Abstract
Immune thrombocytopenic purpura is a disorder characterized by decreased platelet count that may be secondary to infectious or autoimmune etiologies. We present a patient with upper gastrointestinal bleeding complicated by severe thrombocytopenia. Endoscopy revealed gastritis with pathology positive for Helicobacter pylori. Platelet count normalized after triple antibiotic therapy. The precise mechanism by which H. pylori causes immune thrombocytopenic purpura remains unclear; however, there are several plausible mechanisms. This case highlights the importance of keeping H. pylori in the differential in patients presenting with thrombocytopenia.
Keywords: Helicobacter pylori, immune thrombocytopenic purpura
Immune thrombocytopenic purpura (ITP) is a diagnosis of exclusion that is further divided into primary and secondary causes. Secondary causes of ITP include infections, autoimmune diseases such as systemic lupus erythematosus, antiphospholipid antibody syndrome, thyroid disorders, and hepatitis C. We present a patient with ITP found to have Helicobacter pylori gastritis and whose platelets normalized after treatment of H. pylori.
CASE PRESENTATION
A 28-year-old man with no significant past medical history presented to the hospital after three episodes of acute hematemesis. He denied melena/hematochezia, abdominal pain, heartburn, use of nonsteroidal antiinflammatory drugs, or alcohol use. During the previous year, he had gingival bleeding and more noticeable bruising. On admission, he was hemodynamically stable. He was awake, alert, and answered all questions appropriately. Dried blood was noted at his nares bilaterally. The cardiac, pulmonary, and abdominal examinations were unremarkable.
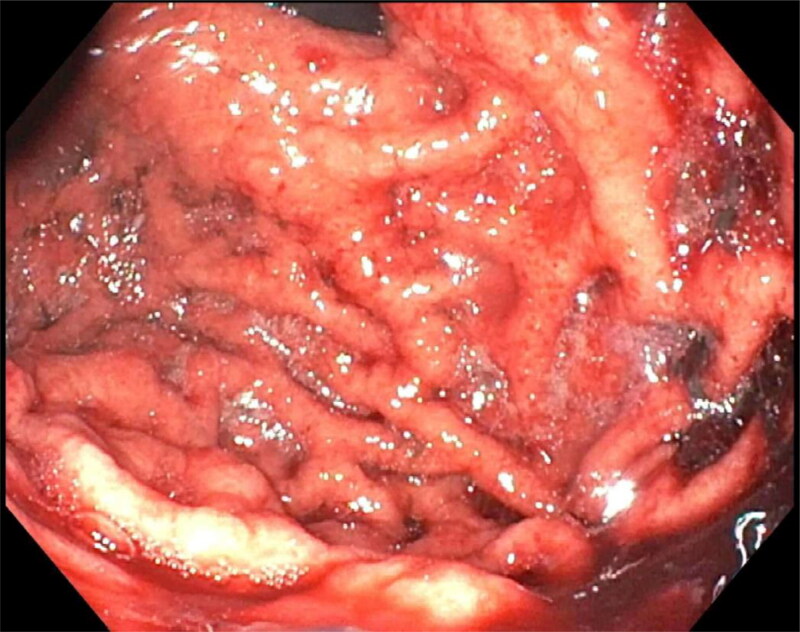
Initial laboratory results showed a platelet count of <3000/µL, leukocyte count of 14,500/µL, and hemoglobin of 11.3 g/dL, with a mean corpuscular volume of 82.8 fL. Lactate dehydrogenase, haptoglobin, iron, and ferritin were normal. An ADAMTS13 level was normal at 6 units/mL. The patient was diagnosed with ITP, given 10 mg of oral dexamethasone, and started on a pantoprazole drip for concern of an acute upper gastrointestinal bleed. His repeat platelet count improved to 7000/µL in 4 hours. Thereafter, he developed another episode of hematemesis with 500 mL of bright red blood. He was taken urgently for upper endoscopy, which demonstrated diffuse erosive gastritis (Figure 1). His bleeding resolved without further episodes of hematemesis. The pantoprazole drip was continued due to the severity of his thrombocytopenia, along with treatment of his ITP, which included intravenous immunoglobulin, with a 1 g/kg one-time dose and daily 40 mg dexamethasone orally for 4 days.
Figure 1.
Endoscopic view of gastric body concerning for diffuse erosive gastritis.
The patient was discharged in stable condition with platelets of 155,000/µL on oral pantoprazole. During his 1-week outpatient follow-up, he again had thrombocytopenia with a platelet count of 42,000/µL. Gastric biopsy results from the upper endoscopy came back positive for H. pylori. He was treated with amoxicillin, clarithromycin, and pantoprazole for 14 days. Due to ongoing thrombocytopenia, he was prescribed a 4-day course of dexamethasone. Subsequent platelet counts at 1 week (75,000/µL), 1 month (221,000/µL), 3 months (192,000/µL), and 6 months (251,000/µL) improved and ultimately normalized. A stool antigen test done 1 month after completing treatment confirmed eradication of H. pylori.
