Abstract
This study demonstrates that significant reproducibility problems can occur during routine use of the Abbott Laboratories LCx assay for Chlamydia trachomatis and Neisseria gonorrhoeae. These problems can go undetected by the quality control procedures outlined in the manufacturer's package insert. We outline here procedures for detecting and preventing contamination and reproducibility problems.
The LCx assay for Chlamydia trachomatis and Neisseria gonorrhoeae (Abbott Laboratories, Abbott Park, Ill.) uses the ligase chain reaction amplification method for the detection of microbial DNA. This method has been demonstrated in numerous reports to have increased sensitivity compared to culture (2, 4, 8, 9). In this study, the reproducibility of the LCx assay was evaluated according to manufacturer's recommendations in a microbiology laboratory.
The Barnes-Jewish Hospital (BJH) microbiology laboratory performs ∼1,700 C. trachomatis and 1,700 N. gonorrhoeae tests each month. Samples are obtained from a variety of geographical locations, including the BJH emergency department, adult and adolescent obstetrics and gynecology clinics, private physicians' offices, and a juvenile detention hall. The majority of specimens are cervical or urethral swabs, with 8% urine specimens. All procedures were performed as outlined in the LCx manufacturer's package insert. In addition to the two Abbott negative control and two calibrator samples provided in the assay kit, positive controls derived from strains of C. trachomatis and N. gonorrhoeae were also prepared and processed as urine samples during each assay.
Abbott recommends that if a test result is in the equivocal range (0.8 to 0.99 sample/cutoff [S/CO] ratio for C. trachomatis and 0.8 to 1.2 S/CO ratio for N. gonorrhoeae) the LCx test should be repeated. If the repeat test S/CO ratio is ≥1.0, the result is considered positive, and if it is <1.0, it is reported as negative. For this study, we examined reproducibility within an expanded equivocal range (0.8 to 2.0 S/CO ratio for C. trachomatis and 0.8 to 3.0 S/CO ratio for N. gonorrhoeae) for a 5-month period. All repeat testing was performed within a 1-week period. Based on preliminary data, approximately 3.5 months after these changes were instituted, the procedure was modified so that all urine samples were tested in duplicate.
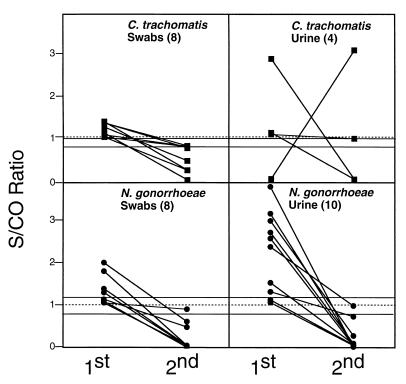
During the study period, 14,420 tests were performed (7,214 C. trachomatis tests and 7,206 N. gonorrhoeae tests). Of these, 181 specimens were tested again. Of the 181, 29 (16%) changed from an initial positive result to a negative result. One additional urine specimen initially tested negative for C. trachomatis but after a culture sample was found to be positive and was called to our attention by the physician, the LCx testing was repeated, and the result was positive. Thereafter, all urine samples were run in duplicate. The data in Fig. 1 illustrate that the magnitude of the reproducibility problem was greater in urine samples compared to swab samples. The mean difference between the first and second result was a 2.0 S/CO ratio for urine samples and a 0.88 S/CO ratio for swab samples. Only 5 (16%) of the 30 discrepant specimens would have been identified by Abbott's recommended equivocal ranges.
FIG. 1.
Difference between initial and repeat testing for C. trachomatis and N. gonorrhoeae using the Abbott LCx. The dotted line represents the cutoff for positive and negative results. The area between the solid lines represents the Abbott Laboratories recommended equivocal range.
Repeated testing of the 29 specimens with initially elevated results suggested that these samples were in fact negative and that contamination or procedural error(s) likely caused the initial result. This suspicion was corroborated when known negative samples, such as urine diluent, were processed as normal urine specimens and occasionally (13%) resulted in S/CO values of >1.00. It should be noted that, during this period, the Abbott negative control and calibrator samples were performing within acceptable limits. Furthermore, monthly monitoring of work surfaces was performed by wipe testing, as outlined in the LCx package insert. Environmental testing was performed after routine cleaning with 20% (vol/vol) sodium hypochlorite solution followed by 70% ethanol, and all counts were negative.
