘reviewed by expert panel’ review status. From these we selected the (i) pathogenic and pathogenic/likely pathogenic variants as the pathogenic set and (ii) benign and benign/likely benign as the benign set. There were 51 benign (genomic coordinate-based; 254 transcript-based) and 17 pathogenic (genomic coordinate-based; n = 68 transcript-based) sSNVs (Supplementary Table S3). We also annotated these ClinVar sSNVs with the precomputed GERP++ scores (http://mendel.stanford.edu/SidowLab/downloads/gerp/) (69).
Splicing dataset comparisons
We downloaded and analyzed a dataset of SNV splicing effects (70) (https://github.com/KosuriLab/MFASS), referenced by genomic coordinates and Ensembl transcript IDs. For comparison with synVep, we downloaded and ran spliceAI (71) (https://github.com/Illumina/SpliceAI) and retrieved CADD-splice (72) annotation from https://cadd.gs.washington.edu/score. spliceAI predictions are composed of probabilities of splice acceptor and donor's gain and loss. Since these outputs are predominantly zero, we took the maximal value for evaluation purpose, as in (72).
Cross-species sequence variation (CSV) analysis
Cross-species variation (CSV) describes the nucleotide difference between the human reference sequence and the ortholog reference sequence of another species. In this study, we selected 20 species to generate CSVs: yeast (Saccharomyces cerevisiae), worm (Caenorhabdiis elegans), fruitfly (Drosophila melanogaster), zebrafish (Danio rerio), xenopus (Xenopus laevis), anole lizard (Anolis carolinensis), chicken (Gallus gallus), platypus (Ornithorhynchus anatinus), opossum (Monodelphis domestica), dog (Canis familiaris), pig (Sus scrofa), dolphin (Tursiops truncatus), mouse (Mus musculus), rabbit (Oryctolagus cuniculus), tree shrew (Tupaia belangeri), tarsier (Carlito syrichta), gibbon (Nomascus leucogenys), gorilla (Gorilla gorilla), bonobo (Pan paniscus), and chimpanzee (Pan troglodytes).
To represent the evolutionary distance of the CSV species to human, we obtained the value in million years since divergence from the TimeTree database (73). Given a human transcript T and its corresponding human gene G, we queried Ensembl BioMart for G’s orthologs in the 20 species, Gorthologs = [Gyeast, Gworm, Gfruitfly, …, Gchimpanzee]. We downloaded all coding DNA sequences (CDS) for these orthologs from Ensembl (release-94) (74). For each gene in Gorthologs, we identified its longest transcript per organism, Torthologs = [Tyeast, Tworm, Tfruitfly, …, Tchimpanzee]. We then used PRANK (75) to generate a multiple sequence alignment (MSA) for each T. PRANK aligns CDSs by first translating them into protein sequences so that gaps tend to be placed between codons, instead of within codons. For each codon in each human transcript, we could identify if other organisms carried the same codon or another, even if the amino acid remained the same. If the codon was different, the corresponding human sSNV was termed a CSV.
Evaluation of synVep predictions according to constraint on coding regions
Constrained regions (76), referenced by genomic coordinates, were downloaded from https://s3.us-east-2.amazonaws.com/ccrs/ccr.html. The constraint of human coding region is measured by percentile (of residuals from a linear regression for distance-to-mutation prediction as computed in (76)), where a high percentile indicates a more constrained region. We annotated the sparsity of sSNVs, i.e. the fraction of observed sSNVs among all possible sSNVs in a region of a certain constraint level, and the median synVep prediction of variants in these regions.
Analysis of sSNVs identified in Qatari Genome
We downloaded all VCF files containing variants identified from the Qatari Genome project (QTRG) (77) from NCBI Sequence Read Archive (78) (https://trace.ncbi.nlm.nih.gov/Traces/sra/?study=SRP061943). We then parsed these VCF files, extracted the variants, and mapped the sSNVs to our observed, singleton, not-seen, and unobservable sets.
RESULTS AND DISCUSSION
Generated sSNVs may be observable in the future
In the absence of a gold-standard experimentally validated data set describing sSNV functional effects, we sought an alternative for the development of our method. We had previously proposed to use sSNVs that have been observed in major sequencing projects versus all other possible human genome sSNVs (the generated set) for method evaluation (17). We collected 72 400 human transcripts with 4 160 063 (n = 1 520 334 genomic coordinate-based) observed sSNVs and 3 438 470 (n = 1 233 878 genomic coordinate-based) singletons (observed in only one individual) from the exome sequencing data of the Genome Aggregation Database (gnomAD exome) (39). We then created a generated set of 57 208 450 (n = 21 314 668 genomic coordinate-based) all possible sSNVs in these transcripts that were not found in gnomAD data. Note that only ∼12% of all sSNVs in our set were ever reported by gnomAD. We annotated these sSNVs with 35 transcript- and variant-specific features, including codon bias, codon autocorrelation, transcript stability, expression level, distance to regulatory sites, predicted protein secondary structures, etc. (Methods; Supplementary Table S2).
While the observed sSNVs are not necessarily functionally neutral, they are at least compatible with life. The generated sSNVs, on the other hand, likely comprise two subtypes: the not-seen sSNVs, which may or may not become observed with more sequencing, and the unobservable ones, which cannot be observed given the contemporary variant-discovery capability. Note that the unobservable character of sSNVs may be due to a broad range of technical and biological reasons such as sequencing (79,80), molecular functional constraints (81), and analytical biases or extreme deleteriousness resulting in early embryonic incompatibility with life (82,83). We also note that in our modeling, the unobservable set may simply be poorly described by our selection of variant features.
We used observed sSNVs as positives in positive-unlabeled learning (PUL) (32–34) to differentiate the not-seen sSNVs (similar to observed) from unobservable ones in the generated (unlabeled) set (Figure 1). At convergence (epoch 63, Methods; Supplementary Figure S1), PUL partitioned all generated sSNVs into unobservable (n = 6 278 254 transcript-based and 2 764 229 genomic coordinate-based; 11%) and not-seen (n = 50 930 196 transcript-based and 19 730 623 genomic coordinate-based; 89%). Additionally, 8% (n = 266 192) of singletons were deemed unobservable by the PUL model, as were 2% of the observed sSNVs (n = 79 639). The latter result highlights the possible insufficiency of our variant descriptors for capturing the complete observable variant diversity, while the former may also indicate sequencing errors. The difference in percentages of variants misidentified by the model (11% of generated versus 2% of observed), however, suggests that deleteriousness of variants also plays a role in defining unobservable variants.
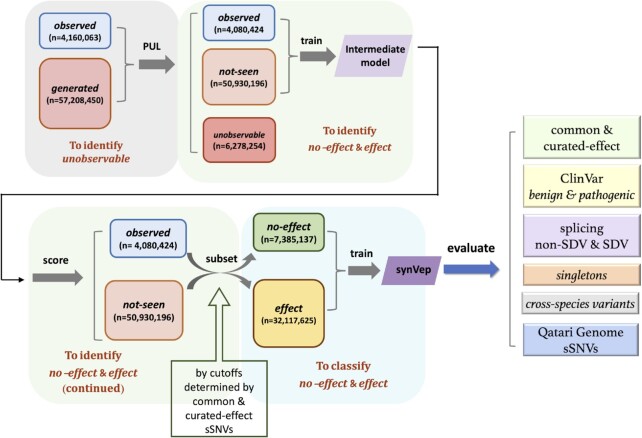
Figure 1.
Pipeline of predictor construction. Starting with 4 160 063 observed and 57 208 450 generated sSNVs, 63 epochs of positive unlabeled learning (PUL) was conducted to separate the generated set into not-seen (observable) and unobservable set (Supplementary text). An intermediate model was trained using the observed and not-seen sets (observed set was up-sampled to equal amount of not-seen variants). The intermediate model's predictions for common and pathogenic sSNVs were used as guideline to set cutoffs assigning no-effect and effect set. The final predictor was trained using the no-effect and effect sets (no-effect set was up-sampled to equal amount of effect sets). After the final synVep model was trained, it was evaluated on independent datasets as shown. Here, singletons are sSNVs found in only one individual in gnomAD; observed are any other sSNVs found in gnomAD; generated are all possible sSNVs, except singletons or observed; unobservable are sSNVs PUL-labeled to be unlike the observed; not-seen are any other generated sSNVs; effect/no-effect are sSNVs that affect/do not affect the function or quantity of a gene product.
Observed and not-seen variant sets contain both no-effect and effect sSNVs
We trained the intermediate model (Figure 1) to recognize observed vs. not-seen sSNVs. The model accurately (F-score = 0.71; Equation 3) recognized the two classes in a previously unseen variant test set (Supplementary Figure S2). It also predicted 9% (9 282 542) of the not-seen sSNVs to be observed (scoring < 0.5), implying that these may be sequenced in the future.
Although large effect sSNVs may be enriched in the not-seen group, the intermediate model cannot be directly used to evaluate effect, because it is only meant to predict whether an sSNV has been observed or not. To build a model for effect evaluation, we leveraged the intermediate model's predictions on common variants excluded from training and the experimentally validated effect sSNVs (curated-effect; Methods; 170 transcript-based sSNVs). While these curated-effect sSNVs are, in fact, observed, their prediction scores were higher than those of the excluded common set (Supplementary Figure S2, Mann–Whitney U test P-value < 2.2e–16). This observation is likely due to the fact that the not-seen set is enriched, while the common variant set is depleted, in large effect sSNVs. We assume this for common variants because large-effect deleterious variants would not become common and large-effect advantageous variants would tend to become wild-type.