DISCUSSION
H. pylori is a urease-positive gram-negative organism associated with gastritis and peptic ulcer disease. H. pylori has also been described as a cause of secondary ITP since 1988, when eight ITP patients had documented improvement in platelet count after eradication of H. pylori.1 A recent literature review of 11 case studies between 2008 and 2018 showed platelet improvements after treating patients with concomitant H. pylori infection.2 However, the normalization of platelet counts following therapy appears to be variable, with reports of complete resolution to no response.3,4 The literature raises the question of whether disease duration or thrombocytopenia severity impacts platelet recovery.
The mechanism of action through which H. pylori infection may lead to ITP remains unclear. One proposed mechanism is that of molecular mimicry. The cytotoxin-associated gene A (CagA) is the fourth most common protein produced by H. pylori, with studies reporting an association between CagA and ITP.5–9 Furthermore, there are reports of a decrease in platelet-associated immunoglobulin G (PAIgG) levels in ITP patients who successfully responded to H. pylori treatment, and it is postulated that there is cross-reactivity between the two, possibly through immune complex formulations.10,11
Another rare reported cause of thrombocytopenia is proton pump inhibitor (PPI) use. However, all such implications come from case reports. A community-based retrospective study of 468 patients did not show an association between ITP and PPIs.12 It is standard clinical practice to use PPIs in the setting of gastrointestinal bleeding.13 Our patient was not on any PPIs prior to admission but was started on pantoprazole due to the acuity and severity of his bleeding. He was transitioned to oral pantoprazole after platelet counts stabilized.
Our patient also responded well to antibiotic therapy, and his platelet counts recovered completely. Although there was an initial response to steroids, his platelet count continued to drop, suggesting a secondary cause of the ITP. After H. pylori treatment, urease breath testing confirmed eradication. His platelet counts remained stable at that time without the need for further steroids, implicating H. pylori as the likely secondary cause of his ITP.
References
- 1.Gasbarrini A, Franceschi F, Tartaglione R, et al. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352(9131):878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 2.Vanegas YAM, Vishnu P.. Management of Helicobacter pylori in patients with immune thrombocytopenia. Hamostaseologie. 2019;39(3):279–283. doi: 10.1055/s-0039-1683974. [DOI] [PubMed] [Google Scholar]
- 3.Zain MA, Zafar F, Ashfaq A, et al. Helicobacter pylori: an underrated cause of immune thrombocytopenic purpura. A comprehensive review. Cureus. 2019;11(9):e5551. doi: 10.7759/cureus.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payandeh M, Raeisi D, Sohrabi N, et al. Poor platelet count response to Helicobacter pylori eradication in patients with severe idiopathic thrombocytopenic purpura. Int J Hematol Oncol Stem Cell Res. 2013;7(3):9–14. [PMC free article] [PubMed] [Google Scholar]
- 5.Jiménez-Soto LF, Haas R.. The CagA toxin of Helicobacter pylori: abundant production but relatively low amount translocated. Sci Rep. 2016;6:23227. doi: 10.1038/srep23227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama M, Kitadai Y, Ito M, et al. Immune response to CagA protein is associated with improved platelet count after Helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura. Helicobacter. 2007;12(1):36–42. doi: 10.1111/j.1523-5378.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 7.Rinaldi M, Perricone C, Ortega-Hernandez OD, et al. Immune thrombocytopaenic purpura: an autoimmune cross-link between infections and vaccines. Lupus. 2014;23(6):554–567. doi: 10.1177/0961203313499959. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YS, Kuang LP, Zhuang CL, et al. Effects of cytotoxin-associated gene A (CagA) positive Helicobacter pylori infection on anti-platelet glycoprotein antibody producing B cells in patients with primary idiopathic thrombocytopenic purpura (ITP). Pak J Med Sci. 2015;31(1):121–126. doi: 10.12669/pjms.311.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Yujiri T, Shinohara K, et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124(1):91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 10.Frydman GH, Davis N, Beck PL, et al. Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: a review and the role of biogeography. Helicobacter. 2015;20(4):239–251. doi: 10.1111/hel.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20(3):714–723. doi: 10.3748/wjg.v20.i3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotan E, Katz R, Bratcher J, et al. The prevalence of pantoprazole associated thrombocytopenia in a community hospital. Expert Opin Pharmacother. 2007;8(13):2025–2028. doi: 10.1517/14656566.8.13.2025. [DOI] [PubMed] [Google Scholar]
- 13.Laine L, Barkun AN, Saltzman JR, et al. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116(5):899–917. doi: 10.14309/ajg.0000000000001245. [DOI] [PubMed] [Google Scholar]