After repeated discussions with Abbott Laboratories and other laboratories performing the LCx assay, it became clear that these types of problems are not uncommon. We concluded that our reproducibility problems originated primarily in the sample processing area. Based on suggestions from Abbott Laboratories and other LCx users, the BJH microbiology laboratory underwent numerous changes in its specimen processing and has incorporated procedures that are not listed in the LCx package insert. The important changes are summarized in Table 1.
TABLE 1.
Recommended guidelines for detecting and preventing reproducibility problems during C. trachomatis and N. gonorrhoeae testing using the Abbott LCx
| Guideline category and step | Description |
|---|---|
| Detection | |
| 1 | Negative controls should be used, processed as samples on every run; sample rates of ≥100 are unacceptable |
| 2 | Utilize a modified equivocal range of 0.8 to 1.5 for C. trachomatis and 0.8 to 2.0 for N. gonorrhoeae; samples in this range should be repeated in duplicate, and results should be discussed with the physician |
| 3 | Monthly reproducibility checks should be done by repeating random samples |
| Prevention | |
| 1 | Change gloves frequently, especially between processing and amplification areas |
| 2 | Pipette tips should never enter any tube more than once (autopipettes can help prevent hand fatigue, e.g., Matrix Technologies Corporation, Hudson, N.H.) |
| 3 | Briefly microcentrifuge all vials before opening them |
| 4 | Utilize a microcentrifuge vial opener for all microcentrifuge tubes and use a separate one for urine samples and amplification vials; after use soak them in 20% (vol/vol) hypochlorite solution |
| 5 | For urine samples, make certain all urine is removed after centrifugation, even if no pellet is visible, but be careful not to dislodge pellet |
| 6 | Clean work area and pipettes frequently with sodium hypochlorite solution |
| 7 | Continuously monitor personnel to ensure proper technique and update training one or two times per year |
Since implementing these changes, the BJH laboratory has had a significant improvement in reproducibility (Table 2). In a 2-month period following implementation of the procedure changes, 7,728 LCx tests were performed (3,891 C. trachomatis and 3,837 N. gonorrhoeae tests). Of these, 39 swab samples were repeated, and 475 urine specimens were performed in duplicate (514 total). The initial result for 13 (2.5%) of the 514 specimens differed from the second result (C. trachomatis, 10 swabs and 1 urine; N. gonorrhoeae, 1 swab and 1 urine). Only 6 of the 13 (46%) discrepant specimens would have been identified by Abbott's recommended equivocal range, which supports the use of an expanded equivocal range. The mean difference between the first and second result was a 0.49 S/CO ratio for swab samples, which was significantly lower (P < 0.05) than before the changes, indicating an increase in precision. The frequency of the reproducibility problems in urine samples was greatly reduced compared to before the processing changes. Although a rare occurrence (2 in 475), processing urine specimens remains a problem. Repeated testing of the C. trachomatis urine specimen with widely discrepant values indicated that this specimen was clearly positive. The low value was likely due to the presence of residual urine inhibitors (1, 3, 5) or aspiration of the centrifuged pellet. Urine specimens should be closely monitored, including repeat testing of all samples if this is a low-volume specimen.
TABLE 2.
Summary of improved reproducibility following procedural changes for the Abbott LCx C. trachomatis and N. gonorrhoeae tests
| Parameter | Results:
|
|
|---|---|---|
| Before changes | After changes | |
| No. of samples | 14,420 | 7,728 |
| No. of repeats | 181 | 514a |
| No. of discrepant results/no. of repeats (%) | 30/181 (17) | 13/514 (2.5) |
| No. of discrepant results detected by the Abbott equivocal range/total no. of discrepant results (%) | 5/30 (17) | 6/13 (46) |
| Mean difference (S/CO ratio [n]) between pairs of results | ||
| Urine | 2.0 (14) | 1.9 (2) |
| Swabs | 0.88 (16) | 0.49 (11)b |
Includes 475 urine specimens that were tested in duplicate.
Differs significantly (P < 0.05) from values before changes.
The BJH laboratory has adopted a policy that a test yielding any pair of results that do not agree should be repeated a third time, and the results should be discussed with the physician. It should be noted that these samples are not routinely confirmed by an alternative amplification method. Therefore, it is impossible to ascertain the true positive or negative status of the specimens in this study.