We excluded 10% (7957) of the common sSNVs from training of the intermediate model for selecting the cutoff of effect/no-effect variants as next described. For training of the final model, we selected as no-effect those sSNVs (both observed and not-seen) scoring below the intermediate model prediction median (0.38) of the excluded common sSNVs; variants scoring above the intermediate model median of the curated-effect sSNVs (score = 0.63) were labeled effect (Supplementary Figure S2). We thus obtained 7 385 137 (2 580 540 observed and 4 804 597 not-seen) no-effect and 32 117 625 (405 170 observed and 31 712 455 not-seen) effect sSNVs. We trained the final model (synVep, Figure 1) to differentiate the no-effect and effect sSNVs (in balanced class training), using a 9:0.5:0.5 split of data for training, validation, and testing purposes (Methods). synVep was accurate (F-score = 0.90; binary score cutoff = 0.5) in evaluating the hold-out test set (369 257 no-effect and 369 257 effect). Note that synVep prediction scores did not correlate with allele frequency (Pearson correlation = 0.02).
Feature importance in discriminating effect
We collected 35 features (Supplementary Table S2; Methods) highlighting the different ways how sSNVs can impact gene function (17). We examined the correlation of feature scores across all sSNVs (Supplementary Figure S3; Methods) and computed feature importance for the final model (Supplementary Figure S4; Methods). Feature scores correlated within the same feature category for some categories (e.g. codon context, codon bias, and expression profile), but not across different categories. The most important feature for our model was codon_mutation (i.e. the wild type/mutant codon pair), which is consistent with our earlier observation that some codons are preferentially mutated in observed sSNVs (17) and with the Karczewski et al. (39) observation that CpG-transitions in the population are closer to saturation than other mutation types. Another codon context feature – next_codon, which is highly correlated with the last_codon and codon_mutation features – was the third most important feature. This reflects the biological importance of codon pairs in modulating translational efficiency (84–86). Codon bias measures (and their changes due to mutation) were also of high importance (starting at second highest rank), in line with the abundant evidence of the relationship between codon composition and a variety of biologically-relevant factors, including gene expression (49,87,88), translational efficiency (14,89,90), and mRNA stability (91–94). Since codon selection modulates translational speed and thus cotranslational folding (95), sSNVs can also affect protein structure (96) without altering protein sequence. We incorporated protein annotations (predicted secondary structure, solvent accessibility, and disorder) as features; curiously, solvent accessibility ranked 8th in importance for the synVep model. Surprisingly, most other features had low importance; including features related to mRNA structure and stability, which are known to be directly influenced by sSNVs (8). This is perhaps due to the fact it is difficult to accurately predict RNA structure/stability for sequences longer than 500 nucleotides (97), and over 74% of transcripts in our data are longer than that.
Note that we did not use conservation as a synVep feature as it is usually the overarching signal of effect for most predictors (6) and we were hoping to capture additional, more subtle, signals orthogonal to those already reported. However, we also evaluated synVep's potential performance loss due to this choice by re-training the final model with an additional conservation feature (we used GERP++ scores for evaluation purposes (69)). This model was not significantly better in discriminating no-effect/effect variants (0.9 versus 0.9 F-score with and without conservation, respectively, in evaluating the test set); we also note that the difference in distribution of conservation scores across the effect and no-effect data sets was minimal (Supplementary Figure S5). Driven by this somewhat unexpected lack of conservation difference between the effect/no-effect sets, we further aimed to validate our set selection at the intermediate model level. We trained an intermediate model using conservation as one of the features. This model identified a new, conservation-included set of effect (n = 6 263 638) and no-effect (n = 32 010 049) sSNVs. This new partition overlapped significantly with the original one (75% of the original sSNVs were present and had identical effect/no-effect labels in both data sets). Moreover, synVep (without the conservation feature) predicted both data sets equally well. Using a balanced test set from the original data, it achieved 89%/90% no-effect precision/recall (Equations 1 and 2) and 90%/89% effect precision/recall; it achieved a similar performance for a balanced subset of the conservation-included effect/no-effect set (87%/88% no-effect precision/recall and 87%/88% effect precision/recall). Given these results, we chose to further continue to exclude conservation from synVep features. This choice makes synVep scores orthogonal to those of other predictors and allows for the further described cross-species variant analysis to be performed.
Predictors identify sSNV effect
To evaluate the performance of synVep, we needed a gold-standard set of designated effect and no effect variants. However, since there is no experimentally validated ‘neutral’ sSNVs, we used the common sSNVs excluded in training as neutrals (no-effect), i.e. as described above we assumed that the majority of common sSNVs have little or no effect. Note that variants with large deleterious effects could not become common, while neutral or weak effect mutations could due to genetic drift (98,99). Note, that the predictors that we discuss below target different classifications of variants (effect, fitness, pathogenicity, etc.) and are therefore not directly comparable on this data set; additionally, some may have used portions of our test set in training, e.g. DDIG-SN’s and FATHMM-MKL’s training sets included common variants from 1000 Genome Project (100), which may overlap with our test set's negative samples.
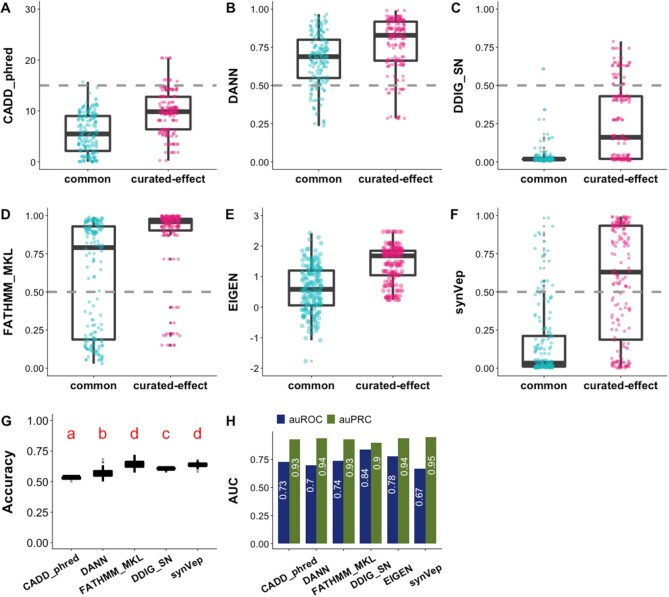
We used the set of curated-effect sSNVs (n = 170) as the effect group and the subset of common sSNVs (n = 9274) as the no-effect group to compare the predictor performances (Figure 3; both sets were excluded from synVep training). In testing, synVep had the highest auPRC but the lowest auROC (Figure 3H). However, at the default binary classification cutoff, synVep and FATHMM-MKL had the highest accuracy (Figure 3G). Importantly, note that most predictors failed to differentiate the two groups of variants at their default cutoff, placing both sets of variants below (CADD, DDIG-SN) or above the default threshold (DANN, FATHMM-MKL). The absence of a well performing standardized cutoff could arguably limit the practical applicability of these predictors in annotation of individual variant effects.
Figure 3.
Predictor performance on common vs. curated-effect sSNVs. Panels A–F show the differential predictions on sets of curated-effect (n = 170) and common sSNVs (randomly selected n = 170) for CADD (phred-like scaled scores), DANN, DDIG-SN, FATHMM-MKL, EIGEN, and synVep, respectively. Gray line indicates scoring cutoff suggested by tool authors. Neither the common set nor the curated-effect set were included in synVep training. Permutation tests show that all predictors give significantly different scores between the effect and common variant sets in every iteration, except for DANN where 11 of 100 comparisons were not significant (P-value > 0.05 after Bonferroni correction). Panel G reports two-class predictor accuracy (Equation 4) on resampled data (100 resampling sets; common set is down-sampled to match the number of curated-effect variants). Predictors with different red letters indicate significant difference by ANOVA test and Tukey's procedure; e.g. CADD’s ‘a’ and DANN’s ‘b’ indicate that CADD’s and DANN’s mean accuracies are significantly different. Panel H reports the performance (auROC and effect auPRC) of each predictor on the left-out common (negative; n = 9274) and curated-effect (positive; n = 170) sSNVs. FATHM, DDIG, and EIGEN auROC and auPRC are significantly different from synVep's (P-value < 0.05; Methods). Note that the performance comparisons here are limited as each predictor targets a different effect (e.g. pathogenicity vs molecular effect) and some methods have used our test set in training.
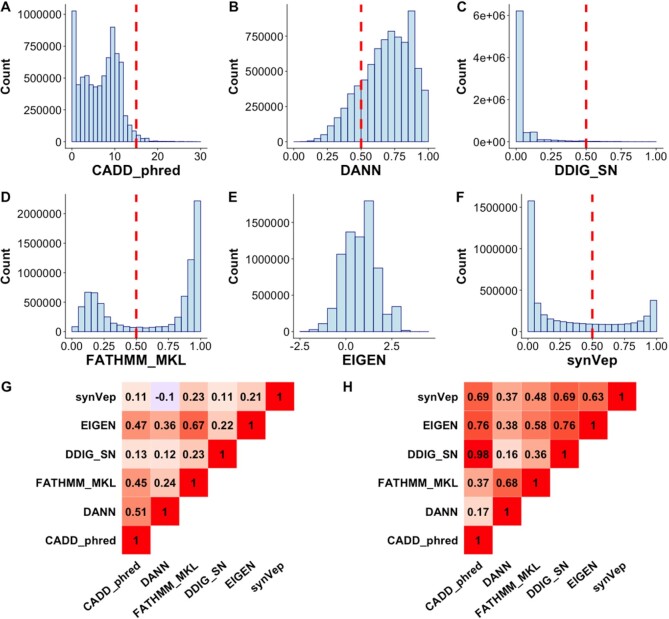
We further retrieved each predictor's predictions for all observed sSNVs. For this set, CADD and DANN predicted that 2% and 83%, respectively, of all observed sSNVs to be deleterious; DDIG-SN and FATHMM-MKL find that 1% and 62%, respectively are pathogenic. Meanwhile, synVep predicts that 31% of all observed sSNVs are effect – a more moderate finding in line with, for example, a fruitfly population study that highlighted ∼22% of four-fold synonymous sites to be under strong negative selection (101). We also examined the correlation of the predicted scores (Figure 4G) and the Fraction of Consensus Binary Prediction (17) (FCBP; Figure 4H) on all 4 160 063 observed sSNVs for all predictors (synVep, CADD, DANN, FATHMM-MKL, DDIG-SN and EIGEN). synVep's scores were poorly correlated with other predictor scores (Pearson correlation ranging from −0.1 [DANN] to 0.23 [FATHMM-MKL]), while binary classification was more similar (FCBP ranging from 0.37 [DANN] to 0.69 [CADD and DDIG-SN]).
Figure 4.
Predictions of effect of observed sSNVs. Panels A–F show predictions on all observed sSNVs (n = 4 160 063) by CADD_phred, DANN, DDIG-SN, FATHMM-MKL, EIGEN and synVep, respectively. Red dashed lines indicate the default predictor cutoff for binary classification. Panel (G) Spearman correlation and (H) fraction of Consensus Binary Prediction (FCBP) highlight similarity and lack thereof among the predictors for all observed sSNVs.
Singletons are more likely than observed to have an effect
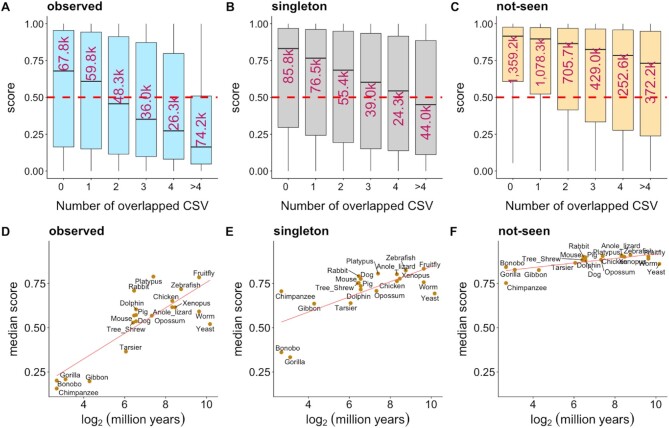
We re-predicted scores of all variants in our data (excluding unobservable) with the final synVep model. As expected, of observed sSNVs, only 31.3% were effect (median score 0.15), while 72.0% of not-seen sSNVs were effect (median score 0.88); singletons were scored/distributed bimodally (Supplementary Figure S6) into the two classes (48.2% effect; median score 0.47). Note that synVep predictions did not appear to be driven by site mutability (putative mutation rate). synVep scores of not-seen sSNVs that share a genomic position with none, 1, or 2 observed variants do not significantly differ from each other (median synVep scores 0.86, 0.94, and 0.88 respectively; Supplementary Figure S7).
Singletons were not included in our training because it is difficult to estimate how many of them are artifacts due to the 0.1–0.6% error rates of next-generation sequencing (102). If the singletons are not artifacts, then they are likely to be individual or ultrarare variants. These are more likely to be effect than higher frequency variants (103,104). An excess burden of ultrarare variants (although not necessarily synonymous) is also often seen in diseases, such as schizophrenia (105–107), Parkinson disease (108), and bipolar disorder (109). In line with these expectations, we found that singletons were, on average, scored higher than observed sSNVs (Supplementary Figure S6), suggesting that singletons are more likely to have an effect than the observed.
Variant effect predictors differentiate benign and pathogenic variants
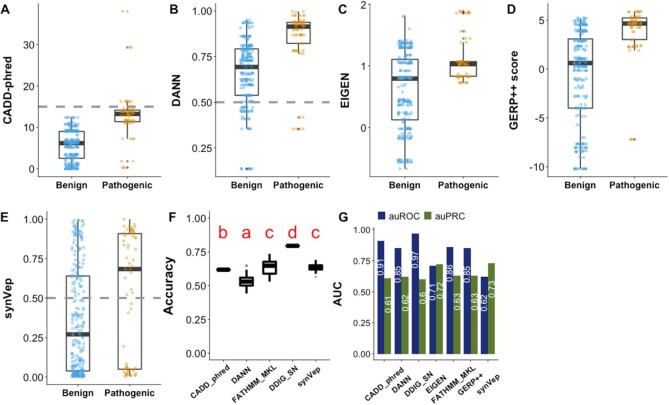
Among the predictors considered in this work, only two (FATHMM-MKL and DDIG-SN) are explicitly aimed to assess variant pathogenicity. To investigate whether predictors for variant functional effect (i.e. not pathogenicity) can identify pathogenic sSNVs, we obtained from ClinVar 17 pathogenic (genomic coordinate-based, 68 transcript-based) sSNVs and 51 benign sSNVs (genomic coordinate-based, 254 transcript-based) variants reviewed by an expert panel. Of these 68 variants, one benign and one pathogenic (genomic coordinate-based, 13 transcript-based) were deemed unobservable by our model and were removed from consideration. These ClinVar sSNVs were also excluded from training of synVep. Note that FATHMM-MKL’s and DDIG-SN’s training/testing data include HGMD-reported variants, which likely overlap with our ClinVar data, thus biasing predictor performance evaluation. All variant-effect predictors, including synVep, assigned higher scores to Pathogenic than Benign variants (Figure 5A–C and E). However, at the default/recommended cutoff, only synVep placed the majority of Benign vs. Pathogenic variants on opposite sides of the cutoff (Pathogenic recall = 0.58; Benign recall = 0.66) and thus attained the highest accuracy overall (Figure 5F).
Figure 5.
Evaluating variant effect predictors using ClinVar data. Benign (negative; n = 254) are variants labeled ‘Benign’ and ‘Benign/Likely benign’ in ClinVar, with ‘Review by expert panel’ as review status. Pathogenic (positive; n = 68) are those labeled ‘Pathogenic’ and ‘Pathogenic/Likely Pathogenic’ in ClinVar, with ‘Review by expert panel’ as review status or with ‘research’ method and at least one publication experimentally validating the effect. Panels A–E show the predictions from for CADD (phred-like scaled scores), DANN, EIGEN, GERP++ score, and synVep, respectively. Grey dashed lines show the method author-recommended cutoffs, where available. Differences between scores of Benign and Pathogenic variants in panels A-E are all statistically significant (one-sided permutation test P-value = 0). Performance boxplots for pathogenicity predictors are excluded to conserve space. Panel F reports two-class predictor accuracy (Equation 4) on resampled data (100 resampling sets; benign set is down-sampled to match the number of pathogenic variants). Predictors with different red letters indicate significant difference by ANOVA test and Tukey's procedure; e.g. CADD’s ‘b’ and DANN’s ‘a’ indicate that CADD’s and DANN’s mean accuracies are significantly different. Panel G reports auROC and auPRC for each predictor; all predictor auROCs and auPRCs are significantly different from that of synVep (P-value < 0.05; Methods). Note that the performance comparisons here are limited as each predictor targets a different effect (e.g. pathogenicity vs molecular effect) and some methods have used our test set in training.
synVep also attained the highest effect auPRC (Figure 5G), suggesting that it can identify disease-causing sSNVs well even though it was not explicitly trained to do so. However, synVep attained the lowest auROC, which may be due to the fact that benign ClinVar variants are actually functionally significant (effect) and are thus predicted by synVep as such but classified as wrong by ClinVar annotation (FP). In our definition, a variant of some effect is not necessarily pathogenic, but pathogenic variants are expected to have effect. Thus, experimentally validated pathogenic variants predicted to be no-effect by synVep are likely errors, but benign variants predicted be effect are possibly correctly identified as having functional impact, which does not necessarily correspond to disease.
Note that because synVep's predictions are transcript-based, they can differ for the same variant across multiple transcripts. Aggregating these predictions to score a variant is not trivial: one can use the mean, maximum, or median scores. A more sophisticated approach would be to weigh the scores from different transcripts by their expression level in multiple tissues. Specifically, if the question is about a disease and if the disease is primarily associated with one tissue, only the transcript most expressed in that tissue can be considered. However, given the complicated regulation and genetic interactions, this idea needs further validation. An evaluation of predictions for the same variant, however, highlights an interesting observation: only 5.1% of not-seen, 5.5% of observed, and 7.4% of singletons had at least one transcript, whose effect prediction differed from others.
All variant-effect predictors, including synVep, assigned higher scores to pathogenic than benign variants (Figure 5A–C and E, all statistically significant, one-sided permutation test P-value = 0). Notably, conservation (GERP++ (69)) carried sufficient signal to recognize pathogenic variants as well, suggesting that these are often found in conserved positions, which may not be the case for variants of less severe effect. Note that all other predictors (CADD, DANN and EIGEN) incorporate GERP++ as a feature, but their auROC and auPRC are not substantially higher (or even lower) than those of GERP++. Highly conserved genomic positions often have experienced extensive purifying selection (110). Therefore, conservation is understandably a commonly used feature for disease variant prioritization (111). However, in the scenario of disease variant prioritization synVep, offers discriminative power independent of conservation, so it may be used in combination with a conservation score or other predictors.
One major challenge in disease variant prioritization is that for complex diseases, causality can rarely be explained by a single variant (112). The utility of variant pathogenicity score is thus questionable: does a high score suggest a high likelihood of an individual developing a disease or a high likelihood of this variant contributing to a disease? Also, would an individual with many predicted-pathogenic variants carry many diseases or be very certain to carry at least one disease? A potential way to solve this puzzle is to establish the variant-disease relationship using the collective effect from the whole variome instead of a single or a few variants. A modification of polygenic risk scoring methods (113,114) to only account for effect variants may represent one approach, although it would be limited by the location of most GWAS SNPs in non-coding regions. Another approach is to unite only the coding variant effects by aggregating all variants per gene to predict disease predisposition (e.g. (115,116)) synVep predictions (as well as those of other predictors) may be plugged in these pipelines to explore the contribution of sSNVs to complex diseases.
synVep highlights correlation between conservation and effect
We annotated all sSNVs as CSVs (cross-species variation) or not (Figure 2; Methods). CSVs are codon differences between the human reference sequence and another species’ ortholog. For example, if the proline-coding codon in a human transcript T is CCC, while the aligned proline codon on T’s chimp ortholog is CCT, then the human sSNV CCC→CCT is considered a chimp-CSV. We thus annotated 15 618 155 unique (only exists in one species) and 35 102 565 non-unique (overlapping across species) CSVs (Supplementary Figure S8). Since less than 10% (7026 of 72 400) of the human transcripts can be mapped to orthologs in all 20 species, we analyzed separately the CSVs in (i) all transcripts (n = 32, 264 860) and (ii) only the transcripts that have orthologs in all 20 species (n = 3 321 574) and that are likely ancient (ancient genes) (117).