The addition of these quality control procedures has serious efficiency and cost implications that must be taken into consideration when selecting this test procedure. The Abbott LCx carousel is capable of holding 24 reaction cells. It is promoted as being able to perform 20 tests per run, with two spaces for negative controls and two spaces for calibrators. The instrument requires that these four controls be performed, and it cannot calculate results without them. Additionally, Abbott suggests that a positive control sample be used, although this is not provided. Furthermore, we have found that the use of two negative control specimens, processed like patient specimens, has helped us detect low levels of contamination. Therefore, a total of seven controls and only 17 patient samples are tested on each run. This decreases efficiency and increases the cost of each patient result.
The Abbott LCx provides a highly sensitive method for the detection of C. trachomatis and N. gonorrhoeae. However, as with all amplification methods, the performance of the assay must be monitored carefully for contamination and reproducibility problems. These problems are not unique to the ligase chain reaction and have been reported for other amplification tests (6, 7). Until more automated procedures evolve, problems such as we have reported here should be anticipated for all amplification assays.
Acknowledgments
We thank all the BJH technologists and, in particular, Sharon Nauman for their hard work and patience during the period of this study. In addition we thank Pat Plier from Abbott Laboratories.
REFERENCES
- 1.Berg E S, Anestad G, Moi H, Storvold G, Skaug K. False-negative results of a ligase chain reaction assay to detect Chlamydia trachomatis due to inhibitors in urine. Eur J Microbiol Infect Dis. 1997;16:727–731. doi: 10.1007/BF01709252. [DOI] [PubMed] [Google Scholar]
- 2.Buimer M, Doornum G J J, Ching S, Peerbooms P G H, Plier P K, Ram D, Lee H H. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by ligase chain reaction-based assays with clinical specimens from various sites: implications for diagnostic testing and screening. J Clin Microbiol. 1996;34:2395–2400. doi: 10.1128/jcm.34.10.2395-2400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chernesky M A, Jang D, Sellors J, Luinstra K, Chong S, Castriciano S, Mahony J B. Urinary inhibitors of polymerase chain reaction and ligase chain reaction and testing of multiple specimens may contribute to lower assay sensitivities for diagnosing Chlamydia trachomatis-infected women. Mol Cell Probes. 1996;11:243–249. doi: 10.1006/mcpr.1997.0109. [DOI] [PubMed] [Google Scholar]
- 4.Kehl S C, Georgakas K, Swain G R, Sedmak G, Gradus S, Singh A, Foldy S. Evaluation of the Abbott LCx assay for detection of Neisseria gonorrhoeae in endocervical swab specimens from females. J Clin Microbiol. 1998;36:3549–3551. doi: 10.1128/jcm.36.12.3549-3551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahony J, Chong S, Jang D, Luinstra K, Faught M, Dalby D, Sellors J, Chernesky M. Urine specimens from pregnant and nonpregnant women inhibitory to amplification of Chlamydia trachomatis nucleic acid by PCR, ligase chain reaction, and transcription-mediated amplification: identification of urinary substances associated with inhibition and removal of inhibitory activity. J Clin Microbiol. 1998;36:3122–3126. doi: 10.1128/jcm.36.11.3122-3126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulcahy G M, Albanese E A, Bachl B L. Reproducibility of the Roche AMPLICOR polymerase chain reaction assay for detection of infection by Chlamydia trachomatis in endocervical specimens. Clin Chem. 1998;44:1575–1578. [PubMed] [Google Scholar]
- 7.Peterson E M, Darrow V, Blanding J, Aarnaes S, De La Maza L M. Reproducibility problems with the AMPLICOR PCR Chlamydia trachomatis test. J Clin Microbiol. 1997;35:957–959. doi: 10.1128/jcm.35.4.957-959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puolakkainen M, Hiltunen-Back E, Reunala T, Suhonen S, Lahteenmaki P, Lehtinen M, Paavonen J. Comparison of performances of two commercially available tests, a PCR assay and a ligase chain reaction test, in detection of urogenital Chlamydia trachomatis infection. J Clin Microbiol. 1998;36:1489–1493. doi: 10.1128/jcm.36.6.1489-1493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stary A, Ching S-F, Teodorowicz L, Lee H. Comparison of ligase chain reaction and culture for detection of Neisseria gonorrhoeae in genital and extragenital specimens. J Clin Microbiol. 1997;35:239–242. doi: 10.1128/jcm.35.1.239-242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]