Figure 2.
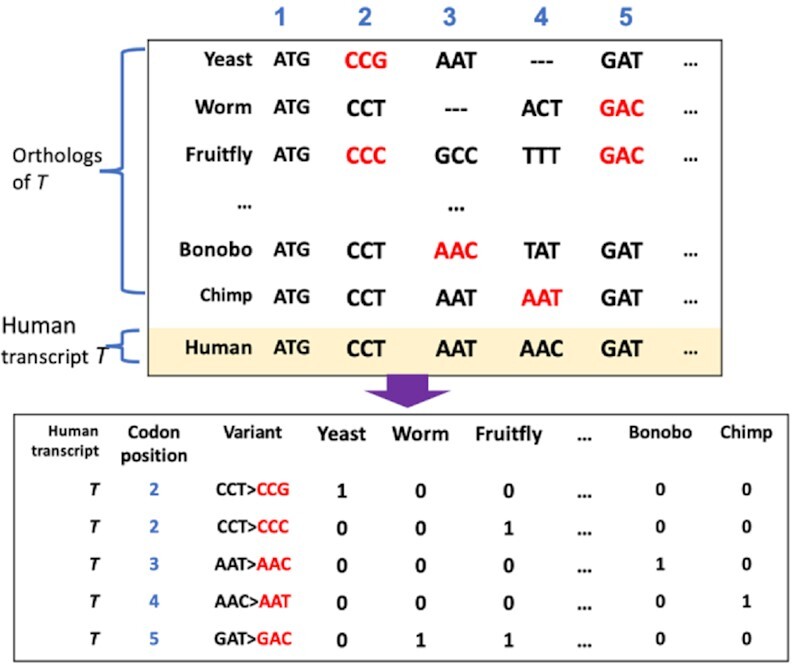
Extraction of cross-species sequence variants (CSV). For each human protein coding transcript T, codon-oriented multiple sequence alignment was performed with 20 species’ longest coding sequencing of the same ortholog as T. The CSV are represented as ‘codon > codon’ format for specific transcript positions and may coincide with human sSNVs.
The distribution of synVep prediction scores for CSVs in the ancient genes and for those in all transcripts were similar (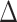 mean = 0.05, Mann–Whitney U test P-value < 2.2e–16), suggesting that synVep’s evaluation of variants does not discriminate by gene age. For all transcripts, observed sSNVs had more CSVs (67%, n = 2 823 142) than did the not-seen variants (53%, n = 26 976 016; Supplementary Figure S9). CSVs overall were predicted less likely to be effect than non-CSV for both ancient and all transcripts (Figure 6A–C; Supplementary Figure S10A–C). While this is in line with the scoring trends of the observed and not-seen variants overall, it also mirrors earlier findings of few CSV nsSNVs corresponding to a known human disease (118–121). synVep scores also trended lower for CSVs whose substituting nucleotide was found in more species, for both ancient (Figure 6A–C) and all transcripts (Supplementary Figure S10A–C). Since the number of CSV species is somewhat indicative of codon conservation, this trend suggests that, although synVep was trained without using conservation features, its predictions still identify conserved codons that are often functionally relevant (122).
mean = 0.05, Mann–Whitney U test P-value < 2.2e–16), suggesting that synVep’s evaluation of variants does not discriminate by gene age. For all transcripts, observed sSNVs had more CSVs (67%, n = 2 823 142) than did the not-seen variants (53%, n = 26 976 016; Supplementary Figure S9). CSVs overall were predicted less likely to be effect than non-CSV for both ancient and all transcripts (Figure 6A–C; Supplementary Figure S10A–C). While this is in line with the scoring trends of the observed and not-seen variants overall, it also mirrors earlier findings of few CSV nsSNVs corresponding to a known human disease (118–121). synVep scores also trended lower for CSVs whose substituting nucleotide was found in more species, for both ancient (Figure 6A–C) and all transcripts (Supplementary Figure S10A–C). Since the number of CSV species is somewhat indicative of codon conservation, this trend suggests that, although synVep was trained without using conservation features, its predictions still identify conserved codons that are often functionally relevant (122).
Figure 6.
Variant effect prediction from the perspective of cross-species variation (CSV). Panels A–C show synVep-predicted scores for variants grouped by the number of species carrying the mutant nucleotide; separately for observed, singletons, and not-seen sets. The red dashed line is synVep's default cutoff for effect and no-effect. The number in each box indicates the number of variants of that group (in thousands). Panels D–F show the median score (y-axis) across species at log2 million years since divergence from common ancestor with human (x-axis) and linear regression trendline (red line) between the two. The Spearman correlations between median synVep score and log2(million years) for panels D–F are 0.68, 0.64 and 0.66, respectively.
To further elucidate the effect of sequence conservation across species, we calculated codon mutation fraction (CMF, Supplementary Equation S9) to describe how common a human's alternative codon is, compared to the reference codon, among the 20 species included for CSV analysis. For example, if in a multiple sequence alignment of the 20 species orthologs, the human CCC codon is aligned to 10 CCC, 5 CCT, and 5 other codons, then the CMF of the corresponding synonymous variant, CCC > CCT, is 5/15 = 0.33. We observed that predicted scores generally decrease with higher CMF (Supplementary Figure S11A–C), indicating that sSNVs with alternative codons commonly present as reference codons among other species have less effect.
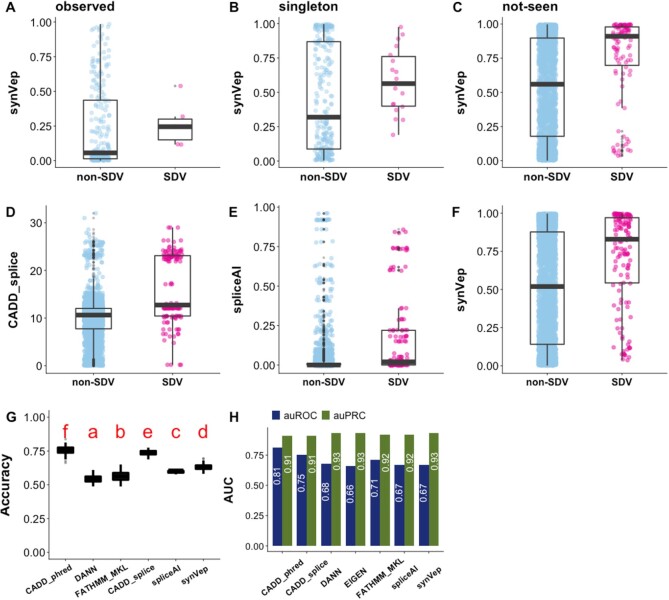
We additionally investigated the relationship between the evolutionary distance of CSV species from human and the effects of the corresponding sSNVs. Since one sSNV can correspond to multiple species CSVs, we only considered CSVs that are uniquely found in one species for this evaluation. The medians of synVep scores of these species-exclusive CSVs in both ancient genes (Figure 6D–F) and all transcripts (Supplementary Figure S10D–F) correlated with the evolutionary distance of the corresponding species to human. However, for ancient genes, the median scores of observed variants unique to further related (i.e. beyond Tarsier) species were in the effect range (synVep > 0.5). Arguably, this means that human sSNVs that introduce nucleotides likely present in recent ancestors tend to be no-effect, while similarity to further removed relatives carries no such benefit (Figure 6D). These findings agree with our recent work on nsSNV CSV analysis (35). We note that species relationship had much less impact on binary effect classification for singleton variants and none for not-seen variants (Figure 6E-F). The same observations could not be made for the all transcript set of variants, where observed and singleton CSVs were predicted to be no-effect for a large portion of species (Supplementary Figure S10D–F). This observation suggests that ancient genes are functionally crucial and have been sufficiently optimized over time to only permit minor levels of variation without impact on functionality.
synVep differentiates splice-disrupting variants
Cheung et al. (70) measured the splice-disrupting effects of genomic variants (3297 transcript-specific sSNVs) and defined a group of large-effect splice-disrupting variants (140 SDV sSNVs). As expected, synVep scores of SDVs were on average higher than those of non-SDVs (Figure 7A–C). Curiously, 140 SDVs comprised only six observed (4.6%) and 18 singletons (13.7%) variants; nine were deemed unobservable (6.4%) and 107 were not-seen (76.4%). The fact that most of SDVs are not-seen reinforces our assumption that not-seen sSNVs are enriched for large-effect deleterious sSNVs that may have been purified.
Figure 7.
Evaluation of synVep, CADD-splice, and spliceAI using large-effect splice-disrupting variants (SDVs) and non-SDVs. SynVep predictions are higher scoring for a set of experimentally determined SDV (positive; n = 140) than non-SDV (negative; n = 3,157) variants across observed (A), singleton (B) and not-seen (C) data sets. Note that non-SDVs may still carry other functional effects. The observed SDVs mispredicted as no-effect highlight the limitations of our training data, although two of the five (40%) observed SDVs are correctly annotated as effect. Panels D–F show the distribution of scores on complete non-SDV and SDV collections predicted by CADD-splice, spliceAI, and synVep, respectively. Boxplots for other non-splicing predictors are not shown to conserve space. Panel G reports predictor two-state accuracy (Equation 4) on resampled data (100 resampling sets; non-SDV set is down-sampled to match the number of SDV variants). Predictors with different red letters indicate significant difference by ANOVA test and Tukey's procedure. Note that DDIG-SN was offline when this dataset was analyzed (e.g. CADD’s ‘f’ and DANN’s ‘a’ indicate CADD’s and DANN’s mean accuracies are significantly different). Panel H reports auROC and auPRC for each predictor; all predictor auROCs and auPRCs are significantly different from that of synVep (P-value < 0.05; Methods). Note that the utility of auROC and auPRC is limited without a pre-defined test set; thus, a cutoff is needed.
We evaluated two state-of-the-art predictors for splicing effect evaluation: CADD-splice (72) and spliceAI (71) on this set of experimentally determined variants (Figure 7D, E). spliceAI is a 32-layer deep learning model that predicts splicing donor/acceptor gain/loss probabilities. CADD-splice was developed based on CADD (23) with the addition of two splicing-specific predictor (MMsplice (123) and spliceAI) outputs as features. MMsplice and spliceAI were selected to be incorporated into CADD-splice because they performed best among several other splicing-specific predictors on the same non-SDV/SDV dataset (not limited to synonymous variants). CADD-splice had the highest accuracy (Figure 7G) and auROC (Figure 7H); meanwhile, the auRPC of all the three predictors are similar (Figure 7H).
Splicing disruption is a well-known and well-studied mechanism of sSNV effect (124). In fact, most of the experimental validations of our curated-effect and ClinVar pathogenic variants refer to elucidating splicing effects (Supplementary Table S1 and S3). Moreover, many cancer driver mutations are found to be splice-disrupting synonymous variants (125). Aside from splicing, experimental validation of variant effect is rare, arguably due to technical challenges (126). Perhaps, since the experimental evidence for splicing disruption is more abundant than non-splicing effects’, the former is considered a major factor in clinical consideration for sSNVs. For example, according to the guidelines from American College of Medical Genetics and Genomics (127), an sSNV is clinically benign if it is not in a conserved position and is predicted to be non-impacting to a splice site (e.g. via GeneSplicer (128), NNsplice (129)). Thus, synVep's ability to identify effect and score sSNVs regardless of their splice effects or conservation makes it an ideal tool for prioritization of all possible variants, regardless of their mechanism or evolutionary evidence of effect.
sSNV effects reflect genomic constraints
Havrilla et al. developed the concept of ‘coding constrained regions’ (CCR) to describe the regional scarcity of protein-changing (missense or loss-of-function) variants in the human genome (76). Here, a region with fewer of these variants observed in the human population has a higher CCR percentile score. For our set of variants, the fraction of observed (number of observed sSNVs divided by all possible sSNVs in this region) negatively correlated (Pearson ρ = −0.61) with CCR percentile (Figure 8A); i.e. higher constraint indicates fewer sSNVs. Furthermore, synVep predictions positively correlated with CCR percentiles for observed (ρ = 0.58, Figure 8B), i.e. lower CCR percentile (less constrained regions) indicated lower (no-effect) synVep scores.
Figure 8.
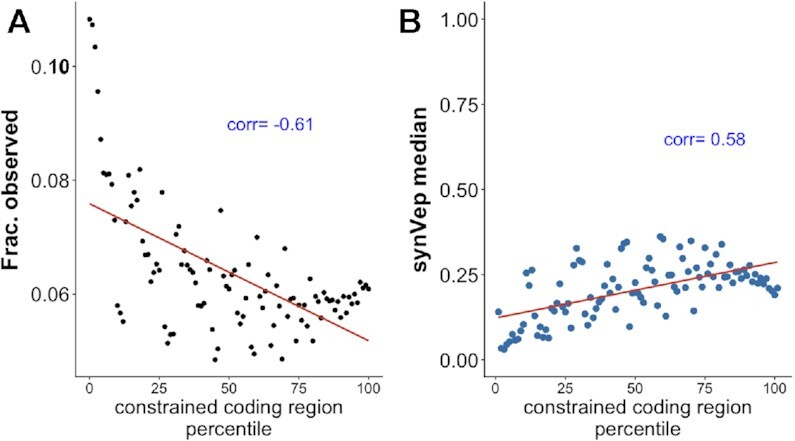
sSNV effect measured by region constraint. Coding constrained regions (CCR) describe the regional scarcity of nsSNVs; higher percentile regions represent have fewer observed nsSNVs. Observed sSNVs are relatively scarce in constrained regions (A), while their median synVep scores are higher (B). Pearson correlation are indicated in blue.
The negative correlation between the fraction of sSNVs and CCR indicates a positive correlation between synonymous mutation rate and missense or loss-of-function mutation rate. This observation is in line with earlier studies (130,131), but raises a question of the utility of Ka/Ks ratio (non-synonymous divided by synonymous mutation rate), which is widely used to measure the strength of evolutionary selection at certain genomic sites (132). The application of the Ka/Ks ratio is based on the assumption that synonymous mutations are neutral and thus Ks can serve as a baseline for Ka. However, it has been demonstrated that a high Ka/Ks can also result from a low Ks due to strong negative selection at the synonymous sites (10,11,133–135). Efforts have been made to improve the utility of Ka/Ks by incorporating codon preference (136–138), but the question remains: how often is the selection at synonymous sites sufficiently underestimated so that Ka/Ks is no longer accurate? Lawrie et al. found that 22% of the fourfold synonymous sites (where the amino acid can be encoded by four codons) in the fruitfly genome are under strong selection (101). Lu and Wu estimated that 90% synonymous differences between human and chimp are deleterious (139). Hellmann et al. estimated that 39% mutations at the human-chimp-diverged non-CpG fourfold synonymous sites have been purified (140). Zhou et al. showed that 9% of all yeast genes and 5% all worm genes undergo purifying selection on synonymous sites (138). In turn, our results show that, excluding unobservable (9.6%), ∼67% of all possible human sSNVs are effect (synVep score > 0.5), but we cannot estimate the strength of selection acting upon these. These findings suggest that Ka/Ks measures of genomic site constraints may be underpowered.
synVep sheds light on future variant discovery and interpretation
Whenever a human genome variant is sequenced, it will automatically be reassigned a class in our collection. Thus, a newly sequenced variant will first become a singleton and may, eventually, be a member of the observed group. An enrichment in observed variants will likely come from large-scale sequencing. The ethnic diversity of gnomAD represents the ethnic diversity in the United States, but not global ethnicity diversity; although only 16% of global population are of European descent (141), 53% of the samples from gnomAD exomes database are (142) are; i.e. there is a significant underrepresentation of sSNVs from other ethnicities. When more diverse genomes are sequenced, will there be a significant addition to the observed set (i.e. significant reduction of the not-seen set)?
To answer this question, we obtained all variants from the Qatar Genome (QTRG) project (77) and mapped them to our set of sSNVs. QTRG comprises 1,376 individuals and may serve as a representative pool of genomic variants in Middle East and north Africa (MENA) area (77); thus, this set is complementary to gnomAD. We identified 526 616 transcript-based sSNVs (n = 192 246 genomic coordinate-based) from QTRG sequencing. Importantly, only 0.6% of the Qatari sSNVs mapped to our unobservable set—a fraction that is lower than the misprediction rate (5%) that we allowed during PUL. Moreover, two thirds of these variants were singletons in QTRG. This observation suggests that our unobservable variants are indeed unlikely to be ever observed in future sequenced human populations. The majority of QTRG sSNVs (81.9%) mapped to our observed set; 4.6% and 12.8% were singleton and not-seen, respectively (Figure 9A). Interestingly, 63.5% and 64.6% QTRG sSNVs mapping to our singleton and not-seen sets, respectively, were singletons in the Qatari cohort. We also found that gnomAD singletons that were present in QTRG, on average, scored higher than QTRG variants overlapping with observed sSNVs (34.7% versus 26.6% effect variants, respectively). This finding further confirms that singletons are more likely to have an effect than observed variants.
Figure 9.
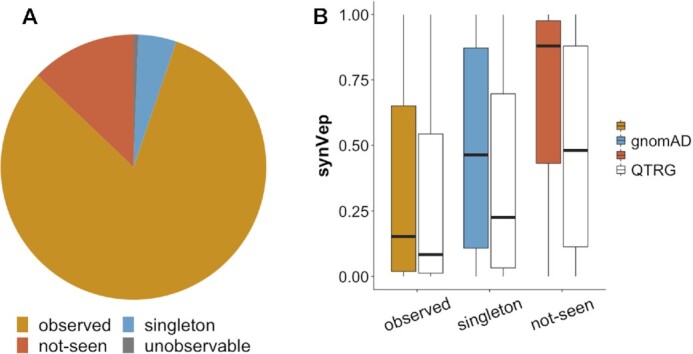
Distributions of the Qatar Genome sSNVs. In both panels, our gnomAD-based observed (orange), singleton (blue) and not-seen (dark orange) sets are highlighted. (A) represents the fraction of the QTRG sSNVs mapped our observed, singleton, not-seen, and unobservable (gray) sets. (B) synVep scores for our (gnomAD-based) variant sets, as well as the scores for QTRG sSNVs (white) mapping to the corresponding gnomAD-classes. Importantly, the synVep scores of QTRG variants that were previously classified as singletons or not-seen score much lower than other variants in the corresponding groups.
How many of the previously not-seen sSNVs are effect? New sSNVs are likely to come from clinical sequencing and could thus could often be deemed disease-associated. We expect, however, that these variants will carry little or no effect. In other words, currently not-seen no-effect observable sSNVs (n = 14 259 180 transcript-based and n = 5 975 076 genomic coordinate-based) are more likely to be discovered in the future than an effect ones—even if a sample is taken from a sick individual. Recall our assumption that the not-seen set is composed of those sSNVs that carry a large effect and have been purified, as well as those that are putatively neutral and will be seen in the future if more sequencing is performed. The synVep scores of the QTRG sSNVs mapping to our not-seen set were, on average, much lower than those of the entire not-seen set (Figure 9B, average synVep score 0.49 versus 0.88, Mann–Whitney U test P-value < 2.2e–16). Similarly, the median synVep score of the 2 469 205 sSNVs not-seen according to gnomAD, but present in dbSNP (143) was lower than for the entire not-seen set (0.47 versus 0.88; P-value < 2.2e–16). These results confirm our assumption, as these newly identified sSNVs are actually observable (not purified) and thus they are generally less likely to have large effect (and thus lower synVep scores). It may also be that the newly identified predicted effect variants (from QTRG, and other sequencing efforts in the future) are the ethnicity-differentiating, i.e. not necessarily affecting overall fitness, but contributing to individual differences (as in e.g. (144)).
CONCLUSION
We developed synVep—a machine learning-based model for evaluating the effect of human sSNVs. Our model does not use disease/deleteriousness-labeled training data. Instead, we used the signals derived from observed (and corresponding generated) sSNVs from large sequencing projects. Our model successfully distinguishes sSNVs with experimentally validated effect, e.g. splice-site disrupters, as well as pathogenic sSNVs. Moreover, our model's predictions of cross-species variants (CSVs) correlate with the evolutionary distance between human and CSV-species. While further experimental validations of effect prediction are necessary, synVep's evaluation on sSNV effect will greatly contribute to our understanding of biological molecular pathways in general, and of pathogenicity pathways in particular.
DATA AVAILABILITY
synVep webserver for online query: https://services.bromberglab.org/synvep; For local run, Python script (https://bitbucket.org/bromberglab/synvep_local) and prediction database (https://zenodo.org/record/4763256) are also available.
Supplementary Material
ACKNOWLEDGEMENTS
We thank our current and former lab members, Dr Yannick Mahlich, Dr Chengsheng Zhu, Dr Maximillian Miller and Dr Yanran Wang (all Rutgers), for all discussions and constructive suggestions. We also thank Kyle Flannery (Rutgers) for the idea of testing our predictions with Qatari Genome variant data. We are also grateful to the Rutgers Office of Advanced Research Computing (OARC) for making high-performance compute resources available to this project, Thomas Pawlowski (Rutgers Office of Information Technology) for setting up the host of synVep webserver, and to the Ensembl team for their help and feedback. Last but not least, we want to thank all researchers and human subjects who made the data and tools used in this study available.
Author contribution: Z.Z. and Y.B. designed the study, evaluated the results, and wrote the manuscript; Z.Z. conducted the study; Z.Z. and A.A. built the webserver.
Contributor Information
Zishuo Zeng, Department of Biochemistry and Microbiology, Rutgers University, New Brunswick, NJ 08873, USA.
Ariel A Aptekmann, Department of Biochemistry and Microbiology, Rutgers University, New Brunswick, NJ 08873, USA.
Yana Bromberg, Department of Biochemistry and Microbiology, Rutgers University, New Brunswick, NJ 08873, USA; Department of Genetics, Rutgers University, Piscataway, NJ 08854, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Z.Z. and Y.B. were supported by the NIH/NIGMS grant R01 [GM115486]; A.A. is supported by the Astrobiology Institute grant [80NSSC18M0093]; Y.B. was also supported by NIH grant R01 [MH115958]. Funding for open access charge: NIH/NIGMS grant R01 [GM115486].
Conflict of interest statement. None declared.
REFERENCES
- 1. Ashley E.A. Towards precision medicine. Nat. Rev. Genet. 2016; 17:507. [DOI] [PubMed] [Google Scholar]
- 2. Visscher P.M., Wray N.R., Zhang Q., Sklar P., McCarthy M.I., Brown M.A., Yang J.. 10 years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 2017; 101:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan B., Kusko R., Xiao W., Zheng Y., Liu Z., Xiao C., Sakkiah S., Guo W., Gong P., Zhang C.et al.. Similarities and differences between variants called with human reference genome HG19 or HG38. BMC Bioinformatics. 2019; 20:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auton A., Abecasis G.R., Altshuler D.M., Durbin R.M., Abecasis G.R., Bentley D.R., Chakravarti A., Clark A.G., Donnelly P., Eichler E.E.et al.. A global reference for human genetic variation. Nature. 2015; 526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ward L.D., Kellis M.. Interpreting noncoding genetic variation in complex traits and human disease. Nat. Biotechnol. 2012; 30:1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu C., Miller M., Zeng Z., Wang Y., Mahlich Y., Aptekmann A., Bromberg Y.. Computational approaches for unraveling the effects of variation in the human genome and microbiome. Annu.Rev. Biomed. Data Sci. 2020; 3:411–432. [Google Scholar]
- 7. Shen H., Li J., Zhang J., Xu C., Jiang Y., Wu Z., Zhao F., Liao L., Chen J., Lin Y.et al.. Comprehensive characterization of human genome variation by high coverage whole-genome sequencing of forty four caucasians. PLoS One. 2013; 8:e59494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sauna Z.E., Kimchi-Sarfaty C.. Understanding the contribution of synonymous mutations to human disease. Nat. Rev. Genet. 2011; 12:683. [DOI] [PubMed] [Google Scholar]
- 9. Stergachis A.B., Haugen E., Shafer A., Fu W., Vernot B., Reynolds A., Raubitschek A., Ziegler S., LeProust E.M., Akey J.M.. Exonic transcription factor binding directs codon choice and affects protein evolution. Science. 2013; 342:1367–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pagani F., Raponi M., Baralle F.E.. Synonymous mutations in CFTR exon 12 affect splicing and are not neutral in evolution. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:6368–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chamary J.V., Parmley J.L., Hurst L.D.. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat. Rev. Genet. 2006; 7:98–108. [DOI] [PubMed] [Google Scholar]
- 12. Meyer I.M. Statistical evidence for conserved, local secondary structure in the coding regions of eukaryotic mRNAs and pre-mRNAs. Nucleic Acids Res. 2005; 33:6338–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duan J., Shi J., Ge X., Dölken L., Moy W., He D., Shi S., Sanders A.R., Ross J., Gejman P.V.. Genome-wide survey of interindividual differences of RNA stability in human lymphoblastoid cell lines. Sci. Rep. 2013; 3:1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah P., Ding Y., Niemczyk M., Kudla G., Joshua. Rate-limiting steps in yeast protein translation. Cell. 2013; 153:1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pechmann S., Frydman J.. Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat. Struct. Mol. Biol. 2013; 20:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stoletzki N., Eyre-Walker A.. Synonymous codon usage in Escherichia coli: selection for translational accuracy. Mol. Biol. Evol. 2006; 24:374–381. [DOI] [PubMed] [Google Scholar]
- 17. Zeng Z., Bromberg Y.. Predicting functional effects of synonymous variants: a systematic review and perspectives. Front. Genet. 2019; 10:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buske O.J., Manickaraj A., Mital S., Ray P.N., Brudno M.. Identification of deleterious synonymous variants in human genomes. Bioinformatics. 2013; 29:1843–1850. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X., Li M., Lin H., Rao X., Feng W., Yang Y., Mort M., Cooper D.N., Wang Y., Wang Y.. regSNPs-splicing: a tool for prioritizing synonymous single-nucleotide substitution. Hum. Genet. 2017; 136:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livingstone M., Folkman L., Yang Y., Zhang P., Mort M., Cooper D.N., Liu Y., Stantic B., Zhou Y.. Investigating DNA-, RNA-, and protein-based features as a means to discriminate pathogenic synonymous variants. Hum. Mutat. 2017; 38:1336–1347. [DOI] [PubMed] [Google Scholar]
- 21. Gelfman S., Wang Q., McSweeney K.M., Ren Z., La Carpia F., Halvorsen M., Schoch K., Ratzon F., Heinzen E.L., Boland M.J.. Annotating pathogenic non-coding variants in genic regions. Nat. Commun. 2017; 8:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi F., Yao Y., Bin Y., Zheng C.-H., Xia J.. Computational identification of deleterious synonymous variants in human genomes using a feature-based approach. BMC Med. Genet. 2019; 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kircher M., Witten D.M., Jain P., O’roak B.J., Cooper G.M., Shendure J.. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014; 46:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rentzsch P., Witten D., Cooper G.M., Shendure J., Kircher M.. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019; 47:D886–D894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quang D., Chen Y., Xie X.. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2014; 31:761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shihab H.A., Rogers M.F., Gough J., Mort M., Cooper D.N., Day I.N., Gaunt T.R., Campbell C.. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics. 2015; 31:1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods. 2014; 11:361. [DOI] [PubMed] [Google Scholar]
- 28. Stenson P.D., Mort M., Ball E.V., Evans K., Hayden M., Heywood S., Hussain M., Phillips A.D., Cooper D.N.. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017; 136:665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landrum M.J., Lee J.M., Riley G.R., Jang W., Rubinstein W.S., Church D.M., Maglott D.R.. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014; 42:D980–D985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bromberg Y., Kahn P.C., Rost B.. Neutral and weakly nonneutral sequence variants may define individuality. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:14255–14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pejaver V., Babbi G., Casadio R., Folkman L., Katsonis P., Kundu K., Lichtarge O., Martelli P.L., Miller M., Moult J.. Assessment of methods for predicting the effects of PTEN and TPMT protein variants. Hum. Mutat. 2019; 40:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu B., Lee W.S., Yu P.S., Li X.. ICML. 2002; 2:Citeseer; 387–394. [Google Scholar]
- 33. Liu B., Dai Y., Li X., Lee W.S., Yu P.S.. Third IEEE International Conference on Data Mining. 2003; IEEE; 179–186. [Google Scholar]
- 34. Fusilier D.H., Montes-y-Gómez M., Rosso P., Cabrera R.G.. Detecting positive and negative deceptive opinions using PU-learning. Inform. Process. Manage. 2015; 51:433–443. [Google Scholar]
- 35. Mahlich Y., Miller M., Zeng Z., Bromberg Y.. Low diversity of human variation despite mostly mild functional impact of de novo variation. Front. Mol. Biosci. 2021; 8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinsella R.J., Kahari A., Haider S., Zamora J., Proctor G., Spudich G., Almeida-King J., Staines D., Derwent P., Kerhornou A.et al.. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database (Oxford). 2011; 2011:bar030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Church D.M., Schneider V.A., Graves T., Auger K., Cunningham F., Bouk N., Chen H.-C., Agarwala R., McLaren W.M., Ritchie G.R.S.et al.. Modernizing Reference Genome Assemblies. PLoS Biol. 2011; 9:e1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang K., Li M., Hakonarson H.. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010; 38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P.et al.. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2019; 581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freedman D., Pisani R., Purves R., Adhikari A.. Statistics. 2007; NY: WW Norton & Company. [Google Scholar]
- 41. Acock A.C., Stavig G.R.. A measure of association for nonparametric statistics. Soc. Forces. 1979; 57:1381–1386. [Google Scholar]
- 42. Fisher R.A. Breakthroughs in Statistics. 1992; Springer; 66–70. [Google Scholar]
- 43. Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N.. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013; 45:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharp P.M., Li W.-H.. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987; 15:1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J. Mol. Biol. 1981; 151:389–409. [DOI] [PubMed] [Google Scholar]
- 46. Karlin S., Mrázek J.. What drives codon choices in human genes. J. Mol. Biol. 1996; 262:459–472. [DOI] [PubMed] [Google Scholar]
- 47. Freire-Picos M.A., Gonzalez-Siso M.I., Rodríguez-Belmonte E., Rodriguez-Torres A.M., Ramil E., Cerdan M.E.. Codon usage in Kluyveromyces lactis and in yeast cytochrome c-encoding genes. Gene. 1994; 139:43–49. [DOI] [PubMed] [Google Scholar]
- 48. Wan X., Xu D., Zhou J.. A new informatics method for measuring synonymous codon usage bias. Intell. Eng. Syst. Artif. Neural Netw. 2003; 13:1–8. [Google Scholar]
- 49. Dos Reis M., Wernisch L., Savva R. Unexpected correlations between gene expression and codon usage bias from microarray data for the whole Escherichia coli K-12 genome. Nucleic Acids Res. 2003; 31:6976–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Team, R.C. R: A Language and Environment for Statistical Computing. 2013; [Google Scholar]
- 51. Supek F., Vlahoviček K.. Comparison of codon usage measures and their applicability in prediction of microbial gene expressivity. BMC Bioinformatics. 2005; 6:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cannarozzi G., Schraudolph N.N., Faty M., Von Rohr P., Friberg M.T., Roth A.C., Gonnet P., Gonnet G., Barral Y.. A role for codon order in translation dynamics. Cell. 2010; 141:355–367. [DOI] [PubMed] [Google Scholar]
- 53. Yevshin I., Sharipov R., Valeev T., Kel A., Kolpakov F.. GTRD: a database of transcription factor binding sites identified by ChIP-seq experiments. Nucleic Acids Res. 2016; gkw951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao H., Sun Z., Wang J., Huang H., Kocher J.-P., Wang L.. CrossMap: a versatile tool for coordinate conversion between genome assemblies. Bioinformatics. 2013; 30:1006–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giudice G., Sánchez-Cabo F., Torroja C., Lara-Pezzi E.. ATtRACT—a database of RNA-binding proteins and associated motifs. Database. 2016; 2016:baw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cáceres E.F., Hurst L.D.. The evolution, impact and properties of exonic splice enhancers. Genome Biol. 2013; 14:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yachdav G., Kloppmann E., Kajan L., Hecht M., Goldberg T., Hamp T., Hönigschmid P., Schafferhans A., Roos M., Bernhofer M.. PredictProtein—an open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014; 42:W337–W343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rost B. Methods in Enzymology. 1996; 266:Elsevier; 525–539. [DOI] [PubMed] [Google Scholar]
- 59. Schlessinger A., Punta M., Yachdav G., Kajan L., Rost B.. Improved disorder prediction by combination of orthogonal approaches. PLoS One. 2009; 4:e4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lorenz R., Bernhart S.H., Zu Siederdissen C.H., Tafer H., Flamm C., Stadler P.F., Hofacker I.L.. ViennaRNA Package 2.0. Algorith. Mol. Biol. 2011; 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sabarinathan R., Tafer H., Seemann S.E., Hofacker I.L., Stadler P.F., Gorodkin J.. RNA snp: efficient detection of local RNA secondary structure changes induced by SNP s. Hum. Mutat. 2013; 34:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. LeCun Y., Bengio Y., Hinton G.. Deep learning. Nature. 2015; 521:436–444. [DOI] [PubMed] [Google Scholar]
- 63. Chen T., Guestrin C.. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2016; ACM; 785–794. [Google Scholar]
- 64. Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V.. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 2011; 12:2825–2830. [Google Scholar]
- 65. Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., Müller M.. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grau J., Grosse I., Keilwagen J.. PRROC: computing and visualizing precision-recall and receiver operating characteristic curves in R. Bioinformatics. 2015; 31:2595–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ionita-Laza I., McCallum K., Xu B., Buxbaum J.D.. A spectral approach integrating functional genomic annotations for coding and noncoding variants. Nat. Genet. 2016; 48:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Landrum M.J., Kattman B.L.. ClinVar at five years: delivering on the promise. Hum. Mutat. 2018; 39:1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Davydov E.V., Goode D.L., Sirota M., Cooper G.M., Sidow A., Batzoglou S.. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput. Biol. 2010; 6:e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cheung R., Insigne K.D., Yao D., Burghard C.P., Wang J., Hsiao Y.-H.E., Jones E.M., Goodman D.B., Xiao X., Kosuri S.. A multiplexed assay for exon recognition reveals that an unappreciated fraction of rare genetic variants cause large-effect splicing disruptions. Mol. Cell. 2019; 73:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jaganathan K., Panagiotopoulou S.K., McRae J.F., Darbandi S.F., Knowles D., Li Y.I., Kosmicki J.A., Arbelaez J., Cui W., Schwartz G.B.. Predicting splicing from primary sequence with deep learning. Cell. 2019; 176:535–548. [DOI] [PubMed] [Google Scholar]
- 72. Rentzsch P., Schubach M., Shendure J., Kircher M.. CADD-Splice—improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Med. 2021; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kumar S., Stecher G., Suleski M., Hedges S.B.. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017; 34:1812–1819. [DOI] [PubMed] [Google Scholar]
- 74. Zerbino D.R., Achuthan P., Akanni W., Amode M.R., Barrell D., Bhai J., Billis K., Cummins C., Gall A., Girón C.G.. Ensembl 2018. Nucleic Acids Res. 2017; 46:D754–D761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Löytynoja A., Goldman N.. An algorithm for progressive multiple alignment of sequences with insertions. Proc. Natl. Acad. Sci. 2005; 102:10557–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Havrilla J.M., Pedersen B.S., Layer R.M., Quinlan A.R.. A map of constrained coding regions in the human genome. Nat. Genet. 2019; 51:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fakhro K.A., Staudt M.R., Ramstetter M.D., Robay A., Malek J.A., Badii R., Al-Marri A.A.-N., Abi Khalil C., Al-Shakaki A., Chidiac O.. The Qatar genome: a population-specific tool for precision medicine in the Middle East. Hum. Genome Variation. 2016; 3:16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leinonen R., Sugawara H., Shumway M., Collaboration I.N.S.D.. The sequence read archive. Nucleic Acids Res. 2010; 39:D19–D21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dohm J.C., Lottaz C., Borodina T., Himmelbauer H.. Substantial biases in ultra-short read data sets from high-throughput DNA sequencing. Nucleic Acids Res. 2008; 36:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rieber N., Zapatka M., Lasitschka B., Jones D., Northcott P., Hutter B., Jäger N., Kool M., Taylor M., Lichter P.. Coverage bias and sensitivity of variant calling for four whole-genome sequencing technologies. PLoS One. 2013; 8:e66621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Forsdyke D.R. Functional constraint and molecular evolution. eLS. 2001; 10.1002/9780470015902.a0029286. [DOI] [Google Scholar]
- 82. Alazami A.M., Awad S.M., Coskun S., Al-Hassan S., Hijazi H., Abdulwahab F.M., Poizat C., Alkuraya F.S.. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol. 2015; 16:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shamseldin H.E., Tulbah M., Kurdi W., Nemer M., Alsahan N., Al Mardawi E., Khalifa O., Hashem A., Kurdi A., Babay Z.. Identification of embryonic lethal genes in humans by autozygosity mapping and exome sequencing in consanguineous families. Genome Biol. 2015; 16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shao Z.-Q., Zhang Y.-M., Feng X.-Y., Wang B., Chen J.-Q.. Synonymous codon ordering: a subtle but prevalent strategy of bacteria to improve translational efficiency. PLoS One. 2012; 7:e33547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tesina P., Lessen L.N., Buschauer R., Cheng J., Wu C.C.C., Berninghausen O., Buskirk A.R., Becker T., Beckmann R., Green R.. Molecular mechanism of translational stalling by inhibitory codon combinations and poly (A) tracts. EMBO J. 2020; 39:e103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gamble C.E., Brule C.E., Dean K.M., Fields S., Grayhack E.J.. Adjacent codons act in concert to modulate translation efficiency in yeast. Cell. 2016; 166:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boël G., Letso R., Neely H., Price W.N., Wong K.-H., Su M., Luff J.D., Valecha M., Everett J.K., Acton T.B.. Codon influence on protein expression in E. coli correlates with mRNA levels. Nature. 2016; 529:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Plotkin J.B., Robins H., Levine A.J.. Tissue-specific codon usage and the expression of human genes. PNAS. 2004; 101:12588–12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pop C., Rouskin S., Ingolia N.T., Han L., Phizicky E.M., Weissman J.S., Koller D. Causal signals between codon bias, mRNA structure, and the efficiency of translation and elongation. Mol. Syst. Biol. 2014; 10:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Qian W., Yang J.-R., Pearson N.M., Maclean C., Zhang J.. Balanced codon usage optimizes eukaryotic translational efficiency. PLoS Genet. 2012; 8:e1002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hia F., Yang S.F., Shichino Y., Yoshinaga M., Murakawa Y., Vandenbon A., Fukao A., Fujiwara T., Landthaler M., Natsume T.. Codon bias confers stability to human mRNA s. EMBO Rep. 2019; 20:e48220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Presnyak V., Alhusaini N., Chen Y.-H., Martin S., Morris N., Kline N., Olson S., Weinberg D., Baker K.E., Graveley B.R.. Codon optimality is a major determinant of mRNA stability. Cell. 2015; 160:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chamary J.-V., Hurst L.D.. Evidence for selection on synonymous mutations affecting stability of mRNA secondary structure in mammals. Genome Biol. 2005; 6:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Duan J., Wainwright M.S., Comeron J.M., Saitou N., Sanders A.R., Gelernter J., Gejman P.V.. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003; 12:205–216. [DOI] [PubMed] [Google Scholar]
- 95. Jacobs W.M., Shakhnovich E.I.. Evidence of evolutionary selection for cotranslational folding. Proc. Natl. Acad. Sci. U.S.A. 2017; 114:11434–11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kimchi-Sarfaty C., Oh J.M., Kim I.-W., Sauna Z.E., Calcagno A.M., Ambudkar S.V., Gottesman M.M.. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007; 315:525–528. [DOI] [PubMed] [Google Scholar]
- 97. Lorenz R., Wolfinger M.T., Tanzer A., Hofacker I.L.. Predicting RNA secondary structures from sequence and probing data. Methods. 2016; 103:86–98. [DOI] [PubMed] [Google Scholar]
- 98. Hughes A.L. Near-neutrality: the leading edge of the neutral theory of molecular evolution. Ann. N. Y. Acad. Sci. 2008; 1133:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Charlesworth B. The effects of deleterious mutations on evolution at linked sites. Genetics. 2012; 190:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Birney E., Soranzo N.. The end of the start for population sequencing. Nature. 2015; 526:52–53. [DOI] [PubMed] [Google Scholar]
- 101. Lawrie D.S., Messer P.W., Hershberg R., Petrov D.A.. Strong purifying selection at synonymous sites in D. melanogaster. PLoS Genet. 2013; 9:e1003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wall J.D., Tang L.F., Zerbe B., Kvale M.N., Kwok P.-Y., Schaefer C., Risch N.. Estimating genotype error rates from high-coverage next-generation sequence data. Genome Res. 2014; 24:1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B.et al.. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016; 536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bloom J.S., Boocock J., Treusch S., Sadhu M.J., Day L., Oates-Barker H., Kruglyak L.. Rare variants contribute disproportionately to quantitative trait variation in yeast. Elife. 2019; 8:e49212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Purcell S.M., Moran J.L., Fromer M., Ruderfer D., Solovieff N., Roussos P., O’dushlaine C., Chambert K., Bergen S.E., Kähler A.. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014; 506:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Teng S., Michonova-Alexova E., Alexov E.. Approaches and resources for prediction of the effects of non-synonymous single nucleotide polymorphism on protein function and interactions. Curr. Pharm. Biotechnol. 2008; 9:123–133. [DOI] [PubMed] [Google Scholar]
- 107. Genovese G., Fromer M., Stahl E.A., Ruderfer D.M., Chambert K., Landén M., Moran J.L., Purcell S.M., Sklar P., Sullivan P.F.. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat. Neurosci. 2016; 19:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bobbili D.R., Banda P., Krüger R., May P.. Excess of singleton loss-of-function variants in Parkinson's disease contributes to genetic risk. J. Med. Genet. 2020; 57:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang D., Cheng L., Qian Y., Alliey-Rodriguez N., Kelsoe J.R., Greenwood T., Nievergelt C., Barrett T.B., McKinney R., Schork N.. Singleton deletions throughout the genome increase risk of bipolar disorder. Mol. Psychiatry. 2009; 14:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cooper G.M., Shendure J.. Needles in stacks of needles: finding disease-causal variants in a wealth of genomic data. Nat. Rev. Genet. 2011; 12:628–640. [DOI] [PubMed] [Google Scholar]
- 111. Eilbeck K., Quinlan A., Yandell M.. Settling the score: variant prioritization and Mendelian disease. Nat. Rev. Genet. 2017; 18:599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. MacArthur D., Manolio T., Dimmock D., Rehm H., Shendure J., Abecasis G., Adams D., Altman R., Antonarakis S., Ashley E.. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014; 508:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Torkamani A., Wineinger N.E., Topol E.J.. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018; 19:581–590. [DOI] [PubMed] [Google Scholar]
- 114. Khera A.V., Chaffin M., Aragam K.G., Haas M.E., Roselli C., Choi S.H., Natarajan P., Lander E.S., Lubitz S.A., Ellinor P.T.. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018; 50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang Y., Bromberg Y.. Identifying mutation-driven changes in gene functionality that lead to venous thromboembolism. Hum. Mutat. 2019; 40:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Raimondi D., Simm J., Arany A., Fariselli P., Cleynen I., Moreau Y.. An interpretable low-complexity machine learning framework for robust exome-based in-silico diagnosis of Crohn's disease patients. NAR Genomics Bioinformatics. 2020; 2:lqaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Capra J.A., Stolzer M., Durand D., Pollard K.S.. How old is my gene. Trends Genet. 2013; 29:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Briscoe A.D., Gaur C., Kumar S.. The spectrum of human rhodopsin disease mutations through the lens of interspecific variation. Gene. 2004; 332:107–118. [DOI] [PubMed] [Google Scholar]
- 119. Waterston R.H., Pachter L.. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002; 420:520–562. [DOI] [PubMed] [Google Scholar]
- 120. Kondrashov A.S., Sunyaev S., Kondrashov F.A.. Dobzhansky–Muller incompatibilities in protein evolution. Proc. Natl. Acad. Sci. U.S.A. 2002; 99:14878–14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Subramanian S., Kumar S.. Evolutionary anatomies of positions and types of disease-associated and neutral amino acid mutations in the human genome. BMC Genomics. 2006; 7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li W., Manktelow E., von Kirchbach J.C., Gog J.R., Desselberger U., Lever A.M.. Genomic analysis of codon, sequence and structural conservation with selective biochemical-structure mapping reveals highly conserved and dynamic structures in rotavirus RNAs with potential cis-acting functions. Nucleic Acids Res. 2010; 38:7718–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cheng J., Nguyen T.Y.D., Cygan K.J., Çelik M.H., Fairbrother W.G., Gagneur J.. MMSplice: modular modeling improves the predictions of genetic variant effects on splicing. Genome Biol. 2019; 20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang G.-S., Cooper T.A.. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 2007; 8:749–761. [DOI] [PubMed] [Google Scholar]
- 125. Supek F., Miñana B., Valcárcel J., Gabaldón T., Lehner B.. Synonymous mutations frequently act as driver mutations in human cancers. Cell. 2014; 156:1324–1335. [DOI] [PubMed] [Google Scholar]
- 126. Weile J., Roth F.P.. Multiplexed assays of variant effects contribute to a growing genotype–phenotype atlas. Hum. Genet. 2018; 137:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E.. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015; 17:405–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pertea M., Lin X., Salzberg S.L.. GeneSplicer: a new computational method for splice site prediction. Nucleic Acids Res. 2001; 29:1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Reese M.G., Eeckman F.H., Kulp D., Haussler D. Improved splice site detection in Genie. J. Comput. Biol. 1997; 4:311–323. [DOI] [PubMed] [Google Scholar]
- 130. Comeron J.M., Kreitman M.. The correlation between synonymous and nonsynonymous substitutions in Drosophila: mutation, selection or relaxed constraints. Genetics. 1998; 150:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wyckoff G.J., Malcom C.M., Vallender E.J., Lahn B.T.. A highly unexpected strong correlation between fixation probability of nonsynonymous mutations and mutation rate. Trends Genet. 2005; 21:381–385. [DOI] [PubMed] [Google Scholar]
- 132. Li J., Zhang Z., Vang S., Yu J., Wong G.K., Wang J.. Correlation between Ka/Ks and Ks is related to substitution model and evolutionary lineage. J. Mol. Evol. 2009; 68:414–423. [DOI] [PubMed] [Google Scholar]
- 133. Hurst L.D., Pál C.. Evidence for purifying selection acting on silent sites in BRCA1. Trends Genet. 2001; 17:62–65. [DOI] [PubMed] [Google Scholar]
- 134. Orban T.I., Olah E.. Purifying selection on silent sites–a constraint from splicing regulation. Trends Genet. 2001; 17:252–253. [DOI] [PubMed] [Google Scholar]
- 135. Parmley J.L., Hurst L.D.. How common are intragene windows with K A>K S owing to purifying selection on synonymous mutations. J. Mol. Evol. 2007; 64:646–655. [DOI] [PubMed] [Google Scholar]
- 136. McVean G.A., Vieira J.. Inferring parameters of mutation, selection and demography from patterns of synonymous site evolution in Drosophila. Genetics. 2001; 157:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nielsen R., Bauer DuMont V.L., Hubisz M.J., Aquadro C.F.. Maximum likelihood estimation of ancestral codon usage bias parameters in Drosophila. Mol. Biol. Evol. 2007; 24:228–235. [DOI] [PubMed] [Google Scholar]
- 138. Zhou T., Gu W., Wilke C.O.. Detecting positive and purifying selection at synonymous sites in yeast and worm. Mol. Biol. Evol. 2010; 27:1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lu J., Wu C.-I.. Weak selection revealed by the whole-genome comparison of the X chromosome and autosomes of human and chimpanzee. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:4063–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hellmann I., Zöllner S., Enard W., Ebersberger I., Nickel B., Pääbo S.. Selection on human genes as revealed by comparisons to chimpanzee cDNA. Genome Res. 2003; 13:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Martin A.R., Kanai M., Kamatani Y., Okada Y., Neale B.M., Daly M.J.. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019; 51:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P.. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020; 581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sherry S.T., Ward M.-H., Kholodov M., Baker J., Phan L., Smigielski E.M., Sirotkin K.. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001; 29:308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bromberg Y., Kahn P.C., Rost B.. Neutral and weakly nonneutral sequence variants may define individuality. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
synVep webserver for online query: https://services.bromberglab.org/synvep; For local run, Python script (https://bitbucket.org/bromberglab/synvep_local) and prediction database (https://zenodo.org/record/4763256) are also